Abstract
Achieving the Joint United Nations Program on human immunodeficiency virus (HIV)/AIDS Fast-Track targets requires additional strategies for mobile populations. We examined trends and socio-demographics of migrants (overseas-born) and Australian-born individuals presenting with late and advanced HIV diagnoses between 2008 and 2017 to help inform public health approaches for HIV testing coverage and linkage to care and treatment.
We conducted a retrospective population-level observational study of individuals diagnosed with HIV in Australia and reported to the National HIV Registry. Annual proportional trends in late (CD4+ T-cell count <350 cells/μL) and advanced (CD4+ T-cell count <200 cells/μL). HIV diagnoses were determined using Poisson regression.
Of 9926 new HIV diagnoses from 2008 to 2017, 84% (n = 8340) were included in analysis. Overall, 39% (n = 3267) of diagnoses were classified as late; 52% (n = 1688) of late diagnoses were advanced. Of 3317 diagnoses among migrants, 47% were late, versus 34% of Australian-born diagnoses (P < .001).
The annual proportions of late (incidence rate ratio [IRR] 1.00; 95% confidence interval [CI] 0.99–1.01) and advanced HIV diagnoses (IRR 1.01; 95% CI 0.99–1.02) remained constant. Among migrants with late HIV diagnosis, the proportion reporting male-to-male sex exposure (IRR 1.05; 95% CI 1.03–1.08), non-English speaking (IRR 1.03; 95% CI 1.01–1.05), and individuals born in countries in low HIV-prevalence (IRR 1.02; 95% CI 1.00–1.04) increased. However, declines were noted among some migrants’ categories such as females, heterosexual exposure, English speaking, and those born in high HIV-prevalence countries.
Late HIV diagnosis remains a significant public health concern in Australia. Small declines in late diagnosis among some migrant categories are offset by increases among male-to-male exposures. Reaching the Fast-Track targets in Australia will require targeted testing and linkage to care strategies for all migrant populations, especially men who have sex with men.
Keywords: advanced diagnosis, HIV, late diagnosis, migrants
1. Introduction
Achieving the Joint United Nations Program on human immunodeficiency virus (HIV)/AIDS (UNAIDS) Fast-Track targets is projected to reduce HIV incidence and mortality, but requires equitable access to HIV testing and treatment services for all population groups.[1,2] Progress made to reach these targets has often not been uniform across population groups.[3] Late HIV diagnosis (CD4+ T-cell count <350 cells/μL), especially among society's most vulnerable, including migrant populations, remains a challenge.[4] Investigating the patterns of late and advanced (CD4+ T-cell count <200 cells/μL) HIV diagnosis in migrants is important to identify gaps in HIV testing and treatment service coverage that inhibit overall HIV prevention and care performance.
Migration involves movement of people across international borders and immigrants are non-nationals who move into a country often for settlement purposes.[5] Patterns and trends in global migration have changed significantly in recent decades, with the number of international migrants increasing to 258 million in 2017.[6] This level of migration has important implications for HIV prevention and treatment strategies. Migrant populations face barriers to accessing health care due to irregular migration (illegal stay), lack of migrant-inclusive health policies, exclusion from government-funded subsidies, cost, and difficulties in navigating health systems.[5,7] Other barriers are stigma and lack of culturally appropriate service options.[8–10] In countries with “universal” health coverage, migrants may not be in a position to prioritize their health and may be unaware of available health services or even excluded from accessing health care.[7,8,11] In Australia, migrants commonly face many of these barriers, reducing their access to timely HIV testing and treatment.[12]
In 2017, 7.3 million (29%) of Australia's estimated resident population were born overseas (migrants), an increase from 5 million (25%) in 2006.[13] Australian citizens and permanent residents are covered by Medicare (the national health insurance scheme), which allows free or subsidized access to general health care. Medicare coverage extends to people from countries with a reciprocal health care agreement (RHCA) with Australia (Belgium, Finland, Italy, Malta, Netherlands, New Zealand, Norway, Ireland, Slovenia, Sweden, and the United Kingdom). However, depending on their classification, many other migrants require private health insurance or must pay the full cost to access health care. Migrants may be eligible for compassionate programs to access HIV care or private health insurance which may require reimbursable upfront payments. Additionally, persons living in rural or remote areas of Australia often have poor health access compared to those in metropolitan areas irrespective of migration status.[14] Lack of health care access is part of the multifactorial reasons that may lead to individuals seeking HIV testing or care late. Delayed access to HIV health care can result in 2 main negative consequences: first, the individual receives delayed diagnosis that affects their health outcomes, and second, the likelihood of onward HIV transmission increases.[15] Recent modeling has indicated more than half of new HIV transmissions in Australia are attributed to undiagnosed HIV, and this proportion has increased over time.[16]
Routine reports present the proportion with late diagnoses over time, but this could mask emerging trends in subpopulations. Annual surveillance reports suggest changing patterns of HIV transmission and risk in Australia. While approximately 65% of HIV diagnoses in Australia were attributed to sex between men in 2017, some jurisdictions are reporting declines in diagnoses among gay and bisexual men who have sex with other men (MSM) but an increase in heterosexual transmissions.[17] In addition, declines in HIV diagnoses and incidence being observed among Australian-born MSM are being offset by increases in diagnoses among MSM born overseas particularly Southeast Asia, Northeast Asia, and Southern and Central Asia.[18,19] Previous research has shown that late HIV diagnosis is common among migrants from high adult HIV-prevalence countries while more recent data from clinics with high caseloads of migrant MSM point to low testing coverage and potentially high rates of undiagnosed HIV in this population group.[20–23] Given the shifting HIV epidemiology, it is becoming increasingly important to monitor late and advanced HIV diagnosis more closely among migrants to help inform responses to the epidemic. This paper examines recent trends in late and advanced HIV diagnosis among a range of migrant subgroups compared to Australian-born people to help inform public health approaches designed to increase HIV testing coverage and linkage to treatment.
2. Methods
We conducted a retrospective population-level observational study of individuals diagnosed with HIV in Australia between January 1st, 2008 and December 31st, 2017.
2.1. Study population and data source
We analyzed Australian HIV notification data as reported to the National HIV Registry at The Kirby Institute at the University of New South Wales (Sydney, Australia), for the period 2008 to 2017 with a focus on identifying changes in trends in late and advanced HIV diagnosed in subgroups of migrants. We analyzed HIV notifications among migrants and Australian-born individuals. People with missing CD4+ T-cell count, sex, and country of birth were excluded from the analysis.
In Australia, HIV is a notifiable disease and recording of patient data is mandatory under jurisdictional and federal legislation. Under procedures described previously, all new diagnoses must be reported to state and territory health authorities by laboratories and/or physicians.[24] State and territory authorities collect enhanced surveillance information for each case and reports this to the National HIV Registry, which is maintained by the Kirby Institute at University of New South Wales, Sydney, on behalf of the Australian Government Department of Health.[25]
2.2. Data collection
Data collected for each HIV notification included year of diagnosis, age at diagnosis, sex, country and country/region of birth, main language spoken at home, area of residence, CD4+ T-cell count at diagnosis or within 3 months, mode of HIV exposure category/likely route of HIV transmission, and jurisdiction of residence at the time of diagnosis.
Using the World Health Organization classification for case definitions and clinical staging of HIV and a later HIV consensus definition for late presentations, we classified HIV notifications with a CD4+ T-cell count <350 cells/μL as late HIV diagnosis and those with CD4+ T-cell count <200 cell/μL as advanced HIV diagnosis (a subset of late diagnoses).[26,27] We did not consider a past HIV testing history when defining late or advanced diagnosis, as this information is unique to Australia and we wanted to be consistent with international classifications.
Country of birth is self-reported to the diagnosing clinician; we used this variable to classify individuals diagnosed with HIV as migrants (born in countries other than Australia) or Australian-born. Based on country of birth, individuals were grouped into region of birth based on the Australian Bureau of Statistics’ (ABS) classification.[13]
Mode of exposure to HIV is self-reported to the diagnosing clinician and classified as male-to-male sex only which occurs in MSM, injecting drug use (IDU) only, male-to-male sex with dual risk of IDU, heterosexual sex only, and other (eg, mother-to-child transmission, direct blood/tissue exposure, and iatrogenic exposure). We collapsed HIV exposure into 3 categories – male-to-male sex only, heterosexual only, and other (all other exposures).
Area of residence in Australia is classified as major cities, inner regional, outer regional, remote, and very remote as per ABS classification. We created a binary variable to classify area of residence as major cities or regional/remote. Main language spoken at home is self-reported to the clinician and categorized as English or otherwise. Country of birth was also used to determine if the individual was from a country with a RHCA with Australia. Temporary migrants eligible for RHCA have similar access to health care as people born in Australia, so we dichotomized countries as having or not having an RHCA with Australia. We also determined if the country of birth had high (≥1%) or low HIV prevalence (<1%) based on 2017 UNAIDS data.[28,29] In this study, the following were high HIV prevalence countries: Angola, Barbados, Botswana, Burundi, Congo, Republic of Ghana, Guinea, Guyana, Jamaica, Kenya, Liberia, Malawi, Mozambique, Russia Federation, Rwanda, Sierra Leone, South Africa, Sudan, Thailand, Togo, Uganda, Tanzania, Zambia, and Zimbabwe.
2.3. Statistical analysis
Descriptive analysis was conducted to describe new HIV diagnoses between 2008 and 2017 stratified by migration status, sex, age, area of residence and region of birth. We calculated the proportion of HIV cases with CD4+ T-cell ≥350, 200 to 349, <350 (late HIV diagnosis), and <200 cells/ μL (advanced HIV diagnosis), and determined proportions of migrants and Australian-born that presented with late and advanced HIV. Binary variables were compared using Chi-squared tests.
Annual proportions of late and advanced HIV diagnoses were compared by migration status, sex, HIV exposure category, RHCA with Australia, area of residence, main language spoken at home, and high/low HIV-prevalence countries.
Annual trends of proportions of late and advanced HIV diagnosis were assessed using Poisson regression models. Poisson regression is a type of generalized linear models and an alternative analysis approach to quantify time trends of relatively rare discrete outcomes and is a useful tool for program evaluation.[30] Data modeled in the Poisson regression were checked for over-dispersion. Time (year) was treated as an independent covariate. Incidence rate ratios (IRR), confidence intervals (CI), and P-values were used to describe the annual trends. The IRR represents the average annual incidence ratio. Proportions of late and advanced HIV diagnoses among migrants and non-migrants of new infections were computed. We used IRR to compare trends in migrants versus Australian-born with late diagnosis, sex, and HIV exposure category.
All analyses were performed using Stata IC 14 (Stata Corp, College Station, TX), with the significance level set at P < .05.
The Alfred Ethics Committee (Project No. 207/17) and the Monash University Human Ethics Committee (Project No. 8987) approved the study. The Communicable Diseases Network Australia gave approval to access non-identifiable National HIV Registry data.
3. Results
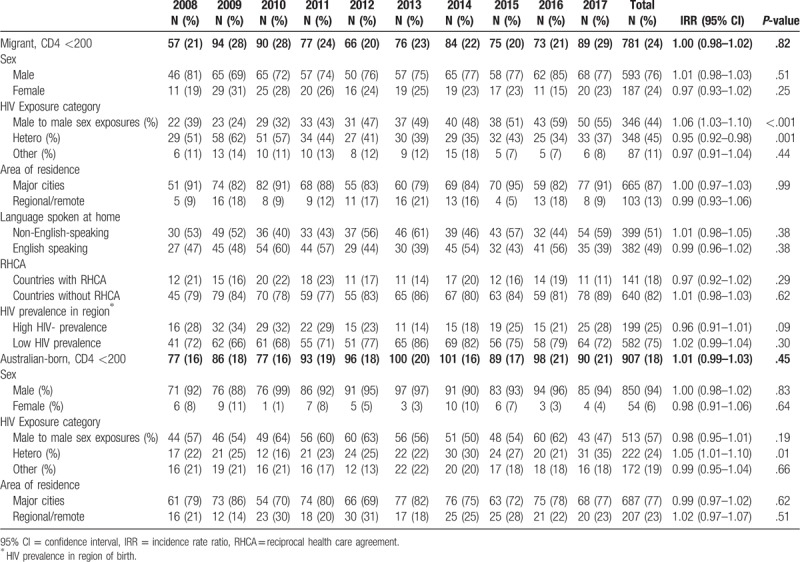
Among 9926 HIV diagnoses notified from 2008 to 2017, 84% (8340) were included in analyses. We excluded individuals with missing CD4+ T-cell count at diagnosis (n = 1401), country of birth (n = 184), sex (n = 1) (Table 1). Most of the individuals with a new HIV diagnosis were male (89%) and living in major cities (85%) (Fig. 1). Individuals with missing CD4+ T-cell count results had similar demographic characteristics to those included in the analysis. Overall, 61% (n = 5073) had CD4+ ≥350 cells/μL, 19% (n = 1579) CD4+ 200 to 349 cells/μL, and 20% (n = 1688) advanced HIV. Thirty-nine percent (n = 3267) had late HIV diagnosis. Of the 3317 HIV diagnoses among migrants, 53% (n = 1754) had CD4+ ≥350 cells/μL, 24% (n = 782) CD4+ 200 to 349 cells/μL, and 24% (n = 781) advanced HIV. Of the 5023 HIV diagnoses among Australian-born, 66% (n = 3319) had CD4+ ≥350 cells/μL, 16% (n = 797) CD4+ 200 to 349 cells/μL, and 18% (n = 907) advanced HIV (Fig. 2). Forty-seven percent (n = 1563) of the migrants had late HIV diagnoses compared to 34% (n = 1704) of Australian-born diagnoses (P < .001). Table 2 further presents CD4+ T-cell categories by sex and migration status. Of the 20 transgender people, 11 (55%) were migrants and 9 (45%) Australian-born (P = .532). Only 9% (n = 1) of the transgender migrants had late HIV diagnosis compared to 33% (n = 3) of Australian-born (P = .192). Most of the late HIV diagnoses among migrants were among people born in Southeast Asia (31%; n = 486), sub-Saharan Africa (17%; n = 264), or North-west Europe (11%; n = 167) (data not shown in table).
Table 1.
Demographics of individuals with new HIV diagnoses by migration status, sex, age, area of residence, and region of birth (2008–17).

Figure 1.
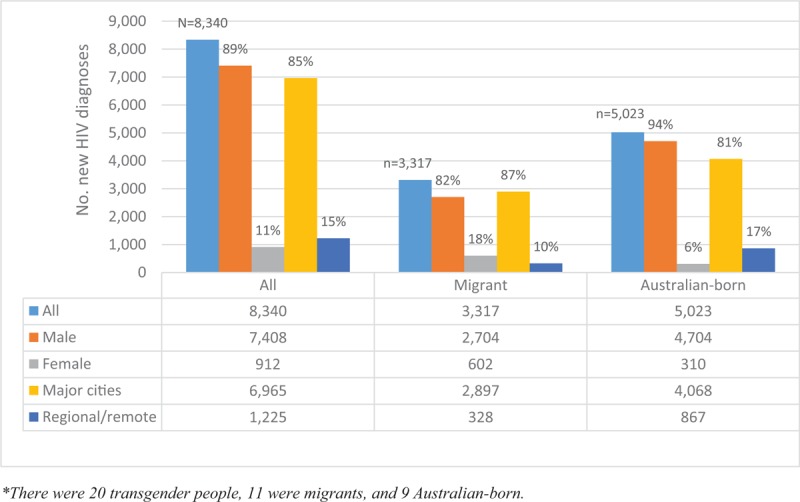
New HIV diagnoses by migration status, sex, and area of residence (2008–17). HIV = human immunodeficiency virus.
Figure 2.
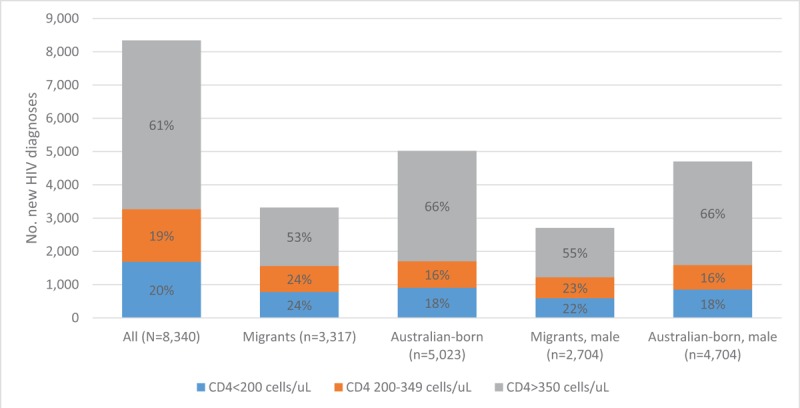
CD4+ T-cell categories of new HIV diagnoses by migration status and sex (2008–17). HIV = human immunodeficiency virus.
Table 2.
CD4+ T-cell categories by sex and migration status (2008–17).

Trends in proportions of CD4+ T-cell ≥350, 200 to 349 cells/μL, and late HIV diagnoses
Table 3 presents the annual trends in the proportions of late HIV diagnoses by migration status, area of residence, main language spoken at home, RHCA with Australia, and high/low HIV-prevalence countries. Figure 3 shows trends in the proportions over time of late HIV diagnoses and CD4+ T-cell categories in migrants compared to Australian-born individuals. Overall, the annual proportion of late HIV diagnoses remained constant over 2008 to 2017 (IRR 1.00; 95% CI 0.99–1.01; P = .79). However, among migrants with late HIV diagnosis, the proportion of men with male-to-male sex as their mode of HIV exposure (IRR 1.05; 95% CI 1.03–1.08; P < .001), individuals whose main language spoken at home is not English (IRR 1.03; 95% CI 1.01–1.05; P = .01), and individuals born in countries in low HIV-prevalence (IRR 1.02; 95% CI 1.00–1.04; P = .01) increased gradually (Figs. 3 and 4 and Table 3). Among migrants with late HIV diagnosis, we identified a decreasing proportions of females (IRR 0.94; 95% CI 0.91–0.98; P = .001), individuals with heterosexual contact as their mode of HIV exposure (IRR 0.94; 95% CI 0.92–0.96; P < .001), those reporting the main language spoken at home was English (IRR 0.97; 95% CI 0.95–0.99; P = .01), born in countries with an RHCA (IRR 0.96; 95% CI 0.92–0.99; P = .03), and individuals born in high HIV-prevalence countries (IRR 0.89; 95% CI 0.86–0.93; P < .001) (Table 3 and Fig. 4). Among people with a late HIV diagnosis who were born in Australia, only the proportion of individuals reporting heterosexual exposure (IRR 1.06; 95% CI 1.02–1.10; P = .001) had an increased (Fig. 4).
Table 3.
Annual trends of late HIV diagnoses by migration status, area of residence, language, RHCA, and HIV-prevalence in region of birth (2008–17).
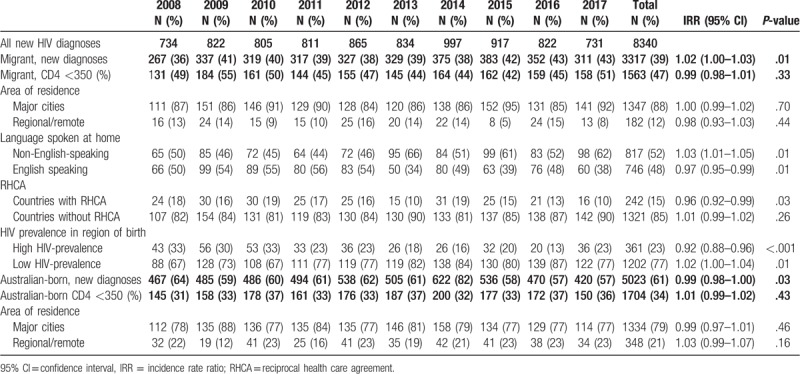
Figure 3.
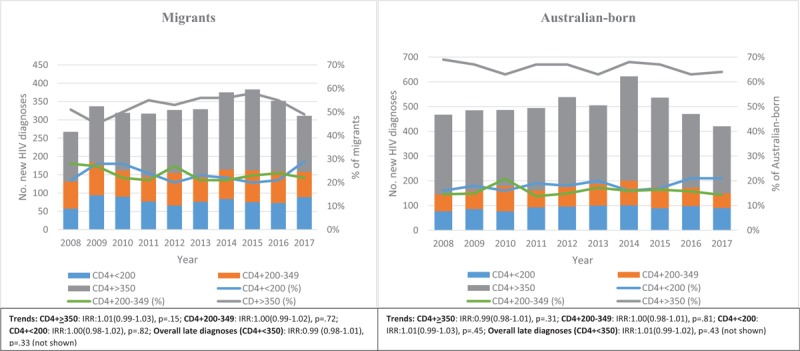
Annual number and percentage distribution of HIV diagnoses (timely [CD4+ >350], late [CD4+ 200–350], and advanced [CD4+ <200]) by migration status (2008–17). HIV = human immunodeficiency virus.
Figure 4.
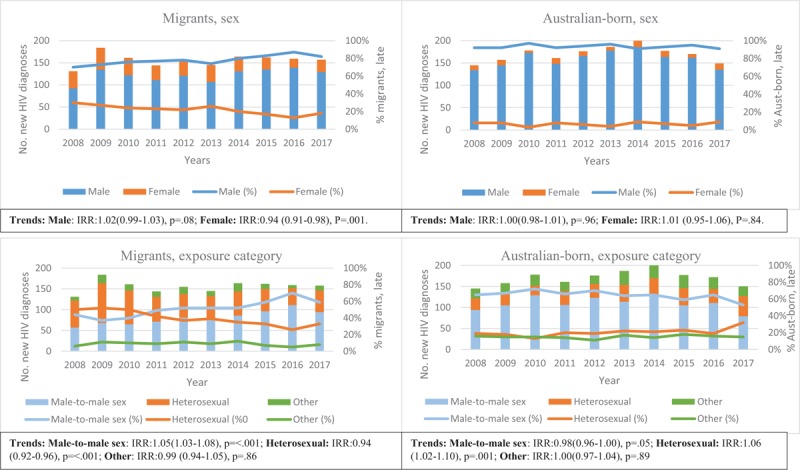
Annual number and percentage distribution of late HIV diagnoses by sex and exposure category in migrants and Australian-born people (2008–17). HIV = human immunodeficiency virus.
3.1. Annual trends of proportion of advanced HIV diagnoses
Table 4 presents annual trends in the proportion of advanced HIV by migration status, sex, HIV exposure category, area of residence, main language spoken at home, RHCA with Australia, and high/low HIV-prevalence countries. Trends of advanced HIV together with other CD4+ T-cell categories are also shown in Figure 3. Similar to late HIV diagnoses, the annual proportions of advanced HIV diagnoses remained constant over 2008 to 2017 (IRR 1.01; 95% CI 0.99–1.02; P = .38) (Fig. 3). Unlike in migrants with late HIV diagnoses, among those with advanced HIV diagnosis the proportion of females (IRR 0.97; 95% CI 0.93–1.02; P = .25) remained stable, but the proportion reporting heterosexual HIV exposure (IRR 0.95; 95% CI 0.92–0.98; P = .001) decreased (Table 4).
Table 4.
Annual trends of advanced HIV diagnoses by migration status, sex, HIV-exposure category, area of residence, language, RHCA, and HIV prevalence in region of birth (2008–17).
4. Discussion
Despite significant investment in health promotion for testing in at-risk groups, scaling up of testing services and increases in the frequency of HIV testing at high-caseload HIV clinics, the annual proportions of individuals diagnosed with late and advanced HIV did not decline in Australia between 2008 and 2017.[31–33] In this period, 40% of individuals diagnosed with HIV in Australia were migrants, and similar to trends in HIV diagnoses overall, there was no significant change in the proportion of migrants diagnosed with late or advanced HIV. We found that most of the late HIV diagnoses among migrants, were people born in Southeast Asia and sub-Saharan Africa. The proportional decreases in late diagnosis seen among females, those reporting heterosexual exposure, individuals born in high HIV prevalence countries, and migrants whose main language spoken at home was English were offset by increases in late diagnoses among migrant MSM and those whose main spoken language at home was not English. The proportion of late diagnosis among Australian-born individuals with heterosexual HIV exposure increased.
Our study identified that 39% of all new HIV diagnoses were late diagnoses, consistent with previous studies in Australia and globally.[21,34,35] However, because we included all new HIV diagnoses irrespective of whether classified as “newly acquired HIV,” the proportion of late diagnosis was higher than what is reported in the surveillance report in Australia. An individual has newly acquired HIV if there is evidence of HIV acquisition in the 12 months before diagnosis.[18] A high proportion of late HIV diagnoses were among migrants, particularly those born in Southeast Asia and sub-Saharan Africa. We found that 47% of migrants had a late HIV diagnosis, similar to the figure produced in an Australian study using routine surveillance data of new non-MSM HIV diagnoses.[36] Our study suggests there is a downwards trend in the proportion of late HIV diagnosis among individuals from high HIV-prevalence countries in Australia, this trend has also been reported in overall new HIV diagnoses among heterosexuals.[37] It is not clear whether migration policies are responsible (ie, whether fewer people with HIV are migrating) or slowing emigration from high HIV-prevalence countries or clinicians increasingly recognizing the need to test people from “traditional” high-prevalence countries and not others. While fewer people from high HIV-prevalence countries now present with late HIV diagnosis, compared to individuals from low-HIV prevalence countries, they are still more likely to present late. This trend has also been shown in previous studies from Australia, Ireland, and the Netherlands.[38–41]
Our findings suggest a shift in the HIV epidemiology of late HIV diagnoses in Australia. While the proportion of late HIV diagnosis declined in some groups, our findings showed an upwards trend in the proportion of late diagnoses among migrants reporting male-to-male sex exposures and Australian-born reporting heterosexual exposure. Medland et al (2018) and Gurnaratnam et al (2019) reported increases in incident HIV cases among newly arrived Asian-born MSM attending a major sexual health service in Melbourne while new HIV infections in other MSM declined.[20,37] Importantly, they found that by the end of the reporting period a significant difference in HIV incidence between the 2 groups had emerged but that previous associations between condom use and number of sex partners were no longer significant. The authors suggested that poor access to biomedical interventions in newly arrived Asian-born MSM helped explain the observed disparities in HIV incidence.[20] While late diagnosis may be occurring in this subpopulation because of health care access barriers due to ineligibility for Medicare other barriers that have been cited include stigma related to HIV, denial, social norms, tradition, and cultural barriers.[42]
In Australia, health care is accessed through Medicare or private health insurance. Migrants on short-term visas (except those from countries with RHCA) are not eligible for Medicare and will be required to pay the cost of health care unless on a private health insurance. The option of having HIV care on private health insurance may need to be negotiated. Migrants wanting to access HIV care services may still find the process of negotiating the options daunting even though compassionate programs may be available for those with no access. Restrictive health policies have been implicated late HIV care presenters among migrants in Ireland, Portugal, and Spain.[8,43,44] In Australia, the role and impact of Medicare ineligibility among migrants at risk of or living with HIV needs further exploration especially among international students who constitute a significant proportion of short term visa holders.
Our findings showing the persistence of late and advanced HIV diagnoses underscore the challenges associated with HIV case finding and reducing undiagnosed HIV. Untreated HIV is a risk for onward transmission, and threatens achievement of the HIV incidence reduction target.[2,4] At this stage of the HIV epidemic and country response, incidence was expected to be declining, including a decline in the proportions with late HIV diagnoses as we approach 2020, the timeline for 90–90–90 targets.[2] Being diagnosed with a CD4+ count <350 cells/μL demonstrates a long period of time with undiagnosed HIV implying possible onward transmission in the community. Among migrants, the time period between arrival and HIV diagnosis is critical especially for those who acquired HIV pre-migration. The latest cascade estimates suggest that Australia has exceeded its 2nd and 3rd “90” of the 90–90–90 targets; however, 27% and 13% of migrants born Southeast Asia and sub-Saharan Africa estimated to be living with HIV were undiagnosed.[18] Evidence from other studies in Australia indicates that migrants are more likely to have lower HIV testing and treatment cascades than nationals.[8,45,46] In order to reach the 95–95–95 targets in 2030, programs must include all vulnerable subpopulations at risk of HIV. While reaching the 95–95–95 targets in 2030 was projected to reduce HIV incidence by 90% compared to 2010, these incidence reductions in Australia are unlikely even if the 95–95–95 targets are reached.[47] This is because more needs to be done regarding prevention strategies (eg, pre-exposure prophylaxis [PrEP]), frequent test for high risk groups and ensuring that no-one is being left behind.
There are some limitations to consider when interpreting these findings. First, we excluded individuals missing baseline CD4+ T-cell counts, area of residence, and country of birth data. Completeness of CD4+ T-cell counts has improved over time, thereby improving the accuracy of our estimates. There were no differences between individuals missing a CD4+ T-cell count and those with complete data hence there was no risk of bias in our trend analyses. Second, we used country of birth as a proxy for migration status, and may have misclassified people born in other countries who may have naturalized (or obtained permanent residency) and we did not report on how long one had lived in Australia. Additionally, we did not have data on whether one reports acquiring HIV pre or post migration which has important implications of prevention strategies for migrants. In a Poisson regression model, patient-years is ideally used as denominator as opposed to the patient counts used in our study. However, the distribution of new HIV diagnoses followed a Poisson distribution which was acceptable. Further research is needed to explore explicit reasons for late HIV diagnosis and any associations between late diagnosis and visa categories, as the latter are not captured in the national dataset.
5. Conclusion
This is the most comprehensive analysis to date of late HIV diagnosis among migrants in Australia. Despite efforts to increase HIV testing in key populations, our results suggest that Australia continues to have a significant proportion of late and advanced HIV diagnoses, with over-representation in migrants and an upwards trend in the proportion of late diagnoses among migrant MSM and Australian-born heterosexuals. Reaching the 95–95–95 targets in Australia will require targeted testing, linkage to care strategies and access to PrEP in migrant populations, with additional efforts for migrant MSM, as well as a renewed focus on strategies to improve testing coverage among Australian-born heterosexuals. There is a need to further explore barriers that migrants and other subpopulations in Australia continue to face in order to ensure that Fast-Track targets are reached.
Acknowledgments
The authors thank Campbell Aitken for editing and proof reading the manuscript and Jonathan King for extracting the data (Permission was granted to be named). We are grateful to jurisdiction HIV surveillance officers who collect all the information reported to the National HIV Registry.
Author contributions
Conceptualization: Tafireyi Marukutira, Rebecca Guy, Danielle Horyniak, Suzanne Mary Crowe, Mark Stoove, Margaret Hellard.
Data curation: Tafireyi Marukutira, Praveena Gunaratnam, Caitlin Douglass, Muhammad S. Jamil, Skye McGregor, Nasra Higgins, Carolien Giele.
Formal analysis: Tafireyi Marukutira, Caitlin Douglass, Tim Spelman.
Methodology: Tafireyi Marukutira, Praveena Gunaratnam, Muhammad S. Jamil, Rebecca Guy, Richard Thomas Gray, Tim Spelman, Carolien Giele, Suzanne Mary Crowe, Mark Stoove, Margaret Hellard.
Project administration: Tafireyi Marukutira.
Supervision: Skye McGregor, Rebecca Guy, Richard Thomas Gray, Tim Spelman, Danielle Horyniak, Suzanne Mary Crowe, Mark Stoove, Margaret Hellard.
Writing – original draft: Tafireyi Marukutira, Caitlin Douglass.
Writing – review and editing: Tafireyi Marukutira, Praveena Gunaratnam, Caitlin Douglass, Muhammad S. Jamil, Skye McGregor, Rebecca Guy, Richard Thomas Gray, Tim Spelman, Danielle Horyniak, Nasra Higgins, Carolien Giele, Suzanne Mary Crowe, Mark Stoove, Margaret Hellard.
Tafireyi Marukutira orcid: 0000-0003-1142-6114.
Footnotes
Abbreviations: ABS = Australian Bureau of Statistics, ART = antiretroviral therapy, CI = confidence interval, HIV = human immunodeficiency virus, IDU = injecting drug use, IRR = incidence rate ratio, MSM = gay and bisexual men who have sex with other men, PrEP = pre-exposure prophylaxis, RHCA = reciprocal health care agreement, UNAIDS = the Joint United Nations Program on HIV/AIDS.
How to cite this article: Marukutira T, Gunaratnam P, Douglass C, Jamil MS, McGregor S, Guy R, Gray RT, Spelman T, Horyniak D, Higgins N, Giele C, Crowe SM, Stoove M, Hellard M. Trends in late and advanced human immunodeficiency virus diagnoses among migrants in Australia; implications for progress on fast-track targets: A retrospective observational study. Medicine. 2020;99:8(e19289).
A poster abstract was presented at the Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine (ASHM) Conference, Sydney, Australia, September 24–26, 2018. Abstract number: 49.
TM is supported by an Australian Government Research Training Program (RTP) Scholarship for his PhD studies. MH receives a Fellowship from the National Health and Medical Research Council of Australia.
MH receives investigator initiated funding from the Gilead Sciences, Abbvie, and GSK for research unrelated to HIV care.
The authors have no conflicts of interest to disclose.
References
- [1].UNAIDS. Understanding Fast-track: Accelerating Action to End the AIDS Epidemic by 2030. 2015;Geneva, Switzerland: UNAIDS, Available at: http://www.unaids.org/sites/default/files/media_asset/fast-track-commitments_en.pdf. Accessed September 16, 2019. [Google Scholar]
- [2].UNAIDS. 90-90-90 An Ambitious Treatment Target to Help End the AIDS Epidemic. 2014;Geneva, Switzerland: UNAIDS, Available at: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf. Accessed September 6, 2019. [Google Scholar]
- [3].Lima VD, St-Jean M, Rozada I, et al. Progress towards the United Nations 90-90-90 and 95-95-95 targets: the experience in British Columbia, Canada. J Int AIDS Soc 2017;20:e25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].UNAIDS. Global AIDS Update 2018: Miles to go- Closing Gaps, Breaking Barriers, Righting Injustices. 2018;Geneva, Switzerland: UNAIDS, Available at: https://www.unaids.org/sites/default/files/media_asset/miles-to-go_en.pdf. Accessed September 6, 2019. [Google Scholar]
- [5].IOM, International Organization for Migration (IOM). Glossary on Migration. International Migration Law. 2011;Available at: https://www.iom.int/key-migration-terms. Accessed June 8, 2019. [Google Scholar]
- [6].IOM, International Organization for Migration (IOM). World Migration Report 2018. 2018;Available at: https://www.iom.int/wmr/world-migration-report-2018. Accessed June 8, 2019. [Google Scholar]
- [7].World Health Organization (WHO). Migrant health in the European Region. WHO Regional Office for Europe: 2016. Available at: http://www.euro.who.int/en/health-topics/health-determinants/migration-and-health/migrant-health-in-the-european-region Accessed September 16, 2019. [Google Scholar]
- [8].Tanser F, Barnighausen T, Vandormael A, et al. HIV treatment cascade in migrants and mobile populations. Curr Opin HIV AIDS 2015;10:430–8. [DOI] [PubMed] [Google Scholar]
- [9].Ridolfo AL, Oreni L, Vassalini P, et al. Effect of legal status on the early treatment outcomes of migrants beginning combined antiretroviral therapy at an outpatient clinic in Milan Italy. J Acquir Immune Defic Syndr 2017;75:315–21. [DOI] [PubMed] [Google Scholar]
- [10].Gray C, Lobo R, Narciso L, et al. Why i can’t, won’t or don’t test for HIV: insights from Australian migrants born in Sub-Saharan Africa, Southeast Asia and Northeast Asia. Int J Environ Res Public Health 2019;16:E1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Demeke HB, Johnson AS, Wu B, et al. Differences between U.S.-born and non-U.S.-born black adults reported with diagnosed HIV infection: United States, 2008-2014. J Immigr Minor Health 2018;21:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Agu J, Lobo R, Crawford G, et al. Migrant sexual health help-seeking and experiences of stigmatization and discrimination in perth, western australia: exploring barriers and enablers. Int J Environ Res Public Health 2016;13:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Australian Bureau of Statistics (ABS). Migration, Australia. In: ABS, editor. Canberra, Australia: 2018. Available at https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3412.02017-18?OpenDocument Accessed September 4, 2019. [Google Scholar]
- [14].Wakerman J, Humphreys JS, Wells R, et al. Primary health care delivery models in rural and remote Australia: a systematic review. BMC Health Serv Res 2008;8:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Enrico Girardi, Caroline A, Sabin, et al. Late diagnosis of HIV infection: epidemiological features, consequences and strategies to encourage earlier testing. J Acquir Immune Defic Syndr 2007;46:S3–8. [DOI] [PubMed] [Google Scholar]
- [16].Gray RT, Wilson DP, Guy RJ, et al. Undiagnosed HIV infections among gay and bisexual men increasingly contribute to new infections in Australia. J Int AIDS Soc 2018;21:e25104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kirby Institute. HIV, Viral Hepatitis and Sexually Transmissible Infections in Australia: Annual Surveillance Report 2017. Sydney: Kirby Institute, UNSW Sydney; 2017. Available at: https://kirby.unsw.edu.au/sites/default/files/kirby/report/SERP_Annual-Surveillance-Report-2017_compressed.pdf Accessed October 2, 2019. [Google Scholar]
- [18].Chow EPF, Medland NA, Denham I, et al. Decline in new HIV diagnoses among MSM in Melbourne. Lancet HIV 2018;5:e479–81. [DOI] [PubMed] [Google Scholar]
- [19].Kirby Institute. HIV, Viral Hepatitis and Sexually Transmissible Infections in Australia: Annual Surveillance Report 2018. Sydney, Australia: Kirby Institute, UNSW Sydney; 2018. Available at: https://kirby.unsw.edu.au/sites/default/files/kirby/report/KI_Annual-Surveillance-Report-2018.pdf Accessed September 2, 2019. [Google Scholar]
- [20].Telfer B, Selvey C, Bowden V, et al. Predictors of Late Diagnosis for People Newly Diagnosed With HIV Infection in NSW. Australian HIV & AIDS Conference 6-8 November 2017; Canberra, Australia. Available at: https://az659834.vo.msecnd.net/eventsairaueprod/production-ashm-public/15613d60eb00463685268ff99ea35377. Accessed June 8, 2019. [Google Scholar]
- [21].Asante A, Körner H, Kippax S. [Accessed September 16, 2019]. Understanding Late HIV Diagnosis Among People From Culturally and Linguistically Diverse Backgrounds (Monograph 7/2009). Sydney: National Centre in HIV Social Research, The University of New South Wales: 2009. Available at: https://www.acon.org.au/wp-content/uploads/2015/04/Understanding-late-HIV-diagnosis-CALD-report-CSRH-2009.pdf. [Google Scholar]
- [22].Lemoh C, Guy R, Yohannes K, et al. Delayed diagnosis of HIV infection in Victoria 1994 to 2006. Sexual Health 2009;6:117–22. [DOI] [PubMed] [Google Scholar]
- [23].Medland NA, Chow EPF, Read THR, et al. Incident HIV infection has fallen rapidly in men who have sex with men in Melbourne, Australia (2013-2017) but not in the newly-arrived Asian-born. BMC Infect Dis 2018;18:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine (ASHM). National HIV Testing Policy. Adopted by the ASHM Board: 2017. Available at: http://testingportal.ashm.org.au/hiv/63-testing-policy/version-tracking Accessed June 8, 2019. [Google Scholar]
- [25].Communicable Diseases Network of Australia (CDNA). Human Immunodeficiency Virus (HIV): CDNA National Guidelines for Public Health Units. 2014. Available at: http://www.health.gov.au/internet/main/publishing.nsf/Content/cdna-song-HIV.htm Accessed September 16, 2019. [Google Scholar]
- [26].World Health Organization (WHO). WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-related Disease in Adults and Children. Geneva, Switzerland: WHO; 2007. Available at: http://apps.who.int/iris/handle/10665/43699 Accessed September 10, 2019 [Google Scholar]
- [27].Antinori A, Coenen T, Costagiola D, et al. Late presentation of HIV infection: a consensus definition. HIV Med 2011;12:61–4. [DOI] [PubMed] [Google Scholar]
- [28].UNAIDS. UNAIDS Data 2018. 2018;Geneva, Switzerland: UNAIDS, Available at: https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf. Accessed June 14, 2019. [Google Scholar]
- [29].UNAIDS. UNAIDS Terminology Guidelines. Geneva, Switzerland: UNAIDS; 2011, revised version. Available at https://www.unaids.org/sites/default/files/media_asset/JC2118_terminology-guidelines_en1.pdf Accessed July 22, 2019. [Google Scholar]
- [30].Kuhn L, Davidson LL, Durkin MS. Use of Poisson regression and time series analysis for detecting changes over time in rates of child injury following a prevention program. Am J Epidemiol 1994;140:943–55. [DOI] [PubMed] [Google Scholar]
- [31].Wilkinson AL, Pedrana AE, El-Hayek C, et al. The impact of a social marketing campaign on HIV and sexually transmissible infection testing among men who have sex with men in Australia. Sex Transm Dis 2016;43:49–56. [DOI] [PubMed] [Google Scholar]
- [32].Hammad Ali, Basil Donovan, Christopher K Fairley, et al. Increasing access by priority populations to Australian sexual health clinics. Sex Transm Infect 2013;40:819–21. [DOI] [PubMed] [Google Scholar]
- [33].Wilkinson AL, El-Hayek C, Spelman T, et al. A ’test and treat’ prevention strategy in Australia requires innovative HIV testing models: a cohort study of repeat testing among ’high-risk’ men who have sex with men. Sex Transm Infect 2016;92:464–6. [DOI] [PubMed] [Google Scholar]
- [34].van der Kop ML, Thabane L, Awiti PO, et al. Advanced HIV disease at presentation to care in Nairobi, Kenya: late diagnosis or delayed linkage to care? – a cross-sectional study. BMC Infect Dis 2016;16:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rice B, Elford J, Yin Z, et al. Trends in HIV diagnoses, HIV care, and uptake of antiretroviral therapy among heterosexual adults in England, Wales, and Northern Ireland. Sex Transm Dis 2014;41:257–65. [DOI] [PubMed] [Google Scholar]
- [36].Peach E, Lemoh C, Stoove M, et al. Aiming for 90-90-90 - the importance of understanding the risk factors for HIV exposure and advanced HIV infection in migrant populations and other groups who do not report male-to-male sex. Sex Health 2018. [DOI] [PubMed] [Google Scholar]
- [37].Gunaratnam P, Heywood AE, McGregor S, et al. HIV diagnoses in migrant populations in Australia-a changing epidemiology. PLoS One 2019;14:e0212268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].O’Connell S, Enkelmann J, Sadlier C, et al. Late HIV presentation - missed opportunities and factors associated with a changing pattern over time. Int J STD AIDS 2017;28:814–21. [DOI] [PubMed] [Google Scholar]
- [39].van Opstal SEM, van der Zwan JS, Wagener MN, et al. Late presentation of HIV infection in the netherlands: reasons for late diagnoses and impact on vocational functioning. AIDS Behav 2018;22:2593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Op de Coul EL, van Sighem A, Brinkman K, et al. Factors associated with presenting late or with advanced HIV disease in the Netherlands, 1996-2014: results from a national observational cohort. BMJ Open 2016;6:e009688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hocking JS, Rodger AJ, Rhodes DG, et al. Late presentation of HIV infection associated with prolonged survival following AIDS diagnosis characteristics of individuals. Int J STD AIDS 2000;11:503–8. [DOI] [PubMed] [Google Scholar]
- [42].Mullens AB, Kelly J, Debattista J, et al. Exploring HIV risks, testing and prevention among sub-Saharan African community members in Australia. Int J Equity Health 2018;17:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ledoux C, Pilot E, Diaz E, et al. Migrants’ access to health care services within the European Union: a content analysis of policy documents in Ireland, Portugal and Spain. Global Health 2018;14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Matlin SA, Depoux A, Schütte S, et al. Migrants’ and refugees’ health: towards an agenda of solutions. Public Health Rev 2018;39:27. [Google Scholar]
- [45].Brown AE, Attawell K, Hales D, et al. Monitoring the HIV continuum of care in key populations across Europe and Central Asia. HIV Med 2018;19:431–9. [DOI] [PubMed] [Google Scholar]
- [46].Reyes-UrueÑA J, Campbell C, Hernando C, et al. Differences between migrants and Spanish-born population through the HIV care cascade, Catalonia: an analysis using multiple data sources. Epidemiol Infect 2017;145:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Scott N, Stoové M, Kelly SL, et al. Achieving 90-90-90 HIV targets will not be enough to achieve the HIV incidence reduction target in Australia. Clin Infect Dis 2018;66:1019–26. [DOI] [PubMed] [Google Scholar]


