Abstract
Direct-acting agents (DAAs) for hepatitis C virus (HCV) treatment are safe and highly effective. Few studies described the sustained virologic response rates of treatment conducted by non-specialists. We performed a systematic review and meta‐analysis to evaluate the effectiveness of decentralized strategies of HCV treatment with DAAs. PubMed, Embase, Scopus and LILACS were searched until March-2019. Studies were screened by two researchers according to the following inclusion criteria: HCV treatment using DAAs on real-life cohort studies or clinical trials conducted by non-specialized health personnel. The primary endpoint was the sustained virologic response rate at week 12 after the end-of-treatment (SVR12), which is binary at the patient level. Data were extracted in duplicate using electronic-forms and quality appraisal was performed with the NIH Quality Assessment Tool. Heterogeneity was assessed by I2 statistics. Random-effects meta-analysis models were used for pooling SVR12 rates. Publication bias was assessed using funnel plots. Among the 130 selected studies, nine papers were included for quantitative synthesis. The quality-appraisal was good for two, fair for three and poor for four studies. The pooled relative risk (RR) of SVR12 was not statistically different between decentralized strategy and treatment by specialists [RR = 1.05; 95% confidence interval (95% CI): 0.98–1.1; I2 = 45% (95% CI: 0–84%), p = 0.145]. SVR12 rate for decentralized HCV treatment was 81% [SVR12 95% CI: 72–89%; I2 = 93% (95% CI: 88–96%)] and 95% [SVR12 95%CI: 92–98%; I2 = 77% (95% CI: 52–89%)] with intention to treat analysis and per-protocol analysis, respectively. SVR12 rates using DAAs managed by non-specialized health personnel were satisfactory and similar to those obtained by specialists. This new delivery strategy can improve access to HCV treatment, especially in resource-limited settings. PROSPERO #: CRD42019122609.
Introduction
Hepatitis C virus (HCV) therapy was revolutionized by use of safe and highly effective direct-acting agents (DAAs) [1, 2]. Treatment with DAAs is associated with reduced risk for mortality and hepatocellular carcinoma and should be considered in all HCV-infected patients [3]. The World Health Organization (WHO) defined strategies to eliminate HCV and to reduce viral hepatitis related deaths by 2030 [4]. However, one of the main barriers to improving HCV care is the lack of an effective linkage-to-care policy for HCV infection involving treatment by non-specialists [5]. In the pegylated interferon (Peg-IFN) era, specialists in gastroenterology or hepatology were the most frequent prescribers of HCV treatment in the United States of America (USA). However, in the last years, HCV treatment managed by infectious disease specialists, internists, general practitioners (GPs) has been increased due to the safety of the new regimens [4, 6]. In order to reach WHO goals in the next decade, non-specialists can play a major role in HCV treatment and long-term follow-up [7].
Several strategies to scale up HCV treatment have been proposed, such as the universal access to highly effective, well-tolerated and affordable regimens. Delivery of treatment using DAAs can involve less trained professionals at lower level health facilities [8]. Implementing new delivery strategies to improve patient access to DAAs in resource-limited settings is crucial to expand testing and treatment to eliminate HCV by 2030 as proposed by the WHO [9]. However, few studies reported relatively low sustained virologic response (SVR) rates (47–66%) of HCV treatment with DAAs conducted by non-specialists in real-world primary care settings [10, 11].
To scale up the decentralization of HCV treatment with DAAs prescribed by non-specialized personnel at primary care settings, evidence synthesis studies need to be conducted to show the effectiveness and safety of these strategies. The aim of this study was to perform a systematic review and meta‐analysis to assess the effectiveness of decentralized strategies of HCV treatment using DAAs.
Materials and methods
Registration, search strategy and eligibility criteria
The protocol of this review is at the international prospective register of systematic reviews (PROSPERO #: CRD42019122609) in the following web address: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42019122609
Literature search strategies were conducted in PubMed, Embase, Scopus, LILACS until March-2019 by experienced researchers, with no language or publication period restrictions (S1 Table). References checking, hand searching and contact with authors were strategies used to identify additional studies. After the removal of duplicate studies, titles and abstracts were independently screened by two trained researchers using the Rayyan QRCI web application (https://rayyan.qcri.org/) [12]. Discordances were solved in a panel with the participation of two experienced reviewers. The decision of an experienced hepatologist solved persistent disagreements. The following pre-specified eligibility criteria was adopted: HCV treatment using DAAs on real-life cohort studies or clinical trials at primary care settings, conducted by non-specialized health personnel (GP, family doctor, or any professional without specialization) with description of SVR rates. Cure of HCV was defined by sustained virologic response 12 weeks after the end-of-treatment (SVR12), which is a binary outcome at the patient-level. A detectable HCV viral load 12 weeks after treatment characterized failure of treatment (no SVR12). Studies were included regardless of presenting a comparison group that received the standard specialized care, managed by hepatologists or infectious diseases specialists. Studies were excluded if the primary endpoint, SVR12, was not showed or disaggregated to allow the assessment of the specific effectiveness of the decentralized treatment delivery strategies (S2 Table).
Data extraction
Study data were extracted in duplicate and managed using electronic data capture tools. Electronic extraction forms were created using REDCap (Research Electronic Data Capture), a secure web-based application designed to support data capture for research studies [13]. SVR12 rates of included studies considering the intention to treat (ITT) and/or the per-protocol analysis were extracted.
Risk of bias assessment
Quality appraisal was performed using the “Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies Personnel” from the National Institutes of Health (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). The 14-item checklist on this form was designed to focus on the key concepts for evaluating the internal validity of a study. The quality of studies was rated as good, fair or poor.
Data analysis
Heterogeneity between the included studies was measured by I2 statistics with the following cut-off points were used to classify heterogeneity: 25–50%, 50–75% and >75% considered as mild, moderate and severe, respectively. The “heterogi” [14] program was used to obtain 95% confidence intervals (95% CI) for I2 in relative risk meta-analysis and “metaan” [15] for meta-analysis of proportions of SVR12. Random effects models using the method of DerSimonian & Laird, with the estimates of heterogeneity being taken from the Mantel-Haenszel models were used to pool the risk ratio (comparison between decentralized versus specialized strategies and among patients with and without cirrhosis) using “metan” command [16]. Maximum likelihood random effects models (ml) were used for pooling SVR12 rate of decentralized HCV treatment strategies and to estimate heterogeneity using “metaan” [15]. We performed subgroup analyses to explore the heterogeneity and to assess how the pre-identified variables affected the pooled estimates. In addition, fixed effects models were planned to be used only after the identification of an absence of heterogeneity using random-effects models. Results with the same data used in “metaan” for meta-analysis of proportion of SVR12 were additionally presented using random effects models (method of DerSimonian and Laird, with the estimate of heterogeneity being taken from the inverse-variance fixed-effect model) with pooled estimate after Freeman-Tukey Double Arcsine Transformation to stabilize the variances, conducted using “metaprop” [17] Stata program. Funnel plots and Egger’s test assessed publication bias and small-study effects, respectively for analysis of relative risks of SVR12 with two groups’ comparison using “metafunnel” [18]. Additional funnel plots were presented considering publication year using “metabias” [19]. The significance level adopted was 5% and statistical analyses were conducted using the “metan” [16] and “metaprop” [17], “metaan”[15], “metafunnel” [18] and “metabias” [19] packages from Stata-SE (2017; StataCorp LP, College Station, TX, USA).
Results
Study characteristics
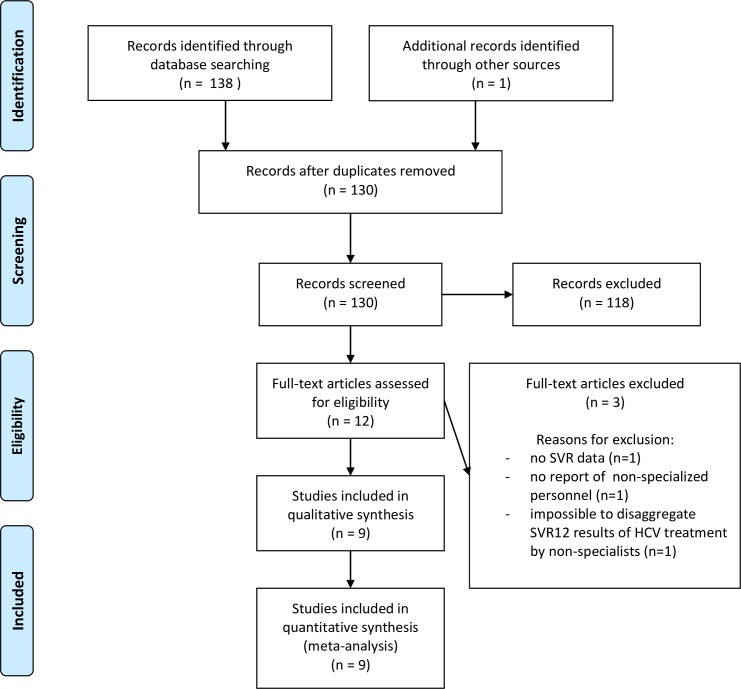
The literature search resulted in 130 unique studies (S1 Table). After the screening of titles and abstracts by two blinded investigators, 12 studies [7, 10, 11, 20–28] were eligible for full-text analysis. The percent agreement and Cohen’s kappa between the two independent reviewers were 95% and 0.69, respectively. Nine papers [10, 11, 20, 22–25, 27, 28] fulfilled our eligibility criteria and were finally included after full-text reading. The flow-chart describes details on the selection process (Fig 1).
Fig 1. Flow diagram of the study selection process.
Overall, 2,099 individuals were treated in the included studies [n = 1,479 by decentralized strategies / n = 620 by specialized delivery of care]. The number of the included patients in the meta-analysis of the proportion of SVR12 with the decentralized strategy was 1,173 for ITT analysis and 917 for per-protocol analysis, considering that some studies showed results of both types of analyses.
Considering the three manuscripts [10, 23, 24] that compared both strategies, HCV treatment with decentralized strategies was delivered for 711 patients, while 620 received specialized care. Among these three studies, only one manuscript had the reporting of SVR12 rates for both ITT and per-protocol analysis [10].
Overall, studies included patients from five countries: USA (n = 3), Australia (n = 3), Pakistan (n = 1), Canada (n = 1), and Rwanda (n = 1). Sofosbuvir (SOF) was the most used DAA, which was present in all included studies regimens. The duration of HCV treatment was for 12 or 24 weeks but was not available for five studies. HCV patients with genotype 1 patients were the most frequently included. Several types of health personnel were used in the decentralization strategies (e.g. mid-level practitioners, social workers, nurses, general practitioners, sexual health physicians, family doctors, and internists) and different types of specialized support (e.g. telephone, electronic messaging, e-mail, education session, remote consultation, pre/post-treatment assessment, and referral to visit by clinical need). Table 1 summarizes the characteristics of included studies.
Table 1. Characteristics of the included studies.
| Study | Country | Period | Regimen | Duration | Genotypes | Types of Analysis | Non-specialized personnel characteristics | Type of support from specialists (when available) | Specialized personnel characteristics (when the comparison is available) |
|---|---|---|---|---|---|---|---|---|---|
| Jayasekera et al 2015[22] | USA | Dec-2013 | SOF /RBV | 12 or 24 weeks | GT1 | ITT and per-protocol | Part-time licensed vocational nurse (mid-level provider) | Pre and post-treatment assessments, and referral by a clinical need | NA |
| GT2 | |||||||||
| GT3 | |||||||||
| GT4 | |||||||||
| Nov-2014 | SOF/SIM | ||||||||
| Capileno et al 2017[20] | Pakistan | Feb-2015 | SOF /RBV | 12 or 24 weeks | GT2 | ITT | Mid-level health practitioners | NA | NA |
| Dec-2015 | GT3 | ||||||||
| GT4 | |||||||||
| Kattakuzhy et al 2017[23] | USA | Jan-2015 | SOF/LDV | 12 weeks | GT1 | ITT | Licensed nurse practitioner or physician board-certified in family or internal medicine | NA | Specialist (infectious diseases or gastroenterology or hepatology) |
| Nov-2015 | |||||||||
| Lasser et al 2017[11] | USA | Mar-2015 | NA | NA | NA | ITT and per-protocol | Primary care physicians | Telephone and electronic messaging | NA |
| Apr-2016 | |||||||||
| Baker et al 2018[27] | Australia | Mar-2016 | SOF/DCV | NA | GT1 | ITT and per-protocol | General practitioners | Consultation with specialist | NA |
| Apr-2016 | SOF/LDV | GT3 | |||||||
| Gupta et al 2018[28] | Rwanda | Feb-2017 | SOF/LDV | 12 weeks | GT1 | ITT and per-protocol | Non-specialist clinicians, internists, general practitioner, nurses and social workers | Supervision and mentoring by one internist with specialized HCV training | NA |
| Sep-2018 | GT4 | ||||||||
| Lee et al 2018[24] | Australia | Feb-2016 | SOF /RBV | NA | GT1 | Per-protocol | General practitioners, sexual health physicians, general physicians, and substance use service | Phone or email support and education sessions | Gastroenterologists |
| SOF/DCV | GT2 | ||||||||
| GT3 | |||||||||
| SOF/LDV | GT4 | ||||||||
| Dec-2017 | Others | ||||||||
| SOF/VEL | |||||||||
| Nouch et al 2018[25] | Canada | Oct-2015 | SOF /RBV | NA | GT1 | ITT and per-protocol | Family doctors | Visit with a specialist when needed | NA |
| SOF/LDV ± RBV | GT2 | ||||||||
| Oct-2017 | GT3 | ||||||||
| SOF/VEL | GT4 | ||||||||
| Wade et al 2018[10] | Australia | July-2015 | SOF /RBV | NA | GT1 | ITT and per-protocol | General practitioners | Remote specialist consultation | Specialist (infectious diseases or gastroenterology or hepatology) |
| SOF/DCV | |||||||||
| SOF/LDV | |||||||||
| Jun-2017 | GT3 |
USA, United States of America; SOF, sofosbuvir; RBV, ribavirin; SIM, simeprevir; LDV, ledipasvir; DCV, daclatasvir; VEL, velpatasvir; NA, not available; GT, genotype; ITT, intention to treat.
Risk of bias assessment
The quality appraisal assessed by NIH Quality Assessment Tool among the included studies was good for two studies [23, 28], fair for three [22, 24, 25], and poor for four studies [10, 11, 20, 27] (S3 Table). Low participation rate, limited sample and absence of sample size calculation were issues for four studies.
Risk of SVR12 of decentralized versus specialized strategies
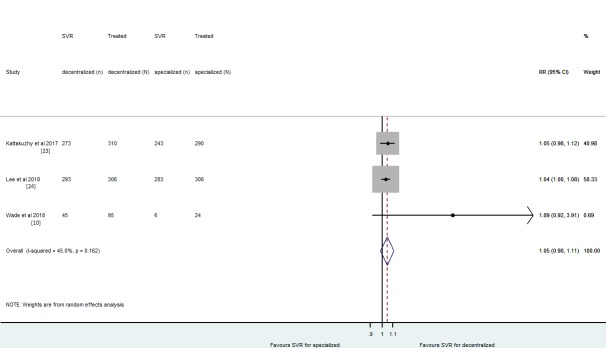
The pooled relative risk (RR) of SVR12 was not statistically different between decentralized strategy and treatment by specialists [RR = 1.05 (95% CI: 0.98–1.1); I2 = 45% (95% CI: 0–84%); p = 0.145]. This synthesis included the three studies [10, 23, 24] that provide data on the comparison of SVR12 between decentralized and specialized strategies. Overall, 1,331 patients were treated in these studies. The pooled results considered all the types of analysis (ITT and per-protocol). Of these, 85.9% (611/711) had SVR12 with decentralized and 85.8% (532/620) with specialized HCV treatment (Fig 2). The funnel plots, even with presence of some asymmetry did not show significant evidence of publication bias, but the small number of studies limits the interpretation. The Egger's test obtained a p-value of 0.07 and an intercept of 1.67 representing no small-study effects (S1 Fig). A funnel plot with each paper´s publication year of was presented additionally (S2 Fig).
Fig 2. The pooled relative risk of SVR12 for decentralized versus specialized strategies were reported by intention-to-treat (Kattakuzhy et al 2017 and Wade et al 2018) or per-protocol analysis (Lee et al 2018).
The effect size of SVR12 rate of decentralized HCV treatment strategies
The pooled SVR12 rate for decentralized HCV treatment was 81% [SVR12 95% CI: 72–89%; I2 = 93% (95% CI: 88–96%)] and 95% [SVR12 95%CI: 92–98%; I2 = 77% (95% CI: 52–89%)] by ITT analysis and by per-protocol analysis, respectively. Heterogeneity was severe among the included studies. The lower individual study point-estimate of SVR12 rate was 47% for results using ITT analysis [10] (Fig 3 and S3 Fig) and the highest were 99% [10, 11] for per-protocol analysis (Fig 4 and S4 Fig). A lower heterogeneity was observed after stratifying studies by risk of bias [I2 = 0% (95% CI: 0–85%) when grouping fair/good quality studies] by ITT analysis. The SVR12 rate was 87% (95%CI: 85–89%) (Fig 3). A total number of 806 of patients were included in the “Good or Fair” quality subgroup for sensitivity analysis (S5 Fig).
Fig 3. The pooled effect size of SVR12 for decentralized strategy with ITT analysis grouped by studies’ quality appraisal.
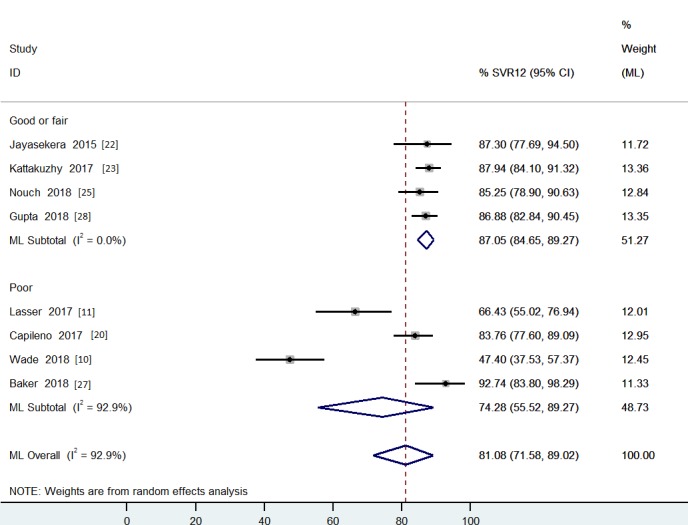
Fig 4. The pooled effect size of SVR12 for decentralized strategy with per-protocol analysis grouped by studies’ country income.
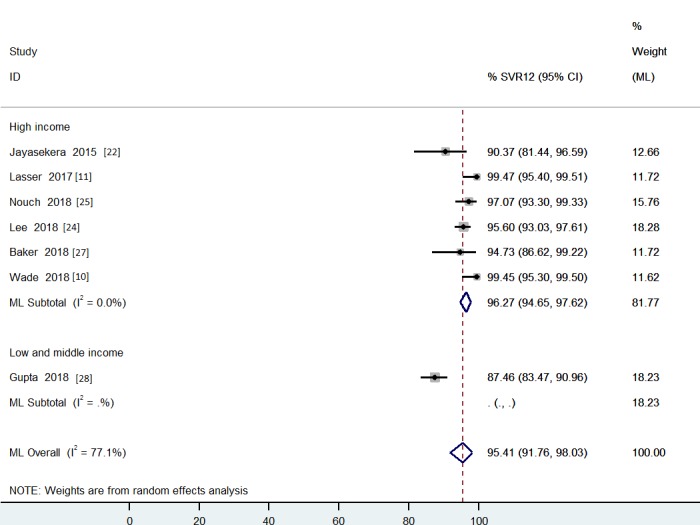
For per-protocol analysis, the heterogeneity was not resolved by subgroup analysis using the levels of quality (S6 Fig). One study from Rwanda [28] had good quality and a lower SVR12 rate in comparison with the others. The heterogeneity decreased when the studies where grouped by levels of country income. For per-protocol analysis, the final pooled SVR12 rate for decentralized HCV treatment was 96% [SVR12 95% CI: 95–98%; I2 = 48% (95% CI: 0–75%)] (Fig 4), including studies with 617 patients in the higher income subgroup (S7 Fig).
In addition, we have found similar results with the same data used for Figs 3 and 4 using random effects models, method of DerSimonian and Laird, with the estimate of heterogeneity being taken from the inverse-variance fixed-effect model with pooled estimates after Freeman-Tukey Double Arcsine Transformation in “metaprop” Stata program (S5 Fig and S7 Fig).
SVR results of patients with and without cirrhosis treated with decentralization
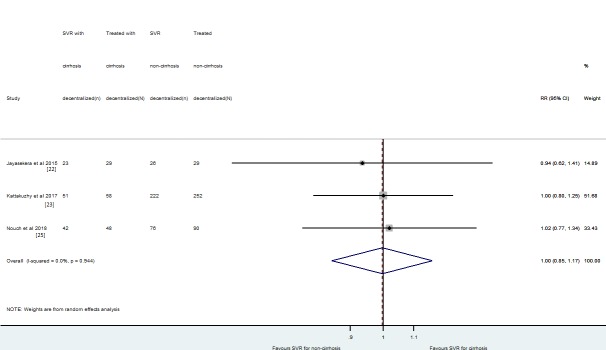
Data extraction on SVR12 rates stratified by absence or presence of cirrhosis was feasible only in three studies using ITT analysis [22, 23, 25]. The SVR12 rates of patients with and without cirrhosis were 85.9% (n = 116/135) and 87.3% (n = 324/371), respectively. The relative risk of SVR12 rate was 1.00 [RR 95% CI: 0.85–1.17; I2 = 0% (95% CI: 0–90%); p = 0.961]. This relative risk represents that SVR12 rate was similar for patients with or without cirrhosis among those who were treated by non-specialists (Fig 5). Albeit with limited interpretation by the small number of studies, funnel plot did not show evidence of publication bias and the Egger's test obtained a p-value of 0.455 and an intercept of -0.63 representing no small-study effects (S8 Fig). An additional funnel plot was presented with publication year information (S9 Fig).
Fig 5. The pooled risk of SVR12 for decentralized strategy, considering patients with cirrhosis versus patients without cirrhosis.
Discussion
The evidence on the effectiveness and safety of DAAs was showed in real-world studies conducted at different settings and subsequent meta-analyses [1, 29–36]. However, to our knowledge, this is the first systematic review and meta-analysis that evaluates the effectiveness of the delivery of HCV treatment with DAAs by non-specialized health personnel at primary care. This meta-analysis highlighted that the HCV treatment using DAAs by non-specialized health personnel was effective and SVR rates were similar to treatment conducted by specialists.
The WHO estimates that up to 71 million people are chronically infected by HCV worldwide [37]. The low screening rates, lack of effective linkage-to-care policies for HCV infection and high drug costs are main barriers for HCV elimination. Many individuals who are unaware that they are HCV positive until the disease progresses into cirrhosis and its complications. HCV cascade can be improved by nation-wide HCV awareness campaign targeting high-risk groups, reduced drugs prices and establishment of multidisciplinary teams to secure linkage to care [38]. Therefore, HCV treatment by non-specialists is a key-strategy to reduce the burden of HCV infection worldwide [39].
Our study reported that SVR12 rates using DAAs by GPs and primary care health personnel are similar to rates obtained by gastroenterologists/hepatologists, reinforcing that this strategy might be implemented in resource-limited setting countries. We acknowledge that patients with cirrhosis should be managed by specialists. Patients with cirrhosis have higher rates of adverse events during treatment [36] and remain at risk of liver-related complications, such as hepatocellular carcinoma after HCV cure [40]. In our systematic review, a small number of patients (n = 134) with cirrhosis were treated by non-specialists. There was a non-significant difference on the SVR rates of patients with versus without cirrhosis treated by non-specialists [RR = 1.0 (95% CI: 0.85–1.17)]. Moreover, the SVR12 was 86.6% for individuals with cirrhosis treated by non-specialists. In our meta-analysis, the presence of cirrhosis has not impacted the primary outcome of HCV treatment. However, patients with cirrhosis should be managed by hepatologist rather than non-specialists due to the higher risk of adverse events during treatment and the need of maintenance of hepatocellular carcinoma screening during a longitudinal follow-up [41]. On the other hand, patients without cirrhosis (METAVIR F≤3) can be managed by GPs or primary care doctors. People with HIV and/or HBV coinfections and other comorbidities (e.g. haemophilia, thalassemia, kidney disfunction) should be referred to specialists [42, 43].
Our meta-analysis has found a considerable difference between ITT and per-protocol results for HCV treatment conducted by non-specialists. Higher lost to follow up (LTFU) is expected at real-world settings, albeit is still possible to find papers reporting similar LFTU between clinical trials and real-world data [44, 45]. Previously, gaps of SVR results for real-world treatment in comparison with clinical trials were due to the LTFU [30]. Moreover, LTFU was related to treatment failure in difficult to treat patients [46]. Future studies should develop and implement new strategies to tackle the LTFU in HCV treatment.
Different decentralization strategies were adopted in the included studies. Only two authors did not mention any type of support offered by specialized personnel [20, 23]. Several categories and combination of health personnel were involved, specialist support or consultancy at distance, training possibilities, and mentoring. Telemedicine support by specialists can be an effective intervention for HCV treatment decentralization to primary care [7].
The main limitations of our study were the high heterogeneity for pooled overall SVR rates and the limited number of studies that compared the decentralized HCV treatment with the standard-of-care. The high heterogeneity could be explained by methodological quality and study design; population and setting characteristics from different countries where the studies were performed, and the variability of specialized support for HCV treatment. As the included studies were investigating the outcomes of new delivery strategies for HCV treatment, with previously unknown effectiveness, the support by specialists could be over implemented due to ethical and/or safety reasons at settings where specialists are available. Apparently, we were able to manage heterogeneity using subgroup analysis considering studies’ quality and income of the country where the studies occurred. Especially in small meta-analyses, it is important to avoid homogeneity assumptions [47]. The report of high heterogeneity rates in our study was an important finding itself. Moreover, the presence of large 95% CI for I2, even after achieving a good point estimate through subgroup analysis showed that reporting confidence intervals is very important [48] and approaching heterogeneity can be more challenging than expected. The small number of included studies is a major limitation of our systematic review. Moreover, the assessment of the publication bias that included the presentation of funnel plots is strongly limited. Funnel plots were shown to illustrate this important issue but cannot be used to conclude that publication bias was absent.
The small number of studies included and the heterogeneity results limit the recommendation of treatment decentralization for all settings, but our meta-analysis results contributes to support strategies of decentralized delivery of treatment for key populations, especially in locations where it is not possible to provide specialized care for all people living with HCV. DAAs prescribed and managed by non-specialized health personnel showed good SVR12 rates. The lack of enough specialists for HCV treatment can be tackled with DAA treatment at primary care settings by non-specialized health personnel. Specialists will be necessary to manage patients with specific clinical conditions (e.g. cirrhosis, coinfections, and comorbidities) to reduce the adverse events and improve the treatment efficacy in these cases [40, 42, 43]. Innovative decentralization strategies could be implemented to improve access to HCV treatment, especially in resource-limited settings, contributing to the achievement of HCV elimination targets.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOC)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq (http://cnpq.br/) through these research grants: Programa Institucional de Bolsas de Iniciação Científica - PIBIC/CNPq (http://cnpq.br/pibic) [RC, LAMMA], Programa Institucional de Bolsas de Iniciação em Desenvolvimento Tecnológico e Inovação - PIBITI/CNPq (http://cnpq.br/pibiti) [RC,IGG] at Fundação Oswaldo Cruz (FIOCRUZ), and Programa de Incentivo a Jovens Pesquisadores at Instituto Nacional de Infectologia Evandro Chagas - INI/FIOCRUZ (https://www.ini.fiocruz.br/) [HP]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wehmeyer MH, Ingiliz P, Christensen S, Hueppe D, Lutz T, Simon KG, et al. Real-world effectiveness of sofosbuvir-based treatment regimens for chronic hepatitis C genotype 3 infection: Results from the multicenter German hepatitis C cohort (GECCO-03). Journal of medical virology. 2018;90(2):304–12. Epub 2017/07/16. 10.1002/jmv.24903 . [DOI] [PubMed] [Google Scholar]
- 2.Welzel TM, Nelson DR, Morelli G, Di Bisceglie A, Reddy RK, Kuo A, et al. Effectiveness and safety of sofosbuvir plus ribavirin for the treatment of HCV genotype 2 infection: results of the real-world, clinical practice HCV-TARGET study. Gut. 2017;66(10):1844–52. Epub 2016/07/16. 10.1136/gutjnl-2016-311609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet (London, England). 2019;393(10179):1453–64. Epub 2019/02/16. 10.1016/s0140-6736(18)32111-1 . [DOI] [PubMed] [Google Scholar]
- 4.WHO. Combating hepatitis B and C to reach elimination by 2030. Advocacy brief 2016. Geneva: World Health Organization; 2016. [Google Scholar]
- 5.Andreone P, Di Marco V, Gaeta GB, Fagiuoli S, Vukotic R, Craxì A. Current and forthcoming perspectives in linkage to care of hepatitis C virus infection: Assessment of an Italian focus group. Digestive and Liver Disease. 2019;51(7):915–21. Epub Epub 2019 Apr 25. 10.1016/j.dld.2019.03.033 [DOI] [PubMed] [Google Scholar]
- 6.Butt AA, Yan P, Lo Re V Iii, Shaikh OS, Ross DB. Trends in Treatment Uptake and Provider Specialty for Hepatitis C Virus (HCV) Infection in the Veterans Affairs Healthcare System: Results From the Electronically Retrieved Cohort of HCV-Infected Veterans (ERCHIVES). Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2019;68(5):857–9. Epub 2018/08/24. 10.1093/cid/ciy697 . [DOI] [PubMed] [Google Scholar]
- 7.Beste LA, Glorioso TJ, Ho PM, Au DH, Kirsh SR, Todd-Stenberg J, et al. Telemedicine Specialty Support Promotes Hepatitis C Treatment by Primary Care Providers in the Department of Veterans Affairs. The American journal of medicine. 2017;130(4):432–8.e3. Epub 2016/12/22. 10.1016/j.amjmed.2016.11.019 . [DOI] [PubMed] [Google Scholar]
- 8.Ford N, Swan T, Beyer P, Hirnschall G, Easterbrook P, Wiktor S. Simplification of antiviral hepatitis C virus therapy to support expanded access in resource-limited settings. Journal of hepatology. 2014;61(1 Suppl):S132–8. Epub 2014/12/03. 10.1016/j.jhep.2014.09.019 . [DOI] [PubMed] [Google Scholar]
- 9.European-Union-HCV-Collaborators. Hepatitis C virus prevalence and level of intervention required to achieve the WHO targets for elimination in the European Union by 2030: a modelling study. The lancet Gastroenterology & hepatology. 2017;2(5):325–36. Epub 2017/04/12. 10.1016/s2468-1253(17)30045-6 . [DOI] [PubMed] [Google Scholar]
- 10.Wade AJ, McCormack A, Roder C, McDonald K, Davies M, Scott N, et al. Aiming for elimination: Outcomes of a consultation pathway supporting regional general practitioners to prescribe direct-acting antiviral therapy for hepatitis C. Journal of viral hepatitis. 2018;25(9):1089–98. Epub 2018/04/17. 10.1111/jvh.12910 . [DOI] [PubMed] [Google Scholar]
- 11.Lasser KE, Heinz A, Battisti L, Akoumianakis A, Truong V, Tsui J, et al. A Hepatitis C Treatment Program Based in a Safety-Net Hospital Patient-Centered Medical Home. Annals of family medicine. 2017;15(3):258–61. Epub 2017/05/10. 10.1370/afm.2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Systematic reviews. 2016;5(1):210 Epub 2016/12/07. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–81. Epub 2008/10/22. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicola O, Matteo B, Julian H, Iain B. HETEROGI: Stata module to quantify heterogeneity in a meta-analysis. S449201 ed: Boston College Department of Economics; 2005. [Google Scholar]
- 15.Kontopantelis E, Reeves D. metaan: Random-effects meta-analysis. Stata Journal. 2010;10(3):395–407. [Google Scholar]
- 16.Harris R, Bradburn M, Deeks J, Harbord R, Altman D, Sterne J. metan: fixed- and random-effects meta-analysis. Stata Journal. 2008;8(1):3–28. [Google Scholar]
- 17.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Archives of Public Health. 2014;72(1):39 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonathan S. METAFUNNEL: Stata module to produce funnel plots for meta-analysis. 2003;(S434101). [Google Scholar]
- 19.Robert MH, Ross JH, Jonathan ACS, Thomas S. METABIAS: Stata module to test for small-study effects in meta-analysis. S404901 ed: Boston College Department of Economics; 2000. [Google Scholar]
- 20.Capileno YA, Van den Bergh R, Donchunk D, Hinderaker SG, Hamid S, Auat R, et al. Management of chronic Hepatitis C at a primary health clinic in the high-burden context of Karachi, Pakistan. PLoS One. 2017;12(4):e0175562 10.1371/journal.pone.0175562 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajarizadeh B, Grebely J, Matthews GV, Martinello M, Dore GJ. Uptake of direct-acting antiviral treatment for chronic hepatitis C in Australia. Journal of viral hepatitis. 2018;25(6):640–8. Epub 2017/12/24. 10.1111/jvh.12852 . [DOI] [PubMed] [Google Scholar]
- 22.Jayasekera CR, Perumpail RB, Chao DT, Pham EA, Aggarwal A, Wong RJ, et al. Task-Shifting: An Approach to Decentralized Hepatitis C Treatment in Medically Underserved Areas. Dig Dis Sci. 2015;60(12):3552–7. 10.1007/s10620-015-3911-6 . [DOI] [PubMed] [Google Scholar]
- 23.Kattakuzhy S, Gross C, Emmanuel B, Teferi G, Jenkins V, Silk R, et al. Expansion of Treatment for Hepatitis C Virus Infection by Task Shifting to Community-Based Nonspecialist Providers: A Nonrandomized Clinical Trial. Ann Intern Med. 2017;167(5):311–8. 10.7326/M17-0118 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee A, Hanson J, Fox P, Spice G, Russell D, Boyd P. A decentralised, multidisciplinary model of care facilitates treatment of hepatitis C in regional Australia. Journal of virus eradication. 2018;4(3):160–4. Epub 2018/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nouch S, Gallagher L, Erickson M, Elbaharia R, Zhang W, Wang L, et al. Factors associated with lost to follow-up after hepatitis C treatment delivered by primary care teams in an inner-city multi-site program, Vancouver, Canada. International Journal of Drug Policy. 2018;59:76–84. 10.1016/j.drugpo.2018.06.019 [DOI] [PubMed] [Google Scholar]
- 26.Read P, Lothian R, Chronister K, Gilliver R, Kearley J, Dore GJ, et al. Delivering direct acting antiviral therapy for hepatitis C to highly marginalised and current drug injecting populations in a targeted primary health care setting. Int J Drug Policy. 2017;47:209–15. 10.1016/j.drugpo.2017.05.032 . [DOI] [PubMed] [Google Scholar]
- 27.Baker D, McMurchie M, Farr V. Hepatitis C in Australia—a role for general practitioners? Medical Journal of Australia. 2018;208(4):190–.e1. [DOI] [PubMed] [Google Scholar]
- 28.Gupta N, Mbituyumuremyi A, Kabahizi J, Ntaganda F, Muvunyi CM, Shumbusho F, et al. Treatment of chronic hepatitis C virus infection in Rwanda with ledipasvir-sofosbuvir (SHARED): a single-arm trial. The lancet Gastroenterology & hepatology. 2019;4(2):119–26. Epub 2018/12/16. 10.1016/s2468-1253(18)30382-0 . [DOI] [PubMed] [Google Scholar]
- 29.Calleja JL, Crespo J, Rincon D, Ruiz-Antoran B, Fernandez I, Perello C, et al. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: Results from a Spanish real-world cohort. Journal of hepatology. 2017;66(6):1138–48. Epub 2017/02/13. 10.1016/j.jhep.2017.01.028 . [DOI] [PubMed] [Google Scholar]
- 30.Haridy J, Wigg A, Muller K, Ramachandran J, Tilley E, Waddell V, et al. Real-world outcomes of unrestricted direct-acting antiviral treatment for hepatitis C in Australia: The South Australian statewide experience. Journal of viral hepatitis. 2018;25(11):1287–97. Epub 2018/06/12. 10.1111/jvh.12943 . [DOI] [PubMed] [Google Scholar]
- 31.Honer Zu Siederdissen C, Buggisch P, Boker K, Schott E, Klinker H, Pathil A, et al. Treatment of hepatitis C genotype 1 infection in Germany: effectiveness and safety of antiviral treatment in a real-world setting. United European gastroenterology journal. 2018;6(2):213–24. Epub 2018/03/08. 10.1177/2050640617716607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong CM, Liu CH, Su TH, Yang HC, Chen PJ, Chen YW, et al. Real-world effectiveness of direct-acting antiviral agents for chronic hepatitis C in Taiwan: Real-world data. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2018. Epub 2018/10/15. 10.1016/j.jmii.2018.09.005 . [DOI] [PubMed] [Google Scholar]
- 33.Ioannou GN, Beste LA, Chang MF, Green PK, Lowy E, Tsui JI, et al. Effectiveness of Sofosbuvir, Ledipasvir/Sofosbuvir, or Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir Regimens for Treatment of Patients With Hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology. 2016;151(3):457–71.e5. Epub 2016/06/09. 10.1053/j.gastro.2016.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marciano S, Haddad L, Reggiardo MV, Peralta M, Vistarini C, Marino M, et al. Effectiveness and safety of original and generic sofosbuvir for the treatment of chronic hepatitis C: A real world study. Journal of medical virology. 2018;90(5):951–8. Epub 2018/01/20. 10.1002/jmv.25033 . [DOI] [PubMed] [Google Scholar]
- 35.Takaguchi K, Toyoda H, Tsutsui A, Suzuki Y, Nakamuta M, Imamura M, et al. Real-world virological efficacy and safety of daclatasvir/asunaprevir/beclabuvir in patients with chronic hepatitis C virus genotype 1 infection in Japan. Journal of gastroenterology. 2019. Epub 2019/03/09. 10.1007/s00535-019-01568-8 . [DOI] [PubMed] [Google Scholar]
- 36.Fernandes FF, Piedade J, Guimaraes L, Nunes EP, Chaves UB, Goldenzon RV, et al. Effectiveness of direct-acting agents for hepatitis C and liver stiffness changing after sustained virological response. Journal of gastroenterology and hepatology. 2019. Epub 2019/05/08. 10.1111/jgh.14707 . [DOI] [PubMed] [Google Scholar]
- 37.WHO. Global Hepatitis Report 2017. Geneva: World Health Organization; 2017 [Google Scholar]
- 38.Kracht PAM, Arends JE, van Erpecum KJ, Urbanus A, Willemse JA, Hoepelman AIM, et al. Strategies for achieving viral hepatitis C micro-elimination in the Netherlands. Hepatology, medicine and policy. 2018;3:12 Epub 2018/10/06. 10.1186/s41124-018-0040-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroeder SE, Pedrana A, Scott N, Wilson D, Kuschel C, Aufegger L, et al. Innovative strategies for the elimination of viral hepatitis at a national level: A country case series. Liver international: official journal of the International Association for the Study of the Liver. 2019;39(10):1818–36. Epub 2019/08/23. 10.1111/liv.14222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fehily SR, Papaluca T, Thompson AJ. Long-Term Impact of Direct-Acting Antiviral Agent Therapy in HCV Cirrhosis: Critical Review. Seminars in liver disease. 2019. Epub 2019/05/02. 10.1055/s-0039-1685538 . [DOI] [PubMed] [Google Scholar]
- 41.Chun HS, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, et al. Design and validation of risk prediction model for hepatocellular carcinoma development after sustained virological response in patients with chronic hepatitis C. European journal of gastroenterology & hepatology. 2019. Epub 2019/09/07. 10.1097/meg.0000000000001512 . [DOI] [PubMed] [Google Scholar]
- 42.Aghemo A, Piroth L, Bhagani S. What do clinicians need to watch for with direct-acting antiviral therapy? Journal of the International AIDS Society. 2018;21 Suppl 2:e25076 Epub 2018/04/11. 10.1002/jia2.25076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rumi MG, Di Marco V, Colombo M. Management of HCV-Related Liver Disease in Hemophilia and Thalassemia. Seminars in liver disease. 2018;38(2):112–20. Epub 2018/06/06. 10.1055/s-0038-1655774 . [DOI] [PubMed] [Google Scholar]
- 44.Sikavi C, Najarian L, Saab S. Similar Sustained Virologic Response in Real-World and Clinical Trial Studies of Hepatitis C/Human Immunodeficiency Virus Coinfection. Dig Dis Sci. 2018;63(11):2829–39. Epub 2018/08/11. 10.1007/s10620-018-5215-0 . [DOI] [PubMed] [Google Scholar]
- 45.Christensen S, Buggisch P, Mauss S, Boker KHW, Schott E, Klinker H, et al. Direct-acting antiviral treatment of chronic HCV-infected patients on opioid substitution therapy: Still a concern in clinical practice? Addiction (Abingdon, England). 2018;113(5):868–82. Epub 2018/01/24. 10.1111/add.14128 . [DOI] [PubMed] [Google Scholar]
- 46.Yek C, de la Flor C, Marshall J, Zoellner C, Thompson G, Quirk L, et al. Effectiveness of direct-acting antiviral therapy for hepatitis C in difficult-to-treat patients in a safety-net health system: a retrospective cohort study. BMC medicine. 2017;15(1):204 Epub 2017/11/21. 10.1186/s12916-017-0969-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kontopantelis E, Springate DA, Reeves D. A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analyses. PLoS One. 2013;8(7):e69930 Epub 2013/08/08. 10.1371/journal.pone.0069930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ (Clinical research ed). 2007;335(7626):914–6. Epub 2007/11/03. 10.1136/bmj.39343.408449.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.