Abstract
Background:
The aim of this meta-analysis was to assess the clinicopathological features and to confirm prognostic value of POLE exonuclease domain mutations (EDM) in endometrial carcinoma patients.
Methods:
The PubMed, Web of Science, the data of China National Knowledge Infrastructure, and Wan fang Medical Network were systematically searched for relevant articles without a cut-off date. The keywords for the search were “endometrial cancer,” “endometrial carcinoma,” “EC,” “POLE mutations,” “POLE exonuclease domain mutations,” “POLE-mutant,” “clinical characteristics” “prognostic.” Pooled hazard ratios (HRs) and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated by using Review manager 5.3 and Stata 14.0 statistical software.
Results:
Six cohort studies assessing 179 EC patients with POLE EDMs were included. The results indicated a favorable progression-free survival in POLE-mutant patients (HR = 0.32; 95% CI: = [0.09–1.18]). Furthermore, the overall survival was great in patients with POLE-mutant (HR = 0.68; 95% CI = [0.41–1.13]). It was shown that a significantly higher incidence of POLE mutations with Federation of International of Gynecologists and Obstetricians (FIGO) I group compared to FIGO II-IV group (pooled ORs: 0.34, 95% CI: [0.12–0.94], P = .04), POLE-mutant EC was not significantly associated with histology (OR = 0.56,95% CI: 0.29–1.23), tumor grade (OR = 1.22,95% CI:0.85–1.74), lymph-vascular space invasion (OR = 0.40,95% 0.06–2.42), depth of myometrial invasion (OR = 0.70,95% CI: 0.41–1.18), lymph node status (OR = 0.41, 95% 0.04–4.50), and European Society for Medical Oncology risk groups (OR = 0.68,95% CI: 0.37–1.26).
Conclusion:
This meta-analysis has confirmed POLE EDMs may serve as a predictive biomarker of favorable prognosis. Further studies are needed to explore the appropriate clinical utility of POLE EDMs in EC.
Keywords: clinicopathological characteristics, endometrial cancer, meta-analysis, POLE mutation, prognosis
1. Introduction
Endometrial cancer (EC) is one of the most prevalent among gynecological malignant tumors, and has shown a steady increase in incidence.[1] Although the majority of women with EC have good outcomes, women with advanced disease or more aggressive subtypes may not be curable even with adjuvant therapy.[2] Traditional classification always yields limited prognostic and therapeutic information.[3,4] Recently, great advances have been made in defining the molecular alterations that contribute towards endometrial tumorigenesis.[5–12] The Cancer Genome Atlas Research Network (TCGA) has proposed that ECs can be divided into 4 categories according to various genetic and epigenetic features: an ultramutated phenotype caused by POLE mutations, a hypermutator phenotype caused by the DNA mismatch repair deficiency leading to microsatellite instability (MSI), a copy number low phenotype, and a copy number high phenotype.[5] Interestingly, investigators found that EC patients with POLE mutations reproducibly demonstrate better prognosis even with poor clinicopathological features.[7,13] And they are also candidates for immune checkpoint inhibitor therapy.[14,15] In this meta-analysis, we aimed to summarize the clinicopathological characteristics and prognostic value of POLE mutations of EC patients.
2. Materials and methods
2.1. Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study does not involve patient consent.
2.2. Search strategy
In order to identify all relevant literatures, a comprehensive literature retrieval was conducted in PubMed, Web of Science, data of China National Knowledge Infrastructure, Wan fang Medical Network independently without a cut-off date. The publication searching terms were as follows: “endometrial cancer,” “endometrial carcinoma,” “EC,” “POLE EDM mutations,” “POLE-mutant,” “clinical characteristics,” “prognosis.” The references of included studies were also screened to identify potential suitable publications. There were no restrictions publication date, and the language was limited to English and Chinese.
2.3. Inclusion and exclusion criteria
Titles and abstracts were reviewed and assessed by 2 reviewers (Ya He and Tian Wang). Full text articles were obtained for potentially eligible publications and eligibility criteria applied independently by 2 authors (He Ya and Tian Wang). Inclusion criteria:
-
(1)
Literatures including POLE-mutant EC patients who were tested by gene sequencing;
-
(2)
At least included 5 POLE-mutant EC cases;
-
(3)
Enough data such as Federation of International of Gynecologists and Obstetricians (FIGO) pathological staging group, differentiation degree, lymphatic vessel invasion group, histology type, depth of myometrial invasion, European Society for Medical Oncology (ESMO) risk stratification can be extracted from the included studies;
-
(4)
Sufficient data to calculate hazard ratio (HR), odds ratios (ORs), and 95% confidence interval (CIs) are available.
Exclusion criteria:
-
(1)
Studies without enough data (the above-mentioned) for calculation;
-
(2)
Patients were not confirmed by EDM sequencing;
-
(3)
Duplicated publications or data;
-
(4)
Papers published in other languages other than English or Chinese.
-
(5)
Commentaries, single case reports, editorials, review articles, letters to the editor, and unrelated articles.
2.4. Data extraction and outcome measures
The data and information from all included studies were independently extracted by 2 investigators. The following information was extracted from each study: first author's name, year of publication, study country, the total number of EC patients and POLE-mutant EC patients, histology type, FIGO stage Grade, lymph-vascular space invasion (LVSI), lymph node status, depth of myometrial invasion, ESMO risk stratification, HR, OR and corresponding 95% CIs. A detailed assessment of potential related references was assessed to determine their qualifications. Any differences between them were discussed with the Corresponding author (Yuanjing Hu).
2.5. Statistical analysis
The statistical heterogeneity among the 6 included publications was evaluated by I2 test. All the data were analyzed by Stata 14.0 and Review Manager 5.3 software (Copenhagen: The Nordic Cochrane Centre, Cochrane Collaboration, 2014). We estimated prognostic effects (overall survival [OS] and progression-free survival [PFS]) with HR, if HR <1 reflects favorable prognostic of POLE-mutant EC patients. All statistical values (pooled HR and OR) were reported with 95% CIs, and the 2-sided P-value threshold for statistical significance was set at .05. Heterogeneity was assessed by I2 inconsistency test and chi-squared-based Cochran Q statistic test; I2 > 50% or Ph < .1 indicated significant heterogeneity, the random-effects model was used, and when I2 < 50% and Ph > .1, the fixed effect model was used.
3. Result
3.1. Characteristic of included studies
In the initial research, a total 184 articles were identified, and 128 articles were reviewed after the removal of duplicates. After reviewing the title, abstract and full text, 6 publications were finally included according to the study inclusion and exclusion criteria. The literature retrieval process is detailed in Figure 1. In this article, a total 2802 EC patients were involved, included 371 POLE-mutant patients. All of the included articles were published in English. All POLE-mutant patients were tested by POLE exonuclease domain mutations (EDM) sequencing. The main characteristics of the included 6 studies are presented in Table 1.
Figure 1.
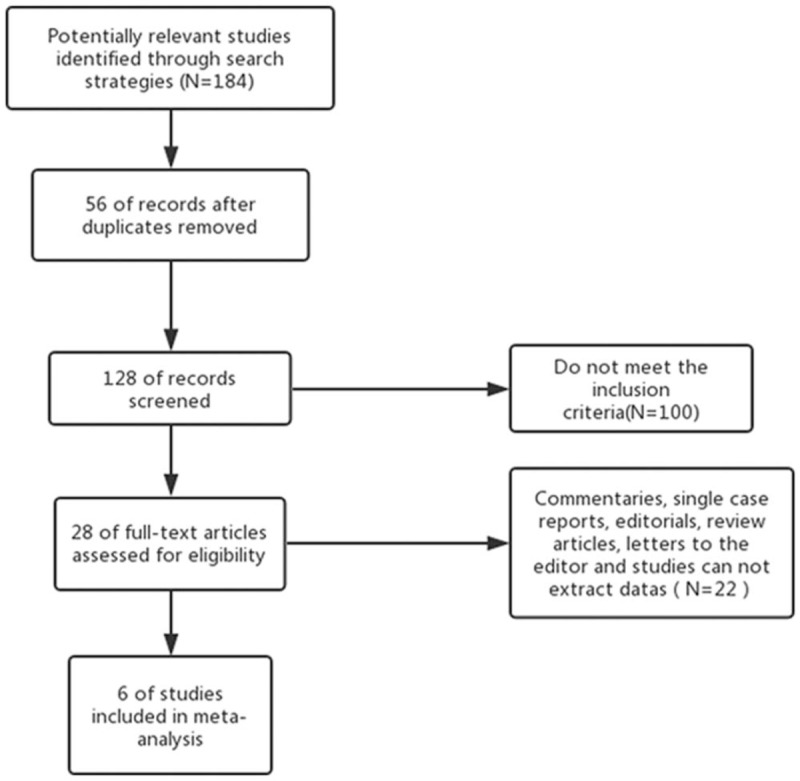
Flowchart showing the literature search and selection process.
Table 1.
The basic characteristics of the included 6 studies.
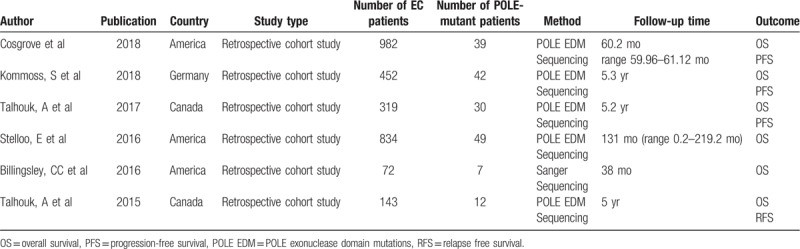
3.2. POLE mutations and OS
Six studies provided the data for OS among 371 POLE-mutant EC patients. When pooling the HR for OS, we found there was no significant heterogeneity between these studies (I2 = 0.00%, Ph = .509), so a fixed effect model was adopted. The estimated HR for OS was 0.68 (95% CI = 0.41–1.13), indicated a favorable OS was associated with POLE-mutant EC patients. The forest plot was shown in Figure 2.
Figure 2.
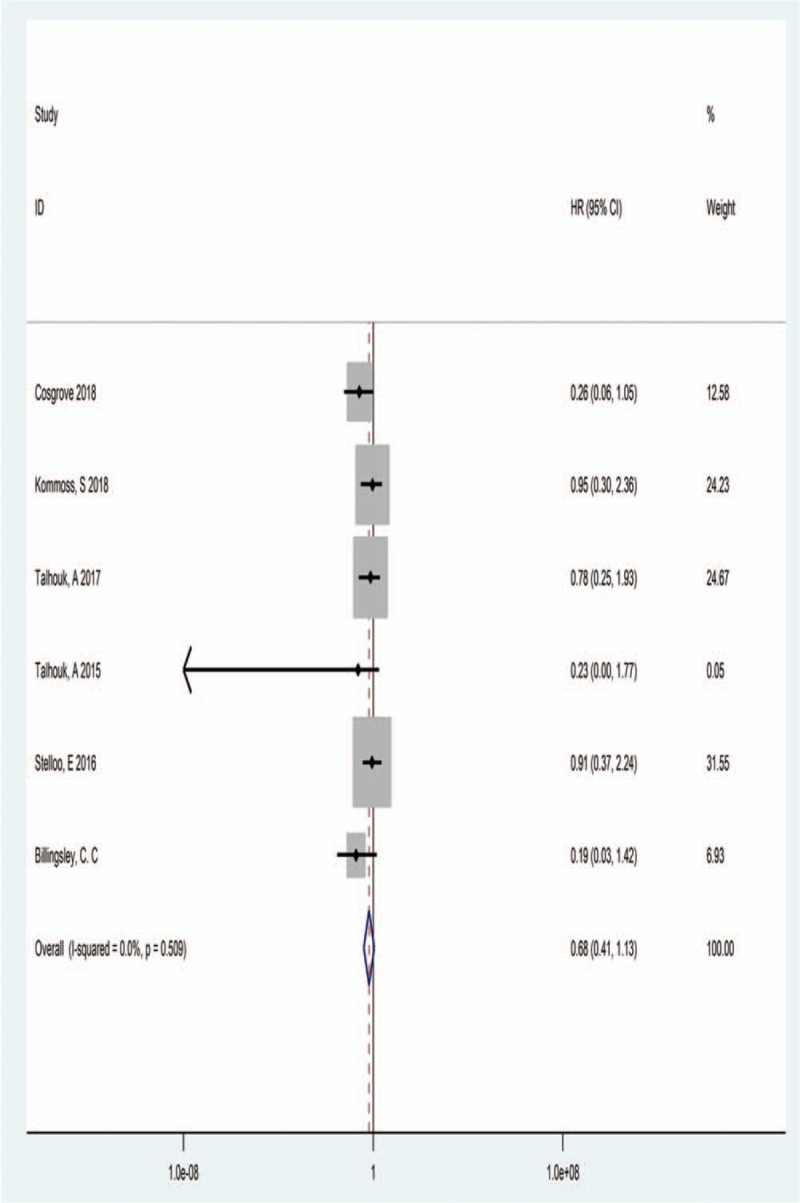
Forest plot of HR for the relationship between POLE mutations and OS. HR = hazard ratios, OR= odds ratios.
3.3. POLE mutations and PFS
Only 2 studies provided appropriate data for analysis of PFS. As showed in Figure 3, there was no severe statistical heterogeneity observed between these studies (I2 = 0.00%, Ph = .458). The fixed effects model was analyzed by the pooled HRs with their corresponding 95% CIs. The overall result of HR was 0.32 (95% CI: 0.09–1.18), indicated a reduced PFS was associated with POLE-mutant EC patients. The results were shown in Figure 3.
Figure 3.
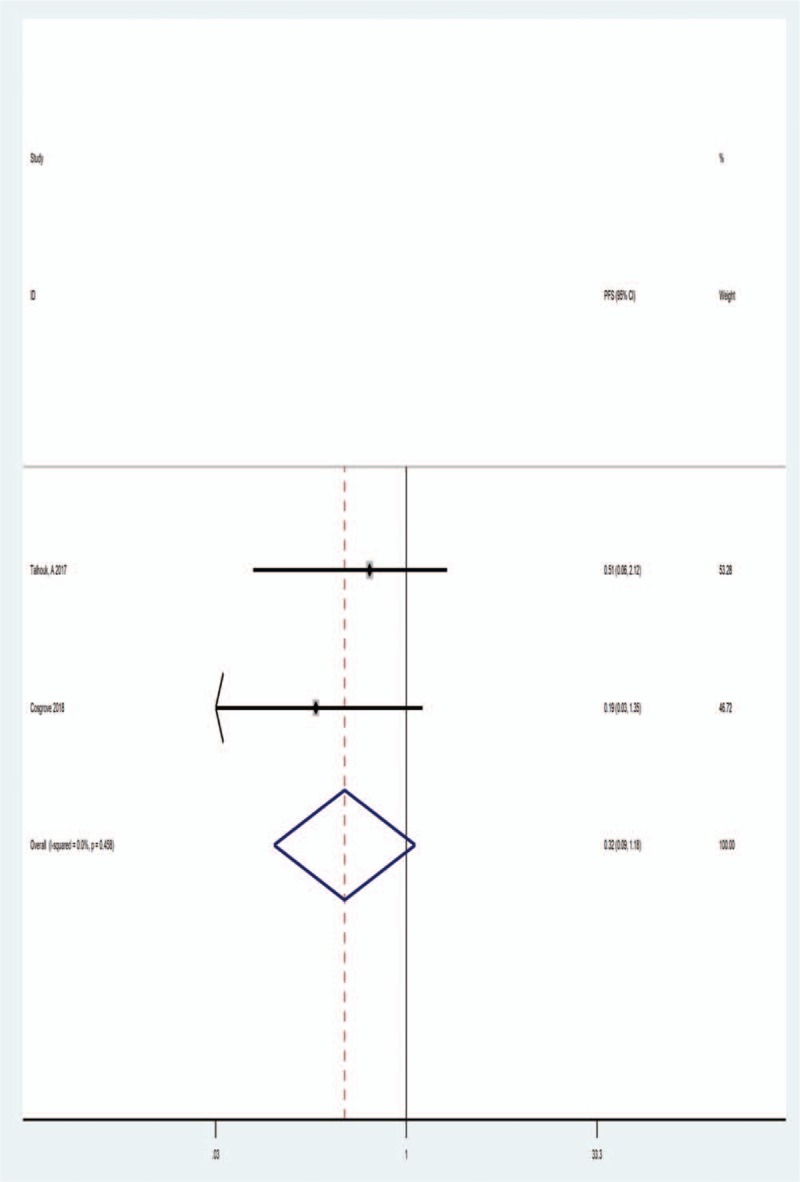
Forest plot of HR for relationship between POLE mutations and PFS. HR = hazard ratios, PFS = progression-free survival.
3.4. Clinicopathological characteristics
We extracted the clinicopathological characteristics from the 6 included studies in order to pool OR value to assess the potential association between POLE mutations with clinical variables. The overall results were shown in Table 2.
Table 2.
Results of association between endometrial cancer with POLE mutations and clinicopathological characteristics.
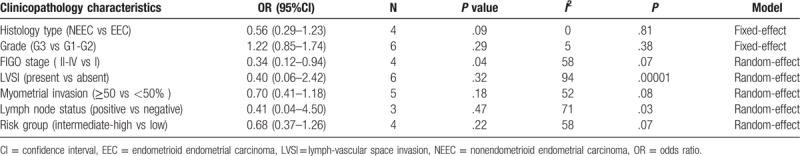
3.5. Histological type in POLE-mutant EC
We can extract histological characteristics from 4 studies, and there was no significant heterogeneity between these studies (I2 = 0%, P = .81). The 95% CI is 0.56 [0.29–1.09], which includes 1, indicated there were no significant between POLE mutations with endometrioid endometrial carcinomas (EECs) and nonendometrioid endometrial carcinomas (NEECs) (shown in Figure 4).
Figure 4.
Histology type in POLE-mutant endometrial cancer.
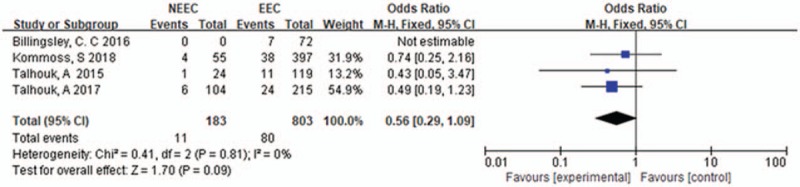
3.6. Tumor grade in POLE-mutant EC
Tumor grade data can be extracted from 6 studies, there was no significant heterogeneity between these studies (I2 = 5%, P = .38), the fixed effects model was used. The pooled OR value was 1.2 [0.85–1.74] (P = .29) demonstrating that there was no significant between G3 and G1-G2 in POLE-mutant EC patients. The result was shown in Figure 5.
Figure 5.
Tumor grade in POLE-mutant endometrial cancer.
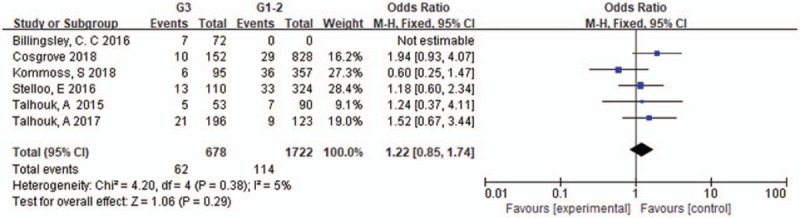
3.7. FIGO stage in POLE-mutant EC
Four articles were selected for analyzing the association of FIGO stage with POLE mutations. As the heterogeneity of these studies was significant (I2 = 58%, P = .07), the random effects model was used. The pooled OR was 0.34 [0.12–0.94] (P = .04), indicating that most of POLE mutations are FIGO I comparing to FIGO II-IV (shown in Figure 6).
Figure 6.
FIGO stage in POLE-mutant endometrial cancer.
3.8. LVSI in POLE-mutant EC
6 studies have been included for analyzing the LVSI in POLE-mutant, there was significant heterogeneity of these studies (I2 = 94%, P < .000011), a random effects model was used, the pooled result was 0.40 [0.06–2.46], P = .32 (shown in Figure 7).
Figure 7.
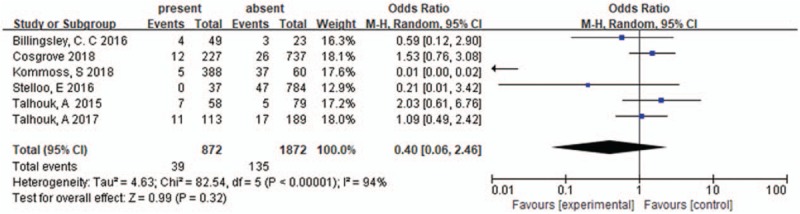
LVSI in POLE-mutant endometrial cancer. LVSI = lymph-vascular space invasion.
3.9. Myometrial invasion in POLE-mutant EC
Five studies were included, the heterogeneity of these studies was significant (I2 = 52%, P = .08), the random effects model was used. The pooled OR of myometrial invasion in POLE-mutants EC was 0.70 [0.41–1.18], P = .18, differences among those studies were not significant. The results were shown in Figure 8.
Figure 8.
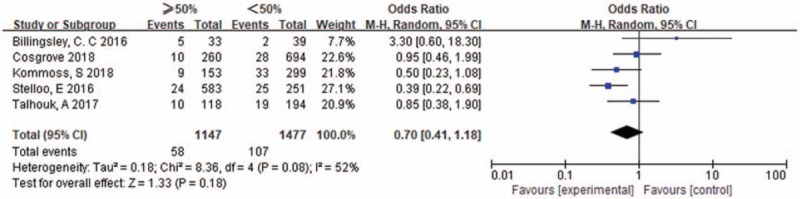
Myometrial invasion in POLE-mutant endometrial cancer.
3.10. Lymph node status in POLE-mutant EC
Three studies were included, the heterogeneity of those studies was significant (I2 = 71%, P = .03), the random effects model was used. The pooled OR of lymph node status in POLE-mutant EC was 0.41 [.04–4.50], P = .47, with no significant differences. The results were shown in Figure 9.
Figure 9.
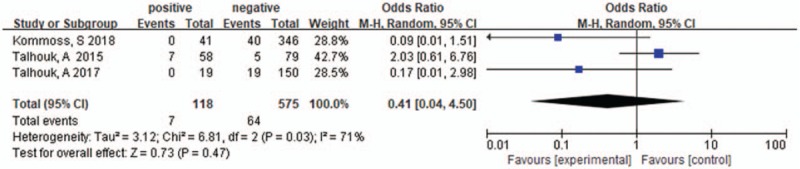
Lymph node status in POLE-mutant endometrial cancer.
3.11. ESMO risk stratification in POLE-mutant EC
Four studies were included, the heterogeneity of those studies was significant (I2 = 58%, P = .07), the random effects model was used. The pooled OR of ESMO in POLE-mutant EC was 0.68 [0.37–1.26], P = .22, with no significant differences. The results were shown in Figure 10.
Figure 10.
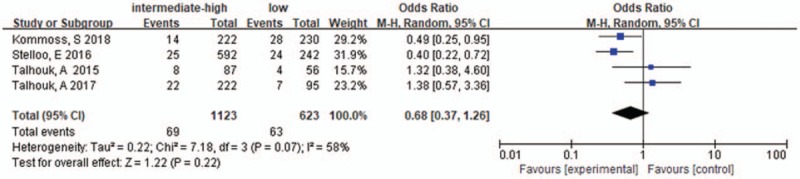
ESMO risk stratification in POLE-mutant endometrial cancer. ESMO = European Society for Medical Oncology.
3.12. The quality of included articles
The methodological quality of the included 6 studies was evaluated by Review manager 5.3, indicating the general quality was good as is shown in Figure 11.
Figure 11.
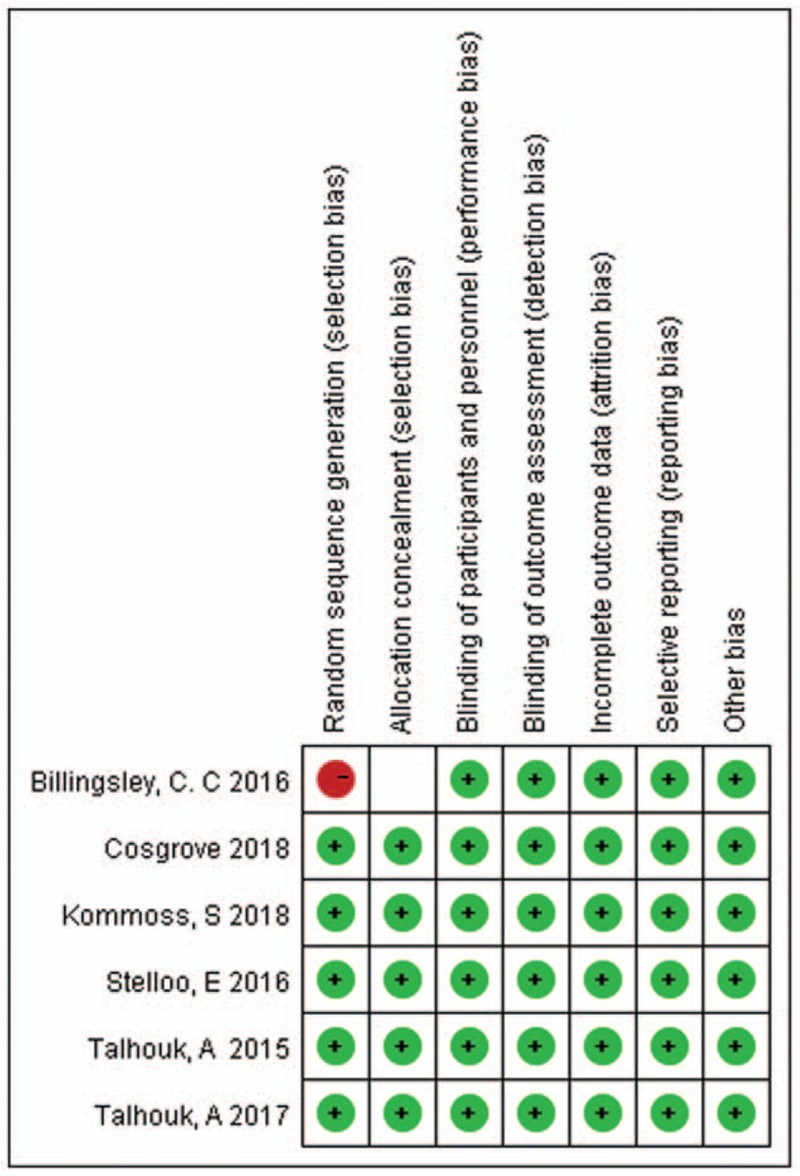
Quality assessment.
3.13. Sensitivity analysis
For meta-analysis of association between the POLE mutations and OS, sensitivity analysis was performed by removing each study in turn from the pooled analysis. We aimed to assess the influence of the removed data set on the overall HR. The result was not significantly influenced by the exclusion of any study, indicating the robustness of the results.
4. Discussion
EC, in particular high-grade cancers, cannot be reliably classified by histomorphology criteria, even by expert pathologists and with the addition of immunohistochemistry.[16–18] In 2013, the multi-institutional TCGA project performed an integrated genomic, transcriptomic, and proteomic analysis of 373 EC patients using massively parallel sequencing and array-based technologies in combination with DNA methylation, reverse phase protein array, and MSI analyses. Based on their results, EC were classified into 4 genomic groups. As for POLE-mutant cohort, an ultramutated subgroup harboring mutations in the EDM of POLE gene, was a new exciting discovery. TCGA describes it characterized by high mutation rates, hotspot mutations and few copy-number aberrations, a high percent of C to A transversions and low percent of C to G transversions, frequently mutated in PTEN, PIK3CA, PIK3CA, FBXW7, KRAS. What's the most important is that these patients have very favorable clinical outcomes, irrespective of high grade and advanced stage. In our meta-analysis, we retrospectively searched previous studies and grossed up the clinicopathology of POLE-mutant patients and prognostic value of POLE mutations. Based on the results, it may be helpful to identify POLE-mutant status at the moment of diagnosis and consider less intensive treatment for patients with POLE-mutations due to the better outcomes reported.
In previous studies, the vast majority of POLE-ultramutated tumor presented at FIGO stage I, grade 3 EEC, rich in tumor-infiltrating lymphocytes and peritumoral lymphocytes, showing marked intertumoral heterogeneity and morphological ambiguity. There are around 8% EC and 15% to 20% of FIGO grade 3 EEC show POLE EDM mutation and an ultratmutated genotype.[5] It is still not clearly understood the reason of the excellent outcomes; however, Meng et al[19] had speculated that this may due to the high mutation burden and increases in base substitution, Howitt et al[20] showed that POLE-mutant EC was associated with high neoantigens and elevated CD8+ tumor infiltrating lymphocytes, which is counterbalanced by overexpression of program death-ligand.
For EC patients, surgical stage is the most important prognostic factor, such as myometrial invasion and lymph node status. Meanwhile, age, histology type, tumor grade, tumor size is also vital prognostic information. In this article; however, we found that POLE-mutant EC presented at FIGO stage I is consistent with previous studies (P = .04). However, we found there were no significant differences in histology type in POLE-mutant EC (11 POLE-mutant NEEC in 183 NEEC, 80 POLE-mutant EEC in 803EEC, P = .09), contrast to formerly reported. And no significant corrections were observed between POLE mutations with histology, tumor grade, LVSI, depth of myometrial invasion, lymph node status, and EMSO risk groups. Currently, risk classifications of EC are mainly based upon clinical and histopathological factors, such as ESMO.[21] In this cohort, the POLE subgroup has an almost equal distribution across risk groups, with a resulting wide variation in therapy. Half of POLE molecular subgroup were identified as “high risk” based on stage, histology, grade.[22] It is clear that there may be both over-treatment and under-treatment of women based solely on application of the ESMO risk-assessment tool. The results of this meta-analysis showed a better OS in POLE-mutant EC patients compared to POLE-wild expression EC, in consistent to previous studies.
Among all the included 137 POLE cases, 90 received adjuvant therapy (data not shown). The high rate of adjuvant therapy is given for the fact that women with POLE mutations had features of poor outcomes. But many studies have suspected that the improved survival seen for POLE-mutant EC patients was due to the high burden these tumor acquire or to combined effects of adjuvant therapy and tumor biology. Nowadays, the POLE mutated cases are mainly detected by next-generation sequencing, but it is not widely available and take weeks, so researchers are working to better characterize POLE mutations in terms of immunophenotype in the future. Cell with POLE-mutations accumulate DNA mutations and can consequently give rise to large numbers of potential neoantigens.
Also, there are some limitations of our meta-analysis. First, the articles we included were small and only 2 studies can be selected for analysis the relationship between POLE mutations with HR of PFS. Second, there was heterogeneity in the articles. The sensitive analysis showed that the results were not significantly influenced by the exclusion any study. When we excluded the study of Talhouk, 2015[22] or Talhouk, 2017,[23] the I2 value raised from .0% to 6.5% and 4.5%, respectively, demonstrating that the heterogeneity is mainly due to these 2 articles. Third, all the articles we included are retrospective studies; therefore, multicenter and high-quality studies are needed to verify our results. One of studies we included selected high-risk EC at first, which may cause a deviation of our study.
In conclusion, we get that POLE EDMs mutations can serve as a biomarker of prognosis, which indicated favorable outcomes for EC. But useful clinical features were not found effectively to identify POLE-mutant patients, excluding early stage EC. Anyway, there are some limitations to our meta-analysis. First, the total sample size was relatively small. Second, as to the collection of POLE EDMs mutations with clinicopathological features, we only found that most of POLE mutant EC patients are in tumor stage I. Third, many studies did not meet the inclusion criterion and were thus excluded, including some contradictory results, which may have caused a deviation of the result of this. Therefore, larger, multicenter, and higher-quality studies with a unified criterion for determining POLE EDMs expression are necessary to validate the results of this study.
Author contributions
Data curation: Ya He.
Formal analysis: Ya He.
Methodology: Tian Wang, Binkai Yang.
Software: Tian Wang, Na Li, Binkai Yang.
Supervision: Yuanjing Hu.
Validation: Yuanjing Hu.
Visualization: Yuanjing Hu.
Writing – original draft: Ya He.
Writing – review and editing: Ya He.
Footnotes
Abbreviations: CI = confidence intervals, EC = endometrial carcinoma, EEC = endometrioid endometrial carcinoma, ESMO = European Society for Medical Oncology, HR = hazard ratios, LVSI = lymph-vascular space invasion, MSI = microsatellite instability, NEEC = nonendometrioid endometrial carcinoma, OR = odds ratios, OS = overall survival, PFS = progression-free survival, POLE EDM = POLE exonuclease domain mutations, TCGA = the Cancer Genome Atlas.
How to cite this article: He Y, Wang T, Li N, Yang B, Hu Y. Clinicopathological characteristics and prognostic value of POLE mutations in endometrial cancer: A systematic review and meta-analysis. Medicine. 2020;99:8(e19281).
This work was supported by the Scientific Research Funding of Tianjin Science and Technology Committee (17ZXMFSY00160).
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].McConechy MK, Talhouk A, Leung S, et al. Endometrial carcinomas with POLE exonuclease domain mutations have a favorable prognosis. Clin Cancer Res 2016;22:2865–73. [DOI] [PubMed] [Google Scholar]
- [3].Gilks CB, Oliva E, Soslow RA. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol 2013;37:874–81. [DOI] [PubMed] [Google Scholar]
- [4].Werner HM, Trovik J, Marcickiewicz J, et al. A discordant histological risk classification in preoperative and operative biopsy in endometrial cancer is reflected in metastatic risk and prognosis. Eur J Cancer 2013;49:625–32. [DOI] [PubMed] [Google Scholar]
- [5].Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stelloo E, Bosse R, Nout RA, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol 2015;28:836–44. [DOI] [PubMed] [Google Scholar]
- [7].McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol 2016;34:3062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stelloo E, Nout RA, Osse EM, et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin Cancer Res 2016;22:4215–24. [DOI] [PubMed] [Google Scholar]
- [9].Alkushi A, Kobel M, Kalloger SE, et al. High-grade endometrial carcinoma: serous and grade 3 endometrioid carcinomas have different immunophenotypes and outcomes. Int J Gynecol Pathol 2010;29:343–50. [DOI] [PubMed] [Google Scholar]
- [10].Salvesen HB, Haldorsen IS, Trovik J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol 2012;13:e353–61. [DOI] [PubMed] [Google Scholar]
- [11].McAlpine JN, Temkin SM, Mackay HJ. Endometrial cancer: not your grandmother's cancer. Cancer 2016;122:2787–98. [DOI] [PubMed] [Google Scholar]
- [12].Church DN, Stelloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst 2015;107:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stelloo E, Bosse T, Nout RA, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol 2015;28:836–44. [DOI] [PubMed] [Google Scholar]
- [14].Mehnert JM, Panda A, Zhong H, et al. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J Clin Investig 2016;126:2334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science (New York, NY) 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Guan H, Semaan A, Bandyopadhyay S, et al. Prognosis and reproducibility of new and existing binary grading systems for endometrial carcinoma compared to FIGO grading in hysterectomy specimens. Int J Gynecol Cancer 2011;21:654–60. [DOI] [PubMed] [Google Scholar]
- [17].Han G, Sidhu D, Duggan MA, et al. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod Pathol 2013;1594–604. [DOI] [PubMed] [Google Scholar]
- [18].Hoang LN, McConechy MK, Köbel M, et al. Histotype-genotype correlation in 36 high-grade endometrial carcinomas. Am J Surg Pathol 2013;37:1421–32. [DOI] [PubMed] [Google Scholar]
- [19].Meng B, Hoang LN, McIntyre JB, et al. POLE exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol Oncol 2014;134:15–9. [DOI] [PubMed] [Google Scholar]
- [20].Howitt BE, Shukla SA, Sholl LM, et al. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol 2015;1:1319–23. [DOI] [PubMed] [Google Scholar]
- [21].Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Radiother Oncol 2015;117:559–81. [DOI] [PubMed] [Google Scholar]
- [22].Talhouk A, McConechy MK, Leung S, et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer 2015;113:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Talhouk A, McConechy MK, Leung S, et al. Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017;123:802–13. [DOI] [PubMed] [Google Scholar]


