Abstract
While high-sensitivity troponin-T (hsTnT) and C-reactive protein (hsCRP) are associated with structural heart disease, we thought to determine whether biomarkers can predict which heart is healthy based on multimodality imaging. Patients from the emergency department with acute chest pain suggestive of acute coronary syndrome undergoing contrast enhanced cardiac CT and stress single photon emission computed tomography (SPECT) myocardial perfusion imaging were included. HsTnT and hsCRP were assessed at time of CT. Imaging data were assessed for coronary atherosclerosis, left ventricular hypertrophy/dysfunction and myocardial perfusion abnormalities. Patients were stratified into those with or without any cardiac findings, who were considered as cardiac healthy. For biomarkers, low cut-off corresponding to good specificity and high cut-off corresponding to good sensitivity for cardiac health were derived. Among 117 patients (52 years, 55 % male), 42 (36 %) were cardiac healthy based on cardiac CT and SPECT imaging. These patients had significantly lower hsTnT and hsCRP levels as compared to those with functional or structural abnormalities (3.58 vs. 5.63 ng/L, p = 0.002; 0.82 vs. 1.93 mg/L, p = 0.0005; respectively). Patients with both low hsTnT (<3.00 ng/L) and hsCRP (<0.45 mg/L) had a probability of 85 % for being cardiac healthy. In contrast, patients with high hsTnT (>7.00 ng/L) and hsCRP (>2.00 mg/L) had 8 % probability for being cardiac healthy. Discriminative capacity of a dual-biomarker strategy was significantly improved as compared to hsTnT or hsCRP alone or to Framingham Risk score (AUC: 0.781 vs. 0.691; vs. 0.678; vs. 0.649; all p ≤ 0.02, respectively). A dual-biomarker strategy of hsTnT and hsCRP is highly discriminative for patients with normal cardiac structure and function and provides incremental value beyond the Framingham risk score.
Keywords: Cardiovascular health, High-sensitivity troponin T, High-sensitivity C-reactive protein, Cardiac computed tomography, Single-photon emission computed tomography
Introduction
It could be argued that research in cardiovascular medicine is more focused to detect/treat disease rather than to define/promote health [1]. A search on pubmed.com (through April, 2012) for the term ‘‘cardiac disease’’ retrieved 9,414 entries while only 268 entries were found for ‘‘cardiac health’’. Because cardiac health, although highly important, is a relatively neglected topic [2, 3], the American Heart Association (AHA) formulated the ‘‘2020 Goals’’ where cardiovascular health of Americans shall be improved by 20 % in 2020. As part of it, the AHA recently introduced a cardiovascular health metric. This metric consists of seven behaviors/factors which should maintain a healthy cardiovascular status [2]. Although a stepwise reduction of death from ischemic heart disease (IHD) was observed with increasing cardiovascular health metric in a national cohort, complying with 6 or more out of 7 health behaviors does prevent IHD death completely (mortality rate of 1–2 % per 1,000 Person-years) [3].
While IHD death reflects the end stage, several clinical and subclinical diseases lead there: epicardial coronary artery disease (CAD) with or without myocardial perfusion abnormalities, micro-vascular disease with myocardial perfusion abnormalities, and diseases primarily affecting the left ventricular (LV) myocardium [4–10]. The total absence of all these diseases, which are associated with poor cardiovascular outcomes, can be used as a definition for cardiac health. A multi-modality approach of anatomic imaging and functional diagnostic testing is required to capture both structural and functional abnormalities. CAD including atherosclerotic plaque and coronary stenosis can be accurately excluded noninvasively by coronary CT angiography [11, 12]. Similar, LV size and regional LV function can be accurately assessed by cardiac CT [13]. Abnormal myocardial perfusion can be accurately quantified by single photon emission computed tomography myocardial perfusion imaging (SPECT-MPI) at rest and stress [14].
For risk stratification, traditional risk factors such as hypertension, smoking, hyperlipidemia are summarized to risk scores such as the Framingham Risk Score (FRS) or the AHA cardiovascular health metric, which predict cardiovascular morbidity and mortality [3, 15]. Blood biomarkers are increasingly used for additional risk stratification although many of them suffer from low specificity [16]. Among the more promising biomarkers are high-sensitivity C-reactive protein (hsCRP), currently used in primary prevention guidance [17] and independently linked to coronary atherosclerosis [18], and high-sensitivity troponin T (hsTnT) which has been linked to cardiac structural disease and adverse outcomes [19, 20].
In this analysis, we used absence of CAD, LV hypertrophy/dysfunction and myocardial ischemia as demonstrated by cardiac CT and SPECT-MPI as a definition for cardiac health and determined whether hsCRP and/or hsTnT are predictive of cardiac health. Our hypothesis was that biomarkers might identify both those patients at low likelihood for substantial cardiovascular disease (‘‘cardiac health’’) as well as those at higher risk for significant structural heart disease.
Methods
Patient population
The study cohort consisted of patients presenting with symptoms suggestive of ACS to the emergency department who were enrolled in the ROMICAT (Rule Out Myocardial Infarction Using Computer Assisted Tomography) trial [21]. Briefly, ROMICAT was a double-blinded, single center prospective observational cohort study of consecutive adult patients with acute chest pain presenting to the Emergency Department (ED). All patients had a low-to-intermediate likelihood for acute coronary syndrome (ACS) based on an initial, inconclusive electrocardiogram and first negative cardiac biomarker. at low-to-intermediate likelihood of acute coronary syndrome (ACS) presenting to the Emergency Department (ED) of Massachusetts General Hospital with acute chest pain without signs of myocardial ischemia on initial electrocardiogram and normal cardiac biomarkers. The institutional review board approved the study protocol and all patients provided written informed consent.
For this analysis, we included only patients who did undergo both—cardiac CT and rest and stress SPECT-MPI. ROMICAT I was a double blinded observational study in which all patients underwent cardiac CT as a research examination. Importantly, caregivers and patients remained blinded to the cardiac CT results excluding the potential for a work-up bias. We excluded patients who developed ACS during index hospitalization, had poor CT image quality, and those who had no biomarker data available.
Single photon emission computed tomography-myocardial perfusion imaging
According to ACC/AHA image acquisition guidelines, rest and stress SPECT-MPI using Tc 99 m sestamibi was performed in a standard 1-day protocol [22]. The patient was first injected with Tc-99m sestamibi at rest and un-gated images were obtained. Subsequently, stress was induced by treadmill exercise or pharmacologically by using adenosine if patient was unable to perform the exercise. Tc 99m sestamibi was injected at peak stress. Gated SPECT images were obtained 45 min later. During stress testing, heart rate, blood pressure, and 12-lead ECG were recorded at baseline and every minute thereafter.
Average isotope doses for sestamibi were in the range of 9–11 mCi for the rest images and 27–33 mCi for the stress images. Beta blockers were held before stress testing for all patients. Patients were instructed not to consume coffee or other products containing caffeine for 24 h prior to the test. A dual-head Siemens gamma camera (E-CAM or C-CAM, Siemens Medical Solutions, Forchheim, Germany) equipped with a low energy, high-resolution collimator (32 views per camera head in 64 × 64 matrix) was used for image acquisition.
Resting and stress SEPCT-MPI were analyzed objectively by two experienced readers (S.U. & W.A.), in semi-quantitative fashion using a standard 17 segment model and a 5-point scoring system using commercially available SPECT image analysis program (4DM, Ann Arbor, MI) [23]. Discrepancies were adjudicated by a third reader (R.B.). Analysis was performed blinded to the results of coronary CT angiography and cardiac biomarkers. Global summed scores were computed for the stress images (Summed Stress Score [SSS], reflecting the combined extent and severity of ischemia plus scar and Summed Difference Score [SDS], reflecting the combined extent and magnitude of myocardial ischemia).
CT angiography
CT imaging was performed using a standard 64-slice multi-detector CT coronary angiography (Sensation 64, Siemens Medical Solutions, Forchheim, Germany) protocol that was acquired at end inspiration and included the administration of sublingual nitroglycerin (0.6 mg) and intravenous beta-blocker (metoprolol 5–20 mg) for those with the baseline heart rate >60 beats per minute and no other contraindications. Details of the protocol are described elsewhere [21]. Reconstructions were performed using retrospective ECG-gated half-scan algorithm for a temporal resolution of 165 ms. The average radiation dose from the coronary CTA was 14.8 ± 3.8 mSv.
For the detection of CAD, axial images were reconstructed using a medium sharp convolution kernel with a slice thickness of 0.75 mm with an increment of 0.4 mm. For each of the coronary segments, coronary atherosclerotic plaque and coronary stenosis (defined as luminal narrowing >50 % diameter) were visually classified as either present or absent as previously described and determined by a two reader consensus using a Leonardo workstation (Siemens Medical Solutions, Forchheim, Germany) as previously validated [21].
For volumetric and functional analysis of the left ventricle, multi-planar reformat image series were reconstructed at 10 % RR-wave interval (resulting in 10 phases) with 1.5 mm slice thickness and 1.5 mm increments of the axial images. Quantitative LV measurements were obtained using Vitrea software (Vital Images, Minnetonka, Minnesota). LV volumes and function were derived by manually corrected semi-automated delineation of the endocardial borders with exclusion of the papillary muscles. LV hypotrophy was defined as body surface area indexed LV mass >89 g/m2 for women and >103 g/m2 for men with relative wall thickness ratio >0.42 for both gender [24]. ‘‘In Space’’ software was used for processing functional analysis (Siemens Medical Solutions). Regional LV dysfunction including wall motion and wall thickening of the myocardium were assessed qualitatively based on the AHA/ACC 17-segment model [23]. Regional LV dysfunction had to be present in at least two contiguous myocardial segments or in one segment visualized in two different views to be considered a true positive finding [25].
Definition of cardiac health
Cardiac health was defined based on three different categories—assessments of the coronary arteries, left ventricle and myocardial perfusion, Table 1. To be considered cardiac healthy, all of the following aspects need to be satisfied: (1) absence of any coronary plaque including any degree of coronary stenosis, (2) absence of regional LV dysfunction and LV hypertrophy, (3) SSS<4, and SDS<1. The cut-points for SSS and SDS were based on current guidelines and safely exclude myocardial ischemia [26]. All patients whom did not satisfy all of the conditions above were considered to have cardiac disease.
Table 1.
Definition cardiac health
| Cardiac healthy | Any cardiac diseased | |
|---|---|---|
| CT: coronary artery assessment | ||
| Any atherosclerotic plaque | Absent | Present |
| Any degree of stenosis | Absent | Present |
| CT: left ventricle assessment | ||
| Regional LV dysfunction | Absent | Present |
| Left ventricular hypertrophy | Absent | Present |
| SPECT: myocardial perfusion | ||
| Summed Stress Score (SSS) | 0–3 | ≥4 |
| Summed Difference Score (SDS) | 0 | ≥1 |
To meet the definition ‘cardiac healthy’ by multi-modality imaging, all six aspects had to be satisfied. For the definition of ‘cardiac diseased’, all parameters were independent of each other in stratifying patients into the three different categories: coronary, left ventricle or perfusion abnormalities
Cardiac serum biomarkers
Blood samples for biomarker testing (hsTnT, hsCRP) were obtained at the time of coronary CT angiography from a peripheral vein and were collected into ethylenediaminetetraacetic acid coated tubes and non-coated tubes. The blood was immediately centrifuged and the aliquoted plasma and serum were stored in microcentrifuge tubes at −80 °C until assayed. Specimens were tested on the first freeze thaw cycle. All analyses were run in a blinded fashion.
For hsTnT level, we used a pre-commercial hsTnT method (Roche Diagnostics, Penzberg, Germany) on an Elecsys® 2010 platform, as described elsewhere [27]. Given enhanced sensitivity, this assay is reported with units of ng/L (rather than ng/mL for cTnT) and the 99th percentile for a normal reference population is reported to be 13 ng/L [27]. The lower level of detection (LOD) was 3 ng/L.
The concentrations of hsCRP was measured nephelometrically on a BN II analyzer (Dade-Behring, Marburg, Germany). In general, the high-sensitivity assay for CRP (unit: mg/L) allows measuring range two orders of magnitude lower than those of the traditional assays [28].
Statistical analysis
Continuous measures were presented as median [interquartile range] and categorical variables as percentages unless otherwise specified. To compare demographics between the groups, Fisher’s exact and Wilcoxon Rank sum test were used. FRS was stratified into low (<10 %), intermediate (10–20 %), and high (>20 %) risk for 10-year coronary heart disease according to the ATP III guidelines [29]. We also performed a sub-analysis, defining ‘‘low risk’’ <6 % and ‘‘intermediate risk’’ as 6–20 % [30, 31].
In order to assess the associations of each individual cardiac finding (CAD, LV hypertrophy/dysfunction, myocardial perfusion abnormalities) with blood biomarkers, separate multivariate linear regressions were performed. While the log-transformed biomarker served as the outcome, the extent to which each disease category contributes to the biomarker level was expressed as partial r2. For this analysis and prior to log-transformation, hsTnT values below the commercial level of detection (LOD, <3 ng/L) were set to the half of LOD (1.5 ng/L) due to the large portion of patients with hsTnT values below LOD. Such a treatment of values below the LOD levels has been described and validated elsewhere [32]. For sensitivity analysis, we restricted the cohort to patients with hsTnT values above LOD.
To determine cut-off values for biomarkers, receiver operating characteristic (ROC) curves were established. For each biomarker, we derived two new cut-offs (one low and one high) from the ROC curve if no established existed: the low cut-off corresponded to a specificity of >70 % and high cut-off corresponded to a sensitivity of >70 % for cardiac health. As established cut-offs, we used 2.00 mg/L for hsCRP (29) since it has been shown as a risk marker for major adverse cardiovascular events, and 3 ng/L for hsTnT, which represents the LOD for the applied assay [27]; we derived the following new cut-offs: low cut-off for hsCRP (0.45 mg/L); high cut-off for hsTnT (7 ng/L). As a result low, intermediate, and high levels were defined for each biomarker and combined for dual biomarker strategy (resulting in nine strata).
Univariate and multivariate logistic regression applying a ‘‘profile penalized likelihood method’’ because of monotone likelihood occurrence was used to determine association between biomarker strata and cardiac health as well to estimate the probability of being cardiac healthy within different strata of biomarkers [33]. AUC were derived and compared between the models using a nonparametric approach closely related to the jackknife technique [34]. To assess the internal validity of predicting cardiac health using the combination of hsTnT/hsCRP and to adjust for overfitting or over-optimism, bootstrap resampling procedures with 1,000 random samples were used.
All of the statistical analyses were performed with SAS software (version 9.2, SAS Institute Inc, Cary, NC). All probability values were two-sided, with a level of significance of <0.05.
Results
From the original ROMICAT cohort (n = 368), we excluded 201 patients who did not undergo SPECT, 31 patients who developed ACS during index hospitalization, 10 patients who had no biomarker data available, and 9 patients with poor CT image quality. Thus, our cohort consisted of 117 patients (55 % male, median age 52 [46–59] years) who were similar to overall population with respect to age (p = 0.38) and FRS (p = 0.78).
One third of patients (n = 42; 36 %) had normal coronary arteries, normal left ventricular size/function and normal myocardial perfusion and were stratified as cardiac healthy (Table 1). Among the remaining 75 patients who had at least one abnormal finding, the majority had CAD (82 %), followed by abnormal myocardial perfusion (20 %), and left ventricular abnormalities (12 % LV hypertrophy and 11 % regional LV dysfunction). Most patients (81 %) had a single finding, 15 % had two findings, and 4 % had CAD, LV hypertrophy/dysfunction and myocardial perfusion abnormalities.
Traditional risk factors and cardiac health
Patient demographics are detailed in Table 2. Cardiac healthy patients were significantly younger (median age 47 [IQR: 42–52] vs. 52 [IQR: 47–62] years, respectively, p = 0.0002) and had less likely hypertension (26 vs. 51 %, respectively, p = 0.01) than patients with disease. The median FRS (10-year risk for myocardial infarction or cardiac death) was also lower in cardiac healthy patients than in those with disease (3.0 % [IQR: 1.0–8.0] vs. 6.0 % [IQR: 2.0–14.0]; p = 0.009). Nearly 80 % of cardiac healthy patients had a low FRS risk. However, more than 50 % of patients with abnormal findings were also considered as low risk, Table 2. Similar results were seen when the modified FRS score was used. Both had moderate discriminatory ability for cardiac healthy patients from those with disease (AUC: 0.649 for FRS stratification based on ATPIII guidelines vs. 0.637, for FRS stratification based on Bethesda Conference, p = 0.65).
Table 2.
Patients’ demographics and biomarker between patients with cardiac health and with any cardiac disease
| All patients | Patients with cardiac health | Patients with cardiac disease | p value | |
|---|---|---|---|---|
| N | 117 | 42 | 75 | N/A |
| Patients’ demographics | ||||
| Age, in years | 52 [46–59] | 47 [42–52] | 54 [48–62] | <0.0001 |
| Male | 55 % (64) | 52 % (22) | 56 % (42) | 0.85 |
| Diabetes | 12 % (14) | 7%(3) | 15 % (11) | 0.37 |
| Hypertension | 42 % (49) | 26 % (11) | 51 % (38) | 0.01 |
| Hyperlipidemia | 44 % (52) | 33 % (14) | 51 % (38) | 0.08 |
| Smoking | 28 % (33) | 21 % (9) | 32 % (24) | 0.29 |
| BMI, in kg/m2 | 28 [25–33] | 27 [24–32] | 29 [26–33] | 0.11 |
| Framingham Risk Score | 0.006 | |||
| Low (<10 %) | 62 % (73) | 81 % (34) | 52 % (39) | |
| Intermediate (10–20 %) | 21 % (24) | 12 % (5) | 25 % (19) | |
| High (>20 %) | 17 % (20) | 7%(3) | 23 % (17) | |
| Biomarkers | ||||
| hsTnT, ng/L | 4.89 [<3.00–7.59] | 3.58 [<3.00–5.67] | 5.63 [3.18–8.71] | 0.002 |
| hsCRP, mg/L | 1.37 [0.63–2.98] | 0.82 [0.38–1.67] | 1.93 [0.83–4.49] | 0.0005 |
Framingham Risk Score was stratified according to the ATP III guidelines. hsCRP denotes highly-sensitive C-reactive protein; hsTnT, highly-sensitive troponin T
Blood biomarkers and cardiac health
Assessing which disease category influenced which biomarker level, hsCRP was more associated with CAD (partial r2: 0.08) and less with myocardial perfusion abnormalities or LV hypertrophy/dysfunction (partial r2: 0.0006 and 0.0005 respectively). In contrast, the association to hsTnT was driven by abnormal myocardial perfusion and LV hypertrophy/dysfunction (partial r2: 0.07 and 0.06, respectively) and less by CAD (partial r2: 0.03). If restricted to patients with hsTnT ≥ 3 ng/L (N = 80), the driving factors were similar, although abnormal myocardial perfusion was less prominent (partial r2: 0.07, 0.04 and 0.01 for LV hypertrophy/dysfunction, abnormal myocardial perfusion, and CAD; respectively).
Overall, cardiac healthy patients, in whom any disease was excluded, had significantly lower median levels of hsCRP and hsTnT as compared to patients with any disease (all p ≤ 0.002, Table 2). When hsCRP and hsTnT stratified based on low and high cut-offs, patients with biomarker values below the low cut-off values had an approximate 6-fold increased odds (OR: 6.64 and 5.68 for hsCRP and hsTnT alone) and for cardiac health as compared to patients above the high cut-off. Similarly comparing patients with intermediate biomarker levels to those with biomarker values above the high cut-off, the odds were 3 times greater to be cardiac healthy (OR: 3.63 and 2.94 for hsCRP and hsTnT alone). These associations remained significant after adjusting for FRS (all p < 0.05; Table 3).
Table 3.
Biomarkers and their association to cardiac health
| Univariate |
Adjusted for FRS |
|||
|---|---|---|---|---|
| OR | p | OR | p | |
| hsTnT | 0.007 | 0.045 | ||
| <3.00 ng/L | 5.68 (1.94–16.59) | 4.10 (1.35–12.46) | ||
| 3.00–7.00 ng/L | 2.91 (1.01–8.35) | 2.46 (0.84–7.23) | ||
| >7.00 ng/L | -ref- | -ref- | ||
| hsCRP | 0.003 | 0.01 | ||
| <0.45 mg/L | 6.61 (2.13–20.49) | 5.63 (1.76–18.00) | ||
| 0.45–2.00 mg/L | 3.63 (1.48–9.31) | 2.94 (1.12–7.71) | ||
| >2.00 mg/L | -ref- | -ref- | ||
All biomarkers were stratified into three categories using two different cut-off values, one with high sensitivity and one with high specificity. hsCRP denotes highly-sensitive C-reactive protein; hsTnT, highly-sensitive troponin T
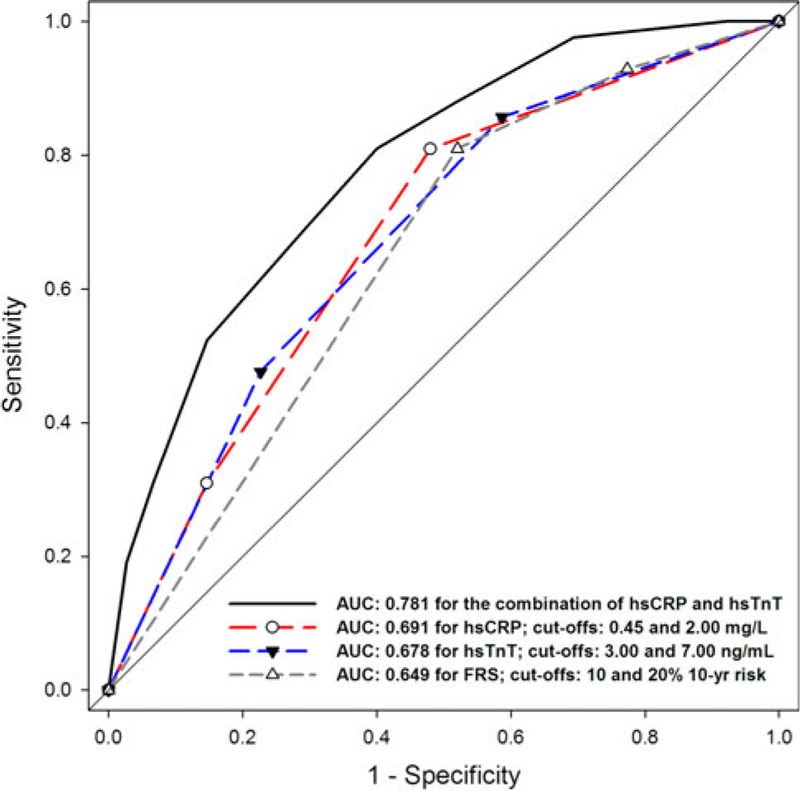
When combining information from hsTnT and hsCRP, the combination demonstrated significantly improved discriminative capacity as compared to a single biomarker or as compared to FRS (AUC of 0.781 for the hsTnT/hsCRP combination vs. 0.691 for hsCRP only, p = 0.002; vs. 0.678 for hsTnT only, p = 0.02; vs. 0.649 for FRS, p = 0.009; Fig. 1). Applying bootstrap simulations, the estimated optimism was low (0.049 ± 0.043) and led to a corrected AUC of 0.804 for the combining of hsTnT and hsCRP in the association to cardiac health.
Fig. 1.
Receiver operating characteristic (ROC) curves for detection of cardiac health. The combination of hsCRP/hsTnT yield improved discriminative capacity as compared to a single biomarker (vs. hsCRP only, p = 0.002; vs. hsTnT only, p = 0.02) and as compared to Framingham Risk score (FRS; p = 0.009). Biomarkers were stratified based on a low and high cut-off (for hsTnT: 3.00 and 7.00 ng/L; for hsCRP: 0.45 and 2.00 mg/L), while the low cut-off corresponded to good specificity, and the high cut-off to good sensitivity for cardiac health. hsCRP denotes highly-sensitive C-reactive protein; hsTnT, highly-sensitive troponin T
Probability for cardiac health based on hsTnT and hsCRP
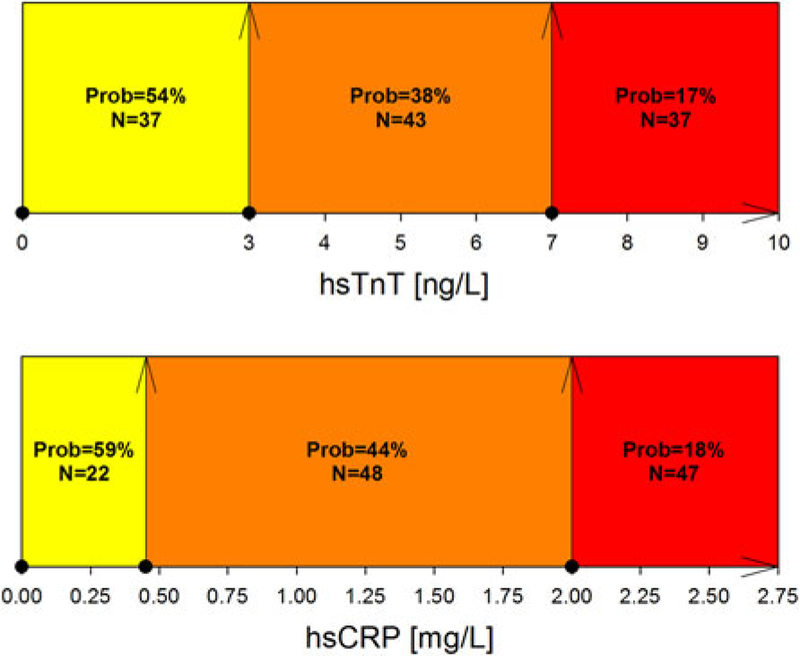
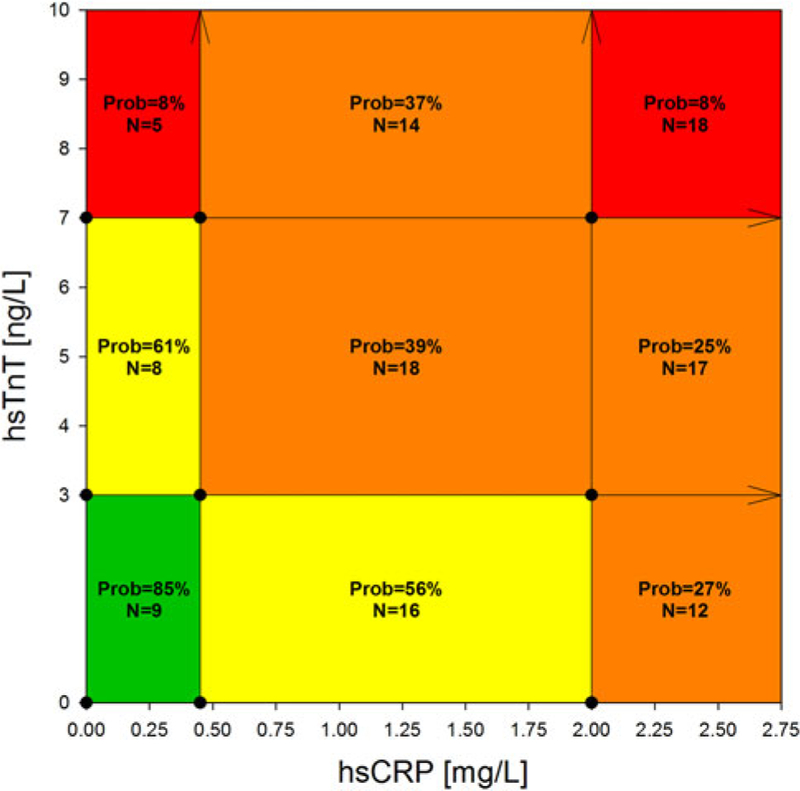
Patients below one of the low biomarker cut-offs had an estimated probability of approximately 55 % for cardiac health (54 % for hsTnT < 3.00 ng/L; 59 % for hsCRP < 0.45 mg/L, Fig. 2), while the probability for cardiac health decreased to 20 % if patients were above one of the high cut-offs (20 % for hsTnT > 7.00 ng/L and to 18 % for hsCRP > 2.00 mg/L, Fig. 2). The probabilities for the biomarker strata for the combination of hsCRP and hsTnT are illustrated in Fig. 3. Patients with both hsTnT < 3.00 ng/L and hsCRP < 0.45 mg/L (N = 9) had an estimated probability of 85 % for cardiac health, while patients with both hsTnT > 7.00 ng/L and hsCRP > 2.00 mg/L (N = 18) had an estimated probability of only 8 % for cardiac health (Fig. 3).
Fig. 2.
Estimated probability for being cardiac healthy based on the combination of hsCRP and hsTnT. Cut-offs from both biomarkers, hsCRP (0.45 and 2.00 mg/L) and hsTnT (3.00 and 7.00 ng/L), were used to stratify patients. For each strata, the probability for being cardiac healthy was estimated. Green indicates an estimated probability of >75 %, yellow of 75–50 %, orange of 50–25 %, and red of <25 % for cardiac health. Each cut-off value was indicated by an arrow. Prob denotes the estimated probability of being cardiac healthy; N, patients in this subgroup
Fig. 3.
Estimated probabilities having cardiac health for single biomarkers. For each stratified category (for hsTnT: <3.00, 3.00–7.00, >7.00 ng/L; for hsCRP: <0.45; 0.45–2.00, >2.00 mg/L; as indicated by the arrows), we estimated the probability of having cardiac health. Green indicates an estimated probability of >75 %, yellow of 75–50 %, orange of 50–25 %, and red of <25 % for cardiac health. Prob denotes the estimated probability of being cardiac healthy; N, patients in this subgroup
Discussion
We focused this analysis on cardiac health defined as the absence of CAD, LV hypertrophy/dysfunction, and myocardial perfusion abnormalities as demonstrated by cardiac CT and SPECT-MPI. Our hypothesis was that biomarkers could be used to exclude structural heart disease as defined by imaging. We determined that a dual biomarker strategy including both hsCRP and hsTnT is highly discriminative for cardiac health and significantly more accurate than single biomarker or FRS.
The early detection of cardiac disease is crucial for effective preventive strategies but is a difficult goal in clinical practice [35]. This was underlined by our findings showing that none of the single traditional risk factors other than age and hypertension were able to predict cardiac health. The widely used FRS was able to distinguish between cardiac healthy patients and those with disease but demonstrated poor specificity (49 %).
In contrast, two studied biomarkers demonstrated significantly lower levels in patients with cardiac health compared to patients with any cardiac disease. Our data suggests that concentrations of hsTnT have the strongest associations with myocardial perfusion abnormalities and LV hypertrophy/dysfunction. Increased hsTnT levels (while still below the 99th percentile) may therefore represent cardio-myocyte stress, ischemia or damage [36, 37]. On the other hand, hsCRP showed the strongest correlation with atherosclerotic plaque by CTA. HsCRP is a marker for inflammation with close links to the presence and severity of atherosclerosis [38].
While the Dallas Heart Study did not assess cardiac health, it provided early evidence that hsTnT is associated with structural heart disease supporting our results [19]. Analogous, population-based cohorts have shown the association of hsCRP with clinically undetected atherosclerosis [39]. We were able to show that the usage of low cut-off values of hsTnT (<3 ng/L) and hsCRP (<0.45 mg/L) corresponded with high specificity for cardiac health, as defined by multi-modality imaging. If hsTnT and hsCRP cut-offs were combined, the discriminative capacity improved significantly. Patients below the low cut-off values of hsTnT and of hsCRP had an 85 % probability of being cardiac healthy. Data from the DETECT study (Diabetes Cardiovascular Risk-Evaluation: Targets and Essential Data for Commitment of Treatment) also showed that a dual-biomarker strategy was superior to a single biomarker in assessing 5,388 individuals without known cardiovascular disease [20]. In this study, cardiac troponin I was measured with a sensitive assay with a LOD of 7 ng/L, which has been shown in our data to be a highly sensitive (85 %) but non specific (42 %) cut-off for cardiac health. In the DETECT study, the combination of cardiac troponin I and hsCRP (c-statistic: 0.844) were incremental to traditional risk factors. However, similar to all prior studies; the outcome was determined to be major adverse cardiac events [20].
While hsTnT is detectable in a significant percentage of clinically ‘‘normal’’ individuals, it is below the LOD in anywhere up to 25 % [20, 40]. These patients have been shown to be at very low risk for acute coronary syndrome when presenting with acute chest pain [20, 40, 41]; our data suggest the mechanism of this reported finding is that patients without detectable hsTnT are exceedingly unlikely to have substantial underlying structural heart disease as determined by imaging. Furthermore, one-third of patients with an acute chest pain syndrome presenting to the ED are considered as cardiac healthy based on multi-modality imaging. To identify these patients early in the triage may be a significant driver for cost saving.
In contrast, we showed that increased levels of hsCRP and/or hsTnT are associated with structural heart disease. It can be hypothesized that the combination of hsTnT/hsCRP with extreme low cut-offs may serve as a gatekeeper for advanced diagnostic testing. Clinical randomized-controlled are needed to verify this hypothesis. It must be further studied whether patients with increased levels of hsTnT benefit more from stress testing given the more strongly association of hsTnT with perfusion abnormalities, and whether diagnostic test demonstrating the coronary morphology may be more appropriate in patients with increased levels of hsCRP given that hsCRP was primarily linked to atherosclerotic disease.
Several limitations must be considered when interpreting our results. Our findings only apply to patients with symptoms suggesting ACS and that further studies are warranted to determine whether the results can be replicated in patients with stable chest pain syndrome. Due to the inclusion criteria for this subanalysis, this cohort represents only a portion of the entire ROMICAT I population with potential for a selection bias. However, the rate of patients with hsTnT < 3.00 ng/L and hsCRP < 0.45 mg/L were similar (7 % in the entire ROMICAT I population vs. 8 % in the subgroup which underwent CTA and SPECT-MPI). Further, cardiac magnetic resonance imaging is accepted as the clinical gold-standard for assessment of LV structure and function but was not used in our study. However, a few studies have shown that cardiac CT assessment of LV structure and function is comparable to magnetic resonance imaging [42]. Similar, quantitative positron emission tomography is potentially superior to SPECT-MPI for the diagnosis of microvascular disease. Finally, no data on patients’ behavior were collected and thwart our ability to assess the incremental value of biomarkers above the AHA cardiovascular health metric.
While our results suggest that patients with a healthy heart can be identified using a dual marker strategy and it raises the question whether biomarkers could improve triage of acute chest pain patients by e.g., serving as a gatekeeper for imaging diagnostics. However, further research in larger observational studies and eventually randomized-controlled trials are necessary to validate our concept of identifying cardiac healthy patients based on biomarkers and to demonstrate the efficacy and effectiveness of potential hypothesis like this one.
Conclusion
A dual-biomarker strategy using hsTnT and hsCRP is highly discriminative to identify patients free of CAD, LV hypertrophy or dysfunction and have normal myocardial perfusion in patients with an acute chest pain syndrome. This dual-biomarker strategy provides incremental value beyond the Framingham risk score to identify cardiac healthy patients.
Acknowledgments
We thank Mr. Eracleo for his kind support. This work was supported by the NIH (R01 HL080053). Drs. Blankstein, Ahmed, and Truong received support from NIH grant T32HL076136. Dr. Truong also received support from NIH grants K23HL098370 and L30HL093896.
Dr. Januzzi has received grant support from Roche Diagnostics, Siemens Diagnostics, and BRAHMS.
Footnotes
Conflict of interest The reagents for high-sensitivity troponin assays were provided free of charge by Roche Diagnostics.
Contributor Information
Christopher L. Schlett, Cardiac MR PET CT Program, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA
Quynh A. Truong, Cardiac MR PET CT Program, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA Cardiology Division, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Waleed Ahmed, Cardiac MR PET CT Program, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA.
Ron Blankstein, Cardiovascular Division, Department of Cardiology, Brigham and Women’s Hospital, Boston, MA, USA.
Maros Ferencik, Cardiac MR PET CT Program, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA; Cardiology Division, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Shanmugam Uthamalingam, Cardiac MR PET CT Program, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA.
Fabian Bamberg, Department of Clinical Radiology, Ludwig-Maximilians University, Munich, Germany.
Wolfgang Koenig, Department of Internal Medicine II-Cardiology, University of Ulm, Ulm, Germany.
James L. Januzzi, Cardiology Division, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
Udo Hoffmann, Cardiac MR PET CT Program, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, 165 Cambridge Street, Suite 400, Boston, MA 02114, USA.
References
- 1.Centers for Disease Control and Prevention (2005) Moving into action: promoting heart-healthy and stroke-free communities (health care leaders). U.S. Department of Health and Human Services, Atlanta [Google Scholar]
- 2.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD (2010) Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation 121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 3.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB (2012) Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA 307(12):1273–1283. doi: 10.1001/jama.2012.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerci MS, Cerci JJ, Cerci RJ, Pereira Neto CC, Trindade E, Delbeke D, da Cunha CL, Vitola JV (2011) Myocardial perfusion imaging is a strong predictor of death in women. JACC Cardiovasc Imaging 4(8):880–888. doi: 10.1016/j.jcmg.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 5.Shaw LJ, Iskandrian AE (2004) Prognostic value of gated myocardial perfusion SPECT. J Nucl Cardiol 11(2):171–185. doi: 10.1016/j.nuclcard.2003.12.004 [DOI] [PubMed] [Google Scholar]
- 6.Marcassa C, Bax JJ, Bengel F, Hesse B, Petersen CL, Reyes E, Underwood R (2008) Clinical value, cost-effectiveness, and safety of myocardial perfusion scintigraphy: a position statement. Eur Heart J 29(4):557–563. doi: 10.1093/eurheartj/ehm607 [DOI] [PubMed] [Google Scholar]
- 7.Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE (2011) Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging 4(1):98–108. doi: 10.1016/j.jcmg.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 8.Yan RT, Bluemke D, Gomes A, Burke G, Shea S, Liu K, Bahrami H, Sinha S, Wu C, Fernandes V, McClelland R, Lima JA (2011) Regional left ventricular myocardial dysfunction as a predictor of incident cardiovascular events MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 57(17):1735–1744. doi: 10.1016/j.jacc.2010.10.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill SF, Sheppard MN (2010) Non-atherosclerotic coronary artery disease associated with sudden cardiac death. Heart 96(14):1119–1125. doi: 10.1136/hrt.2009.185157 [DOI] [PubMed] [Google Scholar]
- 10.Gutstein DE, Fuster V (1999) Pathophysiology and clinical significance of atherosclerotic plaque rupture. Cardiovasc Res 41(2):323–333. doi: 10.1016/S0008-6363(98)00322-8 [DOI] [PubMed] [Google Scholar]
- 11.van der Giessen AG, Toepker MH, Donelly PM, Bamberg F, Schlett CL, Raffle C, Irlbeck T, Lee H, van Walsum T, Maurovich-Horvat P, Gijsen FJ, Wentzel JJ, Hoffmann U (2010) Reproducibility, accuracy, and predictors of accuracy for the detection of coronary atherosclerotic plaque composition by computed tomography: an ex vivo comparison to intravascular ultrasound. Invest Radiol 45(11):693–701. doi: 10.1097/RLI.0b013e3181e0a541 [DOI] [PubMed] [Google Scholar]
- 12.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min JK (2008) Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (assessment by coronary computed tomographic angiography of individuals undergoing invasive coronary angiography) trial. J Am Coll Cardiol 52(21):1724–1732. doi: 10.1016/j.jacc.2008.07.031 [DOI] [PubMed] [Google Scholar]
- 13.Sayyed SH, Cassidy MM, Hadi MA (2009) Use of multidetector computed tomography for evaluation of global and regional left ventricular function. J Cardiovasc Comput Tomogr 3(1 Suppl):S23–S34. doi: 10.1016/j.jcct.2008.10.016 [DOI] [PubMed] [Google Scholar]
- 14.Iida H, Eberl S (1998) Quantitative assessment of regional myocardial blood flow with thallium-201 and SPECT. J Nucl Cardiol 5(3):313–331. doi: 10.1016/S1071-3581(98)90133-7 [DOI] [PubMed] [Google Scholar]
- 15.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97(18):1837–1847. doi: 10.1161/01.CIR.97.18.1837 [DOI] [PubMed] [Google Scholar]
- 16.Gilstrap LG, Wang TJ (2011) Biomarkers and cardiovascular risk assessment for primary prevention: an update. Clin Chem 58:72–82. doi: 10.1373/clinchem.2011.165712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V (2004) C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 350(14): 1387–1397. doi: 10.1056/NEJMoa032804 [DOI] [PubMed] [Google Scholar]
- 18.Bamberg F, Truong QA, Koenig W, Schlett CL, Nasir K, Butler J, Kurtz E, Nikolaou K, Hoffmann U, Januzzi JL Jr (2012) Differential associations between blood biomarkers of inflammation, oxidation, and lipid metabolism with varying forms of coronary atherosclerotic plaque as quantified by coronary CT angiography. Int J Cardiovasc Imaging 28(1):183–192. doi: 10.1007/s10554-010-9773-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK (2010) Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 304(22):2503–2512. doi: 10.1001/jama.2010.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leistner DM, Klotsche J, Pieper L, Stalla GK, Lehnert H, Silber S, Marz W, Wittchen HU, Zeiher AM (2012) Circulating troponin as measured by a sensitive assay for cardiovascular risk assessment in primary prevention. Clin Chem 58(1):200–208. doi: 10.1373/clinchem.2011.174292 [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Sene-viratne SK, Truong QA, Cury RC, Abbara S, Shapiro MD, Moloo J, Butler J, Ferencik M, Lee H, Jang IK, Parry BA, Brown DF, Udelson JE, Achenbach S, Brady TJ, Nagurney JT (2009) Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (rule out myocardial infarction using computer assisted tomography) trial. J Am Coll Cardiol 53(18):1642–1650. doi: 10.1016/j.jacc.2009.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O’Reilly MG, Winters WL, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC (2002) ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol 40(8):1531–1540. doi: 10.1016/S0735-1097(02)02164-2 [DOI] [PubMed] [Google Scholar]
- 23.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105(4):539–542 [DOI] [PubMed] [Google Scholar]
- 24.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, John Sutton M, Stewart W (2006) Recommendations for chamber quantification. Eur J Echocardiogr 7(2):79–108. doi: 10.1016/j.euje.2005.12.014 [DOI] [PubMed] [Google Scholar]
- 25.Seneviratne SK, Truong QA, Bamberg F, Rogers IS, Shapiro MD, Schlett CL, Chae CU, Cury R, Abbara S, Brady TJ, Nagurney JT, Hoffmann U (2010) Incremental diagnostic value of regional left ventricular function over coronary assessment by cardiac computed tomography for the detection of acute coronary syndrome in patients with acute chest pain: from the ROMICAT trial. Circ Cardiovasc Imaging 3(4):375–383. doi: 10.1161/CIRCIMAGING.109.892638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, Friedman J, Diamond GA (1998) Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation 97(6):535–543 [DOI] [PubMed] [Google Scholar]
- 27.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA (2010) Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 56(2):254–261. doi: 10.1373/clinchem.2009.132654 [DOI] [PubMed] [Google Scholar]
- 28.Roberts WL, Sedrick R, Moulton L, Spencer A, Rifai N (2000) Evaluation of four automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Clin Chem 46(4):461–468 [PubMed] [Google Scholar]
- 29.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report (2002). Circulation 106(25):3143–3421 [PubMed] [Google Scholar]
- 30.Greenland P, Smith SC Jr, Grundy SM (2001) Improving coronary heart disease risk assessment in asymptomatic people: role of traditional risk factors and noninvasive cardiovascular tests. Circulation 104(15):1863–1867 [DOI] [PubMed] [Google Scholar]
- 31.Taylor AJ, Merz CN, Udelson JE (2003) 34th Bethesda conference: executive summary—can atherosclerosis imaging techniques improve the detection of patients at risk for ischemic heart disease? J Am Coll Cardiol 41(11):1860–1862. doi: 10.1016/S0735-1097(03)00363-2 [DOI] [PubMed] [Google Scholar]
- 32.Helsel DR (1990) Less than obvious—statistical treatment of data below the detection limit. Environ Sci Technol 24(12): 1766–1774 [Google Scholar]
- 33.Heinze G, Schemper M (2001) A solution to the problem of monotone likelihood in Cox regression. Biometrics 57(1): 114–119 [DOI] [PubMed] [Google Scholar]
- 34.Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143(1):29–36 [DOI] [PubMed] [Google Scholar]
- 35.Integrated management of cardiovascular risk: report of a WHO meeting (2002). World Health Organization, Geneva [Google Scholar]
- 36.Remppis A, Scheffold T, Greten J, Haass M, Greten T, Kubler W, Katus HA (1995) Intracellular compartmentation of troponin T: release kinetics after global ischemia and calcium paradox in the isolated perfused rat heart. J Mol Cell Cardiol 27(2):793–803. doi: 10.1016/0022-2828(95)90086-1 [DOI] [PubMed] [Google Scholar]
- 37.Sabatine MS, Morrow DA, de Lemos JA, Jarolim P, Braunwald E (2009) Detection of acute changes in circulating troponin in the setting of transient stress test-induced myocardial ischaemia using an ultrasensitive assay: results from TIMI 35. Eur Heart J 30(2):162–169. doi: 10.1093/eurheartj/ehn504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Libby P (2002) Inflammation in atherosclerosis. Nature 420(6917):868–874. doi: 10.1038/nature01323 [DOI] [PubMed] [Google Scholar]
- 39.Blaha MJ, Rivera JJ, Budoff MJ, Blankstein R, Agatston A, O’Leary DH, Cushman M, Lakoski S, Criqui MH, Szklo M, Blumenthal RS, Nasir K (2011) Association between obesity, high-sensitivity C-reactive protein >/=2 mg/L, and subclinical atherosclerosis: implications of JUPITER from the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol 31(6):1430–1438. doi: 10.1161/ATVBAHA.111.223768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Everett BM, Cook NR, Magnone MC, Bobadilla M, Kim E, Rifai N, Ridker PM, Pradhan AD (2011) Sensitive cardiac troponin T assay and the risk of incident cardiovascular disease in women with and without diabetes mellitus: the women’s health study. Circulation 123(24):2811–2818. doi: 10.1161/CIRCULATIONAHA.110.009928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Body R, Carley S, McDowell G, Jaffe AS, France M, Cruick-shank K, Wibberley C, Nuttall M, Mackway-Jones K (2011) Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 58(13):1332–1339. doi: 10.1016/j.jacc.2011.06.026 [DOI] [PubMed] [Google Scholar]
- 42.Schlosser T, Mohrs OK, Magedanz A, Voigtlander T, Schmermund A, Barkhausen J (2007) Assessment of left ventricular function and mass in patients undergoing computed tomography (CT) coronary angiography using 64-detector-row CT: comparison to magnetic resonance imaging. Acta Radiol 48(1):30–35. doi: 10.1080/02841850601067611 [DOI] [PubMed] [Google Scholar]