Abstract
Purpose:
Evaluate the toxicity and efficacy of adjuvant temozolomide (TMZ) and irinotecan (CPT-11) for 12 months following concurrent chemo-radiation in newly diagnosed glioblastoma (GBM).
Methods:
Trial RTOG 04-20, a single arm, multi-institutional phase 2 trial was designed to determine the efficacy and toxicity of concomitant TMZ and radiation (RT) followed by adjuvant TMZ combined with CPT-11 given for 12 cycles compared to historical controls of adjuvant TMZ alone given for 6 cycles.
Results:
A total of 170 patients were enrolled, of which 152 were eligible. Adjuvant CPT-11 combined with TMZ was more toxic than expected. A higher rate of hematologic and gastrointestinal toxicities was most frequently noted with the combination regimen compared to adjuvant TMZ alone. Grade 3/4 hematologic toxicity was 38% compared to 14% reported in the Stupp trial. Following an early interim analysis, adjuvant CPT-11 dose was reduced to100 mg/m2 D1 and D15 for the first cycle. CPT-11 dose escalation proceeded over first 3 cycles if tolerated. Median overall survival (OS) for all eligible patients was 16.9 months compared to 13.7 months of the historical control (p=0.03). Post-hoc subgroup analysis suggested an improvement in OS for patients with RTOG recursive partitioning analysis (RPA) Class III although limited to 22 patients (14% of eligible patients).
Conclusions:
Although Irinotecan and TMZ for 12 cycles given after chemoradiation for patients with newly diagnosed GBM significantly improved median survival compared to historical control data at the time the study was conducted, the historical control median survival time of 13.7 months does not represent the current benchmark for this patient population. Treatment intensification does prolong overall survival compared to the current standard.
Summary
RTOG 04-20 tested the adjuvant combination of Irinotecan and temozolomide after initial chemoradiation in a single arm phase II study of newly diagnosed glioblastoma. The doublet regimen given for 12 months is substantially more toxic and does not appear to extend overall survival compared to temozolomide alone given for 6 months, with a potential benefit for the combination regimen only in the best prognostic group, RPA Class III.
Introduction
Standard therapy for newly diagnosed glioblastoma (GBM) remains external beam radiation to 5940cgy and concomitant low dose temozolomide 75 mg/m2 po daily (TMZ) followed by 6 months of adjuvant TMZ given at 150-200 mg/m2 per day on days 1-5 q28 day cycle. An overall survival advantage was demonstrated in patients receiving combined chemo-radiation followed by 6 months of adjuvant TMZ.1 This regimen produces a small number of long term survivors.2 Promoter methylation silencing of the methyl guanine methyltransferase gene (MGMT) was an important molecular marker that correlated with improved survival.3
The median survival for the majority of patients with GBM remains less than 2 years. The addition of chemotherapy lengthens median survival by only approximately 3 months. For the subgroup of MGMT promoter un-methylated tumors, median survival is even lower at approximately 1 year, and two-year survivorship is rare. Clearly, further innovations are required to improve the frequency and durability of treatment response for newly diagnosed GBM.
Irinotecan (CTP-11) crosses the blood brain barrier and has shown modest clinical activity in recurrent malignant gliomas.4,5 Irinotecan is a prodrug, that converts to the biologically active agent, SN-38, which is a potent inhibitor of topoisomerase 1 activity.6-9 It prevents re-ligation of DNA double strands by binding to topoisomerase I-DNA complex, leading to double-strand DNA breaks resulting in tumor cell death and inhibition of DNA replication, transcription and repair.7-9 The rationale for combining temozolomide and Irinotecan was to exploit the differences in the mechanisms of action of each drug as well as their differing toxicities. Preclinical evidence had demonstrated a synergistic effect of the combination of Irinotecan and TMZ in human glioma xenografts.8
Trial RTOG 04-20 was initiated based on the promising results from a phase I clinical trial using this same combination for recurrent gliomas conducted by the North American Brain Tumor Consortium (NABTC).10,11 In the phase I NABTC study, the objective response rate in the trial was 26% including 7 patients with recurrent GBM and 6m PFS was 35%.
This single arm phase II multi-institutional RTOG 04-20 study in newly diagnosed GBM was designed to determine the efficacy and toxicity of adjuvant TMZ combined with Irinotecan given for 12 cycles compared to historical controls of adjuvant TMZ alone given for 6 cycles.
Methods and Materials
Study Population and Eligibility Criteria
Adult patients with newly diagnosed, histologically confirmed, supratentorial GBM and were eligible for study enrollment after recovery from surgery. Institutional Review Boards at all institutions accruing subjects approved protocol study procedures. Signed consent was obtained before enrollment.
Eligibility requirements for RTOG trials including chemoradiation and adjuvant therapy have been described previously .12 The use of enzyme inducing antiepileptic drugs (EIAED) was not permitted; a minimum interval of 14 days from the last dose of EIAED was required prior to initiation of study treatment. Patients receiving steroids were mandated to receive stable or decreasing doses for at least 2 weeks prior to study entry.
A contrast-enhanced MRI or CT was obtained pre and post-operatively (preferably within 72 hours of surgery) prior to study entry, and within 28 days prior to registration.
Radiation Therapy (RT)
Radiation treatment parameters have been described elsewhere.12 A standard radiation dose of 60 Gy was delivered in 2 Gy daily fractions for 30 treatments over 6 weeks. Intensity modulated radiation therapy was not allowed in this study. MRI-guided treatment planning was performed in the majority of cases.
Drug Therapy
TMZ 75mg/m2 po was given daily during radiation therapy. Post-radiation adjuvant chemotherapy therapy was started 4-6 weeks after completion of RT. Initially, the first cycle of Irinotecan was initiated at the full dose of 200mg/m2, but an early interim analysis (see below) identified unacceptable rates of hematologic toxicity, primarily neutropenia and lymphopenia. Therefore, the protocol was amended to lower the initial dose of Irinotecan to 100 mg/m2 with subsequent dose escalation to the full dose of 200 mg/m2 over the next two cycles subject to patient tolerability.
Dose Modification
Dose modification guidelines for subsequent treatment cycles are available in supplemental materials section.
Study Treatment
The study treatment schedule continued without interruption for one year or 12 complete 28-day treatment cycles (whichever is longer) so long as no tumor progression or dose-limiting toxicities occurred.
Evaluation of Response
A neurologic examination was performed once a week during RT, at conclusion of RT, and at every 2-month interval during chemotherapy unless neurologic deterioration required evaluation sooner. A complete physical examination was performed every two months. Laboratory results including CBC and diff were obtained every week during RT and then every 2 weeks during chemotherapy; blood chemistry and anticonvulsant levels were obtained every two months. Mental status was measured by the Mini-Mental Status Exam (MMSE) prior to the start of protocol treatment and during follow-up. Gd-MRIs or contrast CTs of the brain were obtained prior to RT, within four weeks of conclusion of RT, and then every two cycles (2 months) during follow up, and in the event of neurologic deterioration.
This study was one of the first trials employing dose-escalated chemotherapy concurrent with radiation. As a result, the occurrence of pseudo-progression resulting from the increased radio-sensitizing effect of TMZ upon RT had not yet been well described or anticipated. As the trial progressed, investigators were instructed not to remove subjects from study within the first 2-3 months post irradiation without confirmatory Thallium-SPECT, PET or spectroscopic MRI imaging, or neurologic worsening.
Treatment response was defined by clinical and radiological criteria described previously and conforming to current RANO criteria. MRI scans were obtained at baseline and every 2 cycles thereafter. MR and neurological examinations were used to determine treatment response. Patients remained on treatment until tumor progression, development of unacceptable toxicity, or completion of 12 cycles with TMZ and Irinotecan. Failure to return for evaluation due to death related to disease or deteriorating condition was coded as progression.
Statistical Design
This Phase II study sought to determine if RT with concomitant low dose TMZ followed by adjuvant TMZ and Irinotecan improves the overall survival (OS) of newly diagnosed GBM patients. Combining the hazard rates from the EORTC phase III trial of radiation therapy and temozolomide based on the RPA distribution from the ZZZZ GBM database resulted in a median survival time of 13.7 months for the historical control. Assuming an exponential survival, we hypothesized there would be a 35% improvement in median survival time for the experimental therapy, to a median survival time of 18.5 months. This survival improvement corresponds to a 26% relative reduction in monthly hazard rate from 0.051 (historical control) to 0.038, equivalent to a hazard ratio of 0.74 in favor of the experimental therapy. A one-sided log-rank test with a significance level of 0.1 has an 85% power to detect this survival difference with 60 deaths. This required 94 eligible patients to be accrued with at least 18 months of follow-up for each patient. Assuming a 5% ineligibility rate, the target accrual of the study was 99 patients.13
As a secondary endpoint, OS was to be evaluated for patients who started the experimental drug Irinotecan. Patients who discontinued treatment prior to starting Irinotecan were to be declared non-evaluable for this analysis. Because of higher than expected rates of ineligibility and non-evaluability, following initial accrual the study was amended to increase enrollment to ensure a sufficient number of eligible and evaluable patients for this analysis.
Therefore, the target sample size was increased from 99 to 157 patients with an assumed rate of 40% for ineligible or non-evaluable patients.
Statistical Analysis Methods
Frequency tables with counts and percentages were used to describe pretreatment characteristics, adverse events, and compliance review results. Adverse events were graded using CTCAE v 3.0. OS and progression-free survival (PFS) were estimated using the Kaplan-Meier method. An OS event is death due to any cause. A PFS event is death due to any cause or the first progression, whichever comes first. All eligible patients receiving any protocol drug were included in the primary endpoint analysis. Patients not experiencing the event of interest at time of their last follow-up were analyzed as censored observations. When analyzing all eligible patients, OS and PFS were estimated from the date of registration.13 When analyzing only patients who started Irinotecan, OS and PFS were estimated from start of Irinotecan. OS and PFS were also reported by ZZZZ RPA class and compared to historical control data.14
Results
Patient Characteristics
A total of 170 patients were enrolled on study between November 2004 and September 2005 of which 152 patients were eligible. Reasons for ineligibility included: failure to start treatment < 5 weeks following resection (7), use of IMRT (3), use of Gliadel wafers (1), failure to receive treatment due to death, refusal of treatment, rapid clinical decline (4), failure to obtain pre-op scan < 28 days prior to registration (2) and patient withdrawal of consent (1). Patient characteristics are listed in Table 1. The median age of eligible patients was 56 years (range: 20-83). A majority of patients were in RPA Class III/IV (81%), underwent surgical resection (85%), and presented no or only minor neurologic symptoms (88%). Over half of the patients had Zubrod status 1 (54%).
Table 1:
Patient Characteristics
| Eligible (n=152) |
Patients starting Irinotecan (n=95) |
Patients not starting Irinotecan (n=57) |
||||
|---|---|---|---|---|---|---|
| Age | ||||||
| Median | 56 | 55 | 59 | |||
| Range | 20 – 83 | 20 - 76 | 39 - 83 | |||
| n | % | n | % | n | % | |
| 18-49 | 39 | 26 | 28 | 29 | 11 | 19 |
| ≥ 50 | 114 | 74 | 67 | 71 | 46 | 81 |
| Gender | ||||||
| Male | 104 | 68 | 63 | 66 | 41 | 72 |
| Female | 48 | 32 | 32 | 34 | 16 | 28 |
| Zubrod Performance Status | ||||||
| 0 | 70 | 46 | 50 | 53 | 20 | 35 |
| 1 | 82 | 54 | 45 | 47 | 37 | 65 |
| Neurological Function | ||||||
| No symptoms | 36 | 24 | 26 | 27 | 10 | 18 |
| Minor symptoms | 97 | 64 | 57 | 60 | 40 | 70 |
| Moderate (fully active) | 15 | 10 | 9 | 9 | 6 | 11 |
| Moderate (not fully active) | 4 | 3 | 3 | 3 | 1 | 2 |
| Prior Surgery | ||||||
| Biopsy | 23 | 15 | 15 | 16 | 8 | 14 |
| Partial Resection | 65 | 43 | 36 | 38 | 29 | 51 |
| Total Resection | 64 | 42 | 44 | 46 | 20 | 35 |
| RPA Class | ||||||
| III | 22 | 14 | 17 | 18 | 5 | 9 |
| IV | 101 | 66 | 61 | 64 | 40 | 70 |
| V | 29 | 19 | 17 | 18 | 12 | 21 |
| Location(s) of primary | ||||||
| Frontal lobe | 53 | 35 | 31 | 33 | 22 | 39 |
| Temporal lobe | 60 | 40 | 37 | 39 | 23 | 40 |
| Parietal lobe | 66 | 43 | 39 | 41 | 27 | 47 |
| Occipital lobe | 17 | 11 | 10 | 10 | 7 | 12 |
| Deep (basal gangli) | 8 | 5 | 7 | 7 | 1 | 2 |
| Corpus callosum | 4 | 3 | 3 | 3 | 1 | 2 |
| Other | 1 | 1 | 1 | 1 | 0 | 0 |
| Lateralization of tumor | ||||||
| Right side only | 89 | 59 | 54 | 57 | 35 | 61 |
| Left side only | 60 | 40 | 40 | 42 | 20 | 35 |
| Bilateral | 2 | 1 | 0 | 0 | 2 | 4 |
| Unknown | 1 | 1 | 1 | 1 | 0 | 0 |
Fifty-seven (38%) eligible patients did not receive adjuvant Irinotecan while on study. Of these, only 1 received adjuvant temozolomide. Reasons for not giving adjuvant Irinotecan included: evidence of tumor progression (27), patient withdrawal (12), toxicity/complications due to initial therapy (9), death (6), treatment off protocol (2), and unknown (1). A total of 95 patients remained eligible and evaluable for the assessment of the combination of adjuvant TMZ and Irinotecan. No significant differences were noted in pre-treatment characteristics (except for Zubrod status), between patients who received adjuvant Irinotecan and those that did not (Table 1).
Quality Assurance
A quality assurance review of chemotherapy and RT treatment records of all eligible patients was performed for compliance with protocol guidelines. The results of the review are summarized in Table 2. Eighty-nine percent of all eligible patients (135/152) received their RT treatment per protocol guidelines. Sixty-two percent of all eligible patients (94/152) completed their chemotherapy per protocol guidelines without significant modification or delay.
Table 2.
Joint Radiation/Chemotherapy Review
| All Patients | Patients starting Irinotecan |
Patients not starting Irinotecan | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n=152) | (n=95) | (n=57) | ||||||||||||||||
| Chemo Review | Chemo Review | Chemo Review | ||||||||||||||||
| Per Protocol |
Not Per Protocol |
Not Evaluable |
Per Protocol |
Not Per Protocol |
Not Evaluable |
Per Protocol |
Not Per Protocol |
Not Evaluable |
||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Per protocol | 83 | 54 | 50 | 33 | 2 | 1 | 61 | 64 | 31 | 33 | 1 | 1 | 22 | 39 | 19 | 33 | 1 | 2 |
| Acceptable variation | 7 | 5 | 3 | 2 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 5 | 9 | 3 | 5 | 0 | 0 |
| Unacceptable deviation | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 2 | 0 | 0 |
| Incomplete RT (death during RT) | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
| Incomplete RT (progression) | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 |
| Incomplete RT (refusal) | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
Toxicity
Adverse events related to concurrent chemo-radiation for all eligible patients are reported in Table 3. Concurrent TMZ and radiation treatment was relatively well tolerated. Only 9% (13) of eligible patients had any grade 3 or grade 4 hematologic toxicities related to concurrent chemo-radiation; 15% (23) had any grade 3 related non-hematologic; and 3% (4) had grade 4 non-hematologic toxicity. A majority of hematologic adverse events were related to lymphopenia.
Table 3.
Adverse Events Prior to the Start of Irinotecan Attributed as Definitely, Probably, or Possibly Related to Treatment (n=152)
| Grade | |||||
|---|---|---|---|---|---|
| Category | 1 | 2 | 3 | 4 | 5 |
| Allergy/immunology | 2 | 0 | 0 | 0 | 0 |
| Auditory/ear | 3 | 3 | 0 | 0 | 0 |
| Blood/bone marrow | 36 | 11 | 8 | 5 | 0 |
| Cardiac general | 0 | 1 | 0 | 0 | 0 |
| Constitutional symptoms | 54 | 41 | 7 | 1 | 0 |
| Dermatology/skin | 60 | 33 | 1 | 0 | 0 |
| Gastrointestinal | 60 | 25 | 3 | 0 | 0 |
| Hepatobiliary/pancreas | 1 | 0 | 0 | 0 | 0 |
| Infection | 1 | 3 | 2 | 1 | 0 |
| Lymphatics | 3 | 2 | 0 | 0 | 0 |
| Metabolic/laboratory | 31 | 7 | 3 | 2 | 0 |
| Musculoskeletal/soft tissue | 3 | 0 | 1 | 0 | 0 |
| Neurology | 13 | 13 | 5 | 1 | 0 |
| Ocular/visual | 5 | 2 | 0 | 0 | 0 |
| Pain | 22 | 11 | 1 | 0 | 0 |
| Pulmonary/upper respiratory | 1 | 0 | 1 | 0 | 0 |
| Renal/genitourinary | 2 | 0 | 0 | 0 | 0 |
| Sexual/reproductive function | 1 | 1 | 0 | 0 | 0 |
| Syndromes | 0 | 1 | 0 | 0 | 0 |
| Vascular | 0 | 1 | 2 | 0 | 0 |
| Worst non-hematologic | 35 (23%) |
69 (45%) |
23 (15%) |
4 (3%) |
0 (0%) |
| Worst overall | 34 (22%) |
65 (43%) |
28 (18%) |
8 (5%) |
0 (0%) |
Adverse events for patients who received adjuvant Irinotecan and TMZ chemotherapy are reported in Table 4. A substantial increase in hematologic and non-hematologic toxicities was noted in comparison to single agent adjuvant TMZ treatment. Twenty-seven percent of patients (26) receiving adjuvant Irinotecan and TMZ had grade 3 treatment-related hematologic toxicity and 11% (10) had grade 4 treatment-related hematologic toxicity. Thirty-three percent of patients (31) had a treatment-related non-hematologic grade 3 and 4% (4) had grade 4 toxicity.
Table 4.
Adverse Events after the Start of Irinotecan Attributed as Definitely, Probably, or Possibly Related to Treatment (n=95)
| Grade | |||||
|---|---|---|---|---|---|
| Category | 1 | 2 | 3 | 4 | 5 |
| Allergy/immunology | 3 | 1 | 0 | 0 | 0 |
| Auditory/ear | 1 | 3 | 1 | 0 | 0 |
| Blood/bone marrow | 20 | 20 | 26 | 10 | 0 |
| Cardiac arrhythmia | 1 | 0 | 0 | 0 | 0 |
| Cardiac general | 0 | 1 | 0 | 0 | 0 |
| Coagulation | 1 | 0 | 0 | 0 | 0 |
| Constitutional symptoms | 31 | 27 | 15 | 1 | 0 |
| Dermatology/skin | 21 | 16 | 1 | 0 | 0 |
| Endocrine | 3 | 0 | 0 | 0 | 0 |
| Gastrointestinal | 37 | 31 | 10 | 0 | 0 |
| Hemorrhage/bleeding | 1 | 0 | 1 | 0 | 0 |
| Hepatobiliary/pancreas | 1 | 0 | 0 | 0 | 0 |
| Infection | 3 | 4 | 7 | 2 | 0 |
| Lymphatics | 5 | 1 | 0 | 0 | 0 |
| Metabolic/laboratory | 35 | 9 | 7 | 0 | 0 |
| Musculoskeletal/soft tissue | 4 | 5 | 2 | 0 | 0 |
| Neurology | 13 | 16 | 8 | 0 | 0 |
| Ocular/visual | 8 | 3 | 0 | 0 | 0 |
| Pain | 19 | 9 | 2 | 0 | 0 |
| Pulmonary/upper respiratory | 7 | 3 | 3 | 1 | 0 |
| Renal/genitourinary | 3 | 0 | 0 | 0 | 0 |
| Sexual/reproductive function | 0 | 1 | 0 | 0 | 0 |
| Vascular | 1 | 1 | 1 | 1 | 0 |
| Worst non-hematologic | 15 (16%) |
39 (41%) |
31 (33%) |
4 (4%) |
0 (0%) |
| Worst overall | 5 (5%) |
33 (35%) |
39 (41%) |
14 (15%) |
0 (0%) |
Concomitant TMZ with RT increased the likelihood of hematologic toxicity for the initial cycles of adjuvant full dose Irinotecan. A subsequent protocol amendment reduced the Irinotecan dose by 50%, from 200 to 100 mg/m2 for the first cycle following concurrent radiation and TMZ. Modifications to the dosing scheme for the first 3 cycles allowed for dose escalation to the full dose of 200mg/m2 when no significant toxicity occurred. Twenty-one percent of patients starting Irinotecan (20) discontinued Irinotecan due to significant toxicity.
Overall Survival
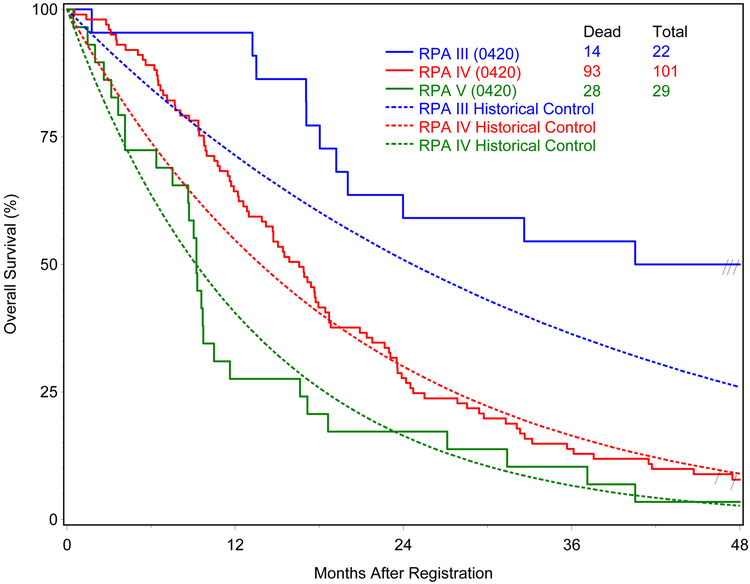
Of the 152 eligible patients, 89% (135) have since died. Median follow-up time for eligible patients still alive was 52.2 months (range: 46.4-58.6). Median survival time for all eligible patients was 16.9 months. For the comparison of OS with the ZZZZ historical data, the analysis showed statistical significance with p=0.03. Median survival time (number of deaths/number of patients) by RPA Class for all eligible patients was III: 45.2 (14/22), IV: 16.6 (93/103), and V: 9.2 (28/29) months. Stupp et al reported median survival time by RPA class in the EORTC phase III trial of radiation therapy combined with temozolomide as17.0, 14.6 and 11.8 months, for RPA Class III-V, respectively.1 Figure 1 shows the OS curves by RPA class with comparison to corresponding curves based on the data from the EORTC trial. Median PFS time for all eligible patients was 6.4 months.
Figure 1.
Overall survival by RPA class compared to historical controls from the ZZZZ database
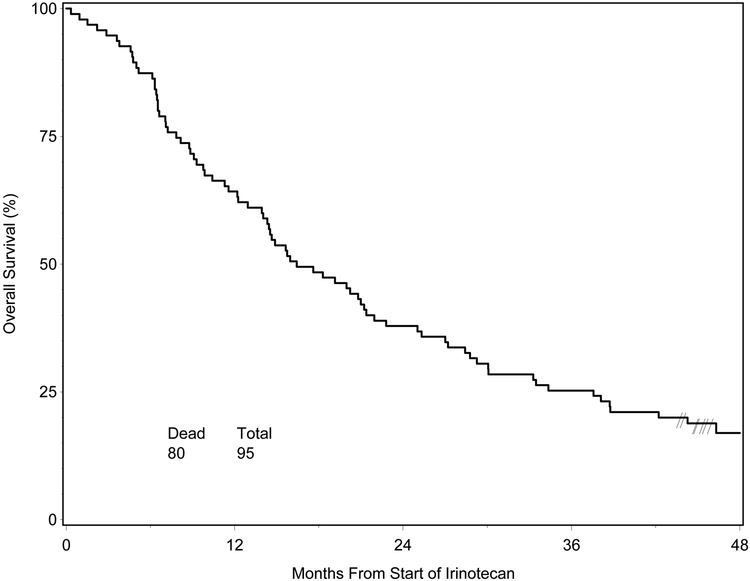
Median survival time for the 95 eligible patients who started Irinotecan was 16.4 months from the start of Irinotecan, and 84% (80) had died. Figure 2 shows the OS curve for this group of the patients. Median survival time by RPA class for this group of patients was 50.8 months for RPA Class III (9/17), 15.6 months (55/61) for RPA Class IV, and 7.2 months (16/17) for RPA Class V. Median PFS time for this group of patients, also calculated from start of Irinotecan, was 6.8 months. Median PFS times were 28.6, 5.3, and 4.2 months for RPA classes III-V, respectively.
Figure 2.
Overall Survival for Patients who Started Irinotecan vs. Historical Control
Discussion
Trial RTOG 04-20, a large, single arm, phase II study of newly diagnosed GBM patients, confirms the increased hematologic toxicity associated with the addition of adjuvant Irinotecan to TMZ in the upfront setting. The rate of acute grades 3-4 hematologic toxicity was 38% for those patients receiving at least one cycle of Irinotecan, and the rate of acute grades 3-4 non-hematologic toxicity rate was 37% for the same cohort.
The combination of Irinotecan and TMZ had previously been studied in a phase 1 trial of recurrent GBM. Prior concomitant TMZ with RT significantly increased the likelihood of hematologic toxicity for the initial cycles of adjuvant full dose Irinotecan and TMZ. Following an early interim data safety analysis, a protocol amendment reduced the initial Irinotecan dose by 50% from 200 to 100 mg/m2 in the first cycle. Dose modifications allowed for dose escalation to the full dose of 200mg/m2 in the subsequent cycles when no significant toxicity occurred.
Median survival time for all eligible patients is 16.9 months, which compares favorably to prior ZZZZ historical controls. Using the ZZZZ historical control of 13.7 months for OS, the p-value associated with OS for all eligible patients is less than the pre-specified significance level, 0.1 (one-sided), suggesting a survival advantage for the adjuvant Irinotecan and TMZ adjuvant regimen. It is important to note that although the ZZZZ historical control data used in the study design was the best and most reliable data at the time, it may not reflect the actual median survival time with current standard treatment in this patient population. If the trial had been designed using a historical control of 16 months for median survival time and resulted in a median survival time of 16.9 months, that would not have reached statistical significance.
Several caveats must be attached to the positive findings. Approximately 40% of all eligible patients received no adjuvant therapy. The additional benefit of the combination regimen was most notable in the small number of ZZZZ RPA Class III patients: 45.2 months (experimental arm) vs. 17 months (historical control arm). The present study did not require MGMT analysis and thus the information is not available for a majority of the patients, including those of the subgroup of RPA Class III. In a small phase II trial of TMZ and Irinotecan given prior to radiation in newly diagnosed GBM patients, combination therapy was poorly tolerated and more toxic, with a median survival of 13.8 months.15 Further molecular analysis including MGMT promoter methylation, is needed to determine whether this patient subset, had better predicted outcomes rather than improved response to treatment.
Optimal duration of adjuvant treatment remains unclear. There is no clear evidence of added benefit from continuing adjuvant temozolomide beyond 6 cycles. YYYY trial, a randomized study of 12 adjuvant cycles of either dose-dense or conventional dose TMZ, reported median PFS and OS of 6.7 and 14.9 for the dose-dense arm and 5.5 and 16.6 months for the conventional dose arm. YYYY trial median survival in the conventional arm is similar to our study, and higher than obtained in the EORTC-NCIC trial. The gains in median survival time may not reflect a benefit from extending adjuvant treatment beyond 6 cycles in all subsets of newly diagnosed GBM patients but an overall improvement in treatment care over time.16
The most problematic issue raised in the present study was the degree to which pseudo-progression may have compromised the results. As the EORTC regimen became standard of care for patients with newly diagnosed GBM, there was a sudden and significant increase in the incidence of transient radiologic worsening noted at the post-radiation MRI scan following concurrent chemo-radiation.17-20 This was not foreseen at the time of the initial study design. It is now common practice for adjuvant trials for newly diagnosed GBM to mandate that treatment change for progression cannot be made within 12 weeks of the end of RT unless there is unequivocal progression of the tumor outside the radiation field. Retrospective analyses of patient series treated with the EORTC regimen suggest that pseudo-progression rates may range from 30-50%, with increased incidence of pseudo-progression associated with MGMT promoter methylation.17-20 It is possible that the increased rate of early tumor progression following chemo-radiation was due to pseudo-progression and not true tumor progression since this was not as well known a phenomena at that time. If a substantial number of early treatment failures were pseudo-progression, the early failure rate may be misleading. Early termination of study treatment and a relatively low number of patients completing protocol therapy may have also compromised treatment efficacy.
Vascular proliferation is a notable feature in GBM and therefore, targeting the vascular endothelial growth factor (VEGF) is a logical treatment approach.21 Treatment intensification with the addition of bevacizumab to the Stupp regimen in the upfront setting has been studied in two, large phase III randomized clinical trials. Both trials failed to demonstrate a benefit in overall survival.22,23 Several trials have evaluated the combination of TMZ and Irinotecan with bevacizumab in the treatment of newly diagnosed GBM. In a single arm phase 2 trial, the addition of bevacizumab to adjuvant TMZ and Irinotecan following standard RT and concomitant TMZ produced a median survival time of 21.2 months and median PFS of 14.2 months.20 A randomized phase 2 trial of neoadjuvant Irinotecan and bevacizumab followed by concomitant TMZ RT and adjuvant Irinotecan and bevacizumab versus TMZ alone in newly diagnosed unresectable GBM patients demonstrated substantial increased toxicity and no benefit in 6 month PFS.24
Conclusions
Although Irinotecan and TMZ for 12 cycles given after chemoradiation for patients with newly diagnosed GBM significantly improved median survival, the historical control median survival time of 13.7 months did not reflect the current standard treatment for this patient population. There was a significant increase in the rate of acute toxicities from the combination regimen. In an unplanned subset analysis, an increase in OS was noted for ZZZZ RPA class III patients compared to historical controls receiving TMZ alone, but these data should only be considered hypothesis generating.
Supplementary Material
Acknowledgments
This project was supported by grants U10CA21661 (RTOG-Ops-Stat), U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology SDMC) from the National Cancer Institute (NCI) and Pfizer.
Footnotes
Conflicts of Interest: Dr. Curran, Hartford, Robins, Schultz, Smith, Wang, Werner-Wasik, and Zhang have nothing to disclose. Dr. Lieberman reports grants from RTOG/NCI, during the conduct of the study; grants from Novacure, grants from Stemline, grants from NCI, grants from Collaborative Ependymoma Research Network, grants from Genentech, outside the submitted work; Dr. Mehta reports personal fees from BMS, personal fees from Celldex, personal fees from Roche, personal fees from Novartis, personal fees from Cavion, grants and personal fees from Novocure, personal fees from Varian, personal fees from Agenus, personal fees from Insys, personal fees from Remedy, grants from Cellectar, personal fees and other from Pharmacyclics, personal fees from Monteris, outside the submitted work; Dr. Tsien reports personal fees from Merck, outside the submitted work.
Previous presentation: Society for Neurooncology
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Eng J Med 2005. March 10;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Roila F, ESMO Guidelines Working Group. Malignant glioma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2008. May;19 Suppl 2:ii83–5. [DOI] [PubMed] [Google Scholar]
- 3.Hegi ME, Diserens AC, Gorlia T, et al. Mgmt gene silencing and benefit from temozolomide in glioblastoma. N Eng J Med 2005;352:997–1003. [DOI] [PubMed] [Google Scholar]
- 4.Reardon DA, Quinn JA, Rich JN, et al. Phase 1 trial of irinotecan plus temozolomide in adults with recurrent malignant glioma. Cancer 2005;104:1478–1486. [DOI] [PubMed] [Google Scholar]
- 5.Gruber ML, Buster WP. Temozolomide in combination with irinotecan for treatment of recurrent malignant glioma. Am J Clin Oncol 2004. February;27(1):33–38. [DOI] [PubMed] [Google Scholar]
- 6.Patel VJ, Elion GB, Houghton PJ, et al. Schedule-dependent activity of temozolomide plus CPT-11 against a human central nervous system tumor-derived xenograft. Clin Cancer Res 2000. October;6(10):4154–4157. [PubMed] [Google Scholar]
- 7.Hsiang YH, Hertzberg R, Hecht S, Liu LF. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem 1985;260:14873–14878. [PubMed] [Google Scholar]
- 8.Houghton PJ, Stewart CF, Cheshire PJ, et al. Antitumor activity of temozolomide combined with irinotecan is partly independent of o6-methylguanine-DNA methyltransferase and mismatch repair phenotypes in xenograft models. Clin Cancer Res 2000;6:4110–4118. [PubMed] [Google Scholar]
- 9.Gupta E, Wang X, Ramirez J, Ratain MJ. Modulation of glucuronidation of sn-38, the active metabolite of irinotecan, by valproic acid and phenobarbital. Cancer Chemother Pharmacol 1997;39:440–444. [DOI] [PubMed] [Google Scholar]
- 10.Prados MD, Yung WKA, Jaeckle KA, et al. Phase I trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortiumst udy Neuro Oncol, 6 (2004), pp. 44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loghin ME, Prados MD, Wen P, et al. Phase 1 study of temozolomide and irinotecan for recurrent malignant gliomas in patients receiving enzyme-inducing antiepileptic drugs: A north american brain tumor consortium study. Clin Cancer Res 2007;13:7133–7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial J Clin Oncol, 31 (2013), pp. 4085–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collett D Modeling survival data in medical research. Chapman & Hall; London; 1994. [Google Scholar]
- 14.Li J, Wang M, Won M, et al. Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma Int J Radiat Oncol Biol Phys, 81 (2011), pp. 623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn JA, Jiang SX, Reardon DA, et al. Phase II trial of temozolomide (tmz) plus (CPT-11) (Irinotecan) in adults with newly diagnosed glioblastoma multiforme before radiotherapy. J Neurooncol 2009;95:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossman SA, Ye X, Piantadosi S, et al. Survival of Patients with Newly Diagnosed Glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res 2010. April 15;16(8):2443–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol 2009;22:633–638. [DOI] [PubMed] [Google Scholar]
- 18.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 2008. May 1;26(13):2192–2197. [DOI] [PubMed] [Google Scholar]
- 19.Brandsma D, Stalpers L, Taal W, et al. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 2008;9:453–461. [DOI] [PubMed] [Google Scholar]
- 20.Vredenburgh JJ, Desjardins A, Reardon DA, et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin Cancer Res 2011;17:4119–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci 2007;8:610–622. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Eng J Med 2014;370:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma N Eng J Med 2014;370:709–722. [DOI] [PubMed] [Google Scholar]
- 24.Chauffert B, Feuvret L, Bonnetain F, et al. Randomized phase II trial of irinotecan and bevacizumab as neo-adjuvant and adjuvant to temozolomide-based chemoradiation compared with temozolomide-chemoradiation for unresectable glioblastoma: Final results of the temavir study from ANOCEF†. Ann Oncol 2014;25:1442–1447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.