Abstract
Microglia are highly heterogeneous and plastic. However, the dynamics of their turnover have been difficult to visualize. A new multicolor reporter system reveals a plastic but stable network of microglia during health and disease.
One of the biggest challenges in the field of microglial research has been understanding how they self-renew at steady state and, more importantly, under pathological conditions. The ability to target microglia versus recruited monocytes in disease conditions remains a central step in devising appropriate therapy. Microglia arise from an embryonic origin that differs from that of peripheral myeloid populations1,2. Unlike monocytes, which are renewed throughout life from bone marrow hematopoietic stem cells, resident microglial cells in the healthy adult brain persist during adulthood via self-renewal, and this occurs without turnover from circulating blood progenitors1,3–5. In this issue of Nature Neuroscience, Tay et al.6 identify the dynamics of microglial renewal in health and disease. Microglia are shown to self-renew stochastically in the healthy brain and expand clonally during pathology. The resulting excess in microglia is resolved by cell egress and programmed cell death.
Manipulation of resident microglia numbers has shown that they recover rapidly from resident proliferative sources after genetic ablation7 or pharmacological depletion8. Thus, microglia resemble other tissue- resident macrophages, which self-renew during homeostasis9. However, mouse models such as Cx3cr1-promoter-based reporter mice have not enabled us to differentiate resident microglia from their progeny or even from their potential precursors, especially in disease3,10,11.
In disease conditions, it has been even harder to distinguish the relative contributions of microglia and recruited monocytes. Ajami et al.3 used a parabiosis-and-irradiation model to label circulating monocytes and found little or no contribution of peripheral monocytes to the microglial population in disease. However, no one has been able to dissect microglial renewal in disease.
Tay et al.6 used an innovative method of fate-mapping brain microglia in vivo to visualize the dynamics with which microglial regulate their cell numbers in response to changes in their microenvironment. The authors found that under physiological conditions, the micro-glial network remains stable, self-proliferating in a stochastic manner at turnover rates that varied by region. However, in an acute model of neurodegeneration, facial nerve axotomy, microglia shifted from random proliferation to selective clonal expansion. The authors also found that the density and distribution of the original microglial network were restored by specific apoptosis.
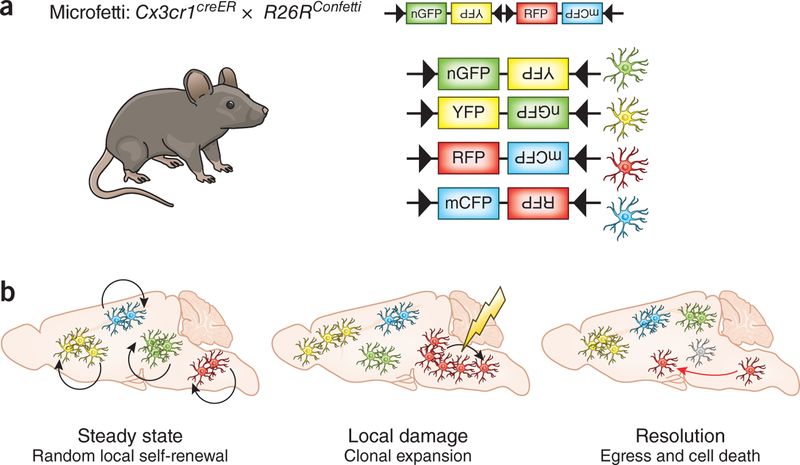
To distinguish microglial subsets in the parenchyma without compromising the blood–brain barrier, the authors generated a myeloid-cell-specific multicolor reporter mouse line by crossing Cx3cr1creER mice with a ubiquitously expressing R26RConfetti model. The use of Cre-ER technology allows the authors to induce recombination leading to the expression of fluorescent proteins at a given time and in a cell-specific manner. In this technique, Cre recombinase is fused to a mutated ligand-binding domain of the human estrogen receptor (ER) that is activated specifically by tamoxifen and not by estradiol. After induction of recombination by tamoxifen injection, CX3CR1+ microglia randomly express one of four possible fluorescent reporter proteins encoded by the Confetti construct, enabling specific, long-term labeling of microglia and their daughter cells (Fig. 1). They named the resulting mouse line Microfetti.
Figure 1.
Microglial network regulation in health and disease. (a) The Confetti reporter strain crossed with a myeloid-cell-specific Cre line generates the Microfetti mouse. It allows random multicolor labeling of microglia with one of four fluorescent proteins: nuclear green fluorescent protein (nGFP), cytoplasmic yellow fluorescent protein (YFP), cytoplasmic red fluorescent protein (RFP) and membrane-localized cyan fluorescent protein (mCFP). (b) This model reveals random local self-renewal of microglia in steady-state conditions. Local damage induces microgliosis through clonal expansion at the site of the lesion, which resolves by egress (red arrow) and apoptosis (gray cell) of excess cells to restore the resting microglia network.
By combining the Microfetti mouse with mathematical modeling, the authors were able to analyze the microglial network during homeostasis and demonstrate considerable microglial stability in the adult brain more than 36 weeks after recombination. In specific niches of cell proliferation, such as the hippocampus and olfactory bulb, the authors found subpopulations of microglia with increased self-renewal. This is in accordance with the findings of Askew et al.5; however, that study compromised the blood–brain barrier and led to the potential infiltration of blood monocytes.
Tay et al.6 also provide insight into the dynamics of the microglia network in vivo during disease and recovery. The authors examined microglial dynamics after facial nerve axotomy, a neurodegenerative model that does not compromise the blood–brain barrier. Using this model, they observed the random self-renewal of steady state microglia shifting toward clonal expansion in response to an acute lesion. Microglia rapidly proliferated and formed a cluster of daughter cells as early as 2 days after the damage, which explains the microgliosis observed after facial nerve axotomy. The Microfetti technique excluded the possibility that microglia were recruited from elsewhere in the brain, as that would have led to a more random distribution of the labeled cells. The resolution of microgliosis was accompanied by a decrease in microglial density. Tay et al.6 suggest that the homeostatic microglial network is restored by a combination of random microglial movement into nearby compartments and local apoptosis in the area of the lesion.
Finally, Tay et al.6 characterized gene expression in clonally expanded microglia during the progression of neural injury. They found that during lesion progression on the way to resolution of microgliosis, the most-modulated microglia functions are related to phagocytosis, cell migration and immune responses such as antigen presentation.
This study provides a new perspective on microglial network regulation in health and especially in disease. Indeed, along with the previous dogma that microglia in rodents are long-lived cells and the recent view that they are self-renewing in steady-state conditions12, Tay et al.6 demonstrate that microglia renewal dynamics are highly dependent on their microenvironment and are region-specific within the CNS. The Microfetti model is a notable tool that confirms microglial plasticity and heterogeneity, and it shows that their density and rate of renewal vary in a context-dependent manner. More importantly, it demonstrates that microglia clonally expand while mounting an inflammatory response to local damage and then die or migrate to resolve the resulting microgliosis.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Ginhoux F et al. Science 330, 841–845 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez Perdiguero E et al. Nature 518, 547–551 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajami B, Bennett JL, Krieger C, Tetzlaff W & Rossi FM Nat. Neurosci 10, 1538–1543 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Kierdorf K et al. Nat. Neurosci 16, 273–280 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Askew K et al. Cell Rep. 18, 391–405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tay TL et al. Nat. Neurosci 20, 793–803 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Bruttger J et al. Immunity 43, 92–106 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Elmore MR et al. Neuron 82, 380–397 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto D et al. Immunity 38, 792–804 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung S et al. Mol. Cell. Biol 20, 4106–4114 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parkhurst CN et al. Cell 155, 1596–1609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry VH & Teeling J Semin. Immunopathol 35, 601–612 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasr S et al. J. Neurosci 31, 13771–13785 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]