Abstract
Backgroud
Resection is still the only potentially curative treatment for patients with intrahepatic cholangiocarcinoma (ICC), but the prognosis remains far from satisfactory. However, the benefit of adjuvant therapy (AT) remains controversial, although it has been conducted prevalently. Hence, a meta-analysis was warranted to evaluate the effect of AT for patients with ICC after resection.
Patients and methods
PubMed, MedLine, Embase, the Cochrane Library, Web of Science were used to identify potentially eligible studies from Jan.1st 1990 to Aug. 31st 2019, investigating the effect of AT for patients with ICC after resection. Primary endpoint was overall survival (OS), and secondary endpoints was recurrence-free survival (RFS). Hazard ratio (HR) with 95% confidence interval (CI) was used to determine the effect size.
Results
22 studies with 10181 patients were enrolled in this meta-analysis, including 832 patients in the chemotherapy group, 309 patients in the transarterial chemoembolization (TACE) group, 1192 patients in the radiotherapy group, 235 patients in the chemoradiotherapy group, and 6424 patients in the non-AT group. The pooled HR for the OS rate and RFS rate in the AT group were 0.63 (95%CI 0.52~0.74), 0.74 (95%CI 0.58~0.90), compared with the non-AT group. Subgroup analysis showed that the pooled HR for the OS rate in the AT group compared with non-AT group were as follows: chemotherapy group was 0.57 (95%CI = 0.44~0.70), TACE group was 0.56 (95%CI = 0.31~0.82), radiotherapy group was 0.71 (95%CI = 0.39~1.03), chemoradiotherapy group was 0.73 (95%CI = 0.57~0.89), positive resection margin group was 0.60 (95%CI = 0.51~0.69), and lymph node metastasis (LNM) group was 0.67 (95%CI = 0.57~0.76).
Conclusion
With the current data, we concluded that AT such as chemotherapy, TACE and chemoradiotherapy could benefit patients with ICC after resection, especially those with positive resection margin and LNM, but the conclusion needed to be furtherly confirmed.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer following hepatocellular carcinoma with a stably growing incidence and mortality[1, 2]. Surgical resection is still the most preferred treatment for patients with ICC, but only 15% of patients have the chance of surgery at initial diagnosis[3–5]. However, the prognosis of patients with ICC after resection remains far from satisfactory with the 5-year survival rate around 30%[6, 7]. Hence, concerns have always been focused on any strategies intended to improve the prognosis.
Various kinds of adjuvant therapies (AT), such as chemotherapy[8–10], radiotherapy[11, 12], transarterial chemoembolization (TACE)[13, 14], and chemoradiotherapy[15] have been conducted prevalently to improve the prognosis of patients after resection, and 21.4%-57.7% of patients were reported to receive AT after resection[14, 16]. However, the benefit of AT remains controversial[8, 9, 12]. Considering that randomized controlled trials or prospective studies evaluating the clinical vale of AT are hard to conduct, a comprehensive systematic review and meta-analysis is needed to confirm it.
Material and method
This study was based on published studies and the informed consent of the patients and the ethical approval were not required. This meta-analysis was conducted according to the preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Literature search
A comprehensive search on the existing published medical literature was conducted by Qiao Ke and Nanping Lin to investigate the value of the AT for patients with ICC after surgical resection. English electronic databases such as PubMed, MedLine, Embase, the Cochrane Library, Web of Science were used to search the literature from Jan.1st 1990 to Aug. 31st 2019. Key words were as follows: (“intrahepatic cholangiocarcinoma” or “ICC” or “iCCA”) AND (“adjuvant therapy” or “transarterial chemoembolization” or “chemotherapy or “radiotherapy” or “chemoradiotherapy”). Any potentially eligible studies were then identified manually through the references of the included studies, reviews, letters and comments.
Selection criteria
Inclusion criteria
i) patients with ICC confirmed by pathology; ii) patients receiving surgical resection; iii) groups must include AT group and non-AT group; iv) outcomes must include the long-term outcomes.
Exclusion criteria
i) patients including gallbladder carcinoma or extrahepatic cholangiocarcinoma; ii) patients receiving neoadjuvant therapy; iii) patients receiving palliative resection; ⅳ) data on the long-term outcomes was not available; ⅴ) studies based on overlapping cohorts deriving from the same center; ⅵ) reviews, comments, letters, case report, and conference abstract.
Of note, considering that the data of most of the American studies came from the national cancer data base (NCDB), we only incorporated the study with longest research span if overlapping cohorts existed among studies.
Intervention
Hepatectomy was conducted with or without lymph node dissection[17, 18], regardless of margin status.
AT was defined as any strategies administrated before recurrence, regardless of TACE, chemotherapy, radiotherapy, and chemoradiotherapy.
Data extraction
Data such as the author’s first name, year of publication, study methods, patient’s characteristic, interventions, and outcomes were extracted and assessed by Qiao Ke and Nanping Lin with predefined forms. The hazard ratios (HRs) of OS or RFS were extracted directedly from the original data or extracted from the Kaplan-Meier curves according to the methods described in detail by Tierney et al[19]. and Parmar et al[20]. In case of disagreement, a third investigator, Manjun Deng, was intervened to reach a conclusion.
Quality assessment
The quality of non-randomized studies was assessed by the modified Newcastle-Ottawa Scale (NOS)[21], and more than 7 stars were defined as high quality, 4~6 star as medium quality, and <4 stars as low quality.
Statistical analysis
The meta-analysis was registered at http://www.crd.york.ac.uk/PROSPERO/ (Review registry 145810) and was performed using Stata 14. Considering all of the included studies were retrospective cohort studies, endpoints in this meta-analysis were evaluated by HRs and 95%CIs using the random-effects model[22, 23]. Subgroup analyses were conducted in the group of different AT strategies, R1 resection, and lymph node metastasis. Sensitivity analysis was conducted to observe whether the present result would be affected by any one study. Publication bias was determined using Begg’s and Egger’s tests, and “trim and fill” method was introduced to check the effect of potentially unpublished studies on the present result.
Results
Base characteristic of the included studies
Totally, 1267 records were excluded from the initially identified 1289 records. 22 studies including 23 cohorts and 10181 patients were enrolled in this meta-analysis[9, 11–14, 16, 24–39]. Groups were classified as follows: 832 patients in the chemotherapy group, 309 patients in the TACE group, 1192 patients in the radiotherapy group, 235 patients in the chemoradiotherapy group, and 6424 patients in the non-AT group. Of note, both adjuvant chemotherapy and adjuvant chemoradiotherapy were evaluated in Sur’s study[33], so the former was defined as Sur 2014a and the latter was defined as Sur 2014b. The search strategies and results were shown in Fig 1.
Fig 1. PRISMA flow diagram showing selection of articles for meta-analysis.
The characteristics and baseline demographic data of the patients in each research were listed in Table 1. Of note, two studies were international multi-centers ones[16, 33]. Details of AT in the included studies were depicted in Table 2. NOS score of each included study was exhibited in Table 3, among of which 20 studies were scored 7–9[9, 11–14, 16, 25–37, 39] and two were scored 5–6[24, 38].
Table 1. Basic characteristics of included studies.
| Study | Country | Design | Period | Primary endpoints | Sex(M/F) | LNM(+/-) | Vascular invasion(+/-) | Resection margin(+/-) | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| Roayaie 1998[24] | US | Single center | 1991–1997 | OS/RFS | NR | 8/8 | NR | NR | 35.7(0.1–73.2) |
| Jan 2005[25] | China | Single center | 1997–2001 | OS | 128/184 | NR | NR | 222/90 | 14.1(1.05–167.6) |
| Jiang 2010[26] | China | Single center | 1998–2008 | OS | 52/38 | 90/0 | NR | NR | 13.2(0.3–123) |
| Shen 2011[27] | China | Single center | 2002–2003 | OS/RFS | 88/37 | 10/115 | 38/87 | NR | 18(3–96) |
| Wu 2012[28] | China | Single center | 2005–2006 | OS | 88/26 | 11/103 | 14/100 | NR | NR |
| Ribero 2012[30] | Italy | Multi-center | 1990–2008 | OS | 243/191 | 113/321 | 211/223 | NR | 36.5 |
| Bhudhisawasdi 2012[29] | Thailand | Single center | 1998–2002 | OS | 116/55 | NR | 145/26 | 141/30 | NR |
| Li 2013[32] | China | Single center | 2000–2011 | OS/RFS | NR | 34/177 | 51/160 | NR | NR |
| Liu 2013[31] | China | Single center | 2005–2011 | OS | 48/33 | 50/31 | NR | NR | NR |
| Sur 2014[33] | US | Multi-center | 1998–2006 | OS | NR | 128/510 | NR | 180/458 | NR |
| Miura 2015[34] | US | Multi-center | 1998–2011 | OS | NR | NR | NR | NR | NR |
| Li 2015[14] | China | Single center | 2008–2011 | OS/RFS | 368/185 | 104/449 | 73/480 | NR | 25.3(2.2–76.2) |
| Okumaura 2016[36] | Japan | Single center | 2004–2015 | OS/RFS | 67/42 | 32/77 | 69/40 | 18/91 | NR |
| Luvira 2016[35] | Thailand | Single center | 2004–2009 | RFS | 26/24 | 18/32 | NR | 27/23 | NR |
| Hammad 2016[12] | US | Multi-center | 1998–2013 | OS | 819/755 | 607/967 | NR | NR | NR |
| Jeong 2017[13] | China | Single center | 2011–2015 | OS | 28/14 | 15/27 | 16/26 | NR | 36(11–65) |
| Tran 2017[39] | US | Multi-center | 2004–2012 | OS | NR | NR | NR | NR | NR |
| Schweitzer 2017[37] | Germany | Single center | 2000–2015 | OS | 111/86 | 45/152 | 44/153 | NR | NR |
| Reames 2017[9] | International | Multi-center | 1990–2015 | OS | 638/516 | 200/954 | 217/805 | 146/992 | NR |
| Zheng 2018[11] | China | Single center | 2007–2016 | OS | NR | 31/18 | 10/39 | NR | NR |
| Lee 2019[16] | US | Multi-center | 2004–2014 | OS | 1315/1498 | 582/2231 | NR | 649/2164 | 25.2(13.2–42) |
| Sahara 2019[38] | International | Multi-center | 1990–2015 | OS | NR | NR | NR | NR | 21.2(11.2–38.9) |
M: male; F: female; LNM: lymph node metastasis; NR: not report; OS, overall survival; RFS, recurrence-free survival.
Table 2. Interventions of adjuvant treatments in the included studies.
| Study | Treatment types | Patients(yes/no) | Regimens |
|---|---|---|---|
| Roayaie 1998[24] | CRT | 9/7 | 5-FU(1000mg/m2)+external beam radiotherapy (40-50GY) |
| Jan 2005[25] | CT | 118/194 | 5-FU+cisplatin+gemcitabine+doxorubicine+oxaliplatin |
| Jiang 2010[26] | RT | 24/66 | external beam radiation(50 Gy) |
| Shen 2011[27] | TACE | 53/72 | 5-FU (500 mg)/ carboplatin (100 mg)+iodized oil (3–5ml)+epirubicin (20 mg)+hydroxycamptothecin (10 mg) |
| Wu 2012[28] | TACE | 57/57 | 5-FU (500 mg)/ carboplatin (100 mg)+iodized oil (3–5ml)+epirubicin (20 mg)+hydroxycamptothecin (10 mg) |
| Ribero 2012[30] | CT | 116/318 | NR |
| Bhudhisawasdi 2012[29] | CT | 54/117 | 5-FU(1000mg/m2)+mitomycin C(10mg/m2) |
| Li 2013[32] | TACE | 68/143 | 5-FU(500 mg)+iodized oil(3–5 ml)+epirubicin (20 mg)+hydroxycamptothecin (10 mg) |
| Liu 2013[31] | CT | 18/63 | 5-FU+cisplatin+gemcitabine+doxorubicine+oxaliplatin |
| Sur 2014a[33] | CT | 75/416 | NR |
| Sur 2014b[33] | CRT | 147/416 | NR |
| Miura 2015[34] | RT | 486/77 | NR |
| Li 2015[14] | TACE | 122/431 | 5-FU(500 mg)+iodized oil(3–5 ml)+epirubicin (20 mg)+hydroxycamptothecin (10 mg) |
| Okumaura 2016[36] | CT | 47/62 | Gemcitabine+ S-1 |
| Luvira 2016[35] | CT | 18/32 | 5-FU(1000mg/m2)+mitomycin C(10mg/m2) |
| Hammad 2016[12] | RT | 525/1049 | NR |
| Jeong 2017[13] | TACE | 9/33 | 5-FU+epirubicin+cisplatin |
| Tran 2017[39] | CRT | 79/170 | NR |
| Schweitzer 2017[37] | CT | 39/158 | gemcitabine (1000 mg/m2)+cisplatin (25mg/m2)+oxaliplatin (100mg/m2) |
| Reames 2017[9] | CT | 347/807 | gemcitabine (1000 mg/m2)+cisplatin (25mg/m2)+oxaliplatin (100mg/m2) |
| Zheng 2018[11] | RT | 26/23 | intensity-modulated radiotherapy(50-60Gy) |
| Lee 2019[16] | CT/CRT | 1189/1624 | NR |
| Sahara 2019[38] | RT | 131/505 | NR |
CRT: chemoradiotherapy; CT: chemotherapy; RT: radiotherapy; TACE, transarterial chemoembolization; NR: not report.
Table 3. Newcastle-Ottawa quality assessment of the included studies.
| Study | Selection | Comparability | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome of interest was presented | Assessment of outcome | Follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | Scores | ||
| Roayaie 1998[24] | ★ | ★ | ★ | ★ | ★ | 5 | |||
| Jan 2005[25] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Jiang 2010[26] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | |
| Shen 2011[27] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | |
| Wu 2012[28] | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |
| Ribero 2012[30] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | |
| Bhudhisawasdi 2012[29] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | |
| Li 2013[32] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Liu 2013[31] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Sur 2014[33] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | |
| Miura 2015[34] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Li 2015[14] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Okumaura 2016[36] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Luvira 2016[35] | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Hammad 2016[12] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Jeong 2017[13] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | |
| Tran 2017[39] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Schweitzer 2017[37] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Reames 2017[9] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Zheng 2018[11] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Lee 2019[16] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Sahara 2019[38] | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
Endpoints
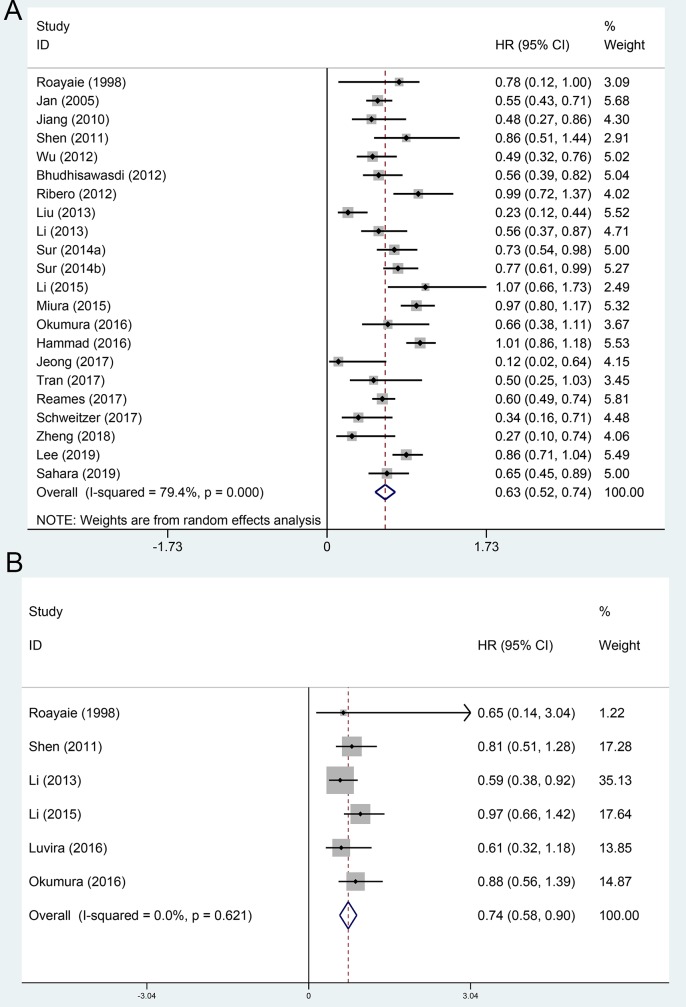
The OS and RFS comparing between AT group and non-AT group were evaluated in 22[9, 11–14, 16, 24–34, 36–39] and 6 included cohorts [14, 24, 27, 32, 35, 36], respectively. Using a random-effect model, the pooled HR for the OS and RFS in the AT group were 0.63 (95%CI 0.52~0.74, Fig 2A), and 0.74 (95%CI 0.58~0.90, Fig 2B), respectively, compared with the non-AT group.
Fig 2. Forest plot of the overall survival and recurrence-free survival rates between adjuvant therapy and operation only.
A, overall survival; B, recurrence-free survival.
Subgroup analysis stratified by different AT strategies
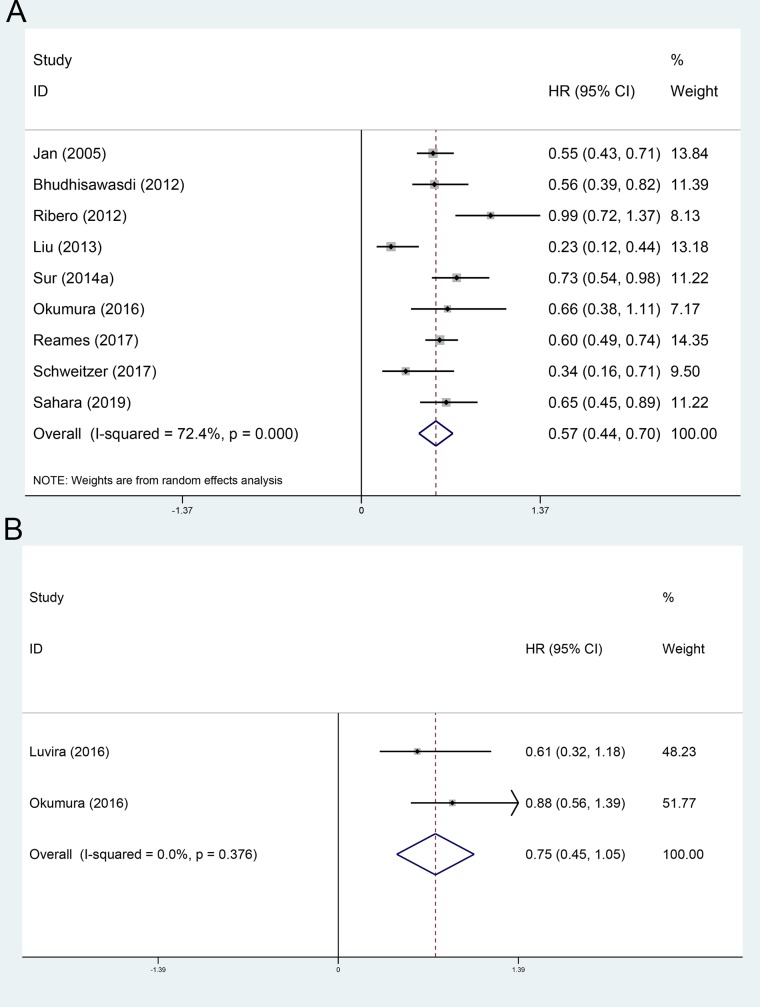
The OS and RFS comparing between adjuvant chemotherapy group and non-AT group were evaluated in 9[9, 25, 29–31, 33, 36–38] and 2[35, 36] included cohorts, respectively. Using a random-effect model, the pooled HR for the OS and RFS in the AT group were 0.57 (95%CI 0.44~0.70, Fig 3A), and 0.75 (95%CI 0.45~1.05, Fig 3B), respectively, compared with the non-AT group.
Fig 3. Forest plot of the overall survival and recurrence-free survival rates between adjuvant chemotherapy and operation only.
A, overall survival; B, recurrence-free survival.
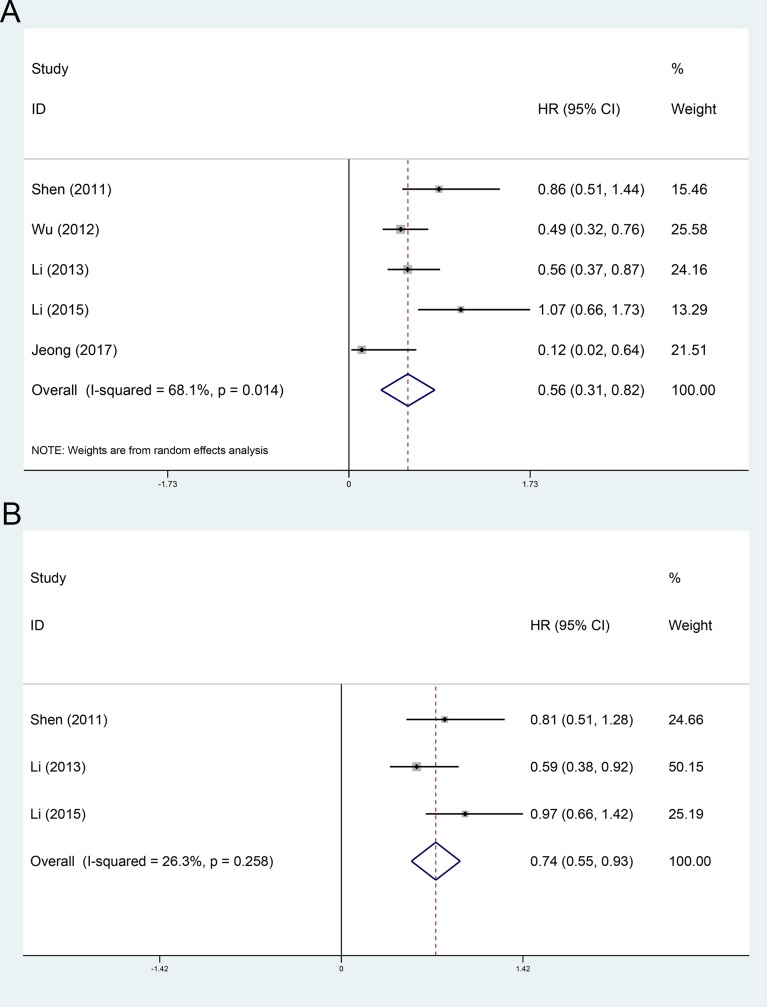
The OS and RFS comparing between adjuvant TACE group and non-AT group were evaluated in 5[13, 14, 27, 28, 32] and 3[14, 27, 32] included cohorts, respectively. Using a random-effect model, the pooled HR for the OS and RFS in the adjuvant TACE group were 0.56 (95%CI 0.31~0.82, Fig 4A), and 0.74 (95%CI 0.55~0.93, Fig 4B), respectively, compared with the non-AT group.
Fig 4. Forest plot of the overall survival and recurrence-free survival rates between adjuvant TACE and operation only.
A, overall survival; B, recurrence-free survival.
The OS comparing between adjuvant radiotherapy group or adjuvant chemoradiotherapy group and non-AT group were evaluated in 4[11, 12, 26, 34] and 3[24, 33, 39] included cohorts, respectively. Using a random-effect model, the pooled HR for the OS in the adjuvant radiotherapy group and adjuvant chemoradiotherapy group were 0.71 (95%CI 0.39~1.03, Fig 5A), and 0.73 (95%CI 0.57~0.89, Fig 5B), respectively, compared with the non-AT group.
Fig 5. Subgroup analysis of OS stratified by adjuvant radiotherapy and chemoradiotherapy.
A, adjuvant radiotherapy; B, adjuvant chemoradiotherapy.
Subgroup analysis stratified by high risk factors
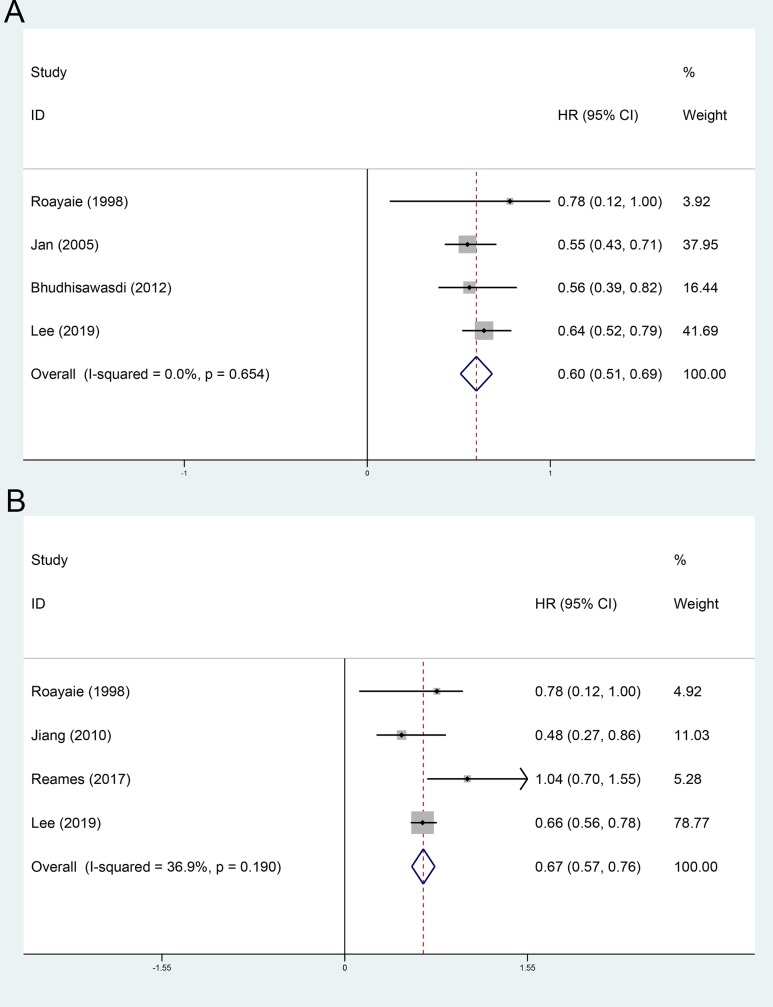
The effect of AT on the patients with positive resection margin was evaluated in 4 included cohorts [16, 24, 25, 29]. Using a random-effect model, the pooled HR for the OS in the AT group was 0.60 (95%CI 0.51~0.69, Fig 6A), compared with the non-AT group. The effect of AT on the patients with LNM was evaluated in 4 included cohorts [9, 16, 24, 26]. Using a random-effect model, the pooled HR for the OS in the AT group was 0.67 (95%CI 0.57~0.76, Fig 6B), compared with the non-AT group.
Fig 6. Subgroup analysis stratified by high risk factors.
A, positive resection margin; B. lymph node metastasis.
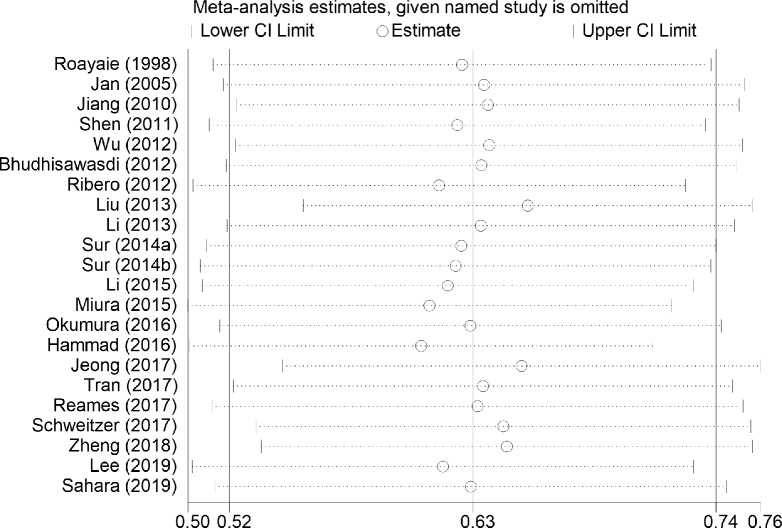
Sensitivity analysis
Sensitivity analysis was conducted in the primary endpoint comparing between AT group and non-AT group, and result showed that the pooled HR for the OS in the AT group did not change substantially after any study was removed compared with the non-AT group (Fig 7), which indicated that the present results in this study were robust.
Fig 7. Sensitivity analysis for overall survival in the included studies.
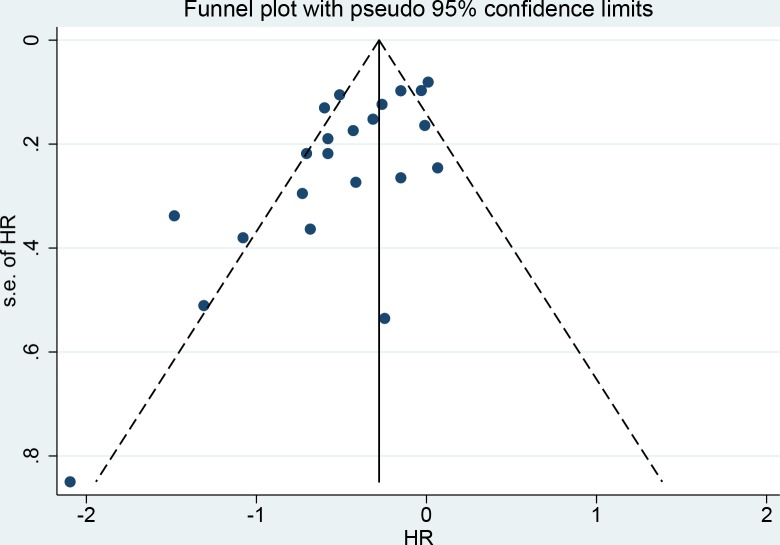
Publication bias analysis
Publication bias analysis was conducted in the primary endpoint comparing between AT group and non-AT group. Asymmetry was observed in the funnel plot (Fig 8) with significant publication bias in the egger’s test (p = 0.004) but not in the Begg’s test (p = 0.09). “Trim and fill” analysis was then conducted, and 5 more studies were found to be potentially unpublished. The adjusted HR for the OS in the AT group was 0.73 (95%CI 0.63–0.85), compared with the non-AT group, indicating that the present result could not be affected by the unpublished studies.
Fig 8. Funnel plot of overall survival in the included studies.
Discussion
The prognosis of patients with ICC after resection is still poor[5, 6], but the benefit of AT has always been questioned in clinical partly because the natural life span is too short and most of the patients have lost the chance of resection at diagnosis[3, 4]. Currently, with the advocation of LND and development of extended resection[40–42], the clinical value of AT should be re-evaluated. This is the first systematic review evaluating the clinical value of AT in the treatment of ICC comprehensively, which included 22 studies with 10181 patients, and results showed that patients could be benefited from AT in a whole. However, in our opinion, identifying the selected patients and choosing the appropriate AT strategy are the keys.
Chemotherapy is first to be administrated in the postoperative adjuvant treatments of ICC, and adjuvant chemotherapy is still the most preferred strategy in clinical up to now with the reported incidence as high as 46.6%[15]. However, the benefit of chemotherapy has been always been questioned mainly because cholangiocarcinoma is not sensitive to chemotherapeutics[43]. In this meta-analysis, adjuvant chemotherapy was confirmed to be associated with improved OS, which was coincident with the previous meta-analysis[10, 44]. In addition, Gemcitabine-based chemotherapy was confirmed to be superior to 5-Fu based chemotherapy in the improvement of prognosis[10, 44].
TACE is conducted widely in the management of ICC, such as adjuvant therapy for patients receiving resection[13, 14], and palliative treatment for unresectable ICC[45, 46]. However, someone argued the benefit of adjuvant TACE for ICC[27], mainly because ICC could metastasize specifically through lymph node. To the best of our knowledge, this is the first meta-analysis confirming the benefit of adjuvant TACE. Reasons are mainly due to that most of the recurrences are still intrahepatic ones[47], but it deserves further validation.
Radiotherapy is playing an increasing important role in the management of ICC with the development of stereotactic body radiotherapy[11]. From the other hand, metastatic lymph node is much more sensitive to radiotherapy[12]. However, the benefit of radiotherapy was not confirmed in this meta-analysis, which deserved our deep rethink. In addition, chemoradiotherapy is being more and more preferred in clinical, because synergistic effect is believed to between radiotherapy and chemotherapy[15]. This is the first meta-analysis identifying the benefit of adjuvant chemoradiotherapy, but either sequential or concurrent chemoradiotherapy deserves further study.
As is known to all, one size does not fit all, so identifying the selected patients who could be benefited from AT is also a big deal. Adjuvant chemotherapy and TACE are found to only benefit patients with “high risk”, such as positive margins[24, 29], LNM[48], and advanced stages[16], but as for radiotherapy it is hard to say. Zheng et al[11] found that adjuvant radiotherapy could benefit patients with narrow surgical margin, but Hammad et al[12] reported that adjuvant radiotherapy could only improve the prognosis of patients with R0 resection rather than those with R1 resection and LNM. Hence, who would be benefited from AT, either with high risk or with low risk, is still a puzzle.
In the recent decades, pathway-targeted therapies made a rapid progress in solid tumors[49, 50]. Previous studies found that approximately 30%~40% of ICC patients exhibited actionable mutations, such as epidermal growth factor receptor (EGFR), and fibroblast growth factor receptor (FGFR) which shed light on the molecular targeted therapies on ICC[51, 52]. From the other hand, next-generation and exome sequencing studies found that 10%~15% of cholangiocarcinoma patients had DNA repair mutations[53], and 40% of cholangiocarcinoma patients had positive programmed cell death receptor 1 (PDL1) expression[54], who might be the potential beneficiaries of immunotherapies. Recent clinical trials have exhibited promising results in the advanced cholangiocarcinoma, which would change the trajectory of ICC management[55, 56]. In future, promises of adjuvant targeted therapies and/or immunotherapies have been expected in the on-going trials.
There were several restrictions of this meta-analysis. First, all the included studies were retrospective ones, indicating an obvious selection and recalling bias. Second, most of the studies were multi-centers or based on the database mainly due to the rare incidence of ICC, which meant that procedure of surgical resection and AT were different and bias was hard to avoid. Third, most of the cofounding factors such as radical resection and LNM were hard to be resorted in the original studies, which would weaken the conclusion. Fourth, RFS was evaluated in only six of the 22 included studies, which was insufficient to evaluate the effect of AT on recurrence. Fifth, considering that the span of the included studies was a little longer (1990~2019), during which the surgical techniques, chemotherapy agents and radiation approaches were different, our conclusion in this study deserved further validation. The last but not the least, publication bias was found in this meta-analysis, although the present result was found to be not changed after “trim and fill” analysis.
Conclusion
With the current data, we concluded that AT would benefit patients with ICC after resection, but it deserved further validation. Considering that not all AT strategies would bring benefit to patients with ICC, and not all patients would be benefited from AT, identifying the potential beneficiaries of different AT is a priority in future.
Supporting information
(DOC)
(DOCX)
Abbreviations
- ICC
intrahepatic cholangiocarcinoma
- AT
adjuvant therapy
- TACE
transarterial chemoembolization
- LNM
lymph node metastasis
- OS
overall survival
- RFS
recurrence-free survival
- HR
Hazard ratio
- OR
odd ratio
- CI
confidence interval
Data Availability
All data generated or analyzed during this study are included in the published articles
Funding Statement
This work was supported by Startup Fund for scientific research, Fujian Medical University (Grant number: 2018QH1195), but no authors in this study received a salary from this fund.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71(1):104–14. 10.1016/j.jhep.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 3.Benson AB, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA, et al. Guidelines Insights: Hepatobiliary Cancers, Version 2.2019. J Natl Compr Canc Netw. 2019;17(4):302–10. 10.6004/jnccn.2019.0019 [DOI] [PubMed] [Google Scholar]
- 4.Merath K, Chen Q, Bagante F, Alexandrescu S, Marques HP, Aldrighetti L, et al. A Multi-Institutional International Analysis of Textbook Outcomes Among Patients Undergoing Curative-Intent Resection of Intrahepatic Cholangiocarcinoma. Jama Surg. 2019:e190571 10.1001/jamasurg.2019.0571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen F, Xie ZH, Xia Y, Wu MC. Progress on surgical treatment of intrahepatic cholangiocarcinoma. Zhonghua Wai Ke Za Zhi. 2019;57(4):241–6. 10.3760/cma.j.issn.0529-5815.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 6.Si A, Li J, Xiang H, Zhang S, Bai S, Yang P, et al. Actual over 10-year survival after liver resection for patients with intrahepatic cholangiocarcinoma. Oncotarget. 2017;8(27):44521–32. 10.18632/oncotarget.17815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Shi SM, Yang H, Yang LX, Wang Z, Li XD, et al. Systemic inflammation score predicts survival in patients with intrahepatic cholangiocarcinoma undergoing curative resection. J Cancer. 2019;10(2):494–503. 10.7150/jca.26890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma KW, Cheung TT, Leung B, She B, Chok K, Chan A, et al. Adjuvant chemotherapy improves oncological outcomes of resectable intrahepatic cholangiocarcinoma: A meta-analysis. Medicine (Baltimore). 2019;98(5):e14013 10.1097/MD.0000000000014013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reames BN, Bagante F, Ejaz A, Spolverato G, Ruzzenente A, Weiss M, et al. Impact of adjuvant chemotherapy on survival in patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis. HPB (Oxford). 2017;19(10):901–9. 10.1016/j.hpb.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 10.Wang ML, Ke ZY, Yin S, Liu CH, Huang Q. The effect of adjuvant chemotherapy in resectable cholangiocarcinoma: A meta-analysis and systematic review. Hepatobiliary Pancreat Dis Int. 2019;18(2):110–6. 10.1016/j.hbpd.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 11.Zheng X, Chen B, Wu JX, Jia AY, Rong WQ, Wang LM, et al. Benefit of adjuvant radiotherapy following narrow-margin hepatectomy in patients with intrahepatic cholangiocarcinoma that adhere to major vessels. Cancer Manag Res. 2018;10:3973–81. 10.2147/CMAR.S172940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammad AY, Berger NG, Eastwood D, Tsai S, Turaga KK, Christian KK, et al. Is Radiotherapy Warranted Following Intrahepatic Cholangiocarcinoma Resection? The Impact of Surgical Margins and Lymph Node Status on Survival. Ann Surg Oncol. 2016;23(Suppl 5):912–20. 10.1245/s10434-016-5560-1 [DOI] [PubMed] [Google Scholar]
- 13.Jeong S, Zheng B, Wang J, Chi J, Tong Y, Xia L, et al. Transarterial Chemoembolization: A Favorable Postoperative Management to Improve Prognosis of Hepatitis B Virus-associated Intrahepatic Cholangiocarcinoma after Surgical Resection. Int J Biol Sci. 2017;13(10):1234–41. 10.7150/ijbs.21149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Wang Q, Lei Z, Wu D, Si A, Wang K, et al. Adjuvant Transarterial Chemoembolization Following Liver Resection for Intrahepatic Cholangiocarcinoma Based on Survival Risk Stratification. Oncologist. 2015;20(6):640–7. 10.1634/theoncologist.2014-0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YK, Hsieh MC, Wang WW, Lin YC, Chang WW, Chang CL, et al. Outcomes of adjuvant treatments for resectable intrahepatic cholangiocarcinoma: Chemotherapy alone, sequential chemoradiotherapy, or concurrent chemoradiotherapy. Radiother Oncol. 2018;128(3):575–83. 10.1016/j.radonc.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 16.Lee GC, Ferrone CR, Tanabe KK, Lillemoe KD, Blaszkowsky LS, Zhu AX, et al. Predictors of adjuvant treatment and survival in patients with intrahepatic cholangiocarcinoma who undergo resection. Am J Surg. 2019;218(5):959–66. 10.1016/j.amjsurg.2019.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, et al. Surgery for cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:143–55. 10.1111/liv.14089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17(8):669–80. 10.1111/hpb.12441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–34. [DOI] [PubMed] [Google Scholar]
- 21.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 22.Deeks JJ, Altman DG. Chapter 9. Analysing Data and Undertaking Meta-Analyses. Cochrane Handbook for Systematic Reviews of Interventions Cochrane Book; 2011. 10.1002/9780470712184.ch9 [DOI] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roayaie S, Guarrera JV, Ye MQ, Thung SN, Emre S, Fishbein TM, et al. Aggressive surgical treatment of intrahepatic cholangiocarcinoma: predictors of outcomes. J Am Coll Surg. 1998;187(4):365–72. 10.1016/s1072-7515(98)00203-8 [DOI] [PubMed] [Google Scholar]
- 25.Jan YY, Yeh CN, Yeh TS, Chen TC. Prognostic analysis of surgical treatment of peripheral cholangiocarcinoma: two decades of experience at Chang Gung Memorial Hospital. World J Gastroenterol. 2005;11(12):1779–84. 10.3748/wjg.v11.i12.1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang W, Zeng ZC, Tang ZY, Fan J, Zhou J, Zeng MS, et al. Benefit of radiotherapy for 90 patients with resected intrahepatic cholangiocarcinoma and concurrent lymph node metastases. J Cancer Res Clin Oncol. 2010;136(9):1323–31. 10.1007/s00432-010-0783-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen WF, Zhong W, Liu Q, Sui CJ, Huang YQ, Yang JM. Adjuvant transcatheter arterial chemoembolization for intrahepatic cholangiocarcinoma after curative surgery: retrospective control study. World J Surg. 2011;35(9):2083–91. 10.1007/s00268-011-1171-y [DOI] [PubMed] [Google Scholar]
- 28.Wu ZF, Zhang HB, Yang N, Zhao WC, Fu Y, Yang GS. Postoperative adjuvant transcatheter arterial chemoembolisation improves survival of intrahepatic cholangiocarcinoma patients with poor prognostic factors: results of a large monocentric series. Eur J Surg Oncol. 2012;38(7):602–10. 10.1016/j.ejso.2012.02.185 [DOI] [PubMed] [Google Scholar]
- 29.Bhudhisawasdi V, Talabnin C, Pugkhem A, Khuntikeo N, Seow OT, Chur-in S, et al. Evaluation of postoperative adjuvant chemotherapy for intrahepatic cholangiocarcinoma patients undergoing R1 and R2 resections. Asian Pac J Cancer Prev. 2012;13 Suppl:169–74. [PubMed] [Google Scholar]
- 30.Ribero D, Pinna AD, Guglielmi A, Ponti A, Nuzzo G, Giulini SM, et al. Surgical Approach for Long-term Survival of Patients With Intrahepatic Cholangiocarcinoma: A Multi-institutional Analysis of 434 Patients. Arch Surg. 2012;147(12):1107–13. 10.1001/archsurg.2012.1962 [DOI] [PubMed] [Google Scholar]
- 31.Liu RQ, Shen SJ, Hu XF, Liu J, Chen LJ, Li XY. Prognosis of the intrahepatic cholangiocarcinoma after resection: hepatitis B virus infection and adjuvant chemotherapy are favorable prognosis factors. Cancer Cell Int. 2013;13(1):99 10.1186/1475-2867-13-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li T, Qin LX, Zhou J, Sun HC, Qiu SJ, Ye QH, et al. Staging, prognostic factors and adjuvant therapy of intrahepatic cholangiocarcinoma after curative resection. Liver Int. 2014;34(6):953–60. 10.1111/liv.12364 [DOI] [PubMed] [Google Scholar]
- 33.Sur MD, In H, Sharpe SM, Baker MS, Weichselbaum RR, Talamonti MS, et al. Defining the Benefit of Adjuvant Therapy Following Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2015;22(7):2209–17. 10.1245/s10434-014-4275-4 [DOI] [PubMed] [Google Scholar]
- 34.Miura JT, Johnston FM, Tsai S, George B, Thomas J, Eastwood D, et al. Chemotherapy for Surgically Resected Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2015;22(11):3716–23. 10.1245/s10434-015-4501-8 [DOI] [PubMed] [Google Scholar]
- 35.Luvira V, Eurboonyanun C, Bhudhisawasdi V, Pugkhem A, Pairojkul C, Luvira V, et al. Patterns of Recurrence after Resection of Mass-Forming Type Intrahepatic Cholangiocarcinomas. Asian Pac J Cancer Prev. 2016;17(10):4735–9. 10.22034/APJCP.2016.17.10.4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okumura S, Kaido T, Hamaguchi Y, Kobayashi A, Shirai H, Fujimoto Y, et al. Impact of Skeletal Muscle Mass, Muscle Quality, and Visceral Adiposity on Outcomes Following Resection of Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2017;24(4):1037–45. 10.1245/s10434-016-5668-3 [DOI] [PubMed] [Google Scholar]
- 37.Schweitzer N, Weber T, Kirstein MM, Fischer M, Kratzel AM, Reineke-Plaass T, et al. The effect of adjuvant chemotherapy in patients with intrahepatic cholangiocarcinoma: a matched pair analysis. J Cancer Res Clin Oncol. 2017;143(7):1347–55. 10.1007/s00432-017-2392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahara K, Tsilimigras DI, Mehta R, Bagante F, Guglielmi A, Aldrighetti L, et al. A novel online prognostic tool to predict long-term survival after liver resection for intrahepatic cholangiocarcinoma: The "metro-ticket" paradigm. J Surg Oncol. 2019;120(2):223–30. 10.1007/s00432-017-2392-8 [DOI] [PubMed] [Google Scholar]
- 39.Tran CH, Zhang Q, Sada YH, Chai C, Curley SA, Massarweh NN. The role of surgery and adjuvant therapy in lymph node-positive cancers of the gallbladder and intrahepatic bile ducts. Cancer. 2018;124(1):74–83. 10.1002/cncr.30968 [DOI] [PubMed] [Google Scholar]
- 40.Bartsch F, Tripke V, Baumgart J, Hoppe-Lotichius M, Heinrich S, Lang H. Extended resection of intrahepatic cholangiocarcinoma: A retrospective single-center cohort study. Int J Surg. 2019;67:62–9. 10.1016/j.ijsu.2019.05.006 [DOI] [PubMed] [Google Scholar]
- 41.Zhang XF, Chakedis J, Bagante F, Chen Q, Beal EW, Lv Y, et al. Trends in use of lymphadenectomy in surgery with curative intent for intrahepatic cholangiocarcinoma. Br J Surg. 2018;105(7):857–66. 10.1002/bjs.10827 [DOI] [PubMed] [Google Scholar]
- 42.Vitale A, Moustafa M, Spolverato G, Gani F, Cillo U, Pawlik TM. Defining the possible therapeutic benefit of lymphadenectomy among patients undergoing hepatic resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2016;113(6):685–91. 10.1002/jso.24213 [DOI] [PubMed] [Google Scholar]
- 43.Shroff RT, Kennedy EB, Bachini M, Bekaii-Saab T, Crane C, Edeline J, et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J Clin Oncol. 2019;37(12):1015–27. 10.1200/JCO.18.02178 [DOI] [PubMed] [Google Scholar]
- 44.Ma KW, Cheung TT, Leung B, She B, Chok K, Chan A, et al. Adjuvant chemotherapy improves oncological outcomes of resectable intrahepatic cholangiocarcinoma: A meta-analysis. Medicine (Baltimore). 2019;98(5):e14013 10.1097/MD.0000000000014013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hyder O, Marsh JW, Salem R, Petre EN, Kalva S, Liapi E, et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol. 2013;20(12):3779–86. 10.1245/s10434-013-3127-y [DOI] [PubMed] [Google Scholar]
- 46.Park SY, Kim JH, Yoon HJ, Lee IS, Yoon HK, Kim KP. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol. 2011;66(4):322–8. 10.1016/j.crad.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 47.Hu LS, Zhang XF, Weiss M, Popescu I, Marques HP, Aldrighetti L, et al. Recurrence Patterns and Timing Courses Following Curative-Intent Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2019;26(8):2549–57. 10.1245/s10434-019-07353-4 [DOI] [PubMed] [Google Scholar]
- 48.Jutric Z, Johnston WC, Hoen HM, Newell PH, Cassera MA, Hammill CW, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB (Oxford). 2016;18(1):79–87. 10.1016/j.hpb.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shroff RT, Yarchoan M, O'Connor A, Gallagher D, Zahurak ML, Rosner G, et al. The oral VEGF receptor tyrosine kinase inhibitor pazopanib in combination with the MEK inhibitor trametinib in advanced cholangiocarcinoma. Br J Cancer. 2017;116(11):1402–7. 10.1038/bjc.2017.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li JH, Ma WJ, Wang GG, Jiang X, Chen X, Wu L, et al. Clinicopathologic Significance and Prognostic Value of Programmed Cell Death Ligand 1 (PD-L1) in Patients With Hepatocellular Carcinoma: A Meta-Analysis. Front Immunol. 2018;9:2077 10.3389/fimmu.2018.02077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jain A, Javle M. Molecular profiling of biliary tract cancer: a target rich disease. J Gastrointest Oncol. 2016;7(5):797–803. 10.21037/jgo.2016.09.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jain A, Kwong LN, Javle M. Genomic Profiling of Biliary Tract Cancers and Implications for Clinical Practice. Curr Treat Options Oncol. 2016;17(11):58 10.1007/s11864-016-0432-2 [DOI] [PubMed] [Google Scholar]
- 53.Ahn DH, Javle M, Ahn CW, Jain A, Mikhail S, Noonan AM, et al. Next-generation sequencing survey of biliary tract cancer reveals the association between tumor somatic variants and chemotherapy resistance. Cancer. 2016;122(23):3657–66. 10.1002/cncr.30247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chun YS, Javle M. Systemic and Adjuvant Therapies for Intrahepatic Cholangiocarcinoma. Cancer Control. 2017;24(3):1145164505 10.1177/1073274817729241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mou H, Yu L, Liao Q, Hou X, Wu Y, Cui Q, et al. Successful response to the combination of immunotherapy and chemotherapy in cholangiocarcinoma with high tumour mutational burden and PD-L1 expression: a case report. BMC Cancer. 2018;18(1):1105 10.1186/s12885-018-5021-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gbolahan O, Hashemi-Sadraei N, O'Neil B. Prolonged Response to Anti-PD-1 Antibody Therapy in Chemotherapy-Refractory Cholangiocarcinoma With High Tumor Mutational Burden. J Natl Compr Canc Netw. 2019;17(6):644–8. 10.6004/jnccn.2019.7304 [DOI] [PubMed] [Google Scholar]