Background
Long considered to be pathogens exclusive to the gastrointestinal tract, astroviruses have been the focus of renewed interest due to the recognition of invasive infection in humans and other mammals, including central nervous system (CNS) infection and viremia[1–4]. While gastrointestinal astrovirus infection is a self-limited disease, six out of ten cases of human meningoencephalitis caused by astroviruses have resulted in death [5–12]. Currently, there are no drugs approved by the US or European regulatory agencies for treatment of astrovirus infections, and there are no published treatment trials of astrovirus infections. It is unclear what role broad-spectrum antivirals may against astroviruses because they have not been empirically tested in vitro or in vivo. In one case of survival from astrovirus encephalitis, the patient received intravenous immunoglobulin, steroids, ribavirin, and pegylated interferon-α2b[5]. In a fatal case, the patient received intravenous immunoglobulin and ribavirin prior to death[6]. Flavonoids and immunomodulatory drugs like interferon-α/β have also demonstrated efficacy in reducing astrovirus replication in cell culture[13–16].
Ribavirin is the prototypical broad-spectrum antiviral as it has multiple mechanisms of action against many RNA viruses, including direct inhibition of the RNA-dependent RNA polymerase (RdRp)[17]. Ribavirin has in vitro activity against noroviruses (10 μM caused 64% reduction in human norovirus replication) and enteroviruses (EC50 range of 106 to >1100μM), members of two viral families that share phylogenetic relationships to the astrovirus RdRp, but the activity of ribavirin against astroviruses has not been quantified[18–20]. Favipiravir also has in vitro antiviral activity against many RNA viruses, including enteroviruses and noroviruses (EC50 range 31–248μM); however, the efficacy of favipiravir against astroviruses has also not been determined[21].
Objectives
We quantified the effect of both ribavirin and favipiravir on the in vitro replication of two astrovirus genotypes, astrovirus VA1 (VA1) and human astrovirus 4 (HAstV4), two strains that have been associated with central nervous system disease in humans[2].
Study Design
Ribavirin (Sigma-Aldrich) was suspended in Dulbecco’s Modified Eagle Media (DMEM, Gibco) with serial dilutions performed to generate dosages over a range of 0.1–1000μM. Due to poor water solubility, favipiravir (Biovision) was suspended in dimethyl sulfoxide (DMSO; Sigma-Aldrich) at 5mM with serial dilutions made into DMEM over a range of 0.1–1000μM. All cells treated with favipiravir had 1% DMSO (v/v) added to each well to normalize to the DMSO concentration present in 1000μM of favipiravir.
Caco-2 cells were grown to confluence in 96-well plates. Both VA1 and HAstV4 stocks were originally isolated from stool specimens and propagated in Caco-2 cells [13, 22]. HAstV4 was pretreated with 100μg/mL of trypsin for 30 minutes as HAstV4 but not VA1 requires trypsin for propagation in cell culture[13, 23]. Cells were infected with a MOI 0.01 of VA1, HAstV4, or Influenza A WSN (IAV) in the presence of ribavirin or favipiravir for one hour, the cells were then washed once, and growth media added to the cells containing ribavirin or favipiravir[22, 24]. IAV was used to confirm the efficacy of both drugs. For VA1, the cells were maintained in growth media containing DMEM with 10% fetal bovine serum (FBS), For HAstV4 and IAV infections, the cells were maintained in DMEM with 3.3μg/mL of trypsin and no FBS. At 72 hours post-inoculation, the supernatant was removed, TRIzol (ThermoFisher) was added to the cells, and RNA extracted using Direct-zol 96-well RNA extraction plates (Zymo Research). VA1, HAstV4, and IAV RNA were quantified using previously published qRT-PCR assays[13, 22, 25]. Viral RNA cycle threshold (CT) values were normalized to the CT values for the housekeeping gene, Ribosomal Protein Lateral Stalk Subunit P0 (RPLP0), using the delta CT method and then normalized to no drug treatment using the delta-delta CT method[26]. Geometric means of the change in RNA relative to no treatment was graphed in Prism 8.1.0 (GraphPad) and compared using one-way or Brown-Forsythe ANOVA tests with post-hoc analysis by Dunnett’s with or without T3 multiple comparisons test. The 50% effective concentration (EC50) values were calculated using Prism. Cells treated with favipiravir or ribavirin in the absence of virus using the aforementioned media conditions for 72 hours were also analyzed by the CellTiter-Glo assay (Promega) for quantification of toxicity. Mean relative luminescence units (RLU) were plotted in GraphPad. Because we did not detect >50% cytotoxicity over the concentrations of the drugs tested, the lower limit 50% cytotoxicity concentration (CC50) value was set at 1000μM for calculation of selectivity indices (SI; CC50 value divided by EC50 value). For all analyses, two independent experiments were conducted with 3 replicates per experiment.
Results and discussion
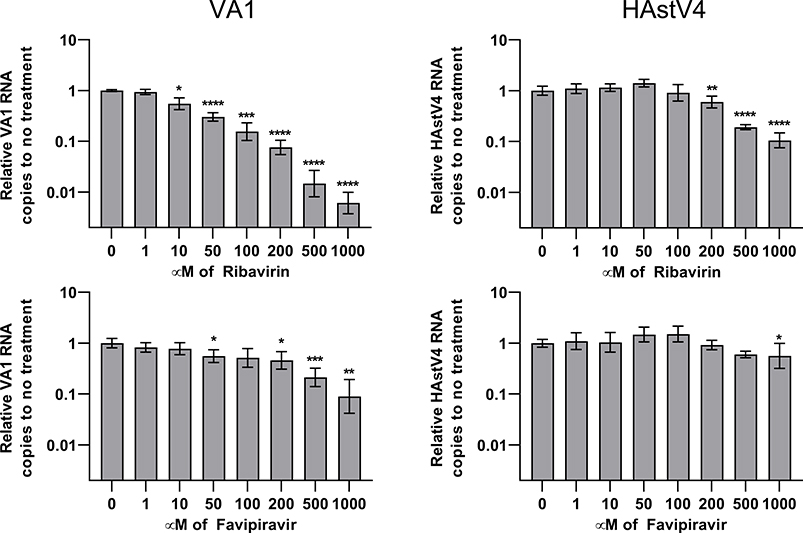
Ribavirin treatment of Caco-2 cells infected with VA1 or HAstV4 caused a 10–100-fold reduction of viral RNA with greater effect on VA1 replication (Figure 1). The EC50 value was also lower for VA1 (154μM) compared to HAstV4 (268μM) (Table 1). Only mild toxicity of ribavirin was observed at 1000μM as a 17–31% decrease in luminescence in the CellTiter-Glo assay was observed (Figure 2; P≤ 0.004). Favipiravir treatment resulted in a 10-fold reduction of VA1 replication, but in contrast, only a 44% reduction of HAstV4 replication was observed at 1000μM (Figure 1). No significant reduction of cell viability was observed at any concentration of favipiravir (Figure 2). We confirmed the activity of our formulations of ribavirin and favipiravir as both significantly inhibited IAV with EC50 values consistent with previously published values (Table 1)[21, 27].
Figure 1:
Quantification of VA1 or HAstV4 RNA upon treatment with ribavirin or favipiravir, normalized to no drug treatment. Error bars represent one geometric standard deviation. *, P≤0.05; **, P≤0.01; ***, P≤0.001; ****, P≤0.0001.
Table 1:
Calculated EC50 concentrations in μM of each virus-antiviral treatment with ±95% confidence intervals and selectivity indices (SI).
| VA1 | HAstV4 | IAV | ||||
|---|---|---|---|---|---|---|
| EC50 | SI | EC50 | SI | EC50 | SI | |
| Ribavirin | 154 μM ± 21 | >6.5 | 268 μM ± 39.5 | >3.7 | 4.54 μM ± 0.97 | >220 |
| Favipiravir | 246 μM ± 76.4 | >4.1 | >1000 μM | 1 | 4.73 μM ± 1.01 | >211 |
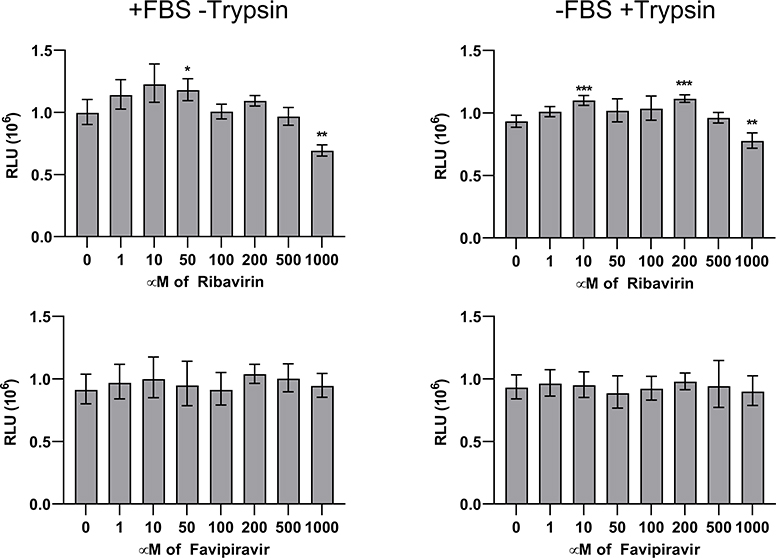
Figure 2:
Measurement of luminescence (RLU, relative luminescence units) for each antiviral drug concentration, reflecting the number of viable cells in each treatment group. Error bars represent one geometric standard deviation. *, P≤0.05; **, P≤0.01; ***, P≤0.001.
The recent recognition of fatal astrovirus infections in humans has highlighted a greater need effective anti-astrovirus therapies. Previously, there was no in vitro data regarding the inhibitory effect of broad-spectrum antivirals on astroviruses. Our results demonstrate that both ribavirin and favipiravir inhibit replication of VA1 and ribavirin inhibits HAstV4 in cell culture. The in vitro EC50 values identified for both drugs have similar activities for other enteric viruses like enteroviruses and noroviruses[18, 19, 21].
Interestingly, very modest inhibition of HAstV4 with favipiravir was observed, despite the drug demonstrating greater activity against VA1 and IAV. The cause of this differential effect of favipiravir could be specific to differences in the RdRp of HAstV4 (AZB52200.1), which shares 52.3% amino acid identity with VA1 (YP_003090286.1). Resistance to favipiravir has been described in other viruses with amino acid substitutions in the RdRp[28]. Alternatively, HAstV4 may utilize unique host factors for replication that are not inhibited by favipiravir.
Currently, there are no animal models of human astrovirus infection, precluding our ability perform in vivo efficacy studies. The ribavirin and favipiravir EC50 concentrations for VA1 are comparable to the EC50 concentrations identified for yellow fever virus (YFV), and both drugs were found to be protective in a hepatitis model of fatal YFV infection[29]. Nonetheless, effective treatment of astrovirus infection of the central nervous system may require penetration of the blood-brain barrier (BBB) by the candidate antiviral. There is conflicting data whether ribavirin is able to penetrate the human BBB while the penetration of favipiravir into the CNS has not been described[30].
We did not observe significant cytopathic effect with our drug concentrations of ribavirin or favipiravir, with or without trypsin/FBS, consistent with other previously published CC50 values that were also >1000μM [18, 19, 21]. We did observe a small increase in cellular adenosine triphosphate (ATP) in the CellTiter-Glo assay in ribavirin treated cells at 10–200μM (Figure 2), consistent with a previous publication[31]. Because of the limits of solubility and the need to maintain sufficient volume of cell culture media, we did not achieve 50% cytopathic effect with the drug concentrations that were tested, so our estimation of the SI is an underestimate. Nonetheless, the calculated SIs indicate the likelihood of a narrow therapeutic window for these drugs in vivo and it could be difficult to achieve serum concentrations for sufficient inhibition of astrovirus replication. In future studies, the possibility of additive or synergistic effects of combination therapy of ribavirin and favipiravir can be evaluated, and the addition of recombinant interferon may also enhance the activity of either drug. Future studies may also lead to identification of novel antivirals that are astrovirus specific.
Highlights.
Ribavirin inhibits replication of astrovirus VA1 and classic human astrovirus 4.
Replication of astrovirus VA1 is reduced by favipiravir
No significant cytotoxicity was detected for favipiravir or ribavirin
Acknowledgements
We would like to thank Carl Kirkwood for providing the classic human astrovirus 4 stock.
Funding information
ABJ is supported in part by the NIH under the grant K08 AI132745. DW is supported in part by NIH grant R21 NS101371. The content is solely our responsibility and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Declaration
None to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bosch A, Pinto RM, Guix S. Human astroviruses. Clinical microbiology reviews. 2014;27:1048–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Reuter G, Pankovics P, Boros A. Nonsuppurative (Aseptic) Meningoencephalomyelitis Associated with Neurovirulent Astrovirus Infections in Humans and Animals. Clinical microbiology reviews. 2018;31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Selimovic-Hamza S, Boujon CL, Hilbe M, Oevermann A, Seuberlich T. Frequency and Pathological Phenotype of Bovine Astrovirus CH13/NeuroS1 Infection in Neurologically-Diseased Cattle: Towards Assessment of Causality. Viruses. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Matias Ferreyra FS, Bradner LK, Burrough ER, Cooper VL, Derscheid RJ, Gauger PC, et al. Polioencephalomyelitis in Domestic Swine Associated With Porcine Astrovirus Type 3. Vet Pathol. 2019:300985819875741. [DOI] [PubMed] [Google Scholar]
- [5].Fremond ML, Perot P, Muth E, Cros G, Dumarest M, Mahlaoui N, Seilhean D, Desguerre I, Hebert C, Corre-Catelin N, Neven B, Lecuit M, Blanche S, Picard C, Eloit M Next-Generation Sequencing for Diagnosis and Tailored Therapy: A Case Report of Astrovirus-Associated Progressive Encephalitis. Journal of the Pediatric Infecctious Diseases Society 2015:1–5. [DOI] [PubMed] [Google Scholar]
- [6].Naccache SN, Peggs KS, Mattes FM, Phadke R, Garson JA, Grant P, et al. Diagnosis of neuroinvasive astrovirus infection in an immunocompromised adult with encephalitis by unbiased next-generation sequencing. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60:919–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Quan PL, Wagner TA, Briese T, Torgerson TR, Hornig M, Tashmukhamedova A, et al. Astrovirus encephalitis in boy with X-linked agammaglobulinemia. Emerging infectious diseases. 2010;16:918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lum SH, Turner A, Guiver M, Bonney D, Martland T, Davies E, et al. An emerging opportunistic infection: fatal astrovirus (VA1/HMO-C) encephalitis in a pediatric stem cell transplant recipient. Transplant infectious disease : an official journal of the Transplantation Society. 2016. [DOI] [PubMed] [Google Scholar]
- [9].Brown JR, Morfopoulou S, Hubb J, Emmett WA, Ip W, Shah D, et al. Astrovirus VA1/HMO-C: an increasingly recognized neurotropic pathogen in immunocompromised patients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60:881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sato M, Kuroda M, Kasai M, Matsui H, Fukuyama T, Katano H, et al. Acute encephalopathy in an immunocompromised boy with astrovirus-MLB1 infection detected by next generation sequencing. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2016;78:66–70. [DOI] [PubMed] [Google Scholar]
- [11].Cordey S, Vu DL, Schibler M, L’Huillier AG, Brito F, Docquier M, et al. Astrovirus MLB2, a New Gastroenteric Virus Associated with Meningitis and Disseminated Infection. Emerging infectious diseases. 2016;22:846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wilson MR, Sample HA, Zorn KC, Arevalo S, Yu G, Neuhaus J, et al. Clinical Metagenomic Sequencing for Diagnosis of Meningitis and Encephalitis. The New England journal of medicine. 2019;380:2327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Janowski AB, Bauer IK, Holtz LR, Wang D. Propagation of astrovirus VA1, a neurotropic human astrovirus, in cell culture. Journal of virology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guix S, Perez-Bosque A, Miro L, Moreto M, Bosch A, Pinto RM. Type I interferon response is delayed in human astrovirus infections. PloS one. 2015;10:e0123087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Marvin SA, Huerta CT, Sharp B, Freiden P, Cline TD, Schultz-Cherry S. Type I Interferon Response Limits Astrovirus Replication and Protects against Increased Barrier Permeability In Vitro and In Vivo. Journal of virology. 2016;90:1988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Superti F, Seganti L, Orsi N, Desideri N, Stein ML, Tinari A, et al. In vitro effect of synthetic flavanoids on astrovirus infection. Antiviral Res. 1990;13:201–8. [DOI] [PubMed] [Google Scholar]
- [17].Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Reviews in medical virology. 2006;16:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dang W, Yin Y, Wang Y, Wang W, Su J, Sprengers D, et al. Inhibition of Calcineurin or IMP Dehydrogenase Exerts Moderate to Potent Antiviral Activity against Norovirus Replication. Antimicrobial agents and chemotherapy. 2017;61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Smee DF, Evans WJ, Nicolaou KC, Tarbet EB, Day CW. Susceptibilities of enterovirus D68, enterovirus 71, and rhinovirus 87 strains to various antiviral compounds. Antiviral Res. 2016;131:61–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wolf YI, Kazlauskas D, Iranzo J, Lucia-Sanz A, Kuhn JH, Krupovic M, et al. Origins and Evolution of the Global RNA Virome. mBio. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Janowski AB, Klein RS, Wang D. Differential In Vitro Infection of Neural Cells by Astroviruses. mBio. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee TW, Kurtz JB. Serial propagation of astrovirus in tissue culture with the aid of trypsin. The Journal of general virology. 1981;57:421–4. [DOI] [PubMed] [Google Scholar]
- [24].Janowski AB, Wang D. Infection and Propagation of Astrovirus VA1 in Cell Culture. Curr Protoc Microbiol. 2018:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kawakami E, Watanabe T, Fujii K, Goto H, Watanabe S, Noda T, et al. Strand-specific real-time RT-PCR for distinguishing influenza vRNA, cRNA, and mRNA. Journal of virological methods. 2011;173:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- [27].Sidwell RW, Bailey KW, Wong MH, Barnard DL, Smee DF. In vitro and in vivo influenza virus-inhibitory effects of viramidine. Antiviral Res. 2005;68:10–7. [DOI] [PubMed] [Google Scholar]
- [28].Delang L, Segura Guerrero N, Tas A, Querat G, Pastorino B, Froeyen M, et al. Mutations in the chikungunya virus non-structural proteins cause resistance to favipiravir (T-705), a broad-spectrum antiviral. The Journal of antimicrobial chemotherapy. 2014;69:2770–84. [DOI] [PubMed] [Google Scholar]
- [29].Julander JG, Shafer K, Smee DF, Morrey JD, Furuta Y. Activity of T-705 in a hamster model of yellow fever virus infection in comparison with that of a chemically related compound, T-1106. Antimicrobial agents and chemotherapy. 2009;53:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rhoades RE, Tabor-Godwin JM, Tsueng G, Feuer R. Enterovirus infections of the central nervous system. Virology. 2011;411:288–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shah NR, Sunderland A, Grdzelishvili VZ. Cell type mediated resistance of vesicular stomatitis virus and Sendai virus to ribavirin. PloS one. 2010;5:e11265. [DOI] [PMC free article] [PubMed] [Google Scholar]