Abstract
BACKGROUND:
Recent studies suggest human neck brown adipose tissue (BAT) to consist of ‘brown adipocyte (BA)-like’ or beige adipocytes. However, little is known about their thermogenic function. Within the beige adipocyte transcriptome, fibroblast growth factor-21 (FGF21) is a gene whose protein product acts as an adipokine, regulating cold-induced thermogenesis in animals. Here, we explored (i) the adipogenic potential, thermogenic function and FGF21 secretory capacity of beige adipocytes derived from human neck fat and (ii) the role of FGF21 in modulating adipose bioenergetics.
METHODS:
Progenitors isolated from human cervical fat were differentiated into adipocytes with either a BA-like or white adipocyte (WA) phenotype. FGF21 secretion was measured by enzyme-linked immuosorbent assay. Real-time PCR/western blotting was used to determine cellular mRNA/protein levels. Extracellular flux bioanalyzer was used to quantify adipocyte oxygen consumption and fatty acid oxidation. Adipocyte heat production was measured by infrared thermography.
RESULTS:
Under hormonal manipulation, primary human neck pre-adipocytes differentiated into adipocytes with either BA-like or WA phenotypes, on gene/protein and functional levels. BA-like cells expressed beige but not classic BA markers. During BA differentiation, FGF21 gene expression and secretion were increased, and were augmented following norepinephrine exposure (a cold mimic in vitro). Differentiated WA expressed β-klotho, a critical co-factor mediating FGF21 action. Treatment of WA with FGF21-induced UCP1 expression and increased oxygen consumption, respiratory uncoupling, norepinephrine-mediated thermogenesis, fatty acid oxidation and heat production, thus recapitulating the association between cold-induced FGF21 secretion and cold-induced thermogenesis in vivo.
CONCLUSION:
Beige adipocytes are thermogenic in humans. FGF21 is a beige adipokine capable of promoting a brown fat-like thermogenic program in WAs.
SIGNIFICANCE:
This study provides first evidence of inducible functional thermogenic beige adipogenesis in human neck fat. FGF21 holds promise as a cold-induced beige adipokine with metabolic benefits of therapeutic relevance through browning of white adipose tissue.
Keywords: fibroblast growth factor 21, FGF21, brown adipose tissue, beige adipose tissue, brite adipose tissue, adipokine, infrared thermography
INTRODUCTION
White adipose tissue (WAT) was once believed to be the only significant adipose depot in adult humans. Advances in positron emission tomography (PET) technology have led to the rediscovery of brown adipose tissue (BAT) in adults, most abundantly located in the cervical-supraclavicular region.1–6 BAT in humans is metabolically active, and greater abundance is associated with lower adiposity and glycemia, suggesting regulatory links with energy metabolism.7 Very recently, developmental molecular tracing experiments in animals have identified a third kind of adipose tissue, consisting of brown adipocyte (BA)-like cells (known as ‘beige’8 or ‘brite’9,10 adipocytes), which can be induced within WAT by cold stimulation.9 Independent laboratories have since discovered ‘beige’ gene expression in human neck ‘BAT’,8,11 suggesting BA-like or beige, rather than classic BAs, are the predominant cell types in human BAT.
Classic brown and beige adipocytes are morphologically indistinguishable. At the basal state, beige adipocytes function similarly to white adipocytes (WAs), with little thermogenic activity.9,12 However, they possess considerable thermogenic potential and can be transformed into ‘BA-like’ cells in a permissive hormonal milieu. Among the various ‘browning’ agents identified,12 fibroblast growth factor-21 (FGF21) is of particular functional and clinical relevance.
FGF21 is a novel hormone that regulates energy homeostasis. Pharmacological FGF21 administration protects animals from obesity and diabetes.13 In rodents, BAT is a source of FGF2114–16 and its secretion is augmented by cold exposure, leading to browning of WAT, thus defining a regulatory role of FGF21 in adaptive thermogenesis.15 Of particular relevance to human BAT is the identification of FGF21 as a beige fat gene and adipocytes with high FGF21 expression also manifest high browning potential.8
To date, evidence supporting metabolic significance of human neck beige fat is associative. Although flurodeoxyglucose uptake in positron emission tomography imaging indicates metabolic activity of beige fat, it is indirect, as fatty acid, rather than glucose, is its chief substrate.17 Whether human beige fat is functional and thermogenic is unclear. In this study, we first demonstrate the capacity of primary human pre-adipocytes harvested from the neck region in differentiating into functional adipocytes with BA-like phenotype expressing beige fat-specific genes. We next show differentiated BA-like cells secrete FGF21. Finally, we reveal FGF21 exposure induces a brown fat thermogenic program in differentiated WAs. This study thus uncovers functional and thermogenic capacity of neck beige adipocytes and provides evidence supporting FGF21 as a thermogenic beige adipokine in humans.
MATERIALS AND METHODS
Primary adipocyte culture
In humans, BAT is most commonly found in cervical-supraclavicular region, which harbor adipogenic precursors.18 We isolated progenitors from stromal vascular fraction of cervical fat during elective thyroid surgery from 17 subjects (age 45 ± 7 years, F = 9), as previously described.18 Cells were cultured in 75 cm3 flasks until 90% confluence in growth medium, which was Dulbecco’s modified Eagle’s medium with 10% newborn calf serum (Invitrogen, Carlsbad, CA, USA), 10 mM HEPES, 33 μM biotin (Sigma, St Louis, MO, USA) and 50 U ml−1 penicillin and 50 mg ml−1 streptomycin. Cells were then trypsinized and plated either in 0.2% gelatin-coated standard 24-well (25 × 104 cells per well) microplates for RNA/protein isolation and/or FGF21 secretion analysis, 24-well (5 × 104 cells per well) microplates (Seahorse Bioscience, North Billerica, MA, USA) for cellular respiration experiments, or 96-well (1 × 104 cells per well) microplates for infrared thermography (IRT) studies. Differentiation of adipocytes was undertaken in microplates.
For differentiation of pre-adipocytes into BA-like cells, they were first switched to induction medium, which was growth medium supplemented with 1 μM dexamethasone (Sigma), 500 um 3-isobutyl-1-methylxanthine (Sigma), 0.85 μM insulin (Invitrogen), 1 nM triiodothyronine (Sigma) and 1 mM rosiglitazone (Sigma). After 2 days, cells were changed to differentiation medium, which was induction medium without dexamethasone but with added 1 nM CL 316243 (Sigma). For WA differentiation, proliferation, induction and differentiation media containing the same basal composition, supplemented with 200 mM indomethacin but without rosiglitazone, triiodothyronine and CL 316243, were used. Medium was changed every other day during proliferative and differentiative phases for both BA and WA, until full differentiation.
FGF21 measurement
FGF21 concentration in culture medium was measured by ELISA (BioVendor, Oxford, UK), according to the manufacturer’s protocol, with intra-assay and inter-assay coefficients of variation of 3.6% and 3.8%, respectively. To mimic the effect of cold exposure in vitro, adipocytes were incubated in 1 um of norepinephrine (Sigma) overnight before FGF21 secretory response analysis.
FGF21 treatment
After differentiation in white adipogenic cocktail for 5 days, differentiated WA were treated for 6 days with 1, 10 or 100 nM recombinant FGF21 protein (Abcam, Cambridge, UK) or vehicle (phosphate-buffered saline), before gene expression analysis to determine optimal FGF21 dosage. WA was treated with 100 nM FGF21 in subsequent cellular respiration experiments.
RNA/protein isolation, real-time quantitative PCR and western blotting
Total RNA/protein from cultured adipocytes was isolated by Trizol reagent (Invitrogen) following the manufacturer’s instruction. One microgram of RNA was reverse-transcribed for quantitative PCR. RNA concentration/ quality was assessed by a NanoDrop spectrophotometer (NanoDrop Technology, Wilmington, DE, USA). For quantification of target gene mRNA levels, reverse-transcriptase PCR was performed on a GeneAmp PCR System 9700 using the Multiscribe Reverse Transcriptase method (Applied Biosystems, Foster City, CA, USA) using 1 mg of RNA. Real-time quantitative PCR was performed to determine complementary DNA content in triplicate on a Step One Plus instrument (Applied Biosystems) using Taqman Gene Expression assays (Applied Biosystems; Supplementary Table). Relative mRNA levels were calculated by the comparative threshold cycle method using PPIA as the housekeeping gene. For western blotting, protein concentrations were determined using BCA Protein Assay Kit (Thermo Scientific Pierce, Rockford, IL, USA). Twenty microgram of protein fractions were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted to a nitrocellulose membrane, and detected by rabbit polyclonal anti-human-UCP1 (U6382; Sigma) and mouse monoclonal anti-human-beta-actin (A5316; Sigma).
Cellular respiration
Oxygen consumption rate (OCR) was measured using the XF24–3 extracellular flux analyzer (Seahorse Bioscience). Four baseline measurements were undertaken before sequential addition of reagents (see next section) for assessment of respiratory uncoupling, norepinephrine-induced thermogenesis and fatty acid oxidation. Measured OCR values were normalized by total protein content per well using BCA Protein Assay Kit (Thermo Scientific Pierce).
Reagents in cellular respiration experiments
Mitochondrial function assessment was performed in XF24–3 Extracellular Flux bioanalyzer (Seahorse Bioscience). Adipocytes were incubated in XF-assay medium containing 25 mM glucose, 1 mM sodium pyruvate and 2mM GlutMAX. Cells were treated with 1 mM oligomycin to block ATP production, rendering uncoupled respiration. Basal and uncoupled (that is, oligomycin-insensitive) respiration rates were normalized by total cell protein. For determination of norepinephrine-induced thermogenesis, 1 um norepinephrine (Sigma) was injected after baseline measurements. OCR was measured every 6-min afterward for six measurements. For determination of fatty acid oxidation, XF Palmitate-bovine serum albumin or bovine serum albumin control was injected at 100 mM after baseline measurements in Krebs-Henseleit buffer medium according to manufacturer’s manual. OCR was measured every 6-min afterward for six measurements to assess cellular response to palmitate.
Infrared thermography
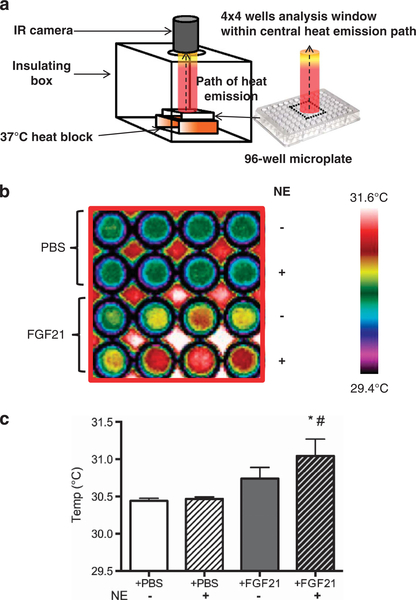
BAT produces heat that can be detected by IRT in humans.19 We therefore measure heat production from cultured adipocytes directly by IRT, as previously described.20 WAs were differentiated and treated with either vehicle (phosphate-buffered saline) or FGF21 in a 96-well microplate, as described under section ‘Primary adipocyte culture’. To mimic the effect of acute cold exposure, adipocytes were treated with 1 uM norepinephrine and then incubated for 10 min at 37 °C, before measuring real-time thermogenesis. IRT was performed by maintaining temperature of the microplate at 37 °C using an insulating system, as shown in Figure 6a. Images were acquired by an infrared camera (FLIR systems 440, Wilsonville, OR, USA), which detects a 7.5–13 μm spectral response with a thermal sensitivity of <0.045 °C, and analyzed using the FLIR Quick Report software (Wilsonville, OR, USA), available at the manufacturer’s website.
Figure 6.
FGF21 increases heat production in WAs. (a) Adipocytes grown in a 96-well microplate were placed on a 37 °C heat block in an insulating polystyrene box, lined with black-colored paper to prevent heat radiation. An infrared (IR) camera was placed 10 cm vertically above microplate through an opening in the insulated-container, capturing the central path of heat emission from the centre of the plate, including 4 × 4 wells, and represents the analysis window displayed in panel b. (b) Infrared thermographic images of adipocytes cultured in microplates treated with vehicle (phosphate-buffered saline, PBS) or FGF21. The temperature scale showed color representation of temperature variation. Norepinephrine (NE) was added to mimic cold exposure. Each column displays adipocytes from one subject (N = 4) treated with either PBS or FGF21, with or without NE. There was a trend of increased heat production by FGF21-treated WA in the basal state (third row in 4 × 4 wells) (P = 0.07), as displayed in graphical format in (c). NE exposure did not alter heat production in untreated adipocytes (second row in 4 × 4 wells). In contrast, heat production increased significantly from FGF21-treated adipocytes on NE exposure (fourth row in 4 × 4 wells). *P<0.01 compared with PBS and #P<0.01 compared with FGF21-treated adipocytes without NE exposure.
Statistics
Statistical analysis was performed using GraphPad Prism 5.0 for Windows (GraphPad Software Inc., La Jolla, CA, USA). Data are expressed as mean±s.d. Paired t-tests were used to compare gene expression between samples. Repeated-measures analysis of variance followed by Bonferroni correction was applied to determine the effect of FGF21 on cellular respiration and heat production. An α error of 0.05 was considered the threshold for statistical significance.
Study approval
The study was approved by the NIDDK-NIAMS institutional review board, and informed consent for the use of surgical discarded material was obtained from all subjects.
RESULTS
Human neck adipogenic precursors differentiate into functional beige adipocytes
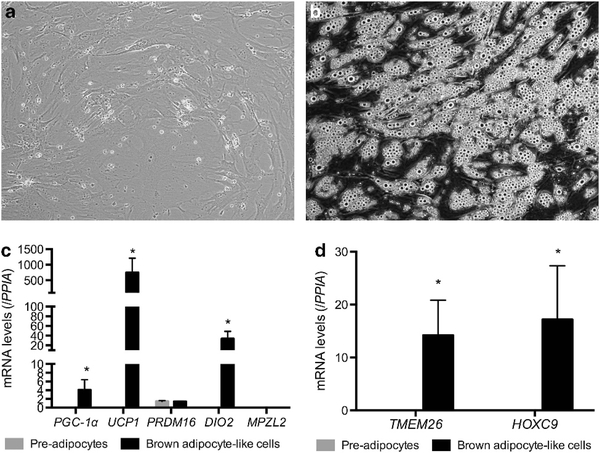
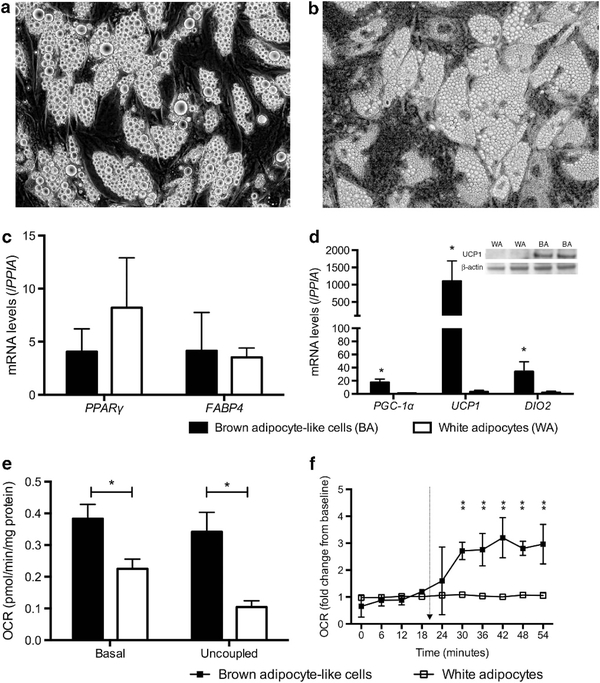
Given the recent identification of beige fat genes in human neck flurodeoxyglucose-avid BAT biopsies,8,11 we first determine whether precursors in human cervical fat differentiate into beige adipocytes. Following isolation of stromal vascular fraction from fat biopsies, fibroblast-like precursors (Figure 1a) differentiated into mature adipocytes with multi-lobulated lipid droplets (Figure 1b) in a brown adipogenic cocktail. Gene expression profiles of pre-adipocytes and differentiated adipocytes were displayed in Figure 1c. Differentiated adipocytes expressed high levels of traditional brown fat genes (UCP1, PGC-1α, PRDM16 and DIO2) but not the novel classic brown fat-specific marker, MPZL2, shown to be expressed only in interscapular BAT in rodents.8 In contrast, the beige fat-specific genes, TMEM26 and HOXC9,8,11 were clearly detectable in differentiated adipocytes (Figure 1d). These results indicate the differentiated BA-like cells were of beige and not classic BA origin.
Figure 1.
Human pre-adipocytes differentiate into BA-like cells. (a) Progenitors isolated from the stromal vascular fraction (SVF) of cervical fat showed fibroblast-like morphology (20 x) in proliferative medium and (b) differentiated into mature adipocytes (20 x) displaying multi- lobulated lipid droplets, (c) expressing high levels of traditional brown fat (UCP1, PGC-Ta, PROMTS and DIO2) but not classic brown fat-specific genes MPZL2 (N = 6). (d) Beige-specific genes were significantly higher in BA-like cells (N = 7). *P<0.01.
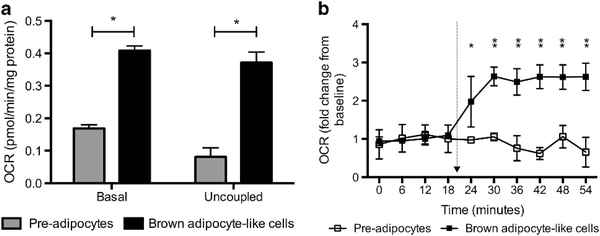
We next characterized the bioenergetics profile of BA-like cells in vitro. Basal OCR was significantly higher (+ 59%, P<0.01) than undifferentiated fibroblast-like cells (Figure 2a). Respiratory uncoupling, the hallmark of BA function, was >4 times greater (P<0.01) in BA-like cells. As norepinephrine is the primary mediator of the cold-induced thermogenesis response in vivo, we mimicked cold exposure by norepinephrine treatment. Norepinephrine-induced thermogenesis was absent in precursors. In contrast, BA-like cells mounted a robust response with nearly 2.5-fold increase (P<0.01) in OCR (Figure 2b).
Figure 2.
Human beige adipocytes (i.e. BA-like cells) are thermogenic with BA-like function. (a) Basal OCR and respiratory uncoupling were significantly higher in differentiated BA-like cells (N = 7). (b) Only differentiated BA-like cells demonstrated norepinephrine-induced thermogenesis. *P<0.01, **P<0.001. Arrow indicates norepinephrine injection.
FGF21 is a beige adipokine in humans
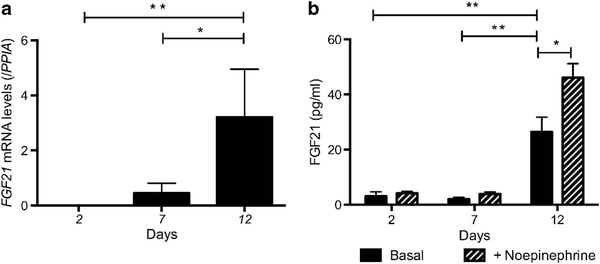
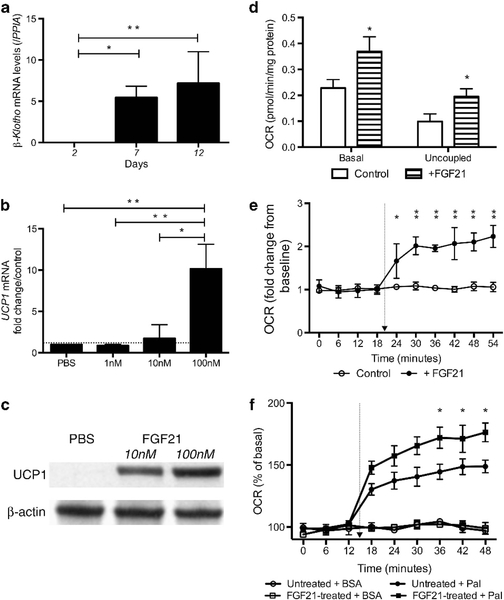
We next examined the expression of FGF21 during BA adipogen- esis. FGF21 was detected after 7 days of differentiation, peaking at day 12 (Figure 3a). FGF21 was absent in culture media in undifferentiated cells and became detectable in culture media of differentiated BA-like cells at day 2, and level increased significantly by day 12 (Figure 3b). On norepinephrine treatment, FGF21 levels rose further, by 77 ± 6% (P<0.01).
Figure 3.
FGF21 is a beige adipokine in humans. (a) Beige adipocytes expressed FGF21 and (b) secreted FGF21 into culture medium, which was further increased following exposure to norepinephrine (N = 4). *P<0.01, **P<0.001.
FGF21 switches on a brown fat-like thermogenic program in human Was
To determine the biological significance of secreted FGF21 from beige adipocytes, we evaluated the effects of FGF21 treatment in WAs. We first differentiated the same progenitors isolated from the stromal vascular fraction of cervical fat in a white adipogenic cocktail; progenitors from the same donor were split and exposed to either a white or brown adipogenic cocktail to permit paired comparison of their final phenotypes, independent of genetic background. The mature WAs exhibited slightly larger lipid droplets than BA-like cells cultured in a brown adipogenic medium (Figures 4a and b). Differentiated WAs expressed similar levels of general adipogenic genes compared with BA-like cells (Figure 4c) but UCP1 expression was negligible in WA compared with BA-like cells (Figure 4d). Accordingly, UCP1 protein was abundant in BA-like cells but undetectable in WA (Figure 4d). On a functional level, basal OCR and respiratory uncoupling of WA were significantly lower than BA-like cells (Figure 4e), and norepinephrine-induced thermogenesis was absent (Figure 4f).
Figure 4.
Comparison between beige (i.e. BA-like cells) and WAs. (a) Differentiated WAs (40 x) displayed similar morphology as (b) beige adipocytes (40 x), other than slightly larger lipid droplets. (c) Expression of general adipogenic genes was not different between beige and WAs, whereas (d) traditional brown fat gene expression was significantly higher in the former (N = 7). UCP1 protein was present in beige but not WAs. (e) Basal OCR and respiratory uncoupling were significantly lower in WAs (N = 6). (f) WAs did not respond to norepinephrine and no thermogenic response was detectable (N = 5). *P<0.01. Arrow indicates norepinephrine injection.
Before we evaluated the effects of FGF21 on WA, we first examined β-klotho expression in WA given the critical role of β-klotho in mediating FGF21 action. Consistent with previous reports,21,22 β-klotho was highly expressed in WA, but not in progenitors (Figure 5a). FGF21 treatment dose-dependently increased UCP1 expression in WA (Figure 5b). UCP1 protein became detectable following FGF21 treatment (Figure 5c). Furthermore, FGF21 treatment raised basal OCR ( + 62%, P<0.01) and respiratory uncoupling ( + 97%, P<0.01;Figure 5d). Although WA was not responsive to norepinephrine stimulation (Figure 4f), FGF21 treatment resulted in significant norepinephrine-induced thermogenesis (Figure 5e).
Figure 5.
FGF21 upregulates brown fat-like thermogenic program in WAs. (a) WAs expressed high level of β-klotho (N = 5). (b) FGF21 treatment increased UCP1 expression dose dependently in WAs (N = 3). *P<0.01. (c) UCP1 was undetectable in untreated WA but became strongly expressed following FGF21 treatment (detectable 3/3 samples at 100 nM and 1/3 at 10 nM). (d) Basal OCR and respiratory uncoupling were higher in FGF21-treated adipocytes (N = 6). FGF21-treated adipocytes displayed (e) significantly greater norepinephrine-induced thermogenesis (N = 5), (f) fatty acid oxidation (N = 4). *P<0.01, **P<0.001. Arrow indicates norepinephrine injection.
FGF21 regulates adipocyte lipid utilization
As lipid is the chief substrate of BAT and FGF21 regulates lipid utilization in vivo, we examined whether FGF21 impacts fatty acid oxidation in vitro. Palmitate increased OCR in both FGF21- and vehicle-treated WA. However, palmitate oxidation was significantly greater in FGF21-treated adipocytes than controls ( + 65 ± 17% vs + 41 ± 13%, P<0.001;Figure 5f).
FGF21-treated adipocytes produce heat
The primary function of BAT is heat production. We evaluated heat production in WA following FGF21 treatment (Figure 6a). Temperature of culture media incubating WA increased following FGF21 treatment compared with control, albeit not reaching statistical significance (P = 0.07; Figures 6b and c). To test the capacity of FGF21 in expanding cold-induced thermogenesis response, WA treated with FGF21 were exposed to the cold mimic, norepinephrine. Temperature of culture media of FGF21-treated WA rose significantly after norepinephrine exposure (P<0.01), whereas temperature was unchanged in media incubating phosphate-buffered saline-treated WA (Figures 6b and c).
DISCUSSION
In this study, we demonstrate that (1) progenitors within human neck fat originate from a non-classic BA lineage, capable in differentiating into beige adipocytes (Figure 1); (2) differentiated beige adipocytes function as thermogenic BA-like cells in vitro (Figure 2); (3) FGF21 expression increases during beige adipogenesis and the protein is secreted into culture medium (Figures 3); (4) FGF induces robust ‘browning’ of human WAs (Figures 5); (5) enhances its oxygen consumptive and fatty acid oxidative capacity (Figure 5) and ultimately (6) increases heat production (Figure 6). Together, our data indicate inducible functional thermogenic beige adipogenesis in human neck fat and provide first evidence that FGF21 is a thermogenic beige adipokine in humans.
Our study reveals novel insight into beneficial FGF21 metabolic actions that could be directly applicable to humans. FGF21 treatment enhances oxidative capacity and respiratory uncoupling of human adipocytes in vitro, inducing a switch from a white to a brown fat-like thermogenic phenotype. These observations are thought provoking, in light of our recent demonstration of the increase in circulating FGF21 levels in humans following a minimal decrease in environmental temperature.23 As FGF21 secretion in beige adipocytes is enhanced by norepinephrine (a cold mimic) in vitro, it is plausible that beige adipocyte-derived FGF21 contributes, at least in part, to cold-induced increase in circulating FGF21 levels in vivo. To date, FGF21 is the only peptide proven to be directly secreted from BAs in rodents.15,16 FGF21 expression increased during beige adipogenesis and the protein was detected in the culture medium, thus providing a proof-of-concept that beige adipocyte-derived FGF21 acts as a paracrine/endocrine factor in humans. One may further hypothesize that FGF21 functions as a beige adipokine (i.e. a ‘beadipokine’) to stimulate browning of WAT, thereby expanding the thermogenic capacity of the individual, as supported by in vitro effects of FGF21 on WAs we observed.
In addition to overall fat browning, FGF21 also enhances fatty acid oxidation, in agreement with the observations in animal models.24 Although multiple organs may contribute to FGF21- induced metabolic effects, our data suggest fat browning with heightened lipid utilization is one mechanism mediating some of the whole body FGF21 effects.
There are several limitations in our study. Our results were based on adipocytes cultured from cervical fat, and their browning potential and FGF21 response may not be generalizable to other fat depots. Our chosen physiologic model is, however, an in vitro model closely resembling human BAT in vivo. It is derived from the same location where human BAT is commonly found, free of genetic or vector-based manipulation. This advantage is offset by limited cellular material available for extensive mechanistic characterization, not encountered in models using animals, cell lines or conventional human subcutaneous fat biopsies, none of which derived from a human BAT location. The uniqueness of our model is further supported by the scarcity of studies using human neck fat in functional studies. Thus far, studies that have examined human neck fat all involved transcriptional and/or morphological analysis without functional characterization.1–6,8,11,18,25,26 As UCP1 mRNA presence does not always represent thermogenesis capacity,27 functional analysis of adipocytes is necessary to determine physiologic significance. A very recent study showed considerable oxygen consumptive capacity of human neck fat.28 It is, however, uncertain whether human beige adipocytes from the neck region are truly thermogenic (that is, capable of heat production). We demonstrated for the first time that beige adipocytes derived from neck fat are functional and indeed produce heat. Furthermore, heat production is augmented by FGF21 treatment, thus indicating FGF21 to be a true thermogenic beige adipokine.
Our experimental model, entailing the induction of functional beige adipogenesis in WAs from the same progenitors is clinically relevant:
First, despite intense interest into the potential anti-obesity role of BAT in humans, and associative evidence from positron emission tomography studies indicating BAT is metabolically active, it is unknown whether human brown/beige adipocytes are in fact functional. We show that not only is beige adipogenesis inducible, the mature adipocytes are functional, and exhibit high oxygen consumptive, fatty acid oxidative and heat-producing capacities. This is first direct proof substantiating metabolic actions of BAT, thus provides impetus for ongoing research into strategies that may harness human BAT function.
Second, the successful induction of brown and WA phenotypes by the respective differentiation cocktail, without altering overall general adipogenic maturation, indicates differentiated beige and WAs are valid in vitro models for investigating human BAT function and the biological effects of browning agents (such as FGF21) on WAT, respectively.
Third, paired derivation of beige and WAs from the same donor indicates substantial plasticity of human adipocytes. Oxygen consumption increased by >50% following FGF21-induced fat browning. If our findings in cervical fat can be translated to other fat depots, the gain in overall adipose thermogenic capacity is sufficient to impact whole body energy expenditure. Given the recent demonstration of cold-induced WA-to-BA transdifferentiation in animals,29 one may argue a similar mechanism underlay the observed FGF21-mediated phenotypic transformation. We cannot exclude the possibility that FGF21 treatment induces clonal expansion of brown adipogenic precursors. It is, however, unlikely as FGF21 treatment commenced in fully confluent cells 5 days post-differentiation. As bipotential precursors capable of BA and WA differentiation have been identified in animals,30 future studies should examine cell surface markers to determine clonality of FGF21-treated adipocytes.
Fourth, cold exposure is the sole intervention to date consistently shown to activate human BAT, but the natural human tendency for thermal comfort limits feasibility of prolonged cold exposure as a BAT-activating strategy. Our data suggest FGF21 may be a cold mimic that boosts whole body BAT abundance.
Finally, the demonstration of FGF21 as a human beige adipokine identifies human BAT as a previously underappreciated organ in energy homeostatic regulation and calls for future investigative studies on BAT as an endocrine organ.
In conclusion, functional thermogenic beige adipogenesis is inducible in humans and FGF21 is a thermogenic beige adipokine. FGF21 is capable of stimulating bioenergetic transformation of WAs into a brown fat phenotype, suggesting cold-induced whole body metabolic changes may be mediated by beige fat-derived FGF21, exploitable in future obesity therapeutics.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Michael Sack and Dr Jie Liu from National Heart, Lung and Blood Institute, for advice on Seahorse bioanalyzer operations, and Dr Douglas Forrest, Laboratory of Endocrinology and Receptor Biology, National Institute of Diabetes and Digestive and Kidney Diseases, for helpful discussion and critical appraisal on the manuscript. Paul Lee is supported by the Australian NHMRC Early Career Fellowship, the RACP Foundation Diabetes Australia Fellowship, and the Bushell Travelling Fellowship. This study was supported by the Intramural Research Program of NIDDK: programs Z01-DK047057–02 and Z01-DK071044.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on International Journal of Obesity website (http://www.nature.com/ijo)
REFERENCES
- 1.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio- Kobayashi J et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009; 58: 1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360: 1500–1508. [DOI] [PubMed] [Google Scholar]
- 3.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009; 360: 1518–1525. [DOI] [PubMed] [Google Scholar]
- 4.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee P, Zhao JT, Swarbrick MM, Gracie G, Bova R, Greenfield JR et al. High prevalence of brown adipose tissue in adult humans. J Clin Endocrinol Metab 2011; 96: 2450–2455. [DOI] [PubMed] [Google Scholar]
- 6.Lee P, Greenfield JR, Ho KK, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2010; 299: E601–E606. [DOI] [PubMed] [Google Scholar]
- 7.Lee P, Swarbrick MM, Ho KK. Brown adipose tissue in adult humans: a metabolic renaissance. Endocr Rev 2013; 34: 413–438. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150: 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic PPAR{gamma} activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classical brown adipocytes. J Biol Chem 2010; 285: 7153–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walden TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs nonrecruited molecular signatures of brown, ‘brite,’ and white adipose tissues. Am J Physiol Endocrinol Metab 2012; 302: E19–E31. [DOI] [PubMed] [Google Scholar]
- 11.Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 2012; 7: e49452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev 2013; 27: 234–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ et al. FGF-21 as a novel metabolic regulator. J Clin Invest 2005; 115: 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, Papavassiliou AG. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol Med 2011; 17: 736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 2012; 26: 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem 2011; 286: 12983–12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 2012; 122: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee P, Swarbrick MM, Zhao JT, Ho KK. Inducible brown adipogenesis of supraclavicular fat in adult humans. Endocrinology 2011; 152: 3597–3602. [DOI] [PubMed] [Google Scholar]
- 19.Lee P, Greenfield JR, Ho KK. Hot fat in a cool man: infrared thermography and brown adipose tissue. Diabetes Obes Metab 2011; 13: 92–93. [DOI] [PubMed] [Google Scholar]
- 20.Paulik MA, Buckholz RG, Lancaster ME, Dallas WS, Hull-Ryde EA, Weiel JE et al. Development of infrared imaging to measure thermogenesis in cell culture: thermogenic effects of uncoupling protein-2, troglitazone, and beta-adrenoceptor agonists. Pharm Res 1998; 15: 944–949. [DOI] [PubMed] [Google Scholar]
- 21.Ding X, Boney-Montoya J, Owen BM, Bookout AL, Coate KC, Mangelsdorf DJ et al. betaKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab 2012; 16: 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R et al. BetaK-lotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA 2007; 104: 7432–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee P, Brychta RJ, Linderman J, Smith S, Chen KY, Celi FS. Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: relationship between FGF21 levels, lipolysis, and cold-induced thermogenesis. J Clin Endocrinol Metab 2013; 98: E98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/ 19 and 21: from feast to famine. Genes Dev 2012; 26: 312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 2009; 23: 3113–3120. [DOI] [PubMed] [Google Scholar]
- 26.Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homoe P, Loft A et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metabolism 2013; 17: 798–805. [DOI] [PubMed] [Google Scholar]
- 27.Nedergaard J, Cannon B. UCP1 mRNA does not produce heat. Biochim Biophys Acta 2013; 1831 : 943–949. [DOI] [PubMed] [Google Scholar]
- 28.Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 2013; 19: 635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 2010; 298: E1244–E1253. [DOI] [PubMed] [Google Scholar]
- 30.Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab 2012; 15: 480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.