Abstract
Optic atrophy type 1 protein (OPA1), a dynamin-related GTPase, that, in addition to mitochondrial fusion, plays an important role in maintaining the structural organization and integrity of the inner mitochondrial membrane (IMM). OPA1 exists in two forms: IMM-bound long-OPA1 (L-OPA1) and soluble short-OPA1 (S-OPA1), a product of L-OPA1 proteolytic cleavage localized in the intermembrane space. In addition to OPA1, the structural and functional integrity of IMM can be regulated by changes in the matrix volume due to the opening/closure of permeability transition pores (PTP). Herein, we investigated the crosstalk between the PTP and OPA1 to clarify whether PTP opening is involved in OPA1-mediated regulation of respiratory chain supercomplexes (RCS) assembly using cardiac mitochondria and cell line. We found that: 1) Proteolytic cleavage of L-OPA1 is stimulated by PTP-induced mitochondrial swelling, 2) OPA1 knockdown reduces PTP-induced mitochondrial swelling but enhances ROS production, 3) OPA1 deficiency impairs the RCS assembly associated with diminished ETC activity and oxidative phosphorylation, 4) OPA1 has no physical interaction with phospholipid scramblase 3 although OPA1 downregulation increases expression of the scramblase. Thus, this study demonstrates that L-OPA1 cleavage depends on the PTP-induced mitochondrial swelling suggesting a regulatory role of the PTP-OPA1 axis in RCS assembly and mitochondrial bioenergetics.
Keywords: mitochondria, respiratory supercomplexes, optic atrophy type 1 protein, mitochondrial swelling, permeability transition pore
1. Introduction
Optic atrophy type 1 protein (OPA1) is a dynamin-related GTPase which located in the inner mitochondrial membrane (IMM). OPA1 exists in two forms in the mitochondria: membrane-bound long-OPA1 (L-OPA1) and soluble short-OPA1 (S-OPA1), a product of L-OPA1 proteolytic cleavage which localizes in the intermembrane space. Four L-OPA1 isoforms contain one (S1) but other four have two sites (S1, S2) for proteolytic cleavage processed by two IMM proteases, YME1L (at S2 site) and OMA1 (at S1 site) (Delettre et al., 2001; Ishihara et al., 2006; Olichon et al., 2002). L-OPA1 regulates mitochondrial fusion, cristae structure, and energetic efficiency (Del Dotto et al., 2017) although the exact roles of individual isoforms and their cleavage have not yet been established. OPA1 preserves the cristae structure and mitochondrial morphology independently from its function in IMM fusion (Frezza et al., 2006; Patten et al., 2014). OPA1 deficiency induced mitochondrial fragmentation and disorganization of the cristae facilitating cytochrome c release (Frezza et al., 2006) whereas OPA overexpression improved cristae shape and ameliorated the phenotype of mitochondrial diseases in mice (Civiletto et al., 2015).
In addition to OPA1, the structural and functional integrity of IMM can be regulated by changes in the matrix volume (Mannella, 2006). Mild changes in mitochondrial volume are associated with regulation of mitochondrial function (Beavis et al., 1985; Halestrap, 1989; Scalettar et al., 1991) whereas excessive swelling leads to mitochondrial dysfunction and cell death. The main mechanism responsible for excessive mitochondrial swelling includes the opening of non-selective channels known as permeability transition pores (PTPs) in the IMM by high mitochondrial Ca2+ and reactive oxygen species (ROS) (Bernardi and Di Lisa, 2015; Halestrap et al., 1998; Kwong and Molkentin, 2015). Although PTP induction is a well-known phenomenon, the molecular identity of the PTPs remains inconclusive. Recent studies from several groups reported that several subunits of the F0F1-ATP synthase (complex V) are involved in PTP formation thus suggesting that F0F1-ATP synthase can serve as a core PTP component (Alavian et al., 2014; Azarashvili et al., 2014; Giorgio et al., 2013). However, this conclusion was later challenged by genetic studies that demonstrated that PTP opening can occur in the absence of these subunits (Carroll et al., 2019; He et al., 2017a; He et al., 2017b). The PTP-induced swelling of mitochondria is driven by an osmotic gradient due to the colloid-osmotic pressure associated with the presence of non-diffusible matrix proteins. The PTP opening requires high Ca2+, depends on pH (low pH inhibits the PTP), mitochondrial membrane potential (ΔΨm, low ΔΨm stimulates the PTP), and the redox state of the cell (oxidative stress favors the PTP) (Halestrap et al., 2004). Ca2+ competes with a variety of inhibitors such as ADP, cyclosporin A, divalent cations (e.g., Mg2+), and protons (Szabo et al., 1992). Mitochondrial PTP-induced swelling diminishes the ETC activity and stimulates ROS production (Batandier et al., 2004; Jang and Javadov, 2017) thereby enhancing mitochondria-mediated cell death (Crompton, 1999; Halestrap et al., 2004).
Cristae remodeling induced by changes in the matrix volume and/or L-OPA metabolism can affect the structural assembly and function of IMM proteins, particularly, respiratory chain supercomplexes (RCS), supramolecular structures that consist of individual ETC complexes in various molar ratios (Wittig et al., 2006). The main RCS, respirasome contains I, III, and IV. Structural and modeling studies demonstrated that assembly of ETC complexes in respirasome facilitates electron transfer to reduce electron leakage, prevents protein aggregation and preserves the structural organization of ETC complexes (Acin-Perez et al., 2008; Bianchi et al., 2004; Chaban et al., 2014; Lapuente-Brun et al., 2013). Disruption of respirasome assembly was observed in Barth syndrome (Huang et al., 2015; McKenzie et al., 2006), Parkinson’s disease (Arthur et al., 2009), diabetes mellitus (Antoun et al., 2015), cardiovascular diseases (Jang et al., 2017; Rosca et al., 2008), and aging (Gomez et al., 2009). Despite extensive studies, the mechanisms of respirasome assembly and physiological role of RCS remain unknown. In addition to preserving the structural organization of individual ETC complexes, mitochondrial cristae shape plays a central role in the maintenance of RCS assembly to provide high respiratory efficacy in mouse fibroblasts (Cogliati et al., 2013). In addition, these studies demonstrated that conditional ablation of OPA1 disrupted cristae shape and RCS assembly although this study did not examine the role of matrix swelling. Our previous studies on isolated cardiac mitochondria (Jang and Javadov, 2017) and an ex-vivo model of heart ischemia-reperfusion (Jang et al., 2017) demonstrated that PTP-induced swelling stimulates dissociation of respirasome.
In this study, we investigated the role of PTP-induced mitochondrial swelling in OPA1-mediated regulation of RCS integrity to determine a cause-and-effect relationship between PTP induction and L-OPA1 proteolytic cleavage. Results showed that PTP-induced swelling of mitochondria stimulates proteolytic cleavage of L-OPA1 in cardiac mitochondria. Genetic depletion of OPA1 reduced PTP opening and increased mitochondrial ROS production associated with diminished ETC activity and oxidative phosphorylation. OPA1 deficiency reduced mitochondrial proteins with high molecular weight and induced disassembly of RCS.
2. Materials and methods
2.1. Animals and cells
Male Sprague-Dawley rats (225–275 g) used in these studies were purchased from Taconic Biosciences (Rensselaer, NY). All experiments were performed according to protocols approved by the University of Puerto Rico Medical Sciences Campus Animal Care and Use Committee and conformed to the National Research Council Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (2011, Eighth Edition). HEK-293 cells were purchased from ATCC (American Type Culture Collection) and cultured in EMEM (ATCC) with 10% fetal bovine serum supplemented with 1% antibiotic solution (Hyclone).
2.2. Isolation of mitochondria
Isolation of mitochondria from rat hearts and HEK cells were described as previously (Jang and Javadov, 2018b). Mitochondria were isolated from intact rat hearts. Briefly, the hearts were surgically removed and washed with ice-cold Krebs-Henseleit buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.25 mM CaCl2, 1.2 mM KH2PO4, 25 mM NaHCO3, 11 mM glucose), then aorta was mounted on a syringe filled with 30 mL of ice-cold Krebs-Henseleit buffer and flushed. After the flush, ventricles were cut and homogenized using Polytron (1,500 rpm, 5 sec) in the ice-cold sucrose buffer (300 mM sucrose, 10 mM Tris-HCl, and 2 mM EGTA, at pH 7.4). The probe of Polytron was pre-cooled on ice prior to the homogenization. The homogenate was centrifuged at 2,000 g for 3 min in the sucrose buffer. The resulted supernatant was centrifuged at 10,000 g for 5 min to sediment the mitochondria. The pellet was washed twice at 10,000 g for 5 min in sucrose buffer. The final pellets containing mitochondria were used for the analysis of mitochondrial PTP opening and OPA1 cleavage.
Isolation of mitochondria from cells was performed as previously with some modifications (Jang and Javadov, 2018b; Kitani et al., 2014). HEK-293 cells of 80–90% confluence were harvested and concentrated in sucrose buffer at 1.5 × 107 cells/mL. The cells were centrifuged at 2,500 g for 5 min and then resuspended in the sucrose buffer using the same concentration by pipetting. After 5 min of incubation on ice, the cells were homogenized with a syringe using a 27G needle with ten swift strokes, then centrifuged at 400 g for 5 min. The supernatant was collected, and the mitochondria were concentrated by centrifugation at 10,000 g for 5 min, and finally dissolved with sucrose buffer.
2.3. Mitochondrial swelling
The swelling of mitochondria as an indicator of PTP opening was determined in isolated heart and HEK-293 cell mitochondria. Isolated mitochondria were monitored by absorbance at 525 nm in the presence or absence of Ca2+. Fresh mitochondria (40 μg) were incubated at 37 °C in the 0.1 mL of the incubation buffer (200 mM sucrose, 10 mM Tris-MOPS, 5 mM α-ketoglutarate, 2 mM malate, 1 mM Pi, 10 μM EGTA-Tris, pH 7.4) using optical flat-bottom 96-well plates (Greiner). The rates of swelling were calculated as the change of absorbance (A525) induced by the programmed addition of Ca2+, in compare to 0.5 μM SfA, which was used to inhibit PTP induced swelling. The swelling assays were performed using CLARIOstar automated microplate reader (BMG Labtech).
2.4. siRNA transfection
siRNA transfections on HEK-293 cells were performed using Lipofectamine™ RNAiMAX (Thermo) and FlexiTube siRNA (Qiagen) according to the manufacturer’s recommendations. The day before transfection, cells were seeded with Opti-MEM™ (Thermo) supplemented with 5% FBS and 1% antibiotic solution (HyClone) at 60–80% confluence. The following day, Lipofectamine RNAiMAX™ and siRNA mixtures were added as manufacturer’s recommendations. Negative control siRNA (Qiagen) was used as a control. siRNA with the following sequences for OPA1 had been used: 5’GCAGCGUUAAGACAUGAAATT/5’UUUCAUGUCUUAACGCUGCAA. Cells were analyzed and harvested 48 h after transfection.
2.5. Mitochondrial ROS production
Freshly isolated mitochondria (40 μg) were incubated at 37 °C in 0.1 mL of the incubation buffer (200 mM sucrose, 10 mM Tris-MOPS, 5 mM α-ketoglutarate, 2 mM malate, 1 mM Pi, 10 μM EGTA-Tris, pH 7.4). Production of H2O2 as an indicator of ROS was measured in isolated mitochondria in the medium containing 0.1 mM Ampliflu™ Red (Sigma), 50 mM sodium phosphate pH 7.4, 0.2 U/mL HRP. A standard curve was generated by adding known concentrations of H2O2. ROS production was monitored in the presence or absence of Ca2+/SfA at an excitation of 560 nm and emission at 590 nm.
2.6. Western blot
By the end of experiments with swelling analysis, mitochondria were collected for quantification of L-OPA1 and S-OPA levels by SDS-PAGE and Western blotting. Briefly, mitochondrial samples underwent swelling analysis were centrifuged at 10,000 g for 5 min, then the pellets were dissolved in the SDS-PAGE sample buffer (25 mM Tris, pH 8.8, 25 mM DTT, 1% SDS, and 0.01% bromophenol blue) and incubated 95°C for 10 min. The samples were loaded into 8% acrylamide gels and running for 90 min at 200 V with Mini-Protean™ system (Bio-Rad). Subsequent transfers and blotting were conducted as per the manufacturer’s recommendations (Bio-Rad). The anti-OPA1 antibody used for the analysis was purchased from Cell Signaling Technologies (#80471). For analysis of ETC complexes, Total OXPHOS Rodent WB Antibody Cocktail (Abcam, #ab110413) was used and incubation conditions were modified to 50°C for 20 min according to the manufacturer’s recommendations. The samples were separated by 15% acrylamide gels. Other immunoblot analyses were performed as the manufacturer’s recommendations (Bio-Rad) using antibodies against cyclophilin D (Abcam, #ab110324), GAPDH (Santa Cruz Biotech, #sc-32233), and ATP5A (Abcam, #ab14748). Images were acquired using the Odyssey CLx Infrared Imaging System (LI-COR Biosciences) and analyzed by ImageJ (NIH).
2.7. ETC complex activities
Enzymatic activity of the ETC complexes I, II, III, and IV was measured according to the procedures described previously (Jang et al., 2017). To measure mitochondrial respiratory complex activities, isolated mitochondria from cells were diluted to 0.1 μg/μL in the buffer containing 2 mM EDTA and 0.01% Triton X-100 and then freeze-thawed two times to give the substrate access to the IMM. All assays were performed using Clariostar (BMG Labtech) at 37°C. Complex I activity was determined by measuring the rotenone-sensitive decrease of the absorbance of NADH at 340 nm. Complex II activity was measured as the velocity of 2,6-dichlorophenolindophenol (DCPIP) reduction by adding succinate, which corresponded to a decrement of absorbance at 600 nm. Complex III activity was determined from the increment of absorbance at 550 nm by the reduction of cytochrome c. Complex IV activity was measured as oxidation of reduced cytochrome c as the decrease of absorbance at 550 nm.
ATP levels of cultured HEK-293 cells were measured using ATP Bioluminescence Assay Kit CLS II (Roche) according to the manufacturer’s recommendations.
2.8. Respiratory chain supercomplexes
Mitochondrial RCS were analyzed by BN-PAGE as previously described with minor modifications (Jang and Javadov, 2018b). Briefly, 60 μg of mitochondrial proteins were dissolved with 50 μL of solubilization buffer (50 mM NaCl, 50 mM imidazole-HCl, 2 mM 6-aminohexanoic acid, 1 mM EDTA) supplemented with 2 μL of 20% digitonin, 0.5 μL protease and phosphatase inhibitor cocktails (Sigma), and 12.5 U Benzonase® (Sigma). Samples were incubated on ice for 20 min and then centrifuged for 20 min at 20,000 g. Supernatants were collected and mixed with 15 μL of sample buffer (50 mM NaCl, 10% glycerol, 0.001% Ponceau S, 50 mM Tris-HCl, pH 7.2). BN-PAGE was performed according to the manufacturer’s recommendations (Invitrogen). The gels were stained by Coomassie brilliant blue G250 and then scanned with the Odyssey CLx Infrared Imaging System (LI-COR Biosciences). The resulting images were analyzed with ImageJ (NIH). Respirasome levels were calculated as integrated pixel densities from the bands containing complexes I, III, and IV, and were normalized to whole lane density using Coomassie-stained blue native gels. The samples were processed as a batch per gel to reduce variations from digitonin (Jang and Javadov, 2018a).
2.9. Co-immunoprecipitation
To determine protein-protein interactions between OPA1 and phospholipid scramblase 3 (PLS3) or mitochondrial ETC complexes, the co-immunoprecipitation analysis was conducted using Dynabeads® as recommended by the manufacturer (Invitrogen). Briefly, mitochondrial protein samples (0.3 mg each) were incubated with 2 μg of OPA1 antibody-conjugated beads overnight at 4°C in phosphate-buffered saline supplemented with Tween 20 (10 mM Na2HPO4, 1.8 mM KH2PO4, 2.7 mM KCl, 137 mM NaCl, 0.02% Tween 20). Protease and phosphatase inhibitor cocktails added in accordance with the manufacturer’s recommendations (Sigma, #P8340 and #P0044). The OPA1 antibodies were purchased from Abcam (ab42364). The immunoprecipitated proteins were dissolved in Laemmli buffer for immunoblot analysis using antibodies against OPA1, PLS3, and mitochondrial ETC complexes. Non-specific IgG was used for assessment of the specificity of OPA1 antibodies for immunoprecipitation.
2. 10. Statistical analysis
Data were analyzed using ANOVA with normality test (Shapiro-Wilk) and pairwise multiple comparison procedures (Holm-Sidak method) in addition to Student’s t-test. Results are presented as mean ± SE. P < 0.05 was considered statistically significant.
3. Results
3.1. OPA1 cleavage is stimulated by mitochondrial swelling
First, we investigated the role of PTP-induced swelling in L-OPA1 proteolytic cleavage using mitochondria isolated from the intact rat heart. Calcium-induced swelling of mitochondria was monitored in the presence or absence of sanglifehrin A (SfA), an inhibitor of cyclophilin D ( a PTP regulator) to determine the contribution of pore opening to L-OPA1 cleavage (Fig. 1A). At the end of each measurement, mitochondria were collected to analyze L-OPA1 and S-OPA1 levels. As shown in Fig. 1B (left panel), immunoblotting revealed two L-OPA1 (bands 1 and 2) and three S-OPA1 (bands 3, 4, and 5) forms in cardiac mitochondria. Mitochondrial swelling induced by 200 μM Ca2+ decreased the level of L-OPA1 by 66% (P<0.01) but the reduction was prevented in the presence of 0.5 μM SfA. Inhibition of L-OPA1 cleavage was consistent with reduced mitochondrial swelling by SfA (Fig. 1B). On the other hand, at high Ca2+ (300 μM), SfA was not able to prevent L-OPA1 cleavage. These data are consistent with previous studies that demonstrated that the PTP can be opened at higher [Ca2+] even in the presence of SfA at the concentration of 1 μM that exhibits maximal inhibition of the pore (Clarke et al., 2002) Addition of tert-butyl hydroperoxide, an organic peroxide which stimulates mitochondrial ROS production (Jang and Javadov, 2017), did not have any significant effect on both OPA1 cleavage and swelling in the absence of Ca2+. Addition of 1 μM alamethicin, a pore-forming ionophore, which permeabilizes membrane and causes maximum mitochondrial swelling, led to L-OPA cleavage and reduced the L-OPA level by 69% (P<0.01) similar to that induced by 200 μM Ca2+ (Fig. 1B) These results demonstrate that the PTP-induced mitochondrial swelling stimulates proteolytic cleavage of L-OPA1.
Fig. 1. Calcium-induced mitochondria swelling results in OPA1 cleavage.
(A) Swelling curves of heart mitochondria induced by Ca2+ in the absence or presence of SfA, tert-butyl hydroperoxide (TBH), and alamethicin (ALM). Mitochondrial swelling was induced by the repetitive addition of 100 μM Ca2+ (arrows on the top). Final concentrations of Ca2+ (in μmol) are shown in brackets for each point. (B) A representative blot of L-OPA1 and S-OPA1 isoforms is shown (left panel). On the right panel, analysis of OPA1 cleavage in heart mitochondria treated with Ca2+ in the absence or presence of SfA, TBH, and ALM. Ca200 and Ca300, 200 and 300 μM Ca2+, respectively, and TBH100 and TBH200, 100 and 200 μM TBH, respectively. *P<0.05, **P<0.01 vs. control (non-treated) mitochondria. n=3 per each group.
3.2. OPA1 knockdown affects mitochondrial swelling and ROS production
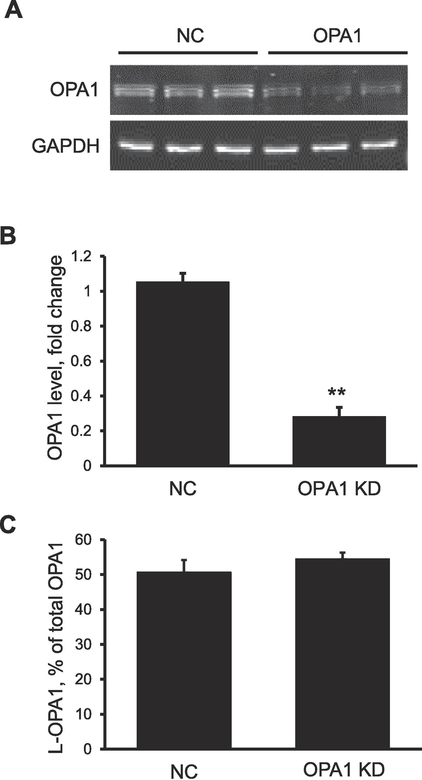
To investigate the effect of perturbation of OPA1 on PTP-stimulated swelling, OPA1 was silenced by siRNA in HEK-293 cells. The silencing resulted in a 74% decrease (P<0.01) in OPA1 protein expression, in contrast to the no significant changes in the proportion of L-OPA1 to total OPA (Fig. 2). As shown in Figure 3A and 3B, mitochondria isolated from OPA1 knockdown (KD) cells showed 17% (P<0.05) less Ca2+-induced swelling compared to negative-control siRNA treated cells. SfA markedly (by 95%; P<0.01) inhibited the swelling confirming its PTP dependence. Less swelling in OPA1 KD mitochondria was not associated with changes in cyclophilin D expression since protein levels of the PTP regulator were not affected in OPA1 siRNA treated cells (Fig. 3C). Analysis of mitochondrial ROS revealed a 20% (P<0.05) increase in Ca2+-induced ROS generation in OPA1 KD cells compared to control cells. Sanglifehrin A reduced Ca2+-induced ROS production in both negative control and OPA1 siRNA-treated cells with no effect on the difference between the two groups (Fig. 3D). Together, the results of these studies show that OPA1 deletion reduces the vulnerability of mitochondria to PTP-induced swelling but increases Ca2+-induced mitochondrial ROS production.
Fig. 2. Knockdown of OPA1 does not induce L-OPA1 proteolytic cleavage.
(A) Immunoblot images of OPA1 from negative control (NC) and OPA1 siRNA treated cells. (B) OPA1 expression normalized to GAPDH. (C) OPA1 cleavage present as the proportion of long-OPA1 to the total OPA1. **P<0.01 vs. NC; n=3 per each group.
Fig. 3. OPA1 knockdown reduces PTP-induced swelling and increases ROS generation in mitochondria.
(A) Swelling curves of mitochondria in the absence (black lines) or presence (grey lines) of SfA in the negative control (NC, solid line) and OPA1 (dashed line) siRNA-treated cells. The arrow represents the addition of 200 μM Ca2+. SfA was added 5 min before Ca2+. Blue lines: no Ca2+ added. (B) Quantification of mitochondrial swelling in NC and OPA1 siRNA treated cells. (C) Analysis of mitochondrial cyclophilin D (CypD) levels from NC and OPA1 siRNA-treated cells. Data were normalized to ATP5A. (D) The effect of swelling on ROS production in mitochondria isolated from NC and OPA1 siRNA-treated cells. *P<0.05 vs. NC; n=3 per each group.
3.3. OPA1 deficiency diminishes ETC activity and oxidative phosphorylation
Reduced swelling and increased ROS production observed in OPA1 silenced mitochondria can be due to inhibition of ETC complexes. Analysis of individual ETC complexes revealed significant alterations in their protein expression and enzymatic activity. Expression of NDUFB8 (complex I subunit), UQCRC2 (complex III subunit), and MTCO1 (complex IV subunit) were reduced 32%, 23% and 24% (P<0.05 for all), respectively, in OPA1 KD cells (Fig. 4A,B). The reductions of subunits expression are apparently due to disassembly of the complexes since the subunits are easily degraded when the target complex is not assembled. Likewise, activities of complexes I and IV were also reduced by 48% and 37%, respectively (P<0.01 for both) in OPA1 KD cells. In addition, OPA1 KD cells contained 38% (P<0.05) less ATP whereas the citrate synthase activity reflecting mitochondria mass was not affected (Fig. 4C–E). These results showed that the perturbation of OPA1 impairs the assembly and activities ETC and diminishes oxidative phosphorylation.
Fig. 4. OPA1 knockdown perturbated ETC activity and OXPHOS.
(A) Representative immunoblot of ETC complexes in the negative control (NC) and OPA1 siRNA treated cells. (B) Protein levels of ETC complexes that were quantified using specific antibodies against the subunits for complexes I (NDUFB8), II (SDHB), III (UQCRC2), IV (MTCO1), and V (ATP5A). Protein levels of complexes were normalized to complex V (ATP5A). (C) Enzymatic activity of ETC complexes. (D) ATP levels. (E) Citrate synthase activity. *P<0.05 vs. NC; n=3 per each group.
3.4. OPA1 knockdown impairs the assembly of RCS
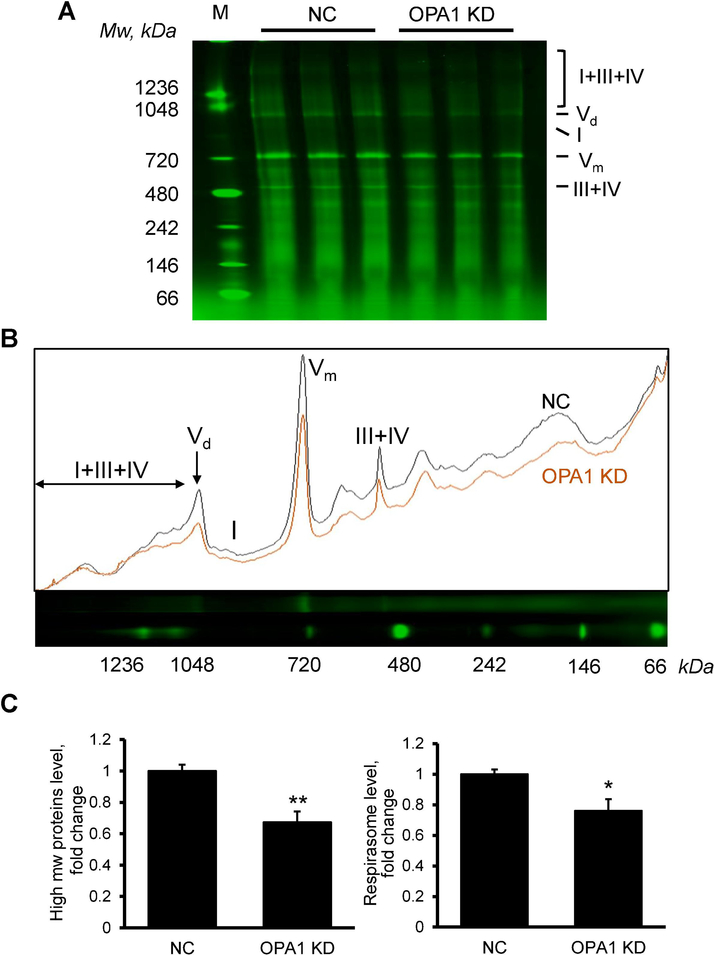
Next, we analyzed the effects of OPA1 depletion on the structural integrity of RCS, particularly respirasome. Digitonin-solubilized fractions of mitochondria from OPA1 or negative control siRNA-treated cells were separated by blue native PAGE (BN-PAGE) and RCS were identified as shown in previous studies (Jang and Javadov, 2017, 2018b; Jang et al., 2017). As shown in Figure 5, OPA depletion significantly reduced (35%, P<0.01) all proteins and protein complexes with high molecular weight (>100 kDa) in mitochondria (Fig. 5C, left panel). We normalized the signals from respirasome by the integrated pixel density of each lane to specifically quantify the RCS level by taking into consideration the reduction of all high molecular weight protein complexes. Results still showed a 24% (P<0.05) decrease of respirasome in OPA1 KD cells in comparison to control cells (Fig. 5C, right panel).
Fig. 5. OPA1 knockdown diminishes respirasome assembly.
(A) Analysis of digitonin-soluble mitochondrial fractions on BN-PAGE. M: Size marker, NC: negative-control siRNA, OPA1 KD: OPA1 siRNA; I+III+IV: respirasome, Vd: complex V dimer, I: complex I, Vm: complex V monomer, III+IV: RCS III+IV. (B) Analysis of BN-image NC: negative-control siRNA, OPA1: OPA1 siRNA. I+III+IV: respirasome, Vd: complex V dimer, I: complex I, Vm: complex V monomer, III+IV: RCS III+IV. (C) Levels of high molecular weight proteins (>100 kDa) and respirasome. Left panel: Proteins with high molecular weight (mw) measured by densitometry of the whole lane. Right panel: respirasome levels normalized by integrated density of whole lanes. *P<0.05; **P<0.01 vs. NC; n=3 per each group.
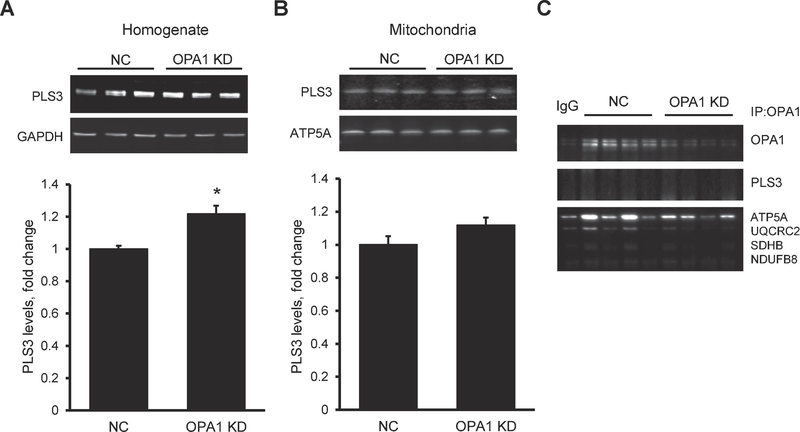
Reductions in all protein complexes with high molecular weight might be due to changes in the metabolism of cardiolipin, which plays a crucial role in the maintenance of the structural integrity of IMM proteins (Chicco and Sparagna, 2007). Indeed, studies from several groups including ours demonstrated a dissociation of RCS in patients with Barth Syndrome (McKenzie et al., 2006) and in tafazzin KD mice (Huang et al., 2015; Jang et al., 2017). One of the important steps in cardiolipin metabolism and signaling is its translocation across the IMM and possible externalization from the IMM to the outer mitochondrial membrane (Chu et al., 2013). Phospholipid scramblase 3 is a mitochondrial isoform of scramblase responsible for intramembrane translocation of phospholipids including cardiolipin in both directions between inner and outer leaflets of the membranes. Most recent studies suggested a possible role of PLS3 in phospholipid trafficking between out and inner mitochondrial membranes (Chu et al., 2013). As shown in Figure 6A and 6B, OPA1 downregulation increased protein expression of PLS3 in homogenate with no effect on mitochondrial PLS3. Furthermore, immunoprecipitation analysis did not reveal any interaction between PLS3 and OPA1 (Fig. 6C).
Fig. 6. OPA1 knockdown neither stimulates PLS3 expression nor OPA1-PLS3 interaction in mitochondria.
(A) Representative images (top panel) and quantitative data (bottom panel) of PLS3 protein levels in cell lysates. (B) Representative images (top panel) and quantitative data (bottom panel) of PLS3 protein levels in mitochondria. (C) Immunoprecipitation analysis of the interaction between OPA1 and PLS3 or ETC complexes in mitochondria. *P<0.05; **P<0.01 vs negative control (NC); n=3 per each group.
Thus, these results demonstrate that OPA deletion significantly reduces all protein complexes with high molecular weight, including RCS in mitochondria. OPA1 deficiency increased PLS3 levels in homogenate, but our result did not show physical interaction between OPA1 and PLS3.
4. Discussion
Although L-OPA1 has been shown to participate in the mitochondrial cristae modulation, the relationship between matrix swelling and L-OPA1 cleavage remains unknown. Here, we demonstrated PTP-induced matrix swelling can stimulate L-OPA1 cleavage. Moreover, the downregulation of OPA1 can induce the disruption of RCS assembly, especially respirasome associated with reduced mitochondrial swelling and ETC activity.
The opening of the PTP is the leading cause of mitochondrial matrix swelling and the earliest and most striking signs of oxidative stress-induced cell injury (Kaasik et al., 2007). Here, we demonstrate that PTP-induced functional and structural alterations in cardiac mitochondria are apparently mediated through the L-OPA1 cleavage. L-OPA1 cleavage has been shown to reduce its activity, enhance mitochondrial fragmentation and impair cardiac metabolism, eventually leading to dilated cardiomyopathy and heart failure in mice (Wai et al., 2015). The dependence of OPA cleavage on the mitochondrial swelling was concluded on the basis of the following observations. First, some basal levels of S-OPA1 can be seen in the control group (Fig.1B, without Ca2+ addition), and S-OPA1 levels were increased with concurrent reduction of L-OPA in the presence of 200 μM Ca2. Second, we confirmed the dependence of L-OPA cleavage on the mitochondrial swelling in experiments with SfA and alamethicin. The effects of 200 μM Ca2+ to induce mitochondrial swelling (Fig. 1A) and L-OPA1 cleavage (Fig. 1B) were prevented in the presence of SfA (Ca200+SfA group). In addition, Ca2+-independent mitochondrial swelling induced by alamethicin, a channel-forming peptide antibiotic that is used for permeabilization of the inner mitochondrial membrane, was associated with L-OPA1 cleavage (Fig. 1B, ALM group). These results demonstrate that L-OPA1 cleavage depends on mitochondrial swelling (PTP-dependent and PTP-independent). The dependence of L-OPA cleavage on the PTP induction can be explained by ΔΨm loss and ATP depletion upon pore opening. Proteolytic degradation of L-OPA occurs at S1 and S2 sites of the protein by proteases OMA1 and YMEL1, respectively. YMEL1-mediated L-OPA1 cleavage is stimulated by increased activity of oxidative phosphorylation and enhances IMM fusion (Mishra et al., 2014). In contrast, L-OPA cleavage by OMA1 is enhanced by mitochondrial dysfunction including ΔΨm loss, reduced respiration rates, and oxidative phosphorylation (Ehses et al., 2009; Head et al., 2009).
Thus, L-OPA1 cleavage by YMEL1 maintains mitochondrial fusion under physiological conditions whereas OMA1-induced cleavage is associated with mitochondrial stress. Our results suggest that the balance between L-OPA1 and S-OPA1 determines the IMM structural and functional integrity and can serve as the intramitochondrial mediators of PTP opening. The loss of L-OPA1 with concomitant accumulation of S-OPA1 induces mitochondrial fragmentation, disturbs cristae morphology and stimulates mitochondria-mediated apoptosis (Anand et al., 2014; Head et al., 2009; Jiang et al., 2014). Similar structural and functional disturbances are observed in response to PTP-induced mitochondrial swelling (Javadov et al., 2011) suggesting that L-OPA cleavage is actually secondary to pore opening. In favor of the role of PTP-induced mitochondrial swelling previous studies using the shrinkage assay confirmed that inhibition of PTP opening by SfA was associated with the reduction in mitochondrial volume (Clarke et al., 2002).
Interestingly, OPA1 downregulation reduced PTP-induced swelling in response to Ca2+ without changes in L-OPA1 cleavage (Fig. 3A,B). The mechanism underlying the low swelling in OPA KD mitochondria is not clear. The low swelling could attenuate, in addition to necrosis, the release of pro-apoptotic proteins (cytochrome c, apoptosis-inducing factor, etc.) from the intermembrane space and thus, prevent caspase-dependent and caspase-independent apoptotic cell death in these cells. L-OPA1 has been shown to modulate cristae organization in response to the cellular metabolic demand (Patten et al., 2014) and thereby, make the IMM more resistant to Ca2+ stress. Likewise, L-OPA1 has been shown to protect mitochondria from respiratory chain inhibition by stabilizing cristae shape (Quintana-Cabrera et al., 2018). We suggest that OPA1 KD results in the loss of its capacity to regulate cristae structure that leads to respirasome disassembly associated with reduced ETC activity and diminished ATP production. In other words, ETC activity is required for correctly assembled RSC, and OPA1 perturbation may have a negative impact on cristae structure which needs to assemble RCS efficiently. Notably, complex I activity and protein expression in OPA1 KD mitochondria was lower than in the control group. Complex I is a regulator of PTP, and inhibition of the complex has been shown to attenuate PTP opening (Jang and Javadov, 2018b; Li et al., 2012). Inhibition of the ETC activity can induce ΔΨm loss, which, in turn, can reduce Ca2+ flux into the mitochondria (Kon et al., 2017) and lead to a lower matrix swelling. One can expect that the number of mitochondria might be increased to compensate for the loss of the expression of ETC subunits. However, we suggest that the time (48 h) after transfection was not sufficient for cells to compensate by increasing mitochondrial number/mass based on the finding that OPA KD had no effect on the activity of citrate synthase (a marker of mitochondrial mass) (Fig. 4E).
Mitochondria isolated from OPA1 KD cells showed increased Ca2+-stimulated ROS production (Fig. 3D). This might be due to reduced complex assembly and perturbated respirasome assembly that could lead to increased ROS generation by ETC complexes. The role of respirasome to facilitate “electron channeling” is disputed (Blaza et al., 2014) and the current between two protein partners in the respirasome decays along with more than 10 nm in the solution (Lagunas et al., 2018). Nevertheless, the respirasome assembly has been suggested to be able to control ROS production (Genova and Lenaz, 2015), and changes in complex I assembly into RSC has been shown to regulate ROS differently in neurons and astrocytes (Lopez-Fabuel et al., 2016). On the other hand, the mitochondria-targeted antioxidant XJB-5–131 prevented oxidative damage of proteins in aged rats with no effect on the respirasome levels (Javadov et al., 2015).
In conclusion, this study revealed that proteolytic cleavage of L-OPA1 depends on the PTP-induced mitochondrial swelling but not ROS. Apparently, L-OPA mediates the effects of matrix swelling to modulate the structural integrity and cristae organization in mitochondria. Increased swelling stimulates the activity of OMA1, a stress-activated metalloprotease, leading to L-OPA1 cleavage that, in turn, impairs the metabolism and function of mitochondria.
Highlights.
PTP-induced mitochondrial swelling stimulates the proteolytic cleavage of L-OPA1 to S-OPA1
OPA1 knockdown reduces PTP-induced mitochondrial swelling but enhances mitochondrial ROS production
OPA1 deficiency impairs the respiratory chain supercomplexes assembly associated with diminished ETC activity and oxidative phosphorylation
OPA1 downregulation increases expression of the phospholipid scramblase 3
Acknowledgments
This study was supported by the National Institute of General Medical Sciences (Grant SC1GM128210 to SaJ) and in part, by the National Center for Research Resources (Grants G12RR-003051 and G12MD007600) of the National Institutes of Health.
Footnotes
Author Disclosure Statement
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, Enriquez JA, 2008. Respiratory active mitochondrial supercomplexes. Mol Cell 32, 529–539. [DOI] [PubMed] [Google Scholar]
- Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA Jr., Jonas EA, 2014. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A 111, 10580–10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, Langer T, 2014. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol 204, 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoun G, McMurray F, Thrush AB, Patten DA, Peixoto AC, Slack RS, McPherson R, Dent R, Harper ME, 2015. Impaired mitochondrial oxidative phosphorylation and supercomplex assembly in rectus abdominis muscle of diabetic obese individuals. Diabetologia 58, 2861–2866. [DOI] [PubMed] [Google Scholar]
- Arthur CR, Morton SL, Dunham LD, Keeney PM, Bennett JP Jr., 2009. Parkinson’s disease brain mitochondria have impaired respirasome assembly, age-related increases in distribution of oxidative damage to mtDNA and no differences in heteroplasmic mtDNA mutation abundance. Mol Neurodegener 4, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarashvili T, Odinokova I, Bakunts A, Ternovsky V, Krestinina O, Tyynela J, Saris NE, 2014. Potential role of subunit c of F0F1-ATPase and subunit c of storage body in the mitochondrial permeability transition. Effect of the phosphorylation status of subunit c on pore opening. Cell Calcium 55, 69–77. [DOI] [PubMed] [Google Scholar]
- Batandier C, Leverve X, Fontaine E, 2004. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J Biol Chem 279, 17197–17204. [DOI] [PubMed] [Google Scholar]
- Beavis AD, Brannan RD, Garlid KD, 1985. Swelling and contraction of the mitochondrial matrix. I. A structural interpretation of the relationship between light scattering and matrix volume. J Biol Chem 260, 13424–13433. [PubMed] [Google Scholar]
- Bernardi P, Di Lisa F, 2015. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J Mol Cell Cardiol 78, 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi C, Genova ML, Parenti Castelli G, Lenaz G, 2004. The mitochondrial respiratory chain is partially organized in a supercomplex assembly: kinetic evidence using flux control analysis. J Biol Chem 279, 36562–36569. [DOI] [PubMed] [Google Scholar]
- Blaza JN, Serreli R, Jones AJ, Mohammed K, Hirst J, 2014. Kinetic evidence against partitioning of the ubiquinone pool and the catalytic relevance of respiratory-chain supercomplexes. Proc Natl Acad Sci U S A 111, 15735–15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J, He J, Ding S, Fearnley IM, Walker JE, 2019. Persistence of the permeability transition pore in human mitochondria devoid of an assembled ATP synthase. Proc Natl Acad Sci U S A 116, 12816–12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban Y, Boekema EJ, Dudkina NV, 2014. Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochim Biophys Acta 1837, 418–426. [DOI] [PubMed] [Google Scholar]
- Chicco AJ, Sparagna GC, 2007. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol 292, C33–44. [DOI] [PubMed] [Google Scholar]
- Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Wang KZQ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, Borisenko G, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Baty C, Watkins S, Bahar I, Bayir H, Kagan VE, 2013. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol 15, 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civiletto G, Varanita T, Cerutti R, Gorletta T, Barbaro S, Marchet S, Lamperti C, Viscomi C, Scorrano L, Zeviani M, 2015. Opa1 overexpression ameliorates the phenotype of two mitochondrial disease mouse models. Cell Metab 21, 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SJ, McStay GP, Halestrap AP, 2002. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem 277, 34793–34799. [DOI] [PubMed] [Google Scholar]
- Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes LC, Perales-Clemente E, Salviati L, Fernandez-Silva P, Enriquez JA, Scorrano L, 2013. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155, 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M, 1999. The mitochondrial permeability transition pore and its role in cell death. Biochem J 341 (Pt 2), 233–249. [PMC free article] [PubMed] [Google Scholar]
- Del Dotto V, Mishra P, Vidoni S, Fogazza M, Maresca A, Caporali L, McCaffery JM, Cappelletti M, Baruffini E, Lenaers G, Chan D, Rugolo M, Carelli V, Zanna C, 2017. OPA1 Isoforms in the Hierarchical Organization of Mitochondrial Functions. Cell Rep 19, 2557–2571. [DOI] [PubMed] [Google Scholar]
- Delettre C, Griffoin JM, Kaplan J, Dollfus H, Lorenz B, Faivre L, Lenaers G, Belenguer P, Hamel CP, 2001. Mutation spectrum and splicing variants in the OPA1 gene. Hum Genet 109, 584–591. [DOI] [PubMed] [Google Scholar]
- Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, Martinou JC, Westermann B, Rugarli EI, Langer T, 2009. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol 187, 1023–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L, 2006. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126, 177–189. [DOI] [PubMed] [Google Scholar]
- Genova ML, Lenaz G, 2015. The Interplay Between Respiratory Supercomplexes and ROS in Aging. Antioxid Redox Signal 23, 208–238. [DOI] [PubMed] [Google Scholar]
- Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P, 2013. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A 110, 5887–5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez LA, Monette JS, Chavez JD, Maier CS, Hagen TM, 2009. Supercomplexes of the mitochondrial electron transport chain decline in the aging rat heart. Arch Biochem Biophys 490, 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, 1989. The regulation of the matrix volume of mammalian mitochondria in vivo and in vitro and its role in the control of mitochondrial metabolism. Biochim Biophys Acta 973, 355–382. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Clarke SJ, Javadov SA, 2004. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res 61, 372–385. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Kerr PM, Javadov S, Woodfield KY, 1998. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta 1366, 79–94. [DOI] [PubMed] [Google Scholar]
- He J, Carroll J, Ding S, Fearnley IM, Walker JE, 2017a. Permeability transition in human mitochondria persists in the absence of peripheral stalk subunits of ATP synthase. Proc Natl Acad Sci U S A 114, 9086–9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Ford HC, Carroll J, Ding S, Fearnley IM, Walker JE, 2017b. Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase. Proc Natl Acad Sci U S A 114, 3409–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM, 2009. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol 187, 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Powers C, Madala SK, Greis KD, Haffey WD, Towbin JA, Purevjav E, Javadov S, Strauss AW, Khuchua Z, 2015. Cardiac metabolic pathways affected in the mouse model of barth syndrome. PLoS One 10, e0128561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Fujita Y, Oka T, Mihara K, 2006. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. Embo j 25, 2966–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Javadov S, 2017. Association between ROS production, swelling and the respirasome integrity in cardiac mitochondria. Arch Biochem Biophys 630, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Javadov S, 2018a. Current Challenges in Elucidating Respiratory Supercomplexes in Mitochondria: Methodological Obstacles. Front Physiol 9, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Javadov S, 2018b. Elucidating the contribution of ETC complexes I and II to the respirasome formation in cardiac mitochondria. Sci Rep 8, 17732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Lewis TS, Powers C, Khuchua Z, Baines CP, Wipf P, Javadov S, 2017. Elucidating Mitochondrial Electron Transport Chain Supercomplexes in the Heart During Ischemia-Reperfusion. Antioxid Redox Signal 27, 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadov S, Jang S, Rodriguez-Reyes N, Rodriguez-Zayas AE, Soto Hernandez J, Krainz T, Wipf P, Frontera W, 2015. Mitochondria-targeted antioxidant preserves contractile properties and mitochondrial function of skeletal muscle in aged rats. Oncotarget 6, 39469–39481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadov S, Rajapurohitam V, Kilic A, Hunter JC, Zeidan A, Said Faruq N, Escobales N, Karmazyn M, 2011. Expression of mitochondrial fusion-fission proteins during postinfarction remodeling: the effect of NHE-1 inhibition. Basic Res Cardiol 106, 99–109. [DOI] [PubMed] [Google Scholar]
- Jiang X, Jiang H, Shen Z, Wang X, 2014. Activation of mitochondrial protease OMA1 by Bax and Bak promotes cytochrome c release during apoptosis. Proc Natl Acad Sci U S A 111, 14782–14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasik A, Safiulina D, Zharkovsky A, Veksler V, 2007. Regulation of mitochondrial matrix volume. Am J Physiol Cell Physiol 292, C157–163. [DOI] [PubMed] [Google Scholar]
- Kitani T, Kami D, Matoba S, Gojo S, 2014. Internalization of isolated functional mitochondria: involvement of macropinocytosis. J Cell Mol Med 18, 1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon N, Satoh A, Miyoshi N, 2017. A small-molecule DS44170716 inhibits Ca(2+)-induced mitochondrial permeability transition. Sci Rep 7, 3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong JQ, Molkentin JD, 2015. Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metab 21, 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunas A, Guerra-Castellano A, Nin-Hill A, Diaz-Moreno I, De la Rosa MA, Samitier J, Rovira C, Gorostiza P, 2018. Long distance electron transfer through the aqueous solution between redox partner proteins. Nat Commun 9, 5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapuente-Brun E, Moreno-Loshuertos R, Acin-Perez R, Latorre-Pellicer A, Colas C, Balsa E, Perales-Clemente E, Quiros PM, Calvo E, Rodriguez-Hernandez MA, Navas P, Cruz R, Carracedo A, Lopez-Otin C, Perez-Martos A, Fernandez-Silva P, Fernandez-Vizarra E, Enriquez JA, 2013. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 340, 1567–1570. [DOI] [PubMed] [Google Scholar]
- Li B, Chauvin C, De Paulis D, De Oliveira F, Gharib A, Vial G, Lablanche S, Leverve X, Bernardi P, Ovize M, Fontaine E, 2012. Inhibition of complex I regulates the mitochondrial permeability transition through a phosphate-sensitive inhibitory site masked by cyclophilin D. Biochim Biophys Acta 1817, 1628–1634. [DOI] [PubMed] [Google Scholar]
- Lopez-Fabuel I, Le Douce J, Logan A, James AM, Bonvento G, Murphy MP, Almeida A, Bolanos JP, 2016. Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc Natl Acad Sci U S A 113, 13063–13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella CA, 2006. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta 1763, 542–548. [DOI] [PubMed] [Google Scholar]
- McKenzie M, Lazarou M, Thorburn DR, Ryan MT, 2006. Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. J Mol Biol 361, 462–469. [DOI] [PubMed] [Google Scholar]
- Mishra P, Carelli V, Manfredi G, Chan DC, 2014. Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab 19, 630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichon A, Emorine LJ, Descoins E, Pelloquin L, Brichese L, Gas N, Guillou E, Delettre C, Valette A, Hamel CP, Ducommun B, Lenaers G, Belenguer P, 2002. The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett 523, 171–176. [DOI] [PubMed] [Google Scholar]
- Patten DA, Wong J, Khacho M, Soubannier V, Mailloux RJ, Pilon-Larose K, MacLaurin JG, Park DS, McBride HM, Trinkle-Mulcahy L, Harper ME, Germain M, Slack RS, 2014. OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. Embo j 33, 2676–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana-Cabrera R, Quirin C, Glytsou C, Corrado M, Urbani A, Pellattiero A, Calvo E, Vazquez J, Enriquez JA, Gerle C, Soriano ME, Bernardi P, Scorrano L, 2018. The cristae modulator Optic atrophy 1 requires mitochondrial ATP synthase oligomers to safeguard mitochondrial function. Nat Commun 9, 3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, Sabbah HN, Hoppel CL, 2008. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res 80, 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalettar BA, Abney JR, Hackenbrock CR, 1991. Dynamics, structure, and function are coupled in the mitochondrial matrix. Proc Natl Acad Sci U S A 88, 8057–8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Bernardi P, Zoratti M, 1992. Modulation of the mitochondrial megachannel by divalent cations and protons. J Biol Chem 267, 2940–2946. [PubMed] [Google Scholar]
- Wai T, Garcia-Prieto J, Baker MJ, Merkwirth C, Benit P, Rustin P, Ruperez FJ, Barbas C, Ibanez B, Langer T, 2015. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science 350, aad0116. [DOI] [PubMed] [Google Scholar]
- Wittig I, Carrozzo R, Santorelli FM, Schagger H, 2006. Supercomplexes and subcomplexes of mitochondrial oxidative phosphorylation. Biochim Biophys Acta 1757, 1066–1072. [DOI] [PubMed] [Google Scholar]