Abstract
This study described the prevalence of adverse pregnancy outcomes (APOs) in Chinese HIV-infected pregnant women, and examined the relationship between maternal HIV infection /HIV-related factors and APOs.
This prospective cohort study was carried out among 483 HIV-infected pregnant women and 966 HIV-uninfected pregnant women. The HIV-infected and HIV-uninfected women were enrolled from midwifery hospitals in Hunan province between October 2014 and September 2017. All data were extracted in a standard structured form, including maternal characteristics, HIV infection status, HIV-related factors and their pregnancy outcomes. APOs were assessed by maternal HIV infection status and HIV-related factors using logistic regression analysis.
The incidences of stillbirth (3.9% vs 1.1%), preterm birth (PTB) (8.9% vs 3.7%), low birth weight (LBW) (12.2% vs 3.1%) and small for gestational age (SGA) (21.3% vs 7.0%) were higher in HIV-infected women than HIV-uninfected women, with adjusted ORs of 2.77 (95%CI: 1.24–6.17), 2.37 (95%CI: 1.44–3.89), 4.20 (95%CI: 2.59–6.82) and 3.26 (95%CI: 3.26–4.64), respectively. No differences were found in neonatal asphyxia or birth defects between HIV-infected and HIV-uninfected groups, with adjusted ORs of 1.12 (95%CI: 0.37–3.43) and 1.10 (95%CI: 0.51–2.39), respectively. Among HIV-infected pregnant women, different antiretroviral (ARV) regimens were significantly associated with stillbirths, but not PTB, LBW or SGA. Compared with untreated HIV infection (10.1%), both mono/dual therapy and HAART were associated with a reduced risk of stillbirths (2.0% and 3.2%, respectively), with an AOR of 0.19 (95%CI: 0.04–0.92) and 0.31 (95%CI: 0.11–0.85), respectively. Initial time of ARV drugs use and HIV infection status of the sexual partner were not associated with maternal APOs.
The findings of this study indicated that maternal HIV infection was associated with significantly increased risks of stillbirth, PTB, LBW and SGA, but not neonatal asphyxia or birth defects. On the condition that most HIV-infected pregnant women started ARV therapy in or after the second trimester, both mono/dual therapy and HAART had a protective effect on stillbirth compared with untreated HIV infection. As some important confounders were not effectively controlled and the specific regimens of HAART were not analyzed, the above findings may have certain bias.
Keywords: adverse pregnancy outcome (APO), antiretroviral, human immunodeficiency virus (HIV), pregnant women
1. Introduction
Human immunodeficiency virus (HIV) infection is a severe public health problem in the world and seriously threatens the health and lives of women and children. In 2018, 37.9 million people were living with HIV worldwide, which consisted of 49.6% of women (15+ years old) and 4.5% of children (< 15 years old).[1] Globally, due to comprehensive interventions against mother-to-child transmission (MTCT), the number of new HIV infections among children under five age declined by 41% from 280,000 in 2010 to 160,000 in 2018.[2] In China, the Prevention of Mother-to-Child Transmission (PMTCT) of HIV program, supported by United Nation Children's Fund, expanded to 1638 counties/districts in 31 provinces in 2014, since the first pilot in Shangcai county in Henan province in 2001, and covered 8 counties in 5 provinces with relatively severe HIV epidemic in 2003.[3] In the past decades, the application of comprehensive interventions on pregnant women and their children led to substantial progress in the implementation of PMTCT in China, as the MTCT rate decreased from 31.8% prior to the program to 5.7% in 2016.[4,5] Especially, the MTCT rates were controlled below 5% in Chinese counties where HIV disease burden was relatively high and the PMTCT program was first implemented.[3]
The main consequences to fetal health following the maternal HIV infection in pregnant women are the vertical transmission of HIV and the potentially increased risks of adverse pregnancy outcomes (APOs), which seriously reduce the birth quality of infants. The widespread implementation of PMTCT has kept the MTCT rate of HIV relatively low, but the situation of APOs associated with maternal HIV infection is not optimistic. Recently, growing attention has been paid to the potentially increased risks of APOs. Many observational studies confirm that maternal HIV infection can increase the risks of stillbirths, neonatal death, low birth weight (LBW), preterm birth (PTB) and small for gestational age (SGA).[6–10] These APOs are also associated with other HIV-related factors, including progression stages of HIV, antiretroviral (ARV) therapy, CD4 cell count, viral load and injection drug use.[11–14] Because of the presence of virus and HIV-related factors, determining the impacts of maternal HIV infection on APOs is particularly challenging and thus, studies should consider the HIV-related factors.
However, the existing studies about the effects of maternal HIV infection on APOs have been mainly conducted in developed countries and Africa, where both medical technology and disease burden are significantly different from China. The PMTCT has been implemented in China for more than ten years, but most of the published Chinese studies focus mainly on the MTCT rate and rarely on the above APOs.[3,15] A few studies conducted in China lack representativeness and persuasiveness because of the small sample sizes, lack of HIV-uninfected control, defective design, and incompleteness of APOs.[16,17] Therefore, this study was aimed to describe the prevalence of APOs in Chinese HIV-infected pregnant women, and to examine the association between maternal HIV infection / HIV-related factors and APOs.
2. Materials and methods
2.1. Study design
This prospective cohort study was carried out among HIV-infected and HIV-uninfected pregnant women with a gestational age ≤28 weeks. The HIV-infected pregnant women were enrolled from midwifery hospitals in Hunan province between October 2014 and September 2017. The HIV-uninfected pregnant women were selected from the same midwifery hospitals by matching their ages and gestational ages with the HIV-infected pregnant women at a ratio of 2:1. All participants were followed up monthly from the first antenatal care visit to 42 days after delivery. To minimize the loss of follow-up, we adopted three methods, including outpatient follow-up by antenatal care providers (the main method), telephone visit and home visit (supplementary methods).
Pregnant women were excluded if any of the following conditions were met:
-
(1)
not living in the study area;
-
(2)
multiple pregnancies;
-
(3)
uncertain status of HIV infection;
-
(4)
miscarriage or elective termination of pregnancy;
-
(5)
no information for birth weight and/or gestational age of infants;
-
(6)
loss to follow-up before the delivery;
-
(7)
inability to provide informed consent.
2.2. HIV testing methods
Maternal HIV testing methods included antibody screening tests and confirmation tests. At first antenatal care, HIV antibodies on peripheral blood samples from pregnant women were screened using enzyme-linked immunosorbent assay (ELISA) and/or chemiluminescence immunoassay (CLIA). The positive results of HIV antibody screening were confirmed using western blot (WB). Details of HIV-related testing strategies for pregnant women were given in National AIDS Testing Technical Specification (2014 Revised Edition). According to HIV antibody test results, the pregnant women with a positive result were enrolled into the HIV-infected group, and those with a negative result were involved into the HIV-uninfected group. Pregnant women with uncertain HIV testing result were excluded from the study.
2.3. Treatment regimens for HIV-infected pregnant women and infants
The National Free AIDS Antiviral Therapy Handbook (Third Edition) recommended that all diagnosed HIV-infected pregnant women should receive ARV therapy, regardless of viral load or CD4 cell level. The 2011 National PMTCT Guidelines divided the use of ARV drugs for pregnant women into prophylactic and therapeutic ARV regimens according to the clinical progression stages of AIDS and/or immunologically. For prophylactic regimens, HIV-infected pregnant women were recommended to receive AZT (azidothymidine) +3TC (lamivudine) + LPV/RTV (lopinavir/ritonavir) or EFV (efavirenz) from the 14th week of gestation to the end of delivery. If HIV-infection was diagnosed at delivery, one dose of NVP (nevirapine) and AZT + 3TC was offered until seven days postpartum. For therapeutic regimens, AZT +3TC +EFV or NVP was recommended for pregnant women. The 2015 National PMTCT Guidelines eliminated such classification of ARV regimens, but recommended that pregnant women upon diagnosis of HIV-infection should be immediately given an ARV therapy of AZT + 3TC +LPV/RTV or TDF (tenofovir) + 3TC + EFV. Pregnant women already receiving ARV therapy before pregnancy were encouraged to maintain their ARV regimens, unless their virus inhibitory effect was unsatisfactory (e.g., the viral load was not less than the minimum limit of detection). HIV-exposed infants whose mothers received ARV therapy during pregnancy and at delivery or postpartum were offered one dose of NVP or AZT daily up to 6 and 12 weeks postpartum, respectively for prophylactic ARV therapy.
2.4. Data Collection
All data were extracted in a standard structured form, including maternal characteristics (age, residence, ethnicity, education level, occupation, gravidity, parity, hemoglobin, pregnant syphilis, HBV infection), HIV infection status, and pregnancy outcomes (mode of delivery, live births, Apgar score at 1 minute, birth defects, infant gender, gestational age at birth, birth weight). For HIV-infected pregnant women, HIV-related factors were also retrieved, such as ARV drugs use, initial time of ARV drugs use, ARV regimens, HIV infection status of sexual partner, CD4 cell count and HIV viral load in the third trimester. At the first antenatal care, syphilis rapid plasma reagin (RPR), treponema pallidum particle assay (TPPA) and hepatitis B surface antigen (HBsAg) tests were performed simultaneously with HIV antibody test. A peripheral blood sample from each pregnant woman before delivery was collected for hemoglobin, CD4 cell count and HIV viral load detection. After birth, every infant was examined by a well-trained pediatrician.
2.4.1. Definition of variables
Ethnicity was classified into two categories: Han and Minorities. Maternal severe anemia was defined as a hemoglobin level <60 g/L. A pregnant woman with positive results of both syphilis RPR and TPPA tests was diagnosed as pregnant syphilis. The positive result of HBsAg test was diagnosed as HBV infection. The initial time of ARV drugs use during pregnancy was classified into three categories, including the first, second and third trimesters, which were defined as 1–13, 14–27, and ≥28 weeks of gestation, respectively. The ARV regimens were divided into mono-therapy, dual-therapy and highly active antiretroviral therapy (HAART) based on the type of ARV drugs, in which HAART was a concomitant use of at least three ARV drugs. According to the degree of immune suppression in HIV-infected pregnant women, CD4 cell count was divided into four scales: <200, 200–349, 350–499, and ≥500 cells/mm3. HIV viral load, which represents the number of copies of the virus in the body and effectively reflects the effect of ARV therapy, was split into three categories: <150 (minimum limit of detection = 150), 150–1000, and ≥1000 copies/ml.
2.4.2. Definition of APOs
Gestation age referred to the duration of pregnancy from the first day of the last menstrual period (LMP) to birth, and ultrasound was used instead if LMP was uncertain. Stillbirth was defined as a fetal death occurring after 20 complete weeks of gestation. Birth < 37 gestational weeks was regarded as PTB, and birth weight <2500 g was considered as LBW. Birth weight <10th percentile was defined as SGA, adjusted for gestational age and gender using Chinese standards (enacted by Chinese neonatologists as a reference for Chinese population).[18] Apgar score referred to the summary of appearance, pulse, grimace, activity and respiration at 1 minute, and neonatal asphyxia was defined as Apgar score ≤7 at 1 minute. Birth defects were classified according to International Classification of Diseases (Tenth Revision).
2.5. Statistical analysis
Data were entered and cleaned on EpiData 3.1 (Jens M.Lauritsen, Michael Bruus and Mark Myatt, Odense, Denmark) and analyzed on SPSS 18.0 (IBM, Chicago, IL). The proportions of categorical variables were compared using the Chi-square test. APOs (stillbirth, neonatal asphyxia, birth defects, PTB, LBW and SGA) were compared between HIV-infected women and uninfected women using adjusted conditional logistic regression. The association between HIV-related factors and APOs was assessed via unconditional logistic regression. The strength of association was evaluated by using odds ratios (ORs) with 95% confidence interval (CI). The potential confounders identified with priori included maternal age, residence, ethnicity, education level, occupation, gravidity, parity, severe anemia, pregnant syphilis and HBV infection. Among HIV-infected women, the HIV-related factors were ARV drugs use, initial time of ARV drugs use, ARV regimens, and HIV infection status of sexual partner. Due to the severe data missing in CD4 cell count and viral load, the specific effects of these two parameters on APOs cannot be assessed. All statistical tests were two-tailed, and the significant level was P < .05.
2.6. Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Hunan Provincial Maternal and Child Health Care Hospital (No. 2015002). The study was conducted in accordance with the Declaration of Helsinki. Strict confidentiality was adhered to during data collection throughout the study. Written informed consents were obtained from all the pregnant women at their first antenatal care visit.
3. Results
Initially, 1750 pregnant women were enrolled between October 2014 and September 2017, including 1075 HIV-uninfected women, 582 HIV-infected women, and 93 not meeting the inclusion criteria (Fig. 1). Of the HIV-uninfected pregnant women, the response rate was 94.9% (1020/1075), and 54 were excluded due to some reasons. Of the HIV-infected pregnant women, the response rate was 91.6% (533/582), and 50 were excluded due to the same reasons. Finally, 1449 pregnant women were included in the final analysis, including 966 HIV-uninfected and 483 HIV-infected pregnant women.
Figure 1.
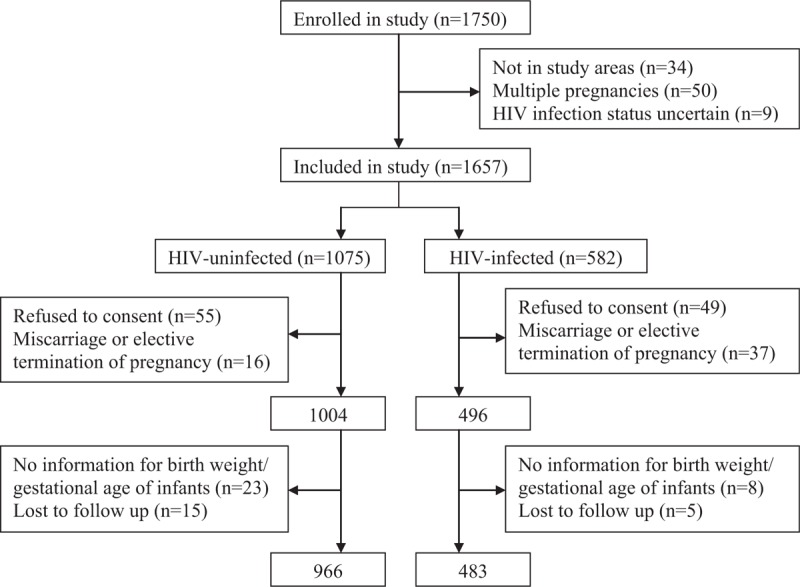
Flow chart of pregnant women selection process. HIV = human immunodeficiency virus.
3.1. Characteristics of participants
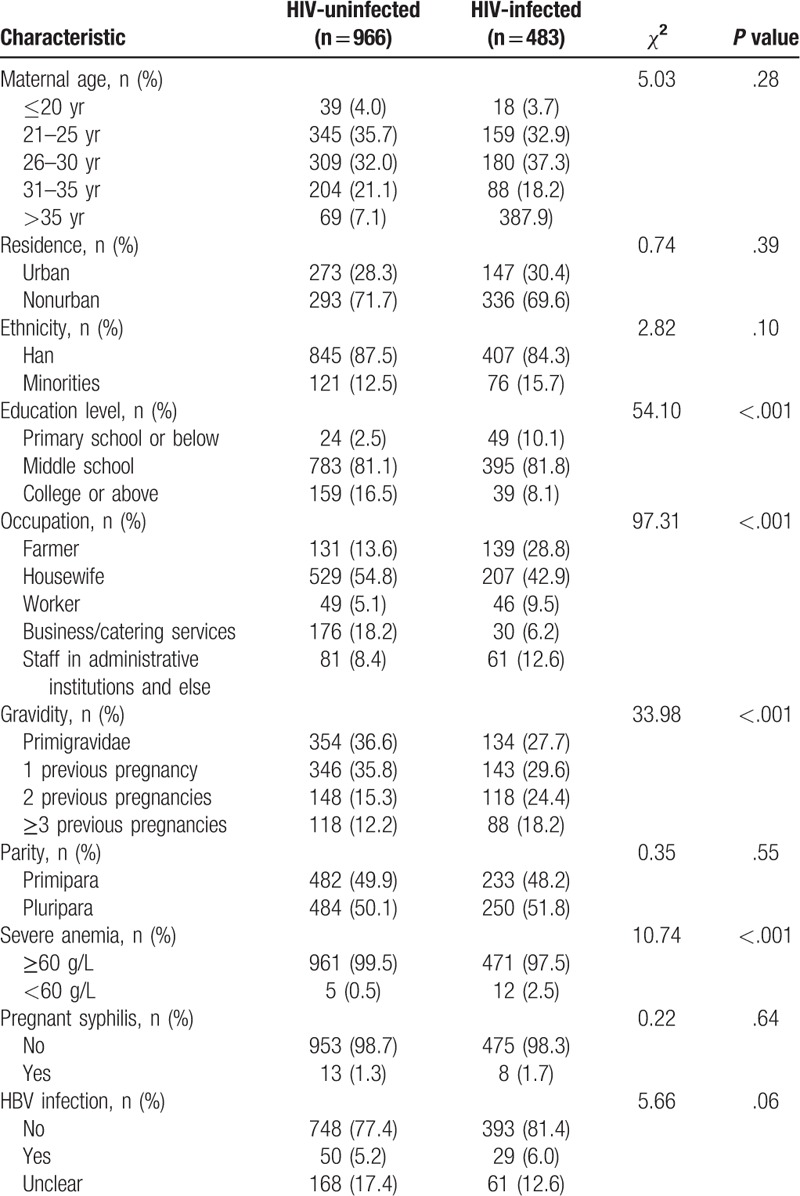
Comparison of maternal characteristics between HIV-uninfected and HIV-infected pregnant women is summarized in Table 1. Significant between-group differences were found in maternal education level, occupation, gravidity and severe anemia, but not in maternal age, residence, ethnicity, parity, pregnant syphilis or HBV infection.
Table 1.
Characteristics of HIV-uninfected and HIV-infected pregnant women.
3.2. HIV infection and APOs
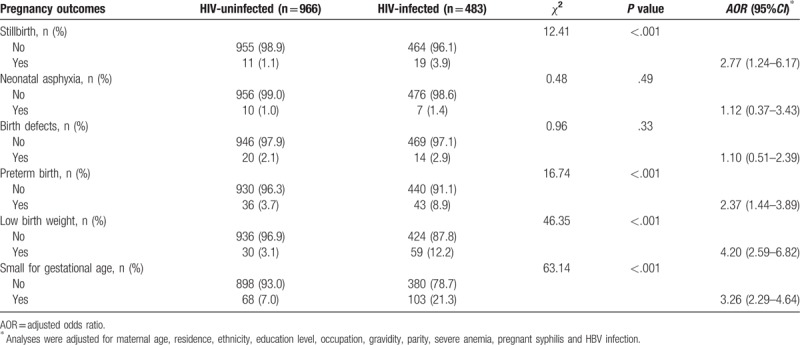
Table 2 shows the APOs for the HIV-uninfected and HIV-infected pregnant women. The infected group compared with the uninfected group was found with significantly more stillbirths (3.9% vs 1.1%), PTB (8.9% vs 3.7%), LBW (12.2% vs 3.1%) and SGA (21.3% vs 7.0%). The proportions of neonatal asphyxia and birth defects were similar between groups (1.4% vs1.0% and 2.9% vs 2.1%, respectively). After adjustment for the potential confounders, HIV infection was significantly associated with an increased risk of stillbirths (adjusted OR (AOR) 2.77, 95%CI: 1.24–6.17), PTB (AOR 2.37, 95%CI: 1.44–3.89), LBW (AOR 4.20, 95%CI: 2.59–6.82) and SGA (AOR 3.26, 95%CI: 2.29–4.64), but not with neonatal asphyxia (AOR 1.12, 95%CI: 0.37–3.43) or birth defects (AOR 1.10, 95%CI: 0.51–2.39).
Table 2.
Adverse pregnancy outcomes for the HIV-uninfected and HIV-infected pregnant women.
3.3. HIV-related factors in HIV-infected pregnant women
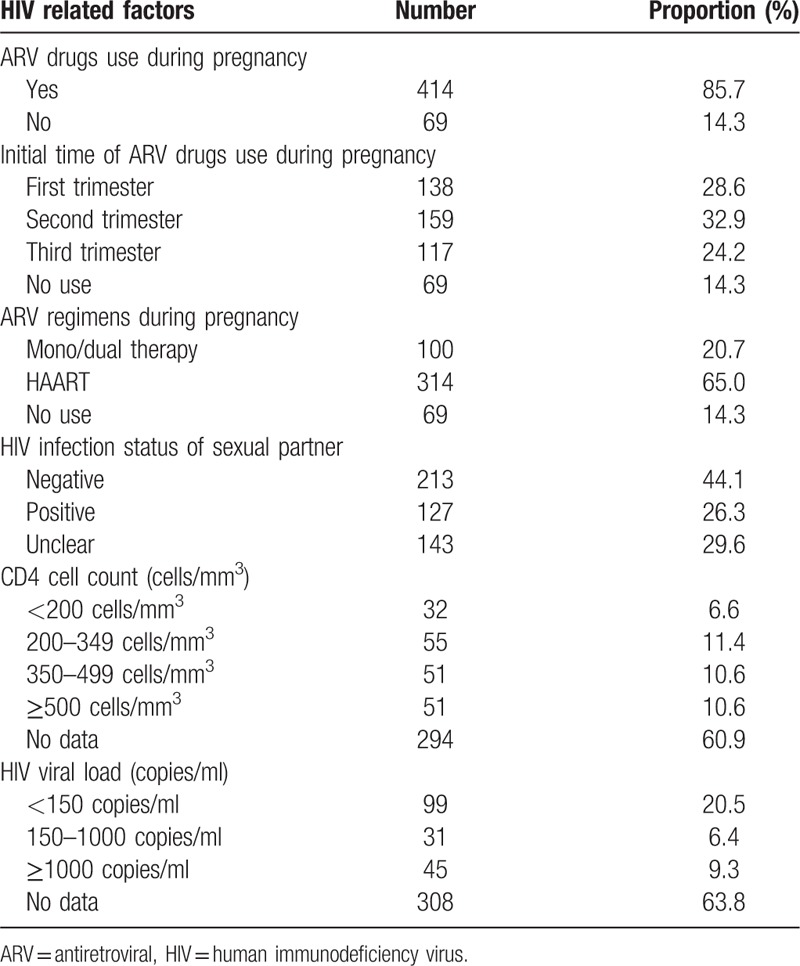
Table 3 shows the HIV-related factors in the 483 HIV-infected pregnant women. Of them, 69 (14.3%) reported no use of ARV drugs during pregnancy, 138 (28.6%) started to use ARV drugs in the first trimester, and 314 (65.0%) received HAART regimens. Among their sexual partners, 127 (26.3%) were diagnosed as HIV-positive. However, only 189 (39.1%) and 175 (36.2%) infected subjects received CD4 cell count testing and virus load testing before delivery, respectively.
Table 3.
HIV related factors of HIV-infected pregnant women (n = 483).
3.4. HIV-related factors and APOs
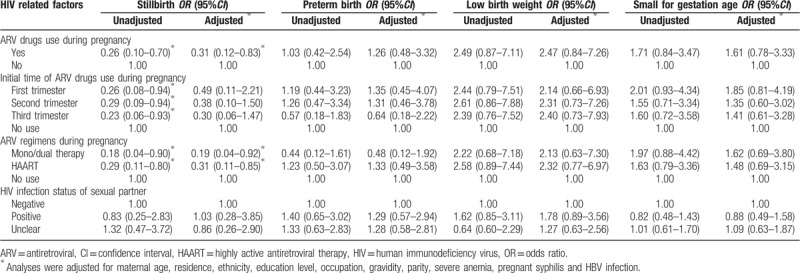
Table 4 shows the proportions of APOs in different HIV-related factors among HIV-infected pregnant women. Maternal ARV drugs use and regimens during pregnancy were significantly associated with stillbirths (Table 5). Compared with untreated HIV infection (10.1%), both mono/dual therapy and HAART were associated with a reduced risk of stillbirths (2.0% and 3.2%, respectively), with an AOR of 0.19 (95%CI: 0.04–0.92) and 0.31 (95%CI: 0.11–0.85), respectively. Initial time of ARV drugs use and HIV infection status of the sexual partner were not associated with maternal APOs.
Table 4.
Adverse pregnancy outcomes in different HIV related factors among HIV-infected women (n = 483).
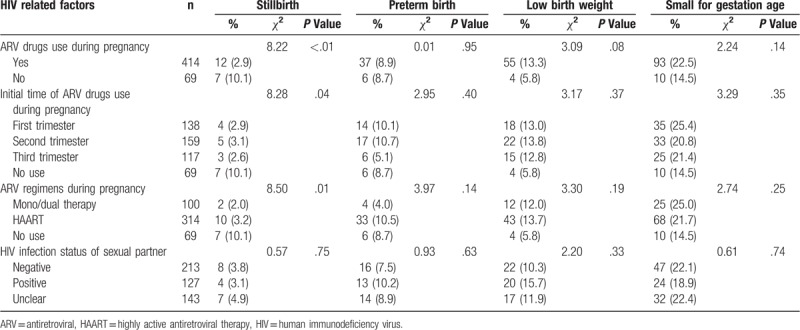
Table 5.
The ORs and 95% CI of HIV related factors on adverse pregnancy outcomes.
4. Discussion
Since the MTCT rates of HIV have dropped to relatively low levels in many countries with the worldwide implementation of PMTCT, studies focus more on the APOs of HIV-infected women. However, these studies about the effects of maternal HIV infection on APOs have been mainly conducted in developed countries and Africa.[6–9] Although a few studies describe a potential association between APOs and HIV infection in China, these studies are retrospective monitoring data analyses without involving HIV-uninfected pregnant women as controls or are cross-sectional studies conducted in ethnic minority areas.[16,17] To our knowledge, there is no prospective cohort study on the association between maternal HIV infection /ARV therapy and APOs in China. Our study fills this gap with midwifery hospital data from Hunan province and provides data for further research of APOs among HIV-infected women in China. Moreover, owing to the high follow-up rates of both groups, this study is not largely affected by follow-up bias.
Multivariate analyses showed HIV-infected pregnant women were more likely to have stillbirth, PTB, LBW and SGA compared to uninfected women, with AORs of 2.77, 2.37, 4.20, and 3.26, respectively; Some previous literatures show similar results.[6,7,10] A prospective cohort study from South Africa involving 1183 HIV-uninfected and 561 infected pregnant women concluded that the risk of stillbirth was doubled in HIV-infected women.[7] A recent meta-analysis of 52 cohort studies involving totally 200,896 (with PTB) and 15,538 (with LBW) HIV-infected women demonstrated that maternal HIV infection was significantly associated with both PTB and LBW, with pooled ORs of 1.56 and 1.64, respectively.[10] A observational study reported that maternal HIV infection was independently associated with SGA, but not PTB, in HIV-exposed infants.[6] One hypothesis is that these APOs may be placentally insufficient due to HIV infection-caused vascular damage or ARV-triggered endothelial injury, which may result in in-utero fetal hypoxia and nutrition deficiency.[19] The impaired immune function of pregnant women is likely another important reason for this relationship. Some studies indicate HIV-infected women are immunocompromised due to the destruction of CD4 cells, which in return makes them more vulnerable to urogenital tract infections that can lead to APOs.[9,20] In addition, the adverse effects of ARV drugs toxicity on pregnancy outcomes cannot be ignored. As reported, ARV therapy may increase the risk of APOs, including stillbirths, LBW, PTB, SGA, and congenital malformations.[11,19,21–24]
The present study showed that maternal HIV infection was not significantly related to neonatal asphyxia. On the contrast, HIV-infected women had higher risk of low Apgar score compared to HIV-uninfected women in Southwest China.[17] The inconsistency among these studies may be attributed to the differences in the threshold value of low Apgar score. Neonatal asphyxia was defined as Apgar score ≤7 at 1 minute in our study, but as low Apgar score <7 at 1, 5, and 10 minutes in Southwest China. In our study, the birth defects in both groups were mainly cardiovascular, craniofacial and musculoskeletal malformations. The prevalence of birth defects among HIV-infected women (2.9%) was not significantly higher compared with HIV-uninfected women and was similar to the level of the general Chinese population (2.5%).[25] Our results are consistent with 3 observational studies that HIV-infected women are not different from HIV-uninfected women in terms of risk for birth defects.[7,12,17]
The ARV therapy rate of HIV-infected pregnant women during pregnancy was 85.7% in our study, which was similar to the level in Tanzania (86.0%)[23] and lower than that in South Africa (98.4%).[8] Although the 2015 National PMTCT guidelines recommended that all HIV-infected pregnant women should initiate ARV therapy during pregnancy, a certain proportion of infected mothers still refused therapy due to psychosocial barriers and stigma for HIV infection and lack of family and social support,[26] and some even deliberately concealed their HIV infection status. All these behaviors delayed the HIV diagnosis and ARV therapy.
Although the beneficial effects of ARV therapy in preventing MTCT are indisputable, many of the published studies show conflicting results on the association between ARV exposure and APOs.[27–30] The present study showed that neither ARV regimens nor initial use time was significantly associated with PTB, LBW or SGA, which is consistent with Malaba's study from South African[27] and Delicio's study from Brazil.[28] In contrast to our findings, several studies suggest HAART can increase the risks of PTB, LBW and SGA.[11,12] And for the initial use time of ARV, a retrospective monitoring data analysis showed that HIV-infected pregnant women starting ARV therapy < 14 gestational weeks versus no treatment were more likely to have PTB, LBW and SGA.[31]
HIV-infected women receiving HAART either before or during pregnancy, compared to the AZT therapy, were prone to higher risks of stillbirth in Botswana.[12] Townsend from the United Kingdom reported similar results as in Botswana that the risk of stillbirth doubled in HAART compared with mono/dual therapy.[21] In our study, both mono/dual therapy and HAART would reduce the risk of stillbirth compared with untreated HIV infection, with AORs of 0.19 and 0.31, respectively. The effect of ARV therapy on stillbirth in our study was contrary to the findings of Botswana and United Kingdom, which may result from the different control groups, since the studies in Botswana and United Kingdom adopted HIV-infected AZT therapy and mono/dual therapy as control groups, respectively, and we used an HIV-infected ARV-unexposed control group. The reason why ARV therapy reduced the risk of stillbirth may be attributed to the therapy effect of ARV, which can control virus replication effectively and reduce the severe adverse effect of HIV infection on embryonic development. Given the diversity and complexity of ARV therapy for HIV infection, many studies focus on the effects of specific ARV regimens (e.g., TDF+FTC+EFV, PI-based regimens, NNRTI-based regimens) on APOs.[22,32,33] Due to the small sample size of HIV-infected pregnant women in our study, the above effects cannot be analyzed, but instead, crude numbers for ARV drugs (mono-therapy, dual-therapy and HAART) were adopted. Thus, larger-size studies are needed to evaluate the APOs among Chinese HIV-infected pregnant women after specific ARV regimens, especially the ARV regimens recommended by National PMTCT Guidelines, and to identify the safest ARV regimens for use by pregnant women during pregnancy.
Evaluation of APOs is complex and should consider maternal nutritional status, lifestyle behaviors (e.g., smoking, alcohol), injection drug, comorbidity, HIV infection, progression stages of HIV, and ARV drugs (e.g., type and initial time of ARV).[7,11–14] The APOs in HIV-infected pregnant women may result from a direct effect of the virus, or from some HIV-related factors (e.g., ARV drugs, immune suppression, viral load, injection drug), or from other non-HIV-related factors.[11,16,28] Studies have confirmed that higher CD4 cell count and lower HIV viral load were protective factors for PTB and LBW,[11,16] and maternal smoking exposure, alcohol, injection drug use, and comorbidity (e.g., gestational diabetes, hypertension, thyroid disorders) were associated with increased risk of APOs.[34–38] Although individual characteristics were controlled in multivariate analyses, the potential confounding cannot be completely ruled out due to the severe data missing in CD4 cell count and viral load, and ignorance of other important factors (e.g., progression stages of HIV, smoking, alcohol, injection drug, comorbidity). The severe data missing was attributed to two reasons: (1) the low compliance of HIV-infected women in ARV therapy and therapeutic effect monitoring; (2) the limitations of HIV laboratory resources and capability in Hunan. Few midwifery hospitals had the capability to detect CD4 cell count and HIV viral load. Especially, viral load detection most relied on provincial/municipal Center for Disease Control and Prevention with a relatively fixed detection time (once half a year), which obviously did not meet the detection requirements of HIV-infected pregnant women and made it difficult to detect the viral load before delivery.
Besides the above confounding bias, our study also had other limitations. First, during the 3 years of prospective observation in this study, the national PMTCT guidelines for ARV therapy had changed in type and time of initiation, and the effect of specific ARV drugs on APOs cannot be controlled instead of crude numbers for ARV drugs (mono/dual therapy or HAART). Second, we were unable to identify whether the abortions were spontaneous or elective due to defects in data collection, so we analyzed neither the rate of spontaneous abortions in the two groups nor the effect of HIV infection on spontaneous abortions. Third, this study only covered one province in China, whether our findings were generalizable to other parts of China needed to be confirmed by multi-center prospective cohort studies.
5. Conclusions
The findings of this study indicated that maternal HIV infection was associated with significantly increased risks of stillbirth, PTB, LBW and SGA, but not neonatal asphyxia or birth defects. On the condition that most HIV-infected pregnant women started ARV therapy in or after the second trimester, both mono/dual therapy and HAART had a protective effect on stillbirth compared with untreated HIV infection. As some important confounders were not effectively controlled and the specific regimens of HAART were not analyzed in the study, the above findings may have certain bias. Larger-size studies are needed to evaluate APOs among Chinese HIV-infected pregnant women on specific HAART regimens, recommended by National PMTCT Guidelines, and to identify the safest HAART regimens for use by pregnant women during pregnancy.
Acknowledgments
We would like to thank all the pregnant women who participated in the study as well as to all researchers from the midwifery hospitals in Hunan province.
Author contributions
Conceptualization: Guangwen Huang, Hua Wang, Huixia Li.
Data curation: Huixia Li, Jiahui Liu, Danfeng Tan.
Formal analysis: Huixia Li, Jianfei Zheng, Juan Xiao.
Funding acquisition: Qun Huang.
Investigation: Jiahui Liu, Danfeng Tan, Jianfei Zheng, Juan Xiao, Na Feng.
Methodology: Huixia Li, Na Feng, Guoqiang Zhang.
Supervision: Guangwen Huang, Hua Wang, Qun Huang.
Writing – original draft: Huixia Li.
Writing – review & editing: Guangwen Huang, Hua Wang, Jiahui Liu.
Footnotes
Abbreviations: AOR = adjusted odds ratio, APO = adverse pregnancy outcomes, ARV = antiretroviral, CI = confidence interval, HAART = highly active antiretroviral therapy, HIV = human immunodeficiency virus, LBW = low birth weight, MTCT = mother-to-child transmission, PTB = preterm birth, SGA = small for gestational age.
How to cite this article: Li H, Liu J, Tan D, Huang G, Zheng J, Xiao J, HuaWang, Huang Q, Feng N, Zhang G. Maternal HIV infection and risk of adverse pregnancy outcomes in Hunan province, China: A prospective cohort study. Medicine. 2020;99:8(e19213).
This study was supported by the National Center for Women and Children's Health, China CDC (grant number 2015FYH016).
The authors report no conflicts of interest.
References
- [1].UNAIDS. UNAIDS data 2019. Available at: https://www.unaids.org/sites/default/files/media _asset/2019-UNAIDS-data_en.pdf [access date July 31, 2019]. [Google Scholar]
- [2].UNICEF. Elimination of mother-to-child transmission. Available at: https://data.unicef.org/topic/hivaids/emtct/ (access date July 31, 2019). [Google Scholar]
- [3].Wang Q, Wang L, Fang L, et al. Timely antiretroviral prophylaxis during pregnancy effectively reduces HIV mother-to-child transmission in eight counties in China: a prospective study during 2004-2011. Sci Rep 2016;6:34526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zeng H, Chow EP, Zhao Y, et al. Prevention of mother-to-child HIV transmission cascade in China: a systematic review and meta-analysis. Sex Transm Infect 2016;92:116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang L. Prevention of mother-to-child HIV transmission. second edBeijing: People's Medical Publishing House; 2018. [Google Scholar]
- [6].Ndirangu J, Newell ML, Bland RM, et al. Maternal HIV infection associated with small-for-gestational age infants but not preterm births: evidence from rural South Africa. Hum Reprod 2012;27:1846–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].González R, Rupérez M, Sevene E, et al. Effects of HIV infection on maternal and neonatal health in southern Mozambique: a prospective cohort study after a decade of antiretroviral drugs roll out. PLoS One 2017;12:e0178134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Santosa WB, Staines-Urias E, Tshivuila-Matala COO, et al. Perinatal outcomes associated with maternal HIV and antiretroviral therapy in pregnancies with accurate gestational age in South Africa. AIDS 2019;33:1623–33. [DOI] [PubMed] [Google Scholar]
- [9].Arab K, Spence AR, Czuzoj-Shulman N, et al. Pregnancy outcomes in HIV-positive women: a retrospective cohort study. Arch Gynecol Obstet 2017;295:599–606. [DOI] [PubMed] [Google Scholar]
- [10].Xiao PL, Zhou YB, Chen Y, et al. Association between maternal HIV infection and low birth weight and prematurity: a meta-analysis of cohort studies. BMC Pregnancy Childbirth 2015;15:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].van der Merwe K, Hoffman R, Black V, et al. Birth outcomes in South African women receiving highly active antiretroviral therapy: a retrospective observational study. J Int AIDS Soc 2011;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis 2012;206:1695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kreitchmann R, Li SX, Melo VH, et al. Predictors of adverse pregnancy outcomes in women infected with HIV in Latin America and the Caribbean: a cohort study. BJOG 2014;121:1501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Aguilar-Zapata D, Piñeirúa-Menéndez A, Volkow-Fernández P, et al. Sociodemographic differences among HIV-positive and HIV-negative recently pregnant women in Mexico City: a case-control study. Medicine (Baltimore) 2017;96:e7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li B, Zhao Q, Zhang X, et al. Effectiveness of a prevention of mother-to-child HIV transmissionprogram in Guangdong province from 2007 to 2010. BMC Public Health 2013;13:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yu L, Li WY, Chen RY, et al. Pregnancy outcomes and risk factors for low birth weight and preterm delivery among HIV-infected pregnant women in Guangxi, China. Chin Med J (Engl) 2012;125:403–9. [PubMed] [Google Scholar]
- [17].Yang M, Wang Y, Chen Y, et al. Impact of maternal HIV infection on pregnancy outcomes in southwestern China - a hospital registry based study. Epidemiol Infect 2019;147:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhu L, Zhang R, Zhang S, et al. Chinese neonatal birth weight curve for different gestational age. Zhonghua Er Ke Za Zhi 2015;53:97–103. [Article in Chinese]. [PubMed] [Google Scholar]
- [19].Snijdewind IJM, Smit C, Godfried MH, et al. Preconception use of cART by HIV-positive pregnant women increases the risk of infants being born small for gestational age. PLoS One 2018;13:e0191389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sebitloane HM, Moodley J. Maternal and obstetric complications among HIV-infected women treated with highly active antiretroviral treatment at a Regional Hospital in Durban, South Africa. Niger J Clin Pract 2017;20:1360–7. [DOI] [PubMed] [Google Scholar]
- [21].Townsend CL, Cortina-Borja M, Peckham CS, et al. Antiretroviral therapy and premature delivery in diagnosed HIV-infected women in the United Kingdom and Ireland. AIDS 2007;21:1019–26. [DOI] [PubMed] [Google Scholar]
- [22].Grosch-Woerner I, Puch K, Maier RF, et al. Increased rate of prematurity associated with antenatal antiretroviral therapy in a German/Austrian cohort of HIV-1-infected women. HIV Med 2008;9:6–13. [DOI] [PubMed] [Google Scholar]
- [23].Li N, Sando MM, Spiegelman D, et al. Antiretroviral therapy in relation to birth outcomes among HIV-infected women: a cohort study. J Infect Dis 2016;213:1057–64. [DOI] [PubMed] [Google Scholar]
- [24].Mehta UC, van Schalkwyk C, Naidoo P, et al. Birth outcomes following antiretroviral exposure during pregnancy: initial results from a pregnancy exposure registry in South Africa. South Afr J HIV Med 2019;20:971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen J, Huang X, Wang B, et al. Epidemiology of birth defects based on surveillance data from 2011-2015 in Guangxi, China: comparison across five major ethnic groups. BMC Public Health 2018;18:1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Geter A, Sutton MY, Hubbard McCree D. Social and structural determinants of HIV treatment and care among black women living with HIV infection: a systematic review: 2005-2016. AIDS Care 2018;28:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Malaba TR, Phillips T, Le Roux S, et al. Antiretroviral therapy use during pregnancy and adverse birth outcomes in South African women. Int J Epidemiol 2017;46:1678–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Delicio AM, Lajos GJ, Amaral E, et al. Adverse effects in children exposed to maternal HIV and antiretroviral therapy during pregnancyin Brazil: a cohort study. Reprod Health 2018;15:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Uthman OA, Nachega JB, Anderson J, et al. Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: a systematic review and meta-analysis. Lancet HIV 2017;4:e21–30. [DOI] [PubMed] [Google Scholar]
- [30].Ejigu Y, Magnus JH, Sundby J, et al. Health outcomes of asymptomatic HIV-infected pregnant women initiating antiretroviral therapyat different baseline CD4 counts in Ethiopia. Int J Infect Dis 2019;82:89–95. [DOI] [PubMed] [Google Scholar]
- [31].Li HX, Zheng JF, Huang GW, et al. Prevalence and associated risk factors on preterm birth, low birth weight, and small for gestational age among HIV-infected pregnant women in Hunan province, 2011-2017. Zhonghua Liu Xing Bing Xue Za Zhi 2018;39:1368–74. [Article in Chinese]. [DOI] [PubMed] [Google Scholar]
- [32].Zash R, Souda S, Chen JY, et al. Reassuring birth outcomes with tenofovir/emtricitabine/efavirenz used for prevention of mother-to-child transmission of HIV in Botswana. J Acquir Immune Defic Syndr 2016;71:428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zash R, Jacobson DL, Diseko M, et al. Comparative safety of antiretroviral treatment regimens in pregnancy. JAMA Pediatr 2017;171:e172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang A, Wang X, Dou L, et al. Incidence of and related risk factors on preterm delivery among HIV-infected pregnant women in China. Zhonghua Liu Xing Bing Xue Za Zhi 2015;36:349–53. [Article in Chinese]. [PubMed] [Google Scholar]
- [35].Smith LK, Draper ES, Evans TA, et al. Associations between late and moderately preterm birth and smoking, alcohol, drug use and diet: a population-based case-cohort study. Arch Dis Child Fetal Neonatal Ed 2015;100:F486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nzelu D, Dumitrascu-Biris D, Nicolaides KH, et al. Chronic hypertension: first-trimester blood pressure control and likelihood of severe hypertension, preeclampsia, and small for gestational age. Am J Obstet Gynecol 2018;218:337.e331–337.e337. [DOI] [PubMed] [Google Scholar]
- [37].Aviram A, Guy L, Ashwal E, et al. Pregnancy outcome in pregnancies complicated with gestational diabetes mellitus and late preterm birth. Diabetes Res Clin Pract 2016;113:198–203. [DOI] [PubMed] [Google Scholar]
- [38].Andersen SL, Olsen J, Wu CS, et al. Low birth weight in children born to mothers with hyperthyroidism and high birth weight in hypothyroidism, whereas preterm birth is common in both conditions: a Danish national hospital register study. Eur Thyroid J 2013;2:135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]


