Abstract
Langerhans cells (LCs) and plasmacytoid dendritic cells (pDCs) play an important role in the cutaneous immune response to viral infection. Verruca vulgaris (VV) is a chronic benign disease caused by human papillomavirus (HPV) infection.
To investigate the possible roles of LCs, pDCs and toll-like receptor (TLR)7/9 signaling pathways in the pathogenesis of VV, we detected the expression of CD1a, CD2AP, CD123, TLR7/9, IFN regulatory factor 7 (IRF7), and interleukin-1 receptor-associated kinase 1 (IRAK1) in VV lesions.
The expression of CD1a, CD2AP, CD123, TLR7/9, IRF7, and IRAK1 in 20 VV lesions was tested by immunohistochemistry. The density and number of stained cells were compared between VV lesions and the perilesional normal skin.
The density and number of CD1a-, CD2AP-, CD123-, TLR9-, and IRAK1-positive cells in the papillary layer of VV lesions were significantly higher than those in the perilesional normal skin (P < .05). There were no significant differences in the density and positive rate of CD1a+ cells in the epidermis and of TLR7+ and IRF7+ cells in the dermis between VV lesions and the perilesional normal skin at the edge (P > .05).
In VV, the number of LCs increases only in the dermis, indicating that LC's antigen-presenting function might not be inhibited. The increased number of pDCs in VV lesions suggests that HPV infection may recruit the pDCs to the virus-infected epithelium. We speculate that the TLR7/9 downstream signaling pathway is not fully activated in VV, leading to difficulty of HPV removal and the relapse of HPV-infected lesions.
Keywords: langerhans cell, plasmacytoid dendritic cell, toll-like receptor, verruca vulgaris
1. Introduction
Verruca vulgaris (VV) is a chronic benign disease caused by human papillomavirus (HPV) infection in the skin or mucosa. The mechanism underlying the recurrence and relapse of VV among healthy populations is unclear. Researchers have speculated that it is probably due to poor local immune function, where it is difficult to establish effective immune responses to eliminate HPV infection.[1]
Dendritic cells (DCs) are currently known to be the most potent professional antigen-presenting cells (APCs) to activate naive T lymphocytes and initiate cellular immune responses. Langerhans cells (LCs) and plasmacytoid dendritic cells (pDCs) are 2 critical DC subgroups. LCs are the most important APCs in the skin. LCs are better at antigen uptake, processing, and handling, and they can present antigens to T helper cells, which are activated by costimulatory signals to initiate the immune response. LCs are critical in immune surveillance and the clearance of HPV.[2] pDCs, also called type-I interferon (IFN)-producing cells, specifically express high levels of toll-like receptor 7 (TLR7) and toll-like receptor 9 (TLR9),[3] through which viral nucleic acid sequences are recognized and TLR-related signal transduction pathways are activated. The phosphorylation and nuclear translocation of the IFN regulatory factor 7 (IRF7) induces a large amount of type-I IFN in pDCs to inhibit the viral replication.[4] PDCs can also mediate immunosuppression by inducing regulatory CD4+CD25+ T lymphocytes.[5]
LCs and pDCs perform important functions in antiviral immune responses; therefore, when infected by HPV, whether the local LCs and pDCs produce an effective anti-HPV immune response is a crucial factor in the onset and progression of VV. However, the expression of LC and pDC markers, and TLR signaling-associated molecules in VV has never been studied. To further reveal the roles and significance of LCs, pDCs, and their TLR signaling pathways in the pathogenesis of VV, the present study detected the molecular marker of LCs (CD1a), the specific surface markers of pDCs (CD2AP and CD123), and the expression of pDC-associated TLR7/9 and the related signaling molecules, such as IRF7 and interleukin-1 receptor-associated kinase 1 (IRAK1), in VV lesions by immunohistochemistry. Our results demonstrate the activity of LCs, pDCs and TLR signaling pathways in the pathogenesis of VV.
2. Materials and methods
2.1. Specimens
Data were collected from 20 VV patients diagnosed by histopathology from January to December 2013 in Ningbo First Hospital and Sir Run Run Shaw Hospital. The authors had access to information that could identify individual participants during data collection. Among them, 9 patients were female, and 11 were male. The age of patients was between 16 and 38, and the disease duration varied from 2 weeks to 10 months. Thirteen specimens of the 20 VV patients had perilesional normal skin at the edge of skin lesions, which were used as control. No patient had diabetes, severe liver or kidney dysfunction, immunodeficiency history, or complications requiring immunosuppressant medication. This study was approved by the relevant ethics committee, and all patients provided consent.
2.2. Reagents
Mouse anti-human CD1a monoclonal antibody, mouse anti-human CD2AP monoclonal antibody, mouse anti-human CD123 monoclonal antibody, mouse anti-human TLR7 monoclonal antibody, mouse anti-human TLR9 monoclonal antibody, mouse anti-human IRF7 monoclonal antibody, and mouse anti-human IRAK1 monoclonal antibody were all purchased from Abcam (Cambridge, United Kingdom). A ready-to-use UltrasensitiveTM SP immune-histochemistry kit and Diaminobenzidine kit were purchased from Beijing Zhongshan Biotechnology Co. Ltd. (PR China).
2.3. Immunohistochemistry
Skin lesion tissues were taken from clinically diagnosed VV patients. After fixation with 4% formaldehyde, the tissues were prepared as wax blocks. Paraffin-embedded specimens were continuously sectioned into 4-μm slices, which were then conventionally dewaxed, hydrated, and immunohistochemically stained. Normal goat serum was used to block the sections at 37°C for 10 minutes, and 1:100 diluted primary antibody was added dropwise for an overnight incubation at 4°C. Biotin-labeled secondary antibody was added dropwise before incubation at 37°C for 30 minutes. Horseradish peroxidase-labeled streptavidin working solution was added dropwise before incubation at 37°C for 30 minutes. Phosphate-buffered saline (PBS) was used to wash the slices between steps. Diaminobenzidine color development, counter-staining, and mounting were performed before the sections were observed under a microscope to determine the results. PBS was used in place of the primary antibody as a negative control.
2.4. Microscopic analysis
CD1a staining: under a light microscope, cells with brown dendrites in the cell membrane and (or) cytoplasm were considered positive cells. CD2AP, CD123, TLR7, TLR9, IRF7, and IRAK1 staining: under a light microscope, cells with yellowish-brown or brown granules in the cell membrane and (or) cytoplasm were considered positive cells. Under a 400× magnification light microscope, 5 random fields of skin lesions that mainly contained epidermis were selected from each section. The number of positive cells in the epidermis and the total number of cells in the epidermis in each field were counted. Then, 5 random fields that mainly contained papillary dermis were selected, and the number of positive dermal cells and the total number of dermal infiltration cells in each field were counted. The counting method for the perilesional normal skin was the same as that for skin lesions. Positive cell density = total number of positive cells in 5 fields/total number of cells in 5 fields. Positive percentage = number of cases with positive cells/total number of cases × 100%.
2.5. Statistical analyses
The detected markers in the 20 specimens of VV skin lesions were compared with the markers in the perilesional normal skin from the 13 of those cases with normal skin at the edge of lesions. The data from the VV skin lesions of those 13 cases were also compared with the data from the perilesional normal skin. SPSS Statistics for Windows, version 18.0 (SPSS Inc., Chicago, IL) was used for statistical analysis. The median M was used for skewed distribution measurements, the rank sum test was used for the comparisons between 2 groups, the X2 test was used to compare counted data, and the significance test level was 0.05. Pearson correlation analysis was used.
3. Results
3.1. Expression of CD1a in VV lesions
Many CD1a+ LC cells were embedded in the spinous cells above the basal layer in both VV lesion and in the perilesional normal skin, mostly in the middle and bottom of the epidermis. The cytoplasm of CD1a+ LCs was brown and irregular with 2 to 4 thin and long protrusions. Some cells showed round and oval shapes (Fig. 1). The CD1a-positive cells (see Table 1) in the 20 cases of VV were compared to those in the 13 cases of perilesional normal skin. The results showed that all 20 cases of warts presented CD1a+ cells in lesional epidermis (100% positive), while 12 of the 13 cases of perilesional normal skin showed CD1a+ cells in the epidermis (92.3% positive). There was no significant difference between the lesional and non-lesional epidermis (P > .05). The median number M of the CD1a+ cell density in the lesional epidermis of the 20 cases of VV was 0.034, which did not differ significantly (P > .05) from that of the 13 cases of normal epidermis (M = 0.033).
Figure 1.

Expressions of CD1a in VV lesions and the perilesional normal skin at the lesion's edge (SP ×400). (A) More CD1a+ dendritic cells with brown cytoplasm were observed in the spinous layer of VV lesions. (B) A number of CD1a+ dendritic cells with brown cytoplasm were observed in the papillary layer of VV lesion. (C) More CD1a+ dendritic cells with brown cytoplasm were observed in the spinous layer of perilesional normal skin of VV lesion. Few CD1a+ dendritic cells with brown cytoplasm were observed in the papillary layer of perilesional normal skin. VV = verruca vulgaris.
Table 1.
Comparison of the density and rate of CD1a-, CD2AP-, and CD123-positive cells in 20 cases of VV lesions and 13 cases of perilesional normal skin of lesions.
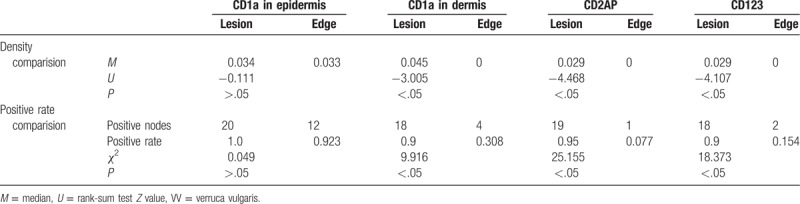
We found that many CD1a+ LCs were present in the papillary dermis of VV lesions, with smaller cell size, more irregular shape, and fewer cell protrusions, compared with epidermal CD1a+ LCs. CD1a+ LCs were rarely observed in the dermis of the perilesional normal skin. There was no significant difference in the morphology of CD1a+ cells between the lesions and the perilesional normal skin. CD1a+ cells were present in the dermis in 18 of the 20 cases of VV lesions, which is a higher frequency than that in the perilesional normal skin (4 of 13 cases, P < .05). Twelve of 13 VV cases showed CD1a-positive cells in the dermis, which was significantly more than in the perilesional normal skin (30.8%) (P < .05).
The dermal CD1a+ cell density M in all 20 cases of VV lesions was significantly higher than that in the 13 cases of perilesional normal skin (P < .05). A paired comparison of the CD1a expression in the skin lesions of the 13 cases of VV with perilesional normal skin was conducted (see Table 2), showing that dermal CD1a+ cell density M of the 13 cases of VV lesions was significantly higher than that of the 13 cases of perilesional normal skin (M = 0) (P < .05).
Table 2.
Comparison of the density and rate of CD1a-, CD2AP-, and CD123-positive cells in 13 cases of VV lesions and in the perilesional normal skin.

3.2. Expression of CD2AP and CD123 in VV lesions
The CD2AP+ cells in the dermis were mononuclear-like cells with brown cytoplasm, and they were mainly located in the papillary layer (see Fig. 2). Our study indicated that of the 20 cases of VV lesions, 19 had CD2AP+ cells (95%), which was significantly more than the frequency in the perilesional normal skin (7.7%) (P < .05). The CD2AP+ cell density M of the 20 cases of VV lesions was significantly higher than that of the perilesional normal skin (P < .05). The paired comparison of the CD2AP expression (see Table 2) showed that all 13 cases with these VV lesions had CD2AP+ cells (100%), which was significantly higher than that in the perilesional normal skin (P < .05). The CD2AP+ cell density M in the 13 cases of VV lesions was 0.03, which was significantly higher than that of the perilesional normal skin (P < .05).
Figure 2.

Expressions of CD2AP in VV lesions and the perilesional normal skin at their edge (SP ×400). (A) Few CD2AP+ monocyte-like cells with brown cytoplasm were observed in the epidermal of VV lesions (B) More CD2AP+ monocyte-like cells with brown cytoplasm were observed in the dermal papillary layer of VV lesion. (C) Few CD2AP+ monocyte-like cells with brown cytoplasm were observed in both epidermal and papillary layer of perilesional normal skin of the lesion. VV = verruca vulgaris.
CD123+ cells were mainly observed in the papillary dermis, and the cytoplasm was brown; no positive cells were observed in the epidermis (see Fig. 3). We found that of the 20 cases of VV skin lesions, 18 had CD123+ cells, which was a significantly higher occurrence than in the perilesional normal skin (P < .05) (see Table 1). The CD123+ cell density M of the 20 cases of VV lesions was significantly higher than that of the perilesional normal skin (P < .05). We also found that of the 13 cases of VV lesions with perilesional normal skin at the edge, 12 cases presented CD123+ cells (92.3%), which was a significantly higher frequency than that in the perilesional normal skin (P < .05) (see Table 2). The CD123+ cell density M of the 13 cases of VV lesions was also significantly higher than that of the perilesional normal skin (P < .05).
Figure 3.

Expressions of CD123+ in VV lesions and the perilesional normal skin at the edge (SP ×400). (A) Few CD123+ monocyte-like cells with brown cytoplasm were observed in the epidermal of VV lesions. (B) Most CD123+ monocyte-like cells with brown cytoplasm were observed in the dermal papillary layer of VV lesion. (C) Few CD123+ monocyte-like cells with brown cytoplasm were observed in the epidermal and papillary layer of perilesional normal skin of VV lesion. VV = verruca vulgaris.
3.3. Expression of TLR9 and TLR7 in VV skin lesions
The TLR9+ cells were mononuclear-like cells with brown cytoplasm, and these cells were mainly located in the papillary dermis (Fig. 4). The TLR9 expression (see Table 3) in the 20 cases of VV was compared with that of the 13 cases with perilesional normal skin. Of the 20 cases of VV skin lesions, 12 cases had TLR9+ cells (60%), which was significantly more than in the perilesional normal skin (7.7%) (P < .05). The TLR9+ cell density M of the 20 cases of VV lesions was higher than that of perilesional normal skin (P < .05). A paired comparison was performed for the TLR9 expression (see Table 4) in the skin lesions of the 13 cases of VV with perilesional normal skin and in the perilesional normal skin, which showed that of the 13 cases of VV lesions 9 had TLR9+ cells, which was significantly more frequent than in the perilesional normal skin (P < .05). The TLR9+ cell density M of the 13 cases of VV lesions was also higher than that of the perilesional normal skin (P < .05). Additionally, TLR9 expression was observed in the cytoplasm of epidermal keratinocytes, which were mainly found in the spinous layer, but the staining was weaker.
Figure 4.
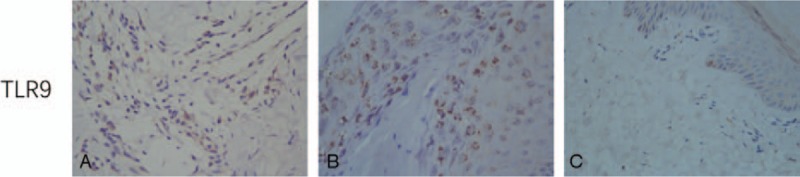
Expressions of TLR9 in VV lesions and the perilesional normal skin at the edge (SP ×400). (A) Many TLR9+ keratinocytes with brown cytoplasm were observed in epidermis of VV, mainly in the spinous layer. (B) More TLR9+ monocyte-like cells with brown cytoplasm were observed in the dermal papillary layer of VV lesion. (C) Small numbers of TLR9+ monocyte-like cells with brown cytoplasm were observed in the papillary layer of perilesional normal skin of VV lesion. VV = verruca vulgaris.
Table 3.
Comparison of the density and rate of TLR9-, TLR7-, IRF7-, and IRAK1-positive cells in 20 cases of VV lesions and 13 cases of perilesional normal skin of lesions.

Table 4.
Comparison of the density and rate of TLR9-, TLR7-, IRF7-, and IRAK1-positive cells between 13 cases of VV lesions and the perilesional normal skin.
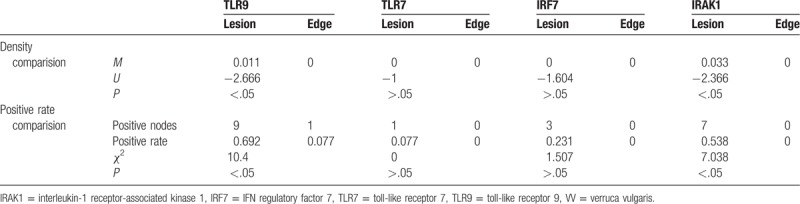
A few TLR7+ cells were observed in the papillary dermis. The cytoplasm was stained brown, and no obvious positive cells were observed in the epidermis (see Fig. 5). The TLR7 expression (see Table 3) was compared between the skin lesions from the 20 cases of VV and the perilesional normal skin from the 13 of those cases with normal skin at the edge of lesions. The comparison showed that 2 of the 20 cases of VV lesions had TLR7+ cells, which was not significantly different from the frequency in the perilesional normal skin (0) (P > .05). The TLR7+ cell density M of the 20 cases of VV lesions was not significantly different from that of the perilesional normal skin (P > .05). A paired comparison was performed to evaluate the TLR7 expression (see Table 4) in the skin lesions and the perilesional normal skin from the 13 of those cases with normal skin at the edge of lesions. This comparison showed that of the 13 cases of VV skin lesions, 1 case had TLR7+ cells, which was not significantly different from the results for the perilesional normal skin (P > .05). The TLR7+ cell density M of the 13 cases of VV lesions was not significantly different from that in the perilesional normal skin (P > .05).
Figure 5.

Expressions of TLR7 in VV lesions and the perilesional normal skin at the edge (SP ×400). (A) Few TLR7+ cells with brown cytoplasm were observed in the epidermal of VV lesions (B) A small number of TLR7+ cells with brown cytoplasm were observed in the papillary layer of VV lesion. (C) Few TLR7+ cells were observed in the epidermal and few TLR7+ cells with brown cytoplasm in the papillary layer of perilesional normal skin at the edge of VV. VV = verruca vulgaris.
3.4. Expression of IRF7 and IRAK1 in VV skin lesions
A few IRF7+ cells were observed in the papillary dermis, and no positive cells were observed in the epidermis (see Fig. 6). The IRF7 expression (see Table 3) in the 20 cases of VV lesions and in the perilesional normal skin of 13 VV lesions was compared, showing that 5 of the 20 cases of VV lesions had IRF7+ cells, which was not significantly different from the frequency in the perilesional normal skin (P > .05). The IRF7+ cell density M of the 20 lesions of VV was not significantly different from that of perilesional normal skin (P > .05). A paired comparison was performed to evaluate the IRF7 expression (see Table 4) in the lesions and the perilesional normal skin from the 13 cases of VV with normal skin at the edge. The comparison showed that 3 of the 13 cases of VV skin lesions had IRF7+ cells, which was not significantly different from the result in the perilesional normal skin (P > .05). The IRF7+ cell density M of the 13 cases of VV lesions was not significantly different from that of the perilesional normal skin (P > .05).
Figure 6.

Expressions of IRF7 in VV lesions and the perilesional normal skin at their edge (SP ×400). (A) Few IRF7+ cells with brown cytoplasm were observed in epidermal of VV lesion. (B) Few IRF7+ cells with brown cytoplasm were observed in the papillary layer of VV lesion. (C) Few IRF7+ cells with brown cytoplasm in both epidermal and the papillary layer of perilesional normal skin of VV lesion. IRF7 = IFN regulatory factor 7, VV = verruca vulgaris.
A few IRAK1+ cells were observed in the papillary dermis, and the cytoplasm was stained brown; no positive cells were observed in the epidermis (see Fig. 7). The IRAK1 expression (see Table 3) was compared between the 20 cases of VV and the 13 of those cases that had perilesional normal skin at the edge of the skin lesions. The comparison showed that 10 of the 20 cases of VV lesions had IRAK1+ cells, which was a significantly higher frequency than that in the perilesional normal skin (P < .05). The IRAK1+ cell density M of the 20 cases of VV lesions was also higher than that of the perilesional normal skin (P < .05). A paired comparison of the IRAK1 expression (see Table 4) was performed using the skin lesions of the 13 cases of VV with perilesional normal skin from the 13 cases of VV with normal skin at the edge, showing that 7 of the 13 cases of VV lesions had IRAK1+ cells, which was a significantly higher occurrence than that in the perilesional normal skin (P < .05). The IRAK1+ cell density M of the 13 cases of VV lesions was significantly higher than that of the perilesional normal skin (P < .05).
Figure 7.
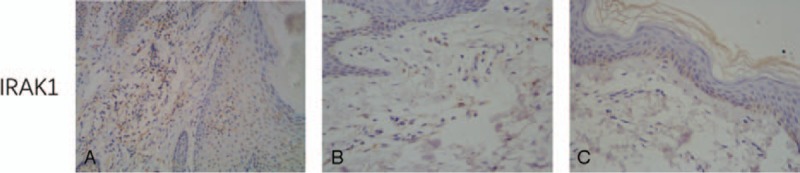
Expressions of IRAK1 in VV lesions and the perilesional normal (SP ×400). (A) Few IRAK1+ cells with brown cytoplasm in epidermal of perilesional normal skin of VV lesion. (B) A small number of IRAK1+ cells with brown cytoplasm were observed in the dermal papillary layer of VV lesion. (C) Few IRAK1+ cells with brown cytoplasm in both epidermal and the papillary layer of perilesional normal skin of VV lesion. IRAK1 = interleukin-1 receptor-associated kinase 1, VV = verruca vulgaris.
3.5. Correlation analysis
In the 20 cases of VV lesions, the CD2AP+ and CD123+ cell densities exhibited a significant positive correlation (correlation coefficient r = 0.864, P < .05) (Fig. 8). This finding suggests high consistency between the CD123 and CD2AP expression in VV lesions; therefore, both CD123+ and CD2AP+ cells can be considered pDCs. The pDC density in VV lesions has a non-linear correlation with the LC density in the epidermis (r = 0.235, P > .05) (Fig. 9). This result suggests that pDCs and LCs play separate roles in the local immune response in VV.
Figure 8.
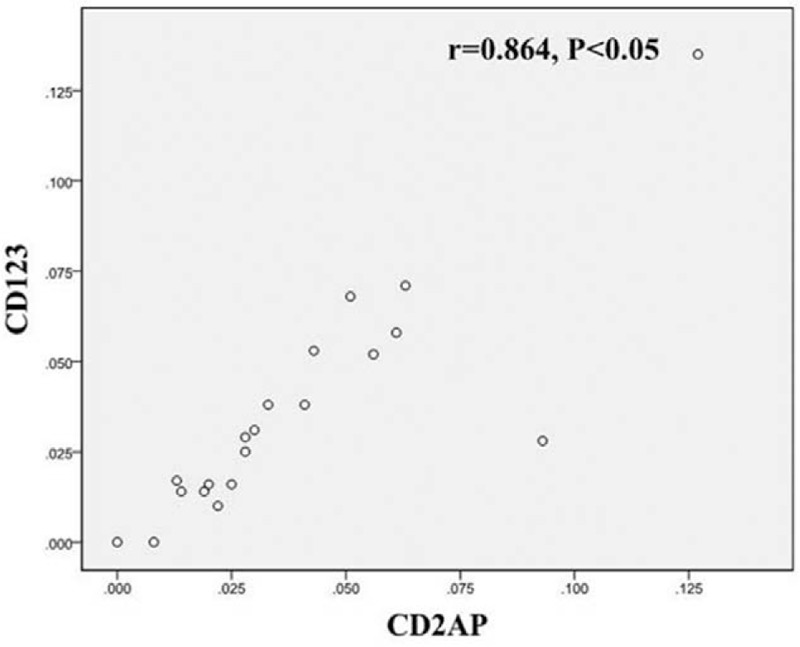
Correlation between CD2AP+ and CD123+ cells in VV lesions. VV = Verruca vulgaris.
Figure 9.

Correlation between pDCs and LCs in VV lesions. LCs = Langerhans cells, pDCs = plasmacytoid dendritic cells, VV = verruca vulgaris.
4. Discussion
VV is mainly caused by infection of HPV-1, 2, 4, 27, 57, and 63; however, VV is not curable by CO2 laser, liquid nitrogen, and other therapies. Inadequate host immune response to HPV infection is thought to be the main mechanisms of the relapse of VV. LCs are specialized epidermal APCs, and they play a crucial role in immune surveillance, which directly affects the clearance of HPV from the epithelium. CD1a is one of the most specific markers on LCs; only human epidermal LCs can express CD1a.[6] It was shown that CD1a+ LCs in the epidermis of condylomata acuminata decreased significantly compared with those in the perilesional normal skin.[7] However, our previous study demonstrated that the CD1a+ LC density in both epidermis and dermis of condyloma acuminatum lesions remained unchanged.[8] With immunohistochemical staining, the present study finds that the morphology and expression density of CD1a+ LCs in the epidermis of VV lesions do not significantly differ from those of the perilesional normal skin, whereas the expression density and positive percentage of CD1a+ LCs in the dermis are significantly higher than those of the perilesional normal skin. We speculate that in VV, LCs migrate to the dermis to perform antigen-presenting functions. Whether the LCs that migrate to the dermis can stimulate T lymphocytes to initiate an immune response is still unclear.
pDCs, another subgroup of DC in skin, are key players in the initiation and regulation of immune responses. CD123 and CD2AP are specific surface markers for these DCs.[9] TLRs are important pattern recognition receptors in innate immunity. TLR signaling results in the activation of nuclear factor κB (NF-κB), which triggers the production of a variety of antimicrobial and proinflammatory cytokines and chemokines.[10–12]
pDCs specifically express high levels of TLR7 and TLR9,[13] which can trigger signal transduction through a MyD88-dependent pathway to activate NF-κB or the interferon regulatory factor family (IRFs) to regulate the expression of inflammatory cytokines and type I IFN, thereby generating an antiviral response.[14] Some of the major molecules involved in TLR7/9 signal transduction pathways in pDCs are IRAK1 and IRF7.[15] Upon infection by herpes simplex virus[16] and cytomegalovirus,[17] pDCs mediate and secrete a large amount of type I IFN via the TLR signaling pathway to inhibit viral replication. In contrast, the TLR signaling pathway is not effectively activated in HPV16 infection,[18] leading to persistent viral infection.
Our results revealed that the expression of CD123 and CD2AP in VV lesions was highly correlated; therefore, CD123+ and CD2AP+ cells can both be considered as pDCs. The densities and percentages of CD123+ pDCs and CD2AP+ pDCs in VV lesions were significantly higher than those in the perilesional normal skin, suggesting that the quantity of pDCs in VV lesions was slightly increased. Saadeh et al also have reported that pDCs constitute a central component of the inflammatory host response in inflamed warts.[19] We speculate that HPV infection affects the quantity of pDCs in local skin and that pDCs may be involved in the local anti-HPV infection immune response.
Our results demonstrated that the density and percentage of TLR9+ cells in the dermis of VV lesions were higher than in the perilesional normal skin. TLR9 could also express in fibroblast cells, and other monocyte-like cells such as DCs. Ku et al previously reported TLR9 was weakly expressed in normal skin but strongly expressed in VV lesions.[20] As the TLR9+ and TLR7+ cells observed in the dermis were mostly monocyte-like cells, and far more TLR9 and TLR7 is expressed in pDCs than in DCs, this finding indicated a possible increase in the number of TLR9-expressing pDCs. In addition, TLR9 expression can be observed in the keratinocytes of each epidermal layer of VV skin lesions, but mainly in the spinous layer, implying that keratinocytes can also express TLR9. Our study also demonstrated that the density and percentage of TLR7+ cells in VV lesions do not significantly differ from those of the perilesional normal skin, suggesting that TLR7 is not upregulated in VV lesions and that the TLR7 secretion function of pDC is not significantly activated. It is hypothesized that HPV infection may partially inhibit pDC function, which would be related to the recurrence and relapse of VV. Moreover, the density and percentage of IRAK1+ cells in VV lesions are higher than those in the perilesional normal skin. In contrast, the density and percentage of IRF7+ cells remained unchanged from those of the perilesional normal skin, implying that the downstream pathway of TLR9 may be to some extent activated. However, those marker's mRNA expression studies are required to further confirm the TLR signaling pathway mechanism in VV. Our previous studies found that the density and percentage of IRAK1+ and IRF7+ cells in condyloma acuminata skin lesions were not significantly different from those of the perilesional normal skin.[21] This study also found a nonlinear correlation between the pDC and LC densities, implying that the roles of pDCs and LCs in the local immune response in VV might not be relevant.
5. Conclusion
In summary, the number of LCs increases only in the dermis of VV lesions, indicating that LC's antigen-presenting function might not be inhibited. The expression of pDCs, TLR9 and IRAK1 in VV skin lesions are upregulated, but not IRF7. We speculated the TLR7/9 downstream signaling pathway is not completely and effectively activated; thus, pDCs are not able to establish a sufficient and effective anti-HPV immune response, thereby triggering a persistent HPV infection with a clinically long disease duration that is prone to relapse. Meanwhile, how HPV affects pDC function and the downstream transduction of the TLR pathway requires further study.
Author contributions
Conceptualization: Xiaoxia Zhu, Hao Cheng.
Data curation: Xiaoxia Zhu, Qiang Zhou.
Formal analysis: Xiaoxia Zhu, Qiang Zhou.
Funding acquisition: Rui Han, Hao Cheng.
Investigation: Yi Tang.
Methodology: Rui Han.
Project administration: Xiaoxia Zhu.
Resources: Rui Han, Qiang Zhou, Hao Cheng.
Software: Yi Tang, Xiaoxia Zhu, Rui Han.
Validation: Yi Tang.
Visualization: Rui Han.
Writing – original draft: Yi Tang.
Writing – review & editing: Hao Cheng.
Footnotes
Abbreviations: DCs = dendritic cells, HPV = human papillomavirus, IFN = regulatory factor (IRF) 7, IRAK1 = interleukin-1 receptor-associated kinase 1, LCs = langerhans cells, PBS = phosphate-buffered saline, pDCs = plasmacytoid dendritic cells, TLR7 = toll-like receptor 7, TLR9 = toll-like receptor 9, VV = verruca vulgaris.
How to cite this article: Tang Y, Zhu X, Han R, Zhou Q, Cheng H. Expression of langerhans cell and plasmacytoid dendritic cell markers, and toll-like receptor 7/9 signaling pathway proteins in verruca vulgaris lesions. Medicine. 2020;99:8(e19214).
YT and XZ contributed equally to this work.
This work was supported by the fund of Health and Family Planning Commission of Zhejiang Province (2017KY215 and 2019PY001).
The authors have no conflicts of interest to disclose.
References
- [1].Zhou Q, Zhu K, Cheng H. Toll-like receptors in human papillomavirus infection. Arch Immunol Ther Exp (Warsz) 2013;61:203–15. [DOI] [PubMed] [Google Scholar]
- [2].Yan L, Woodham AW, Da Silva DM, et al. Functional analysis of HPV-like particle-activated Langerhans cells in vitro. Methods Mol Biol 2015;1249:333–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol 2008;8:594–606. [DOI] [PubMed] [Google Scholar]
- [4].Honda K, Yanai H, Negishi H, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 2005;434:772–7. [DOI] [PubMed] [Google Scholar]
- [5].Sharma MD, Baban B, Chandler P, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest 2007;117:2570–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Feng JY, Peng ZH, Tang XP, et al. Immunohistochemical and ultrastructural features of Langerhans cells in condyloma acuminatum. J Cutan Pathol 2008;35:15–20. [DOI] [PubMed] [Google Scholar]
- [7].Pan YZ, Wang HL, Wang F, et al. Changes in distribution and ultrastructure of Langerhans cells in condyloma acuminatum tissues, and analysis of the underlying mechanism. Dermatologica Sinica 2013;31:120–5. [Google Scholar]
- [8].Zhang X, Wang J, Qian W, et al. Detection of Langerhans cells and plasmacytoid dendritic cells in condyloma acuminatum lesions. Chin J Dermatol 2015;48:343–5. [Google Scholar]
- [9].Dzionek A, Fuchs A, Schmidt P, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol 2000;165:6037–46. [DOI] [PubMed] [Google Scholar]
- [10].Rock FL, Hardiman G, Timans JC, et al. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci U S A 1998;95:588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature 2000;406:782–7. [DOI] [PubMed] [Google Scholar]
- [12].Ulevitch RJ, Mathison JC, da Silva Correia J. Innate immune responses during infection. Vaccine 2004;22 Suppl 1:S25–30. [DOI] [PubMed] [Google Scholar]
- [13].Jarrossay D, Napolitani G, Colonna M, et al. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol 2001;31:3388–93. [DOI] [PubMed] [Google Scholar]
- [14].Boo KH, Yang JS. Intrinsic cellular defenses against virus infection by antiviral type I interferon. Yonsei Med J 2010;51:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Beutler BA. TLRs and innate immunity. Blood 2009;113:1399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Donaghy H, Bosnjak L, Harman AN, et al. Role for plasmacytoid dendritic cells in the immune control of recurrent human herpes simplex virus infection. J Virol 2009;83:1952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Varani S, Cederarv M, Feld S, et al. Human cytomegalovirus differentially controls B cell and T cell responses through effects on plasmacytoid dendritic cells. J Immunol 2007;179:7767–76. [DOI] [PubMed] [Google Scholar]
- [18].Hirsch I, Caux C, Hasan U, et al. Impaired Toll-like receptor 7 and 9 signaling: from chronic viral infections to cancer. Trends Immunol 2010;31:391–7. [DOI] [PubMed] [Google Scholar]
- [19].Saadeh D, Kurban M, Abbas O. Plasmacytoid dendritic cells and type I interferon in the immunological response against warts. Clin Exp Dermatol 2017;42:857–62. [DOI] [PubMed] [Google Scholar]
- [20].Ku JK, Kwon HJ, Kim MY, et al. Expression of Toll-like receptors in verruca and molluscum contagiosum. J Korean Med Sci 2008;23:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rock FL, Hardiman G, Timans JC, et al. The expression of TLR7/9 and associated signaling pathway proteins in condyloma accuminatum lesions. Chin J Exp Clin Virol 2016;95:26–9. [Google Scholar]


