Abstract
Background
Although genetic features vary across ethnicities, few genome-wide association studies (GWAS) have reported the genetic determinants of liver enzyme expression. This study was aimed to evaluate the associations of genome-wide single nuclear polymorphisms (SNPs) with the liver enzymes in a Korean population.
Methods
We performed a GWAS to identify genetic loci influencing liver function, as measured by concentrations of alkaline phosphatase (ALP), alanine transaminase (ALT), gamma-glutamyl transferase (GGT) and total bilirubin (BIL) in in Korean study participants.
Results
A total of 6,488 subjects (4,457 in the discovery and 2,031 in the validation set) were included. The mean subject age was 50.0±10.6 years (male, 53.7%). Among a total of 546,738 SNPs tested, rs651007 and rs579459 located in the ABO gene showed strong associations with ALP (P = 1.63×10−8 and 5.61×10−8, respectively [discovery set]; P = 4.08×10−15 and 9.92×10−16, respectively [validation set]). Additionally, rs5751901 and rs2006092, which are located in the GGT1 gene, showed strong associations with GGT (P = 6.44×10−15 and 1.26×10−15, respectively [discovery set]; P = 4.13×10−10 and 5.15×10−11, respectively [validation set]). Among the 13 SNPs that showed genome-wide significance with total bilirubin levels, rs10929302 and rs6742078 showed the most significant association (P = 3.08×10−64 and 2.05×10−62, respectively [discovery set]; P = 1.33×10−116 and 2.24×10−118, respectively [validation set]). No genome-wide significant associations was found for ALT.
Conclusions
We demonstrated that ABO, GGT1 and UGT1A family were associated with ALP, GGT and BIL, respectively in Korean population. These findings differ from reported results in GWAS in European populations in terms of associated genes and locations, suggesting different genetic mechanisms of liver enzyme regulation according to ethnicity.
Introduction
Liver function tests (LFTs) are commonly used in clinic approaching hepatobiliary disease. These tests offers clinical information on the presence, severity and mortality of liver disease, and differential diagnosis of other diseases. They are also necessary for monitoring and determining of response to treatment [1, 2]. However, careful interpretation is required because LFT could appear normal in advanced liver disease and abnormal LFTs could reflect other disease such as cardiovascular disease, diabetes, or metabolic syndrome [3, 4].
Plasma levels of liver enzymes vary from person to person, which is affected by both environmental and genetic factors. The hereditability of LFT levels are estimated from 22–60% [5, 6], suggesting a genetic role in interpretation of the results. Single-nucleotide polymorphism (SNP) is useful to analyze genetic elements of clinical features. Several genome-wide association studies (GWAS) were reported to find out genetic mechanisms of LFT variations. Most frequently reported gene associated with gamma-glutamyl transferase (GGT) is GGT1, which is involved in glutathione metabolism. HNF1A gene, related to lipid metabolism and inflammation, is also reported [7–9]. PNPLA3 gene, involved in energy mobilization and storage of adipocyte, has been reported several times to be related to both aspartate aminotransferase (AST) and alanine-aminotransferase (ALT) [7–11]. CHUK gene, related to glucose and lipid metabolism, is also known to be significant gene influencing ALT level [7, 8]. In addition, ALPL, GPLD1, and JMJD1C-REEP3 gene and ABO locus are appeared to have significant relationship with alkaline phosphatase (ALP) level [7]. For bilirubin, associations with various SNPs in UGT1A1 gene is reported. UGT1A1 is involved in conjugation of bilirubin and polymorphism of UGT1A1 is associated with Gilbert syndrome [12–15]. Other various genes associated with liver function tests has been reported, however the result varies according to the study population.
Although genetic features are known to differ between ethnicities, few studies on Asians have been published. Korea is endemic area of hepatitis B virus and incidence of nonalcoholic fatty liver disease (NAFLD) is increasing. Therefore, understanding genetic features of LFT in Korean population is important. This study was aimed to evaluate the associations of genome-wide SNPs with the liver enzymes in a Korean population.
Patients and methods
Study population
For our study, we analyzed the database from a previously described GENIE cohort [16]. Briefly, 8,000 people donated blood samples during a routine health check-up program at Seoul National University Hospital Healthcare System Gangnam Center between January 2014 and December 2014, and their blood samples were stored at a biorepository with their informed consent.
Subjects with significant alcohol intake (>20 g/day for males and >10 g/day for females) were excluded (n = 842). We also excluded 248 individuals who were positive for hepatitis B virus and 48 subjects who were positive for hepatitis C virus. Additionally, subjects with missing information were excluded from the study. This study was approved by the Institutional Review Board of the Seoul National University Hospital with a waiver of informed consent (No 1908-126-105).
Clinical and laboratory assessments
The methods employed in this study have been described in detail elsewhere [17]. Briefly, each subject completed a past medical history questionnaire and underwent anthropometric assessment. The body weight and height of each subject were measured using a digital scale. Body mass index (BMI) was calculated as the ratio of weight (kg) to area (m2, estimated from height). Waist circumference was measured at the midpoint between the lower costal margin and the anterior superior iliac crest by a well-trained person. Systolic blood pressure and diastolic blood pressure were each measured twice, and their mean values were reported. After an overnight 12h period, blood samples were collected from the antecubital vein of each individual. The laboratory evaluations included serum ALT, AST, ALP, GGT, total bilirubin (BIL) total cholesterol, triglyceride, fasting glucose, hepatitis B surface antigen and antibody to hepatitis C virus. Hypertension was defined as having systolic blood pressure ≥140 mmHg, having diastolic blood pressure ≥90 mmHg or using of anti-hypertensive medication. Diabetes mellitus was defined as either a fasting serum glucose level of ≥126 mg/dL or the use of anti-diabetic medication.
Genome-wide genotyping and quality control
Genomic DNA was isolated from venous blood samples and 200 ng of DNA from each patient was genotyped using Affymetrix Axiom® Customized Biobank Genotyping Arrays (Affymetrix, Santa Clara, CA, USA). The PLINK program (version 1.90, Free Software Foundation Inc., Boston, MA, USA) was used for quality control procedures. Samples meeting any of the following criteria were removed: (i) gender inconsistency, (ii) call rate ≤ 97% and (iii) related and cryptically related individuals (identical by descent >0.185). SNPs were filtered if (1) the call rate was <95%, (2) the minor allele frequency (MAF) was ≤ 0.01, or (3) there was a significant deviation from the Hardy-Weinberg equilibrium permutation test (P<1x10-4).
Statistical analysis
Linear-regression analyses assuming and additive genetic model was used on serum ALP, ALT, GGT and total bilirubin using PLINK software package to test the association of liver enzymes with SNPs in the genome. Age, and gender were used as covariates. The R statistical software package (version 3.1.1, R development Core Team; R Foundation for Statistical Computing, Vienna, Austria) was used for the statistical analysis and to draw the Manhattan plot of–log10. The quantile-quantile plots were generated using R statistics package to assess the overall significance of the genome-wide associations. The LocusZoom (http://csg.sph.umich.edu/locuszoom) was used to graphically display the genome-wide association scan results. In the regional plot, hg19/1000 Genomes Nov 2014 ASN was used as the reference panel for linkage disequilibrium [18].
The results were verified using discovery and validation sets. We divided the enrolled population into 2 groups based on the time of enrollment. Samples from subjects who enrolled from January, 2014 to October, 2014 were used as the discovery set, and samples from subjects who enrolled in the subsequent months were used as the validation set. SNPs that had a P-value of less than 1x10-7 (by the Bonferroni correction method) in the discovery set were re-evaluated in the validation set. Comparisons of continuous variables between the two groups were performed using Student’s t-test, and categorical variables were compared using a chi-square test or Fisher’s exact test. P-values of less than 0.05 in the validation set were considered significant.
Results
Study population
A total of 6,488 subjects were included. The mean subject age was 50.0±10.6 years, and 53.7% of the subjects were male. Based on the definition described in the methods, there were 4,457 samples in the discovery set and 2,031 samples in the validation set. The characteristics of the study population are described in Table 1. The subjects in the validation set showed greater proportions of male, higher diastolic pressure, BMI ALP, ALT, GGT and fasting glucose (P <0.05).
Table 1. Baseline characteristics of the study population.
| Discovery set (N = 4,457) |
Validation set (N = 2,031) |
P-Value | |
|---|---|---|---|
| Age (years) | 50.1±10.6 | 50.0±10.5 | 0.102 |
| Male, n (%) | 2344(52.6%) | 1140(56.1%) | 0.008 |
| Diabetes mellitus, n (%) | 254 (5.7) | 135 (6.6) | 0.136 |
| Hypertension, n (%) | 821(18.4) | 398(19.6) | 0.261 |
| Systolic blood pressure (mmHg) | 114.6±13.3 | 115.3±13.1 | 0.052 |
| Diastolic blood pressure (mmHg) | 74.6±11.3 | 75.9±10.3 | <0.001 |
| Body mass index (kg/m2) | 22.9±3.0 | 23.1±3.0 | 0.002 |
| Waist circumference (cm) | 81.1±11.9 | 81.4±11.0 | 0.435 |
| alkaline phosphatase (mg/dL) | 52.7±16.0 | 55.3±15.9 | <0.001 |
| ALT (IU/L) | 22.1±16.0 | 23.1±16.9 | 0.015 |
| Total bilirubin (mg/dL) | 0.92 ± 0.90 | 0.94±0.82 | 0.398 |
| GGT (mg/dL) | 29.7± 28.1 | 32.0±32.0 | 0.005 |
| Total cholesterol (mg/dL) | 192.6±33.7 | 194.1±34.3 | 0.096 |
| Triglyceride (mg/dL) | 103.3±70.6 | 105.4±71.7 | 0.265 |
| Fasting glucose (mg/dL) | 97.1±15.3 | 98.7±18.4 | <0.001 |
Data are shown as the mean ± SD.
ALT; alanine-aminotransferase, AST; aspartate aminotransferase, GGT; gamma-glutamyl transferase
Genome-wide association of live enzymes
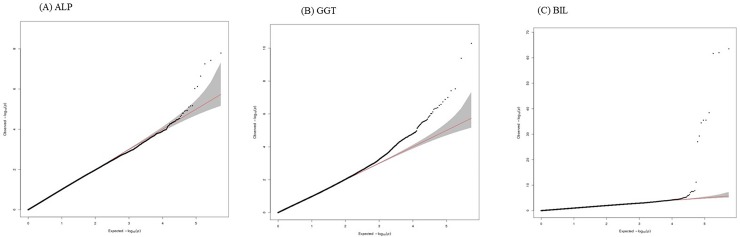
A total of 546,738 autosomal SNPs were tested for the association analysis. Quantile–quantile plots (Fig 1) revealed the presence of a substantial number of SNPs associated with ALP, GGT, ALT and BIL levels at a genome-wide significance level (P < 10−7).
Fig 1. Quantile–quantile plots of association results for GGT (A), ALP (B) and BIL (C).
X-axis and Y-axis indicate the negative log-scale of expected p-values of each SNP and the negative log-scale of the observed p-values, respectively. A straight line indicates the expected results under Hardy-Weinberg equilibrium.
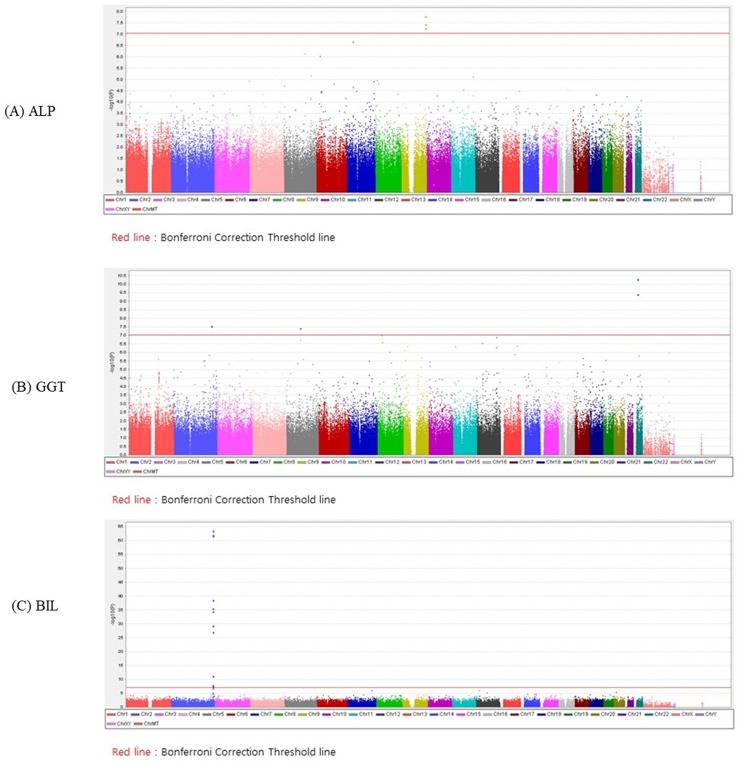
We first evaluated genome-wide associations in the discovery set, with a significance threshold of P < 1x10-7 as the threshold after adjusting for age and gender. Fig 2 shows Manhattan plots showing SNPs that were associated with liver enzymes (A) ALP (B) GGT and (C) BIL. After joint analysis of discovery and replication set, 3 for ALP, 2 for GGT and 13 for BIL reached genome-wide significance (Table 2). In the discovery set of the GWAS, 11 SNPs were significantly associated with ALP and we performed a validation test, 3 remained significant in the validation set. Among them, 2 SNPs, namely, rs651007 and rs579459 located in the ABO gene showed strong associations with ALP (P-values, discovery set = 1.63 x10-8, and 5.61 x10-8, respectively; validation set = 4.08 x10-15, and 9.92 x10-16, respectively). In the regional plot in chromosome 9 is provided in Fig 3(A), which shows rs579459 as the top SNP and nearby SNPs with the level of high linkage disequilibrium (LD) with rs579459.
Fig 2. Manhattan plot of genome-wide association signals with liver in the discovery set.
(A) ALP (B) GGT and (C) BIL. In the Manhattan plot, the x-axis represents the SNP markers on each chromosome. The y-axis shows the -log10 p-value (logistic regression). The red horizontal line represents the genome-wide significance threshold, and the blue horizontal line represents the genome-wide suggestiveness threshold.
Table 2. Genetic loci associated with liver enzymes at p < 1 × 10−7 in the GWAS of Korean.
| Discovery | Validation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Chr | Position# | Nearest Genes | Risk Allele | MAF | B(SE) | P-value | MAF | B(SE) | P-value |
| ALP | ||||||||||
| rs651007 | 9 | 136153875 | ABO | T | 0.244 | -3.045 (0.537) | 1.63×10−8 | 0.254 | -2.825 (0.358) |
4.05×10−15 |
| rs1053878 | 9 | 136131651 | ABO | A | 0.233 | -3.029 (0.549) |
3.78×10−8 | 0.240 | -2.827 (0.367) |
1.57×10−14 |
| rs579459 | 9 | 136154168 | ABO | C | 0.246 | -2.925 (0.537) |
5.61×10−8 | 0.255 | -2.885 (0.358) |
9.92×10−16 |
| GGT | ||||||||||
| rs2006092 | 22 | 24995668 | GGT1,SNRPD3 | G | 0.342 | 6.604 (1.000) |
5.15×10−11 | 0.340 | 4.759 (0.594) |
1.26×10−15 |
| rs5751901 | 22 | 24992266 | GGT1,SNRPD3 | C | 0.342 | 6.604 (0.991) |
4.13×10−10 | 0.338 | 4.773 (0.610) |
6.44×10−15 |
| T.Bilirubin | ||||||||||
| rs10929302 | 2 | 234665782 | UGT1A6,UGT1A10,UGT1A9,UGT1A4,UGT1A5,UGT1A8,UGT1A3,UGT1A7 | A | 0.131 | 0.273 (0.016) |
3.08×10−64 | 0.126 | 0.256 (0.011) |
1.33×10−116 |
| rs4148325 | 2 | 234673309 | UGT1A6,UGT1A1,UGT1A10,UGT1A9,UGT1A8,UGT1A4,UGT1A5,UGT1A3,UGT1A7 | T | 0.131 | 0.272 (0.016) |
1.01×10−62 | 0.125 | 0.256 (0.011) |
3.07×10−115 |
| rs6742078 | 2 | 234672639 | UGT1A6,UGT1A1,UGT1A10,UGT1A9,UGT1A8,UGT1A4,UGT1A5,UGT1A3,UGT1A7 | T | 0.13 | 0.272 (0.016) |
2.05×10−62 | 0.125 | 0.261 (0.011) |
2.24×10−118 |
| rs3755319 | 2 | 234667582 | UGT1A6,UGT1A10,UGT1A9,UGT1A4,UGT1A5,UGT1A8,UGT1A3,UGT1A7 | C | 0.289 | 0.156 (0.012) |
3.04×10−36 | 0.282 | 0.154 (0.008) |
2.26×10−75 |
| rs1105880 | 2 | 234601965 | UGT1A6,UGT1A10,UGT1A9,UGT1A8,UGT1A7 | G | 0.264 | 0.157 (0.012) |
3.21×10−36 | 0.247 | 0.177 (0.008) |
1.18×10−91 |
| rs6759892 | 2 | 234601669 | UGT1A6,UGT1A10,UGT1A9,UGT1A8,UGT1A7 | G | 0.264 | 0.155 (0.012) |
3.60×10−35 | 0.247 | 0.176 (0.009) |
1.30×10−90 |
| rs7586110 | 2 | 234590527 | UGT1A10,UGT1A9,UGT1A8 | G | 0.245 | 0.148 (0.013) |
5.13×10−30 | 0.227 | 0.159 (0.009) |
3.84×10−69 |
| rs4148323 | 2 | 234669144 | UGT1A6,UGT1A1,UGT1A10,UGT1A9,UGT1A8,UGT1A4,UGT1A5,UGT1A3,UGT1A7 | A | 0.204 | 0.15 (0.014) |
9.09×10−28 | 0.186 | 0.157 (0.010) |
9.50×10−59 |
| rs36075906 | 2 | 234436069 | USP40 | T | 0.271 | 0.085 (0.012) |
7.05×10−12 | 0.253 | 0.064 (0.009) |
2.53×10−13 |
| rs13009407 | 2 | 234652347 | UGT1A6,UGT1A10,UGT1A9,UGT1A4,UGT1A5,UGT1A8,LOC100286922,UGT1A3,DNAJB3,UGT1A7 | G | 0.030 | 0.188 (0.033) |
1.40×10−8 | 0.032 | 0.286 (0.022) |
1.22×10−39 |
| rs28899170 | 2 | 234604230 | UGT1A6,UGT1A10,UGT1A9,UGT1A8,UGT1A7 | A | 0.035 | 0.172 (0.031) |
2.99×10−8 | 0.034 | 0.208 (0.021) |
3.25×10−23 |
| rs4663580 | 2 | 234293861 | DGKD | T | 0.067 | 0.125 (0.022) |
3.08×10−8 | 0.057 (0.017) |
0.088 | 1.18×10−7 |
| rs6758317 | 2 | 234168951 | ATG16L1 | T | 0.129 | 0.093 (0.017) |
3.24×10−8 | 0.131 | 0.06 (0.011) |
1.01×10−7 |
#Genomic position is based on NCBI build 37
P values are adjusted for age, sex and body mass index. An additive genetic model was used.
Chr, chromosome; MAF, minor allele frequency; ALT, alanine-aminotransferase; GGT, gamma-glutamyl transferase
Fig 3. Regional association plots of (A) ALP (B) GGT and (C) BIL.
The purple diamonds indicate the associated SNP according to joint analyses. Nearby SNPs are color-coded according to the level of linkage disequilibrium with the top SNP. The left y-axis shows the significance of the association on a -log10 p-value (logistic regression), and the right y-axis shows the recombination rate across the region. Estimated recombination rates from the 1000 Genomes Project Asian base data and hg19 database16 are plotted with the blue line to reflect the local linkage disequilibrium structure.
When examining the association with the serum GGT levels, 2 SNPs were significantly associated in the discovery set, 2 remained significant with the validation set. Among them, 2 SNPs, namely, rs5751901 and rs2006092 located in the GGT1 gene showed strong associations with GGT (P-values, discovery set = 6.44 x10-15, and 1.26 x10-15, respectively; validation set = 4.13 x10-10, and 5.15 x10-11, respectively). In the regional plot in chromosome 22 is provided in Fig 3(B), which shows rs2006092 as the top SNP and nearby SNPs with the level of high LD with rs2006092.
Among the 13 SNPs that showed genome-wide significance with total bilirubin levels, rs10929302 showed the most significant association (P-values, discovery set = 3.08 x10-64, and validation set = 1.33 x10-116). The second most significant variants was rs6742078 (P-values, discovery set = 2.05 x10-62, and validation set = 2.24 x10-118), which was in high LD (r2 > 0.9) with UGT1A cluster. Highly significantly associated SNPs were located within discrete regions of the genome [Fig 3(C)]. No SNP with serum levels of ALT was associated with genome-wide significance in this study.
Discussion
In this study, we investigated associations between liver function tests and relevant genetic loci based on GWAS. We found respectable genetic differences in this Korean population compared to previously reported European populations. First, we found that the ABO locus affected serum ALP levels. We also found that the GGT1 and SNRPD3 genes affected serum GGT levels, and UGT1A6 and UGT1A1 influenced the levels of BIL in this Korean population.
In this study, rs651007 and rs579459 in ABO gene showed significant associations with serum ALP levels. Although other various SNPs (rs657152 in European population [7], rs550057 in Japanese population [19]) within ABO locus on chromosome 9q34.13 were reported previously, an association between these two SNPs and ALP levels was novel. Whitfield et al. reported that about 15% of the genetic variance in ALP activity was associated with ABO blood group polymorphism in the twin study [20]. Although the exact mechanistic relationship between the ABO gene and ALP levels is not yet known, this relationship may depend on genetically determined variations in isoenzyme proportions among different blood types. Interestingly, the intestinal ALP in the serum is strongly involved in chylomicron formation and fatty acid metabolism might change among ABO blood group types [21]. The rs651007 and rs579459, found in this study, have been previously known to be associated with cardio-embolic stroke and large-artery atherosclerosis [22, 23]. Rs579459 is also has been found to be associated with low density lipoprotein levels and venous thromboembolism [24, 25]. Association of orthologous ALPL gene with ALP level was also reported in European population, which was not significant in Korean population [7].
GGT is found in the liver and biliary epithelial cells, is sensitive to hepatobiliary injury. The genetic influence for GGT estimates range 32–69% [26]. In this study, rs5751901 and rs2006092 showed significant associations with serum GGT levels. The relationship between rs5751901 and serum GGT level has been already reported in European population. It is associated with 0.21 standard deviation increase of GGT1 level [27]. In Japanese population, GGT level is associated with rs5751902, which is very close to rs5751901 in our data. This shows racial differences of regulating serum GGT levels [9]. These SNPs presumably regulate gene expression of GGT1 [28]. GGT1 on chromosome 22 encodes GGT, which transfers glutamyl groups linked through the gamma-carboxylic acid from peptides. It is involved in regeneration of intracellular glutathione and protection against oxidative stress [29]. Previously, several studies has reported significant associations between HNF1A gene on chromosome 12 and serum GGT levels [7, 28], however it was not significant in this study. Rs2006092, the location is close to rs5751901, revealed to be novel regarding to the significant association with GGT. Nonetheless, the specific role of rs2006092 and its relationship of two SNPs in GGT1 locus need to be elucidated.
The levels of serum bilirubin are elevated in various diseases including jaundice and hemolytic disorders and the bilirubin concentration is under strong genetic regulation [6]. In American and European populations, UGT1A1 locus (rs6742078) and SLCO1B1 locus (rs4149056) were reported as most significant loci of serum bilirubin level [30]. In our study, rs41409056 in SLCO1B1 locus, showed marginal significance with p-value = 0.0007376. In a Korean GWAS study, rs11891311 and rs4148323 variations in UGT1A1 and the SLCO1B3 variant (rs2417940), influenced the bilirubin levels [15]. Consistently, we found a significant association rs4148323 variations in UGT1A1 (p-value, 9.09E-28) and a Japanese study also reported the independence of UGT1A1*6 (rs4148323) [31]. It is most common nonsynonymous change in East Asian population and it reduces glucuronidation activity, leading to hyperbilirubinemia [32]. However, the rs2417940 in SLCO1B3 variant did not reach genome-wide significance in this study. Top two SNPs selected in this study were rs10929302 and rs6742078. In agreement with our results, the rs10929302 which is reported to have an association with UGT1A1*93 in Korean population [33]. Another SNP, rs6742078, located in the intron1 of UGT1A1 [34], was previously reported in a Reykjavik study cohort [30] and Korean population [15], supporting to our results. The UGT1A region includes nine highly similar protein-coding and four non-coding genes [35], and the SNPs that showed significant associations with serum BIL levels in this study are all located in the UGT1A region.
In European population, CPN1 and PNPLA3 were associated with liver enzymes [7, 8]. In Japanese population, rs2896019 in PNPLA3 was reported [9] and the same result was published in Mexican-American ancestry GWAS [36]. However, previous GWAS study based on Korean children showed that three novel loci; ST6GALNAC3, ADAMTS9, and CELF2 genes that were multiply associated with levels of AST and ALT [37]. These results suggest differences in regulating mechanism of liver enzymes between children and adults. However, we did not see any genome‐wide significant associations with ALT. This is most likely due to heterogeneity of the study population and sample sizes.
This is the first study that analyzed GWA of various liver function tests and SNPs in Korean population. Since the sample size is large and it was confirmed in a large number of validation set, the result has sufficient statistical power. However, this study has several limitations. First, study participants were healthy adults who voluntarily underwent health check-up at a single center. There could be regional and economical selection bias compared with entire Korean population. This could be the reason of different results compared with the study of Kang et al [15]. Second, the population of validation set is in same participants of discovery set. In the future, a validation study should be performed using a different population set. Finally, although liver enzymes can be influenced by alcohol intake, we could not quantitatively adjusted the amount of alcohol intake in this study.
In conclusion, ABO, GGT1 and UGT1A gene were associated with serum concentrations of ALP, GGT and BIL, respectively in Korean population. These findings differ with respect to the identified genes and locations from prior published findings in European populations, suggesting that genetic determinants of liver enzyme levels may vary across ethnicities.
Supporting information
(XLSX)
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine-aminotransferase
- AST
aspartate aminotransferase
- BIL
total bilirubin
- BMI
Body mass index
- GGT
gamma-glutamyl transferase
- GWAS
Genome-wide association study
- LFT
liver function test
- SNP
single-nucleotide polymorphisms
Data Availability
All relevant data are within the paper and supporting table.
Funding Statement
Funding organization: DNA Link. Jong-Eun Lee declares to be CEO of a company of DNA link. Eunsoon Shin declare a paid employee of a company of DNA link.The funder provided support in the form of salaries for authors [J.E. Lee and E. Shin], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Lazo M, Selvin E, Clark JM. Brief communication: clinical implications of short-term variability in liver function test results. Ann Intern Med. 2008;148(5):348–52. Epub 2008/03/05 10.7326/0003-4819-148-5-200803040-00005 . [DOI] [PubMed] [Google Scholar]
- 2.Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342(17):1266–71. Epub 2000/04/27 10.1056/NEJM200004273421707 . [DOI] [PubMed] [Google Scholar]
- 3.Monami M, Bardini G, Lamanna C, Pala L, Cresci B, Francesconi P, et al. Liver enzymes and risk of diabetes and cardiovascular disease: results of the Firenze Bagno a Ripoli (FIBAR) study. Metabolism. 2008;57(3):387–92. Epub 2008/02/06 10.1016/j.metabol.2007.10.015 . [DOI] [PubMed] [Google Scholar]
- 4.Killip T, Payne MA. High Serum Transaminase Activity in Heart Disease—Circulatory Failure and Hepatic Necrosis. Circulation. 1960;21(5):646–60. 10.1161/01.Cir.21.5.646 WOS:A1960WH70400002. [DOI] [PubMed] [Google Scholar]
- 5.van Beek JH, de Moor MH, de Geus EJ, Lubke GH, Vink JM, Willemsen G, et al. The genetic architecture of liver enzyme levels: GGT, ALT and AST. Behav Genet. 2013;43(4):329–39. Epub 2013/04/13 10.1007/s10519-013-9593-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bathum L, Petersen HC, Rosholm JU, Hyltoft Petersen P, Vaupel J, Christensen K. Evidence for a substantial genetic influence on biochemical liver function tests: results from a population-based Danish twin study. Clin Chem. 2001;47(1):81–7. Epub 2001/01/10. . [PubMed] [Google Scholar]
- 7.Yuan X, Waterworth D, Perry JR, Lim N, Song K, Chambers JC, et al. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am J Hum Genet. 2008;83(4):520–8. Epub 2008/10/23 10.1016/j.ajhg.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers JC, Zhang W, Sehmi J, Li X, Wass MN, Van der Harst P, et al. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat Genet. 2011;43(11):1131–8. Epub 2011/10/18 10.1038/ng.970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamatani Y, Matsuda K, Okada Y, Kubo M, Hosono N, Daigo Y, et al. Genome-wide association study of hematological and biochemical traits in a Japanese population. Nat Genet. 2010;42(3):210–5. Epub 2010/02/09 10.1038/ng.531 . [DOI] [PubMed] [Google Scholar]
- 10.Kollerits B, Coassin S, Kiechl S, Hunt SC, Paulweber B, Willeit J, et al. A common variant in the adiponutrin gene influences liver enzyme values. J Med Genet. 2010;47(2):116–9. Epub 2009/06/23 10.1136/jmg.2009.066597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53(6):1883–94. Epub 2011/03/08 10.1002/hep.24283 . [DOI] [PubMed] [Google Scholar]
- 12.Coltell O, Asensio EM, Sorli JV, Barragan R, Fernandez-Carrion R, Portoles O, et al. Genome-Wide Association Study (GWAS) on Bilirubin Concentrations in Subjects with Metabolic Syndrome: Sex-Specific GWAS Analysis and Gene-Diet Interactions in a Mediterranean Population. Nutrients. 2019;11(1). Epub 2019/01/10 10.3390/nu11010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G, Ramos E, Adeyemo A, Shriner D, Zhou J, Doumatey AP, et al. UGT1A1 is a major locus influencing bilirubin levels in African Americans. Eur J Hum Genet. 2012;20(4):463–8. Epub 2011/11/17 10.1038/ejhg.2011.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin JP, O'Donnell CJ, Schwaiger JP, Cupples LA, Lingenhel A, Hunt SC, et al. Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation. 2006;114(14):1476–81. Epub 2006/09/27 10.1161/CIRCULATIONAHA.106.633206 . [DOI] [PubMed] [Google Scholar]
- 15.Kang TW, Kim HJ, Ju H, Kim JH, Jeon YJ, Lee HC, et al. Genome-wide association of serum bilirubin levels in Korean population. Hum Mol Genet. 2010;19(18):3672–8. Epub 2010/07/20 10.1093/hmg/ddq281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choe EK, Lee Y, Cho JY, Choi SH, Park B, Lee JE, et al. Search for genetic factor association with cancer-free prostate-specific antigen level elevation on the basis of a genome-wide association study in the Korean population. Eur J Cancer Prev. 2018;27(5):453–60. Epub 2017/05/05 10.1097/CEJ.0000000000000359 . [DOI] [PubMed] [Google Scholar]
- 17.Chung GE, Kim D, Kwak MS, Yang JI, Yim JY, Lim SH, et al. The serum vitamin D level is inversely correlated with nonalcoholic fatty liver disease. Clin Mol Hepatol. 2016;22(1):146–51. Epub 2016/04/06 10.3350/cmh.2016.22.1.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genomes Project C, Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467(7319):1061–73. Epub 2010/10/29 10.1038/nature09534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masuda M, Okuda K, Ikeda DD, Hishigaki H, Fujiwara T. Interaction of genetic markers associated with serum alkaline phosphatase levels in the Japanese population. Hum Genome Var. 2015;2:15019 Epub 2015/01/01 10.1038/hgv.2015.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitfield JB, Martin NG. Determinants of variation in plasma alkaline phosphatase activity: a twin study. Am J Hum Genet. 1983;35(5):978–86. Epub 1983/09/01. [PMC free article] [PubMed] [Google Scholar]
- 21.Nakano T, Shimanuki T, Matsushita M, Koyama I, Inoue I, Katayama S, et al. Involvement of intestinal alkaline phosphatase in serum apolipoprotein B-48 level and its association with ABO and secretor blood group types. Biochem Biophys Res Commun. 2006;341(1):33–8. Epub 2006/01/18 10.1016/j.bbrc.2005.12.145 . [DOI] [PubMed] [Google Scholar]
- 22.Williams FM, Carter AM, Hysi PG, Surdulescu G, Hodgkiss D, Soranzo N, et al. Ischemic stroke is associated with the ABO locus: the EuroCLOT study. Ann Neurol. 2013;73(1):16–31. Epub 2013/02/06 10.1002/ana.23838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling X, Zheng Y, Tao J, Zheng Z, Chen L. Association study of polymorphisms in the ABO gene with ischemic stroke in the Chinese population. BMC Neurol. 2016;16(1):146 Epub 2016/08/21 10.1186/s12883-016-0671-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43(4):333–8. Epub 2011/03/08 10.1038/ng.784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruzelius M, Strawbridge RJ, Tregouet DA, Wiggins KL, Gertow K, Sabater-Lleal M, et al. Influence of coronary artery disease-associated genetic variants on risk of venous thromboembolism. Thromb Res. 2014;134(2):426–32. Epub 2014/04/22 10.1016/j.thromres.2014.03.054 . [DOI] [PubMed] [Google Scholar]
- 26.Rahmioglu N, Andrew T, Cherkas L, Surdulescu G, Swaminathan R, Spector T, et al. Epidemiology and genetic epidemiology of the liver function test proteins. PLoS One. 2009;4(2):e4435 Epub 2009/02/12 10.1371/journal.pone.0004435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melzer D, Perry JR, Hernandez D, Corsi AM, Stevens K, Rafferty I, et al. A genome-wide association study identifies protein quantitative trait loci (pQTLs). PLoS Genet. 2008;4(5):e1000072 Epub 2008/05/10 10.1371/journal.pgen.1000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Middelberg RP, Benyamin B, de Moor MH, Warrington NM, Gordon S, Henders AK, et al. Loci affecting gamma-glutamyl transferase in adults and adolescents show age x SNP interaction and cardiometabolic disease associations. Hum Mol Genet. 2012;21(2):446–55. Epub 2011/10/20 10.1093/hmg/ddr478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Forman HJ, Choi J. Gamma-glutamyl transpeptidase in glutathione biosynthesis. Methods Enzymol. 2005;401:468–83. Epub 2006/01/10 10.1016/S0076-6879(05)01028-1 . [DOI] [PubMed] [Google Scholar]
- 30.Johnson AD, Kavousi M, Smith AV, Chen MH, Dehghan A, Aspelund T, et al. Genome-wide association meta-analysis for total serum bilirubin levels. Hum Mol Genet. 2009;18(14):2700–10. Epub 2009/05/06 10.1093/hmg/ddp202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urawa N, Kobayashi Y, Araki J, Sugimoto R, Iwasa M, Kaito M, et al. Linkage disequilibrium of UGT1A1 *6 and UGT1A1 *28 in relation to UGT1A6 and UGT1A7 polymorphisms. Oncol Rep. 2006;16(4):801–6. Epub 2006/09/14. . [PubMed] [Google Scholar]
- 32.Jinno H, Tanaka-Kagawa T, Hanioka N, Saeki M, Ishida S, Nishimura T, et al. Glucuronidation of 7-ethyl-10-hydroxycamptothecin (SN-38), an active metabolite of irinotecan (CPT-11), by human UGT1A1 variants, G71R, P229Q, and Y486D. Drug Metab Dispos. 2003;31(1):108–13. Epub 2002/12/18 10.1124/dmd.31.1.108 . [DOI] [PubMed] [Google Scholar]
- 33.Kim JY, Cheong HS, Park BL, Kim LH, Namgoong S, Kim JO, et al. Comprehensive variant screening of the UGT gene family. Yonsei Med J. 2014;55(1):232–9. Epub 2013/12/18 10.3349/ymj.2014.55.1.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oussalah A, Bosco P, Anello G, Spada R, Gueant-Rodriguez RM, Chery C, et al. Exome-Wide Association Study Identifies New Low-Frequency and Rare UGT1A1 Coding Variants and UGT1A6 Coding Variants Influencing Serum Bilirubin in Elderly Subjects: A Strobe Compliant Article. Medicine (Baltimore). 2015;94(22):e925 Epub 2015/06/04 10.1097/MD.0000000000000925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang W, Fu YP, Figueroa JD, Malats N, Garcia-Closas M, Chatterjee N, et al. Mapping of the UGT1A locus identifies an uncommon coding variant that affects mRNA expression and protects from bladder cancer. Hum Mol Genet. 2012;21(8):1918–30. Epub 2012/01/10 10.1093/hmg/ddr619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young KA, Palmer ND, Fingerlin TE, Langefeld CD, Norris JM, Wang N, et al. Genome-Wide Association Study Identifies Loci for Liver Enzyme Concentrations in Mexican Americans: The GUARDIAN Consortium. Obesity (Silver Spring). 2019;27(8):1331–7. Epub 2019/06/21 10.1002/oby.22527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park TJ, Hwang JY, Go MJ, Lee HJ, Jang HB, Choi Y, et al. Genome-wide association study of liver enzymes in korean children. Genomics Inform. 2013;11(3):149–54. Epub 2013/10/15 10.5808/GI.2013.11.3.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and supporting table.