Abstract
Rationale
Cystic fibrosis (CF) lung disease progressively worsens from infancy to adulthood. Disease-driven changes in early CF airway fluid metabolites may identify therapeutic targets to curb progression.
Methods
CF patients aged 12–38 months (n=24; 3/24 later denoted as CF screen positive, inconclusive diagnosis) received chest computed tomography scans, scored by the PRAGMA-CF method to quantify total lung damage (PRAGMA-%Dis) and components such as bronchiectasis. Small molecules in bronchoalveolar lavage fluid (BALF) were measured with high-resolution, accurate-mass metabolomics. Myeloperoxidase was quantified by ELISA and activity assays.
Results
Increased PRAGMA-%Dis was driven by bronchiectasis and correlated with airway neutrophils. PRAGMA-%Dis correlated with 104 metabolomic features (p<0.05, q<0.25). The most significant annotated feature was methionine sulfoxide, a product of methionine oxidation by myeloperoxidase-derived oxidants. We confirmed the identity of methionine sulfoxide in BALF and used reference calibration to confirm correlation with PRAGMA-%Dis (Spearman’s ρ=0.582, p=0.0029), extending to bronchiectasis (PRAGMA-%Bx; ρ=0.698, p=1.5×10−4), airway neutrophils (ρ=0.569, p=0.0046) and BALF myeloperoxidase (ρ=0.803, p=3.9×10−6).
Conclusions
BALF methionine sulfoxide associates with structural lung damage, airway neutrophils and myeloperoxidase in early CF. Further studies are needed to establish whether methionine oxidation directly contributes to early CF lung disease and explore potential therapeutic targets indicated by these findings.
Keywords: Innate immunity, Oxidative stress, Orbitrap, CFSPID, Hypochlorous acid
“Take home” summary statement
Identifying molecules associated with early cystic fibrosis lung disease may lead to new means of limiting progression. We found that airway fluid methionine sulfoxide produced by myeloperoxidase associates with lung disease in CF patients aged 1–3 years.
Short sentence
Methionine oxidation is associated with airway myeloperoxidase activity and structural damage in infants with CF.
Introduction
Cystic fibrosis (CF) is a multi-organ disease caused by genetic mutations affecting expression, stability, regulation and/or function of the cystic fibrosis transmembrane conductance regulator (CFTR) anion channel protein. CF is characterized by progressive bronchiectasis resulting in respiratory failure, the primary cause of mortality (1). During childhood, CF airways exhibit neutrophilic inflammation, infections and impaired mucociliary clearance (2). Inflammation may be key to lung disease development due to its early onset, before routine detection of pathogens. Indeed, presence of neutrophil elastase (NE) in airway fluid was the strongest predictor of development of persistent bronchiectasis in a study of young children with CF (3). Neutrophil release of primary granules, which contain NE, myeloperoxidase (MPO) and other proteins capable of injuring airway mucosa, is a plausible mechanism of CF airway disease pathogenesis (4). Recognizing the earliest onset of inflammation in CF and underlying mechanisms is key to limiting disease progression.
Airway lining fluid, chiefly regulated by epithelia, could play major roles in promoting CF airway inflammation (5, 6). CFTR regulates airway fluid composition and volume directly and through interaction with other proteins, such as the epithelial sodium channel (ENaC) (1). When CFTR function is insufficient, airway fluid may change in ways that promote disease, either providing direct pro-inflammatory cues or injuring airway cells (7). Incoming neutrophils, via intense metabolic and effector activities, may further alter CF airway fluid. Due to the complexity of human airway physiology, unbiased methodologies such as untargeted metabolomics may serve to identify unanticipated biochemical changes driving pathogenesis.
Sensitive chest computed tomography (CT) techniques have been developed to detect early lung disease in CF patients. One such method, the Perth-Rotterdam Annotated Grid Morphometric Analysis for CF (PRAGMA-CF), can quantify bronchiectasis, mucus plugging, air trapping, and other airway abnormalities in CF patients younger than 6 years (8). Lung imaging may be combined with bronchoalveolar lavage (BAL) to survey airway fluid and cells to find associations with structural lung disease. For example, protein-bound glutathione from BAL fluid (BALF) was previously associated with higher risk of developing bronchiectasis (9). Untargeted analyses have identified dozens of candidate metabolite biomarkers for the detection of CF lung disease (10). Transition from biomarkers to mechanisms of pathology may lead to new therapeutic strategies to treat early lung disease in CF.
Here we hypothesized that metabolites in early CF BALF linked to disease progression should correlate with PRAGMA-CF. We applied high-resolution, accurate-mass mass spectrometry (HRMS)-based metabolomics to identify correlations in CF children who underwent prospective BAL and chest CT. We confirmed the identity of the most significant identified metabolite (methionine sulfoxide) and quantified MPO, an enzyme from neutrophils capable of generating it through production of strong oxidants.
Materials and Methods
Study design
The study was performed as part of the Inflammatory Markers in Broncho-Alveolar Lavage Fluid for Lung Disease in Infants with Cystic Fibrosis (I-BALL) study (clinicaltrial.gov identifier, ). It is a translational, exploratory and observational study of infants with CF identified by newborn screening in which BALF, peripheral blood, chest CT and clinical follow-up data are prospectively collected. The IRB of the Erasmus MC approved the study (IRB protocol NL49725.078.14), and all parents signed informed consent.
Sample collection
Bronchoscopy and CT were scheduled prospectively following enrollment and took place within 3 consecutive days on average. Patients fasted overnight prior to the BAL procedure, taking place under general anesthesia. Twenty-four right middle lobe BAL samples were collected from CF patients aged 12–38 months. One ml/kg body weight sterile saline was instilled and recovered three times and research samples pooled from second and third aliquots were placed on ice for no more than 2 h before BALF was isolated by centrifugation at 330 g and 4 °C for 5 min, then stored at −80 °C. The first BAL aliquot was used for clinical pathology, including bacterial culture and cell counts. Free-breathing chest CT scan were acquired without anesthesia using Siemens SOMATOM® Force ultra-fast scanner. PRAGMA-CF was used for quantitative scoring of percentages of bronchiectasis (PRAGMA-%Bx) and total lung disease (PRAGMA-%Dis) (8). CT scans were interpreted by a single observer blinded to other study information at the time of scoring. %Dis was a composite of PRAGMA-%Bx, mucus plugging and bronchial wall thickening. Additional demographics and findings are in Table 1.
Table 1.
Patient characteristics and clinical findings
| Childrena (n) | 24 |
| Age (months) | 31 ± 11 |
| Female:Male (n) | 16:8 |
| F508del homozygous (%) | 50.0 |
| Sweat chloride (mmol/l) | 92±28 |
| Pancreatic insufficiency (%) | 87.5 |
| Using antibiotics at BAL (%) | 91.2 |
| Past antibiotic coursesb | 2.9 ± 1.9 |
| Secondhand smoke (%) | 29.2 |
| BAL neutrophilsc (%) | 27.7 ± 18.9 |
| BAL macrophagesc (%) | 64.6 ± 19.7 |
| BAL lymphocytesc (%) | 7.2 ± 4.7 |
| BAL eosinophilsc (%) | 0.5 ± 0.8 |
| Positive BAL culture (%)c | 39.1 |
| Positive for S. aureus (%)c | 26.1 |
| Positive for P. aeruginosa (%)c | 4.3 |
Data are mean ± SD or a percentage.
All subjects received prospective BAL and CT procedures.
Number of antibiotic courses received (oral and/or i.v., including prophylaxis) 12 months prior to the prospective visit (n=18).
n=23.
Metabolomics
Metabolites were extracted from BALF by 1:2 mixture with acetonitrile (ACN) plus internal standards, carried out on ice for 30 minutes followed by vortexing and centrifugation at 16,000 g and 4 °C for 10 min. Supernatant was maintained on an autosampler held at 4 °C and analyzed via Q Exactive High Field (Thermo) hybrid mass spectrometer. Intensities of detected mass-to-charge (m/z) features were extracted using apLCMS 6.3.3 (11), evaluated for data quality using xMSanalyzer 2.0.8 (12), and pre-filtered as detailed in the Online Supplement. Significant features were annotated using METLIN (13). Standard Reference Material (SRM) 1950 (National Institute of Standards and Technology) was analyzed in parallel to the BALF samples as a quality control and reference standard.
MPO assay
Abundance and activity of MPO were quantified in an assay adapted from Chapman et al. (14). Briefly, MPO is immunocaptured, and its abundance and activity are measured by ELISA and Amplex Red oxidation, respectively. Assay lower limits of quantification of 1.0 ng/ml (Amplex Red) and 0.13 ng/ml (ELISA) were established from three independent assays.
Statistics
Metabolomics data analysis, including log2 transformation, quantile normalization and Pearson’s correlation with p-value calculation and false discovery rate-adjusted q-value calculation, was performed using an in-house R package (https://github.com/kuppal2/xmsPANDA). We selected q<0.25 for multiple comparisons adjustment of metabolomics results due to the convolution inherent to electrospray ionization mass spectra (multiple signals can be observed per compound). Additional analysis was performed using MetaboAnalyst (15) and ggplot2 (16). MPO assays were calibrated by SoftMax Pro v. 7.0.3 (Molecular Devices). Prism 7 (GraphPad) was used to calculate Spearman correlations and Mann-Whitney U test, and to generate figures.
Additional methods are located in the Supplementary Information document accompanying this manuscript.
Results
Patient characteristics and CT determination of lung disease
Patient characteristics are shown in Table 1. Fifty percent of patients were homozygous for F508del, and all but one carried at least one copy of the allele. Three genotyped with an R117H-7T allele and sweat chloride <60 mmol/l were re-designated as CF screened positive, inconclusive diagnosis (CFSPID; highlighted in red in barplot and scatterplot figures; range of sweat chloride, 24–55 mmol/l) (17). Over 90% of patients were on antibiotics at the time of the procedure, and less than half of patients were positive for pathogenic organisms by BAL culture.
PRAGMA-CF analysis was conducted on CT scans acquired at 1, 2 or 3 years of age. PRAGMA-CF analysis identified the presence of bronchiectasis in most of the patients (PRAGMA-%Bx; 0 to 3.74%), while the composite score (PRAGMA-%Dis) ranged from 0.82 to 7.47%. Clinical variables, such as gender, F508del homozygosity, and positive BAL culture, did not significantly influence PRAGMA-%Dis, or we did not consider them because data distributions were unevenly skewed (e.g., pancreatic insufficiency).
Metabolomics
A list of 11,188 m/z-by-retention time features was initially recovered from BALF samples (Supplementary File 1), and a working feature table of 1,798 was produced by applying thresholds for intensity, technical precision and missing values. One sample was determined to have an aberrant number of missing values and removed from the study (Supplementary Figure 1A–B). Data were then log2-transformed and quantile normalized prior to testing (Supplementary Figure 1C). Ultimately, 190 features were significant at p<0.05, and 104 of these passed q<0.25 multiple comparisons-adjusted threshold (results in Supplementary File 2). Significant features were annotated in METLIN (13), and we assigned 30 annotations matching a total of 22 unique metabolites (Figure 1 and Table 2). In cases of multiple ions belonging to the same metabolite, data for the ion of highest average intensity is shown.
Figure 1. Accurate mass metabolite annotations of m/z features significantly associated with PRAMGA-%Dis.
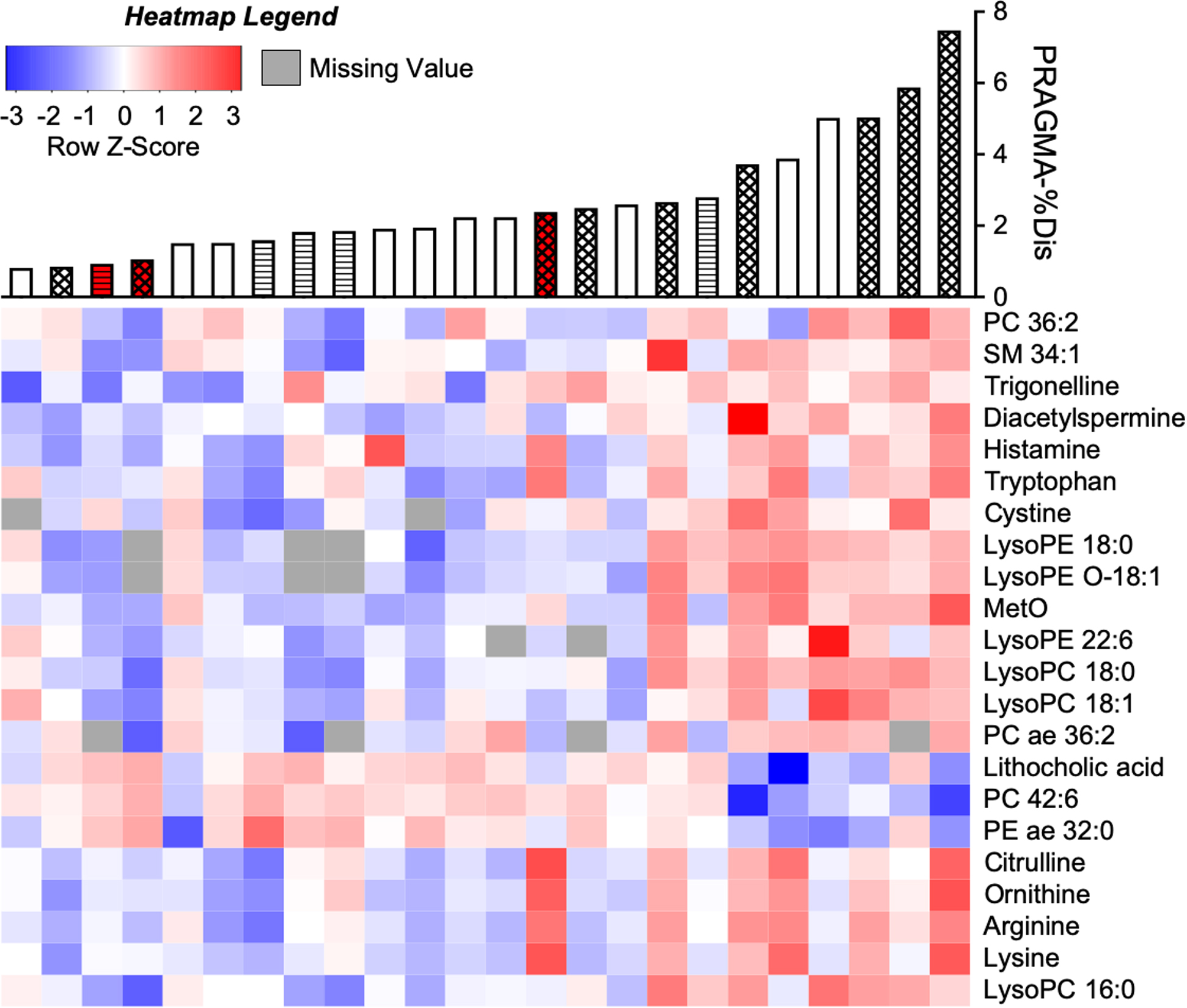
Intensities of m/z-by-retention time features measured from CF BALF by HRMS were log2-transformed, quantile-normalized and correlated with PRAGMA-%Dis using a linear model. Of 104 significant (p<0.05, q<0.25) features, 30 were annotated to accurate-mass matches using METLIN (13) followed by isotope analysis. These corresponded to 22 unique metabolites after deconvolution of co-eluting compounds (see also Table 2; and the complete dataset given in Supplementary File 2). The m/z feature with highest average intensity for each of the 22 metabolites was plotted on a heatmap, autoscaled to Z-scores and coded blue-(most negative)-to-red (most positive). Missing values are represented as gray. Columns were sorted from left-to-right by ascending PRAGMA-%Dis and rows were hierarchically clustered by metabolite intensities. Accurate mass metabolite matches are indicated to the right of the heatmap. PRAGMA-%Dis values corresponding to each patient are shown by a barplot above the heatmap. Bar color corresponds to disease state (white = CF, red = CFSPID). Bar fill corresponds to BAL microbe culture (no fill = no history of positive pathogen culture, diagonal lines = negative for pathogens at time of BAL, but a prior BAL was positive, and hatched = positive pathogen culture at time of BAL).
Table 2.
Metabolites correlated with PRAGMA-%Dis in untargeted analysis.
| m/z | Time (sec) | Metabolite | Adduct | Error (ppm) | r | p-value | q-value |
|---|---|---|---|---|---|---|---|
| 166.0533 | 281 | Methionine sulfoxide | [M+H] | 0 | 0.648 | 5.59E-5 | 0.0273 |
| 862.6339 | 137 | PC 42:6 | [M+H] | 2 | −0.357 | 9.89E-5 | 0.0273 |
| 524.3711 | 270 | LysoPC 18:0 | [M+H] | 0 | 0.669 | 3.57E-4 | 0.0407 |
| 287.2442 | 320 | Diacetylspermine | [M+H] | 0 | 0.318 | 6.36E-4 | 0.0425 |
| 522.3556 | 270 | LysoPC 18:1 | [M+H] | 0 | 0.599 | 2.69E-3 | 0.1272 |
| 175.1190 | 305 | Arginine | [M+H] | 0 | 0.254 | 2.78E-3 | 0.1272 |
| 496.3397 | 274 | LysoPC 16:0 | [M+H] | 0 | 0.598 | 2.86E-3 | 0.1272 |
| 133.0972 | 308 | Ornithine | [M+H] | 0 | 0.304 | 2.98E-3 | 0.1277 |
| 482.3240 | 196 | LysoPE 18:0 | [M+H] | 0 | 0.579 | 3.70E-3 | 0.1428 |
| 466.3288 | 192 | LysoPE O-18:1 | [M+H] | 0 | 0.640 | 4.80E-3 | 0.1586 |
| 147.1128 | 311 | Lysine | [M+H] | 0 | 0.197 | 6.72E-3 | 0.1920 |
| 176.1030 | 257 | Citrulline | [M+H] | 0 | 0.307 | 8.68E-3 | 0.2061 |
| 112.0869 | 303 | Histamine | [M+H] | 0 | 0.284 | 9.54E-3 | 0.2152 |
| 808.5817 | 215 | PC 36:2 | [M+Na] | 1 | 0.161 | 0.0127 | 0.2314 |
| 415.2607 | 222 | Lithocholic acid | [M+K] | 0 | −0.193 | 0.0141 | 0.2314 |
| 526.2913 | 192 | LysoPE 22:6 | [M+H] | 2 | 0.310 | 0.0159 | 0.2431 |
| 660.5332 | 157 | PE ae 32:0 | [M+H-H2O] | 0 | −0.049 | 0.0183 | 0.2456 |
| 772.6232 | 218 | PC ae 36:2 | [M+H] | 2 | 0.678 | 0.0186 | 0.2456 |
| 205.0971 | 182 | Tryptophan | [M+H] | 0 | 0.309 | 0.0198 | 0.2467 |
| 703.5729 | 247 | SM 34:1 | [M+H] | 2 | 0.150 | 0.0205 | 0.2467 |
| 241.0311 | 306 | Cystine | [M+H] | 0 | 0.420 | 0.0207 | 0.2467 |
| 138.0550 | 267 | Trigonelline | [M+H] | 0 | 0.153 | 0.0221 | 0.2467 |
Metabolites p<0.05, q<0.25 are shown following deconvolution to remove redundant features that correspond to the same chemical. In such cases, the m/z feature with the highest mean intensity was selected.
The most significant annotated feature matched to methionine sulfoxide (MetO; Table 2). Several metabolites corresponded to pathways of arginine metabolism (including arginine, citrulline, ornithine, diacetylspermine) or to glycerophospholipids and lysolipids. Others included lithocholic acid, trigonelline and several other amino acids. Most were positively associated with PRAGMA-%Dis, but three – phosphatidylcholine (PC) 42:6, phosphatidylethanolamine acyl/ether (PE ae) 32:0, and lithocholic acid – were inversely associated. Twelve unidentified features ranging from 675–861 m/z and co-eluting at 330 sec showed strong positive correlations with PRAGMA-%Dis (Supplementary File 2; r > 0.6, p ≤ 0.001 for all), but these produced no reasonable database matches and were not further assessed.
Identification and reference calibration of methionine sulfoxide
We sought to confirm the accurate mass annotation of MetO (Figure 1 and Table 2; representative trace in Supplementary Figure 2A). We synthesized isotopically enriched 13C5,15N-L-MetO and spiked this into pooled CF BALF and SRM 1950 to identify MetO by stable isotope co-elution and MS/MS. Naturally occurring and isotopically enriched MetO gave rise to the same five fragments with appropriate mass shifts in the isotopically enriched reagent (Supplementary Figures 2B–C), providing confirmation of the 2D structure of MetO in both BALF and SRM 1950 (Supplementary Figure 2D).
We calibrated MetO and methionine in SRM 1950 to enable reference calibration of the BALF samples and calculate the percentage of MetO relative to the sum of MetO and Met (% OxMet). SRM 1950 was calibrated at 20.68 μM methionine (93% of the NIST-certified reference value, 22.3 μM) and 1.22 μM MetO, corresponding to 5.6% OxMet. MetO was then reference calibrated in BALF and ranged from 24 to 1,031 nM (mean 174±240 nM), while % OxMet ranged from 3.8 to 62.7% (18.8±14.8%). After calibration, MetO remained significantly correlated with PRAGMA-%Dis using non-parametric Spearman’s correlation (Figure 2A).
Figure 2. Correlations of structural lung disease, methionine oxidation and myeloperoxidase in early CF.
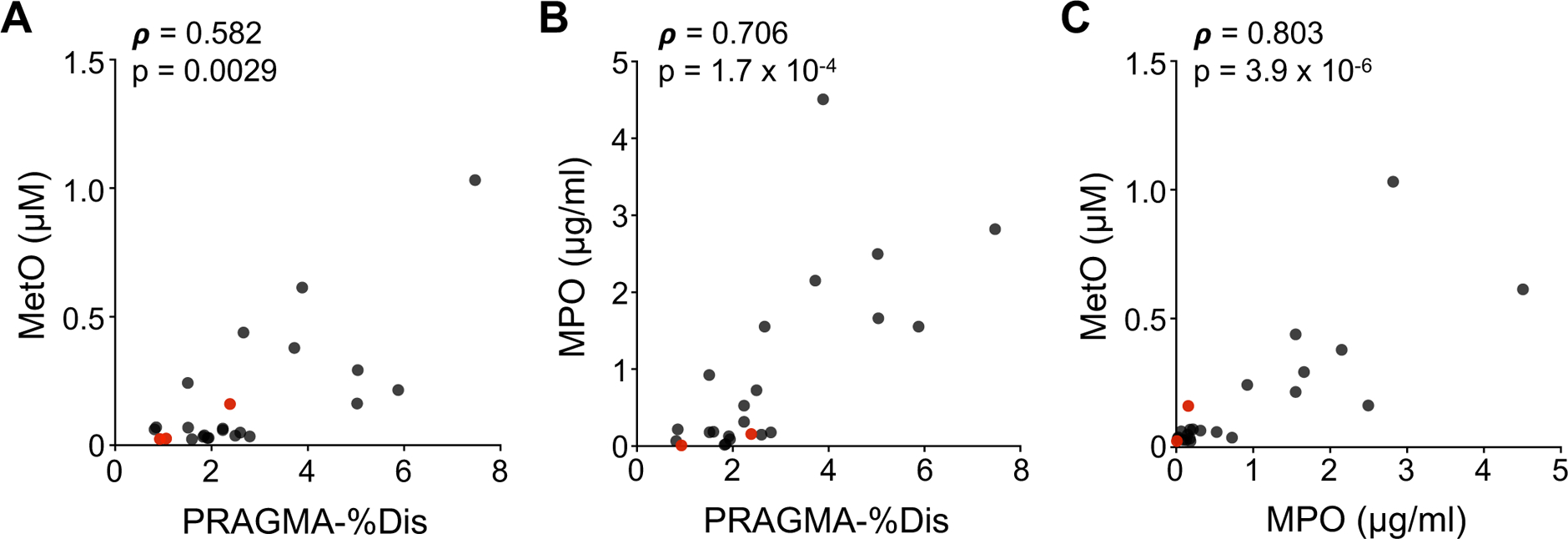
Spearman correlations were analyzed for pairwise combinations of PRAGMA-%Dis (A and B), MetO (measured by HRMS with reference calibration; A and C) and MPO (measured by ELISA; B and C). The correlation results (rho estimate, ρ, and p-value) are inset for each scatterplot. CFSPID patients, identified by newborn screening with later inconclusive diagnosis of disease, are shown in red.
Quantification of MPO in CF BALF
MetO is produced by the reaction of Met with myeloperoxidase (MPO)-derived oxidants hypochlorous (HOCl) and hypobromous acid (HOBr) (18). MPO was detected in BALF and exhibited the same immunoreactivity as in blood neutrophils (Supplementary Figure 3). We determined the catalytic activity and protein abundance of MPO in CF BALF to evaluate its potential relationships with PRAGMA-%Dis and MetO. MPO ranged from 0.0087 to 4.5 μg/ml in CF BALF (mean 0.90±1.17 μg/ml), except for one CFSPID sample in which none was detected. MPO distribution was non-normal and was similar when normalized to protein (Supplementary Figure 4A). BALF MPO was active in an Amplex Red-based peroxidase assay and none of the samples exhibited low activity percentage relative to ELISA results (Supplementary Figure 4B–C). BALF MPO correlated strongly with PRAGMA-%Dis (Figure 2B), as well as MetO (Figure 2C).
Relationships of MetO and MPO with bronchiectasis and neutrophils
We sought to understand the specific contributions of bronchiectasis (PRAGMA-%Bx) and the percentage of airway neutrophils to MetO, % OxMet and MPO. We performed pairwise Spearman correlations for these variables (Table 3). For each of the variables, including airway neutrophils, PRAGMA-%Bx resulted in a stronger correlation than PRAGMA-%Dis. The strongest observed correlation between two independent variables was that of % OxMet and MPO.
Table 3.
Pairwise correlations of variables associated with lung disease.
| PRAGMA-%Bx | PMNs (%) | MetO (nM) | %OxMet | MPO (μg/ml) | |
|---|---|---|---|---|---|
| PRAGMA-%Dis | n = 24 | n = 23 | n = 24 | n = 24 | n = 23 |
| ρ = 0.726 | ρ = 0.502 | ρ = 0.582 | ρ = 0.437 | ρ = 0.706 | |
| p = 5.8 × 10−5 | p = 0.0146 | p = 0.0029 | p = 0.0286 | p = 1.7 × 10−4 | |
| PRAGMA-%Bx | n = 23 | n = 24 | n = 24 | n = 23 | |
| ρ = 0.6742 | ρ = 0.698 | ρ = 0.665 | ρ = 0.752 | ||
| p = 4.2 × 10−4 | p = 1.5 ×10−4 | p = 3.9 × 10−4 | p = 3.5×10−5 | ||
| PMNs (%) | n = 23 | n = 23 | n = 22 | ||
| ρ = 0.569 | ρ = 0.594 | ρ = 0.656 | |||
| p = 0.0046 | p = 0.0028 | p = 0.0092 | |||
| MetO (nM) | n = 24 | n = 23 | |||
| ρ = 0.860 | ρ = 0.803 | ||||
| p = 7.2 × 10−8 | p = 3.9 × 10−6 | ||||
| %OxMet | n = 23 | ||||
| ρ = 0.837 | |||||
| p = 6.4 × 10−7 |
Pairwise Spearman correlation results are shown for the listed variables. Note that the variable pairs of PRAGMA-%Dis and PRAGMA-%Bx and that of MetO and %OxMet are not independent (PRAGMA-%Bx contributes directly to PRAGMA-%Dis, and MetO contributes directly to %OxMet).
Robustness of key correlations
To ensure robustness of key correlations, we analyzed a subset of patients by excluding three CFSPID-designated samples and five others for whom clinical complications prevented the completion of BAL and CT procedures within 28 consecutive days. After removing the total eight samples, MetO remained significantly correlated with PRAGMA-%Bx (Supplementary Figure 5B) and MPO (Supplementary Figure 5C), but the significant association with PRAGMA-%Dis was lost (Supplementary Figure 5A). Because the strength of the subset correlation (ρ=0.4353) was comparable to the original (ρ=0.5817), decreased power probably explains the lack of significance. MPO remained significantly correlated with PRAGMA-%Dis (Supplementary Figure 4D) and PRAGMA-%Bx (Supplementary Figure 4E).
Global correlations
To compare the global correlations of MetO, MPO and PRAGMA-CF to clinical variables, we prepared a Spearman’s correlation matrix (Figure 3). We considered BAL immune cell percentages, BALF total protein, patient age, sweat chloride, and the number of antibiotic (Abx) courses. The three strongest correlations with PRAGMA-%Dis were MPO, MetO, and % BAL macrophages (ρ=−0.5824, p=0.0036) and with PRAGMA-%Bx were MPO, MetO and % BAL neutrophils. BAL % neutrophils and BAL % macrophages exhibited a strong inverse relationship (ρ=−0.9683, p=3.9×10−14). Age and BALF protein also had positive correlations with PRAGMA-%Dis and PRAGMA-%Bx. BAL % eosinophils, BAL % lymphocytes, sweat chloride and the number of antibiotic courses in the past 12 months were not correlated with other variables.
Figure 3. Methionine oxidation, airway MPO and neutrophils are strong correlates of bronchiectasis in early CF.
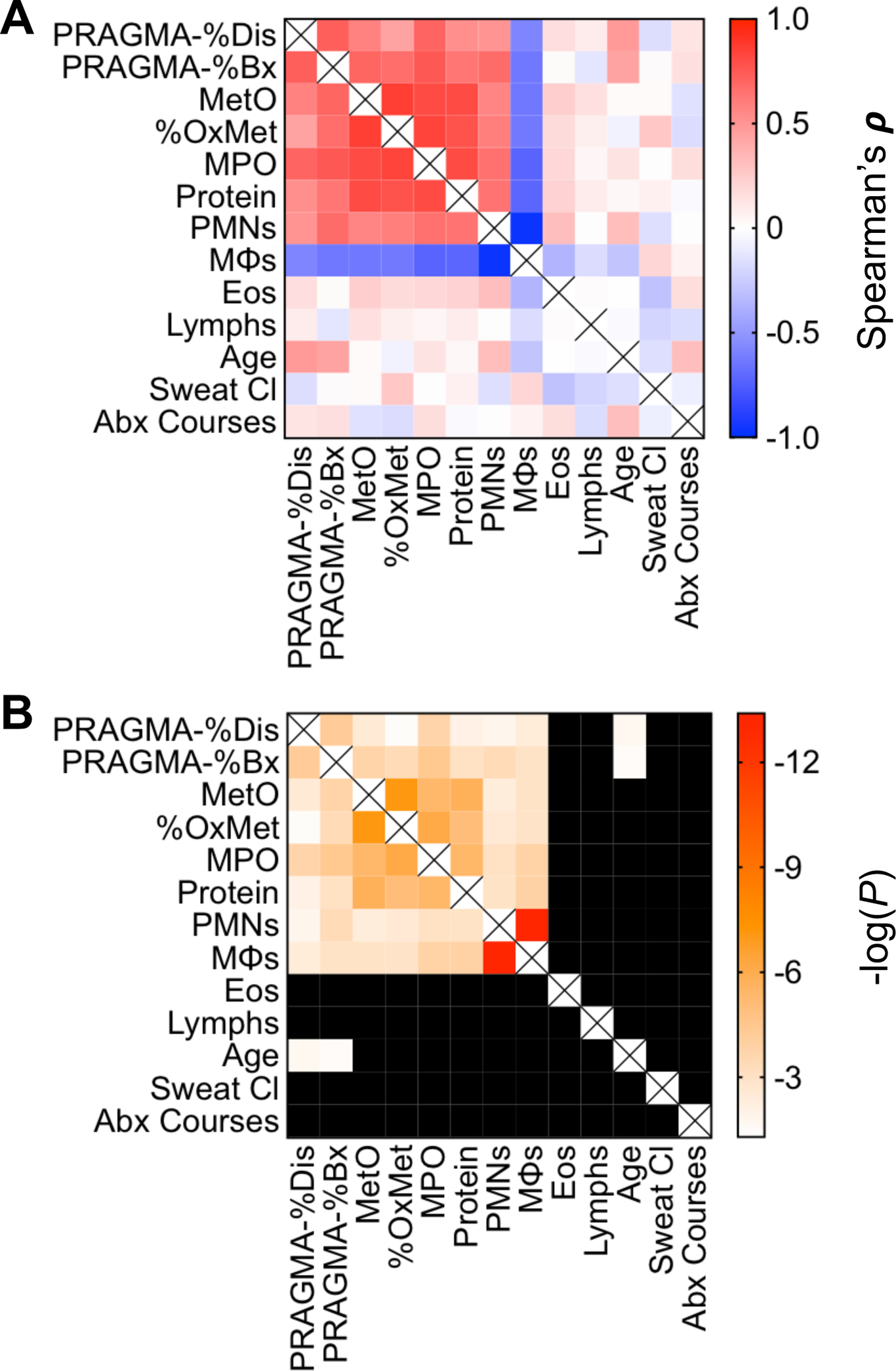
We tested the Spearman correlations of PRAGMA-%Dis and PRAGMA-%Bx with BALF measures (MetO, % OxMet, MPO, total protein), BAL cell populations [percentages of neutrophils (PMNs), macrophages (MΦ), lymphocytes (Lymphs) and eosinophils (Eos)], patient age (months), patient sweat chloride (mM) and the total number of antibiotic courses received by patients in the prior 12 months. (A) Blue-to-red heatmap of Spearman’s ρ for the indicated variables. Red = positive correlation, blue = negative correlation. The intensity of the color indicates the strength of the relationship. (B) Matching single-gradient white-to-red heatmap of p-values (expressed as −log(P)). Intensity of the red color indicates greater statistical significance. Black cells indicate results of p > 0.05 for the relevant pairwise correlation. Cells denoted by (X) indicate where rows and columns for the same variable meet.
Discussion
Taken together, our results are consistent with the early emergence of neutrophilic influx, granule exocytosis and oxidizing activity of secreted MPO early in CF pathology, particularly bronchiectasis (scheme in Figure 4). The contribution of neutrophilic inflammation to CF lung disease is well recognized, yet exact mechanisms of pathogenesis are not fully understood. Interventions directly targeting inflammation in CF are currently limited to high-dose ibuprofen, and no FDA-approved therapies specifically target airway neutrophils (19). Such an intervention might be most effective and have the longest-lasting benefits if administered in earliest stages of CF (20). It is not yet known how CFTR modulators will influence inflammation in the lungs, and these drugs are not yet available for young children.
Figure 4. Roles of myeloperoxidase and methionine sulfoxide in the development of early CF bronchiectasis.
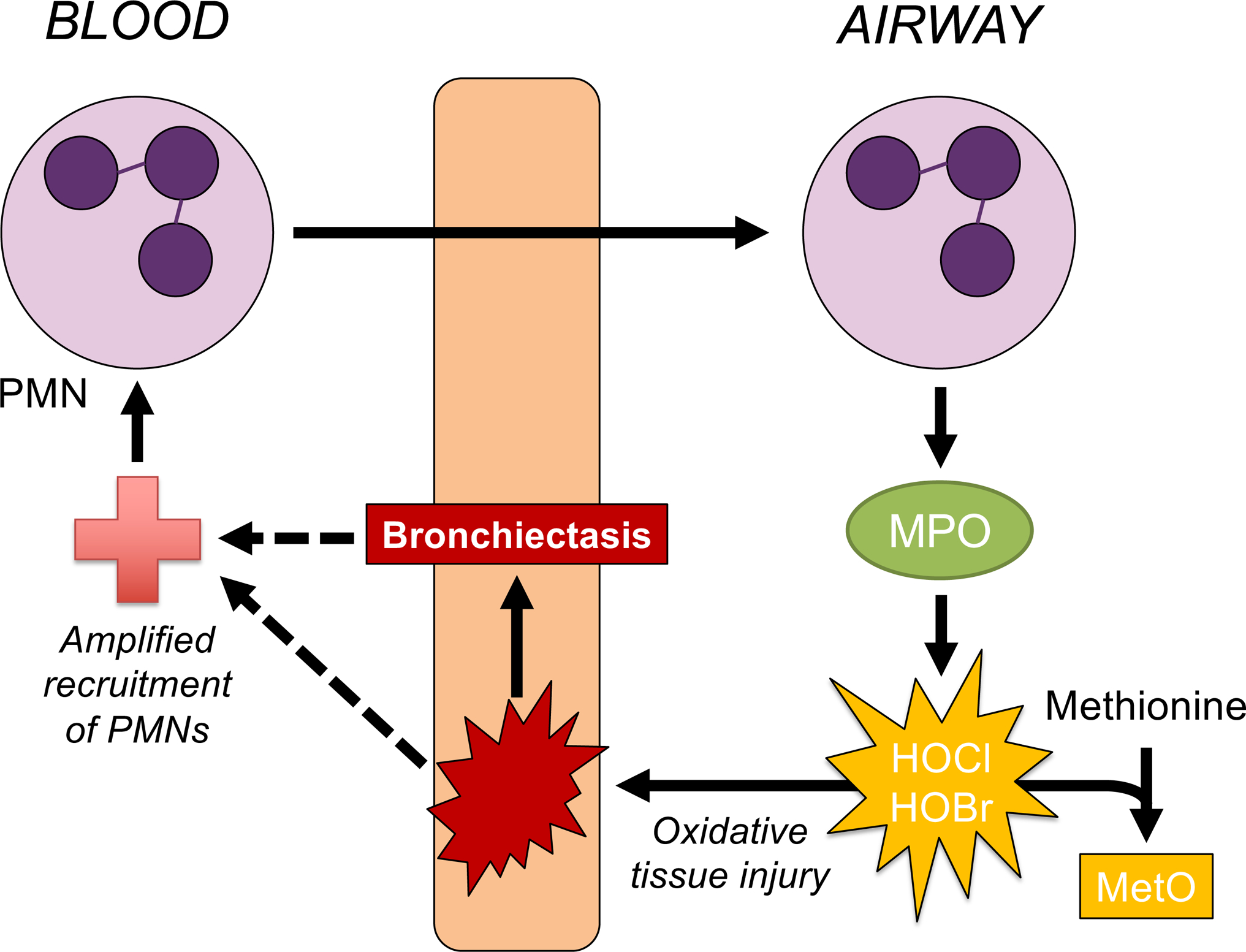
Neutrophil (PMN) recruitment to airways may occur in early CF without an obvious pathogenic stimulus. In the current study, methionine sulfoxide (MetO) in early CF BALF was correlated strongly with CT scores of bronchiectasis. Following influx of neutrophils that secrete myeloperoxidase (MPO) into the lumen, oxidizing activity of MPO may generate MetO from methionine. MPO generates hypochlorous and hypobromous acid, which cause rapid, frequently irreversible damage to an array of biomolecules including tissue macromolecules. The oxidizing activity of MPO provides a common link between MetO and tissue injury which may lead to bronchiectasis. These processes may be insufficiently resolved in early CF and contribute to a feed-forward mechanism underlying sustained PMN recruitment. Whether MetO may directly participate in the promotion of CF airway disease is unclear at this time.
The present study demonstrates the potential importance of methionine oxidation by MPO in early CF lung disease. Airway MetO and % OxMet strongly correlated with MPO, indicating that MPO is responsible for the MetO detected. Biomarker studies have indicated that MPO does generate HOCl and HOBr in CF airways (21). Distinct oxidants with diverse reactivity profiles are generated in the airways that may serve to help or harm the host, with HOCl and HOBr associated with lasting molecular damage (22). These oxidants have exceptional reduction potentials and rapidly oxidize methionine (18, 23), while weaker oxidants and those that are kinetically restrained, such as hypothiocyanous acid and hydrogen peroxide (respectively), react much more slowly with methionine, so that their contributions to its oxidation in vivo may effectively be nil (24, 25). The strong correlation observed for MPO and % OxMet in this cohort support the notion that MPO generates significant HOCl and HOBr in CF airways at a young age. Importantly, MPO may do this regardless of the source of H2O2, which is generated in vivo from multiple physiological processes (26). Although phagocyte NADPH oxidase 2 is a likely contributor to neutrophil-derived hydrogen peroxide (via superoxide), other sources of H2O2 should not be ruled out.
While the influence of MPO is well recognized in CF airway pathophysiology (9, 21), this study is, to the best of our knowledge, the first time that MetO and % OxMet have been identified as correlates of bronchiectasis and airway neutrophils in young children with CF, which we discovered using an unbiased metabolomics approach. Of note, untargeted mass spectral data may incur multiple comparison penalties that are ultimately too conservative, as multiple interdependent spectra can arise from individual chemicals in the samples (27), resulting in a higher likelihood of type II error (28). Recognizing this, we used a moderate multiple comparisons threshold of q<0.25 for untargeted mass spectral data. However, we note that multiple metabolites including MetO would have surpassed more stringent thresholds. Although our data suggest that methionine is one of the most sensitive airway metabolites to the advancement of neutrophilia and bronchiectasis in early CF, additional studies are needed to validate the importance of methionine oxidation in early CF. In addition to these findings, our study confirms the relationship of % OxMet and MPO in CF BALF previously identified by Dickerhof and co-workers (9).
To maximize clinical utility, airway MetO and % OxMet measurements should be extended in future studies to include less invasive samples, such as exhaled breath condensate (EBC), sputum and tracheal aspirates. While we anticipate that sputum and tracheal aspirates will be sufficiently concentrated to perform robust metabolomics research, methodological innovations to improve sensitivity for dilute EBC samples are a necessity. Fortunately, mass spectrometry has already been demonstrated to be amenable to CF EBC metabolite detection (29). Standardized collection in accordance with best clinical practice is also crucial, particularly in early stage pediatric studies where reproducible sample acquisition can be challenging.
Correlations of MetO and % OxMet with bronchiectasis are consistent with previously hypothesized redox dysregulation and oxidative stress in CF. Much attention has been placed on decreased abundance and accelerated oxidation of glutathione in CF airways (9, 30, 31). By contrast, we found that cystine (the homodimeric disulfide of cysteine) was positively correlated with PRAGMA-%Dis. Because the samples were not treated to preserve thiols, the result may be indicative of the total cysteine and cystine pool rather than the oxidized form alone. This indicates that glutathione and cysteine likely support divergent redox signaling pathways in airways, much like other physiological compartments (32). We did not detect the irreversibly oxidized glutathione sulfonamide in the current study, which may be due to methodological differences and/or reflect its low abundance, possibly limited by its stringent reaction (3 mol of HOCl or HOBr react with 1 mol glutathione) (33).
The association of MetO and early CF bronchiectasis could result from multiple pathophysiological processes. Excessive and sustained neutrophil transmigration is associated with progressive airway damage (34), and is a necessary precursor to the accumulation of luminal MPO required for efficient methionine oxidation by HOCl/HOBr. However, neutrophils secrete several other potentially damaging factors, and MetO could represent multiple pathways of damage by proxy. For example, MPO release is generally concomitant with that of neutrophil elastase (4). However, additional experiments are needed to establish the relationship of these enzymes in early CF. In particular, future studies will assess whether MPO-dependent MetO production may be readily measurable in infants with CF while elastase activity may not, due to airway antiprotease shield that counterbalances elastase effectively early on in the disease process (3).
In contrast to MetO, which associated more strongly with PRAGMA-%Bx than PRAGMA-%Dis, methionine was the opposite: associated more strongly with PRAGMA-%Dis than with PRAGMA-%Bx (however, neither association was as strong as those of MetO; data not shown). This reversal may reflect association of methionine with different disease processes not directly related to MPO activity, such as bulk movement of metabolites into the airway lumen with neutrophils, or the methionine salvage pathway. The methionine salvage pathway, in which methionine and adenine are recovered from byproducts of polyamine biosynthesis, has been implicated in worse lung function in CF by genome-wide association and transcriptomic studies indicating potential roles for AMD1, MTAP and APIP (35–37). Airway methionine oxidation might promote increased expression of this pathway, and such potential for pathological cross-talk should be considered in future studies. Additional studies are also needed to determine the extent to which the steady-state redox potential of methionine is actively maintained in airways, including individual fates of the MetO stereoisomers, methionine-S-sulfoxide and methionine-R-sulfoxide. These are reduced back to methionine by distinct enzymes (38), although mammals do not encode an efficient free methionine-(R)-sulfoxide reductase (39).
Inflammation in CF may be present from an extremely early age, provoked by inherent epithelial defects (7). A recent study in children under 18 years of age showed a decreased rate of annual lung function decline and increased long-term survival in high dose ibuprofen-exposed patients (40). The effects of high-dose ibuprofen may include inhibition of neutrophil oxidative burst (41), which would in turn limit production of HOCl and HOBr from MPO. Many compounds have been investigated as candidate MPO inhibitors, including 2-thioxanthines and acetaminophen (42, 43). Such drugs may find utility in limiting early CF lung disease, but this requires further testing. Other interventions directly targeting key reactive species such as HOCl may also have potential to limit airway disease caused by hypohalous acids (44–47). Strategies to limit oxidative imbalance in CF should take the species of oxidants generated and their respective roles into account, as oxidants serve diverse and critical roles in host defense and redox signaling processes (22, 26).
We included three patients with R117H-7T mutations, designated CFSPID after initial enrollment via newborn screening, in the study. Interestingly one of the patients showed significant inflammation, heightened MetO and increased PRAGMA-CF scores on chest CT, despite having a mutation which is considered as not causing CF. Therefore, PRAGMA-CF and BAL studies that are useful in identifying lung disease processes in CF appear to have some applicability to CFSPID as well. Future studies may identify environmental and genetic factors that promote neutrophilic lung disease in otherwise healthy CFSPID and/or in well-controlled CF.
In conclusion, we present data that demonstrate the importance of methionine oxidation via MPO in early CF airway disease. MetO, % OxMet and MPO strongly correlated with PRAGMA-%Dis and PRAGMA-%Bx, sensitive measures of early lung disease and bronchiectasis. Our initial discovery was made through an unbiased metabolomics method and confirmed using targeted analyses. MetO and MPO should be further studied in pathological mechanisms underlying early onset CF airway disease, which may lead to novel biomarkers and targets for therapy.
Supplementary Material
Acknowledgments
We thank the patients and their families for consenting to participate in this study. We acknowledge research coordinators E. Nieuwhof, E. van der Wiel and B. Manai (Erasmus MC-Sophia, Rotterdam, The Netherlands) for their support in recruiting patients. We thank M.W.H. Pijnenburg, J.C. de Jongste, L. Duijts, S. Kloosterman and I.M. de Kleer (Erasmus MC-Sophia, Rotterdam, The Netherlands) for performing bronchoscopies. We thank M. Kemner (Erasmus MC-Sophia, Rotterdam, The Netherlands) for her guidance with PRAGMA-CF scoring. We thank Nina Dickerhof (University of Otago, Christchurch, New Zealand) for insightful technical discussions about the MPO assay method.
Funding and author contributions:
Supported by NHLBI F32 HL132493 (J.D. Chandler); Emory Children’s CF and Airways Disease Research Center Startup Fund (J.D. Chandler and M.B. Kilgore); CFF P30 MCCART15R0 (C. Margaroli); NHLBI R01 HL126603 and Common Fund for Metabolomics Supplement HL126603-02S1 (R. Tirouvanziam, A.J. Taurone, L. Peng, H. Horati, H.M. Janssens, B.J. Scholte and M. Veltman); Dutch Cystic Fibrosis Foundation (NCFS) grant HIT-CF 1 and 2 (H. Horati, M. Veltman, B.J. Scholte, H.A.W.M. Tiddens and H.M. Janssens); ERARE INSTINCT (M. Veltman and B.J. Scholte); and P30 ES019776, S10 OD018006 and R01 ES023485 (H.K. Liu, K. Uppal, Y-M. Go and D.P. Jones).
Experimental conception and design: RT, HMJ, BJS, JDC. Performed experiments: JDC, HH, CM, MBK, MV. Interpreted results and provided critical support: JDC, HH, CM, MBK, HKL, BJS, HAWMT, AJT, LP, LG, KU, YMG, HMJ, DPJ, RT. Prepared manuscript: JDC. Reviewed or edited and approved manuscript: All authors.
Footnotes
This article has supplementary material available from erj.ersjournals.com.
References
- 1.Elborn JS. Cystic fibrosis. Lancet 2016;388:2519–2531. [DOI] [PubMed] [Google Scholar]
- 2.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of Cystic Fibrosis Lung Disease. N Engl J Med 2015;372:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, Murray CP, Stick SM, AREST CF Investigators. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med 2013;368:1963–1970. [DOI] [PubMed] [Google Scholar]
- 4.Margaroli C, Tirouvanziam R. Neutrophil plasticity enables the development of pathological microenvironments: implications for cystic fibrosis airway disease. Molecular and Cellular Pediatrics 2016;3:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartl D, Gaggar A, Bruscia E, Hector A, Marcos V, Jung A, Greene C, McElvaney G, Mall M, Döring G. Innate immunity in cystic fibrosis lung disease. J Cyst Fibros 2012;11:363–382. [DOI] [PubMed] [Google Scholar]
- 6.Forrest OA, Ingersoll SA, Preininger MK, Laval J, Limoli DH, Brown MR, Lee FE, Bedi B, Sadikot RT, Goldberg JB, Tangpricha V, Gaggar A, Tirouvanziam R. Frontline Science: Pathological conditioning of human neutrophils recruited to the airway milieu in cystic fibrosis. J Leukoc Biol 2018;16:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montgomery ST, Mall MA, Kicic A, Stick SM. Hypoxia and sterile inflammation in cystic fibrosis airways: mechanisms and potential therapies. Eur Respir J 2017;49:1600903–13. [DOI] [PubMed] [Google Scholar]
- 8.Rosenow T, Oudraad MCJ, Murray CP, Turkovic L, Kuo W, de Bruijne M, Ranganathan SC, Tiddens HAWM, Stick SM, Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF). PRAGMA-CF. A Quantitative Structural Lung Disease Computed Tomography Outcome in Young Children with Cystic Fibrosis. Am J Respir Crit Care Med 2015;191:1158–1165. [DOI] [PubMed] [Google Scholar]
- 9.Dickerhof N, Pearson JF, Hoskin TS, Berry LJ, Turner R, Sly PD, Kettle AJ, AREST CF. Oxidative stress in early cystic fibrosis lung disease is exacerbated by airway glutathione deficiency. Free Radic Biol Med 2017;113:236–243. [DOI] [PubMed] [Google Scholar]
- 10.Esther CR, Turkovic L, Rosenow T, Muhlebach MS, Boucher RC, Ranganathan S, Stick SM, AREST CF. Metabolomic biomarkers predictive of early structural lung disease in cystic fibrosis. Eur Respir J 2016;48:1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu T, Park Y, Johnson JM, Jones DP. apLCMS--adaptive processing of high-resolution LC/MS data. Bioinformatics 2009;25:1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T, Jones DP. xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC Bioinformatics 2013;14:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guijas C, Montenegro-Burke JR, Domingo-Almenara X, Palermo A, Warth B, Hermann G, Koellensperger G, Huan T, Uritboonthai W, Aisporna AE, Wolan DW, Spilker ME, Benton HP, Siuzdak G. METLIN: A Technology Platform for Identifying Knowns and Unknowns. Anal Chem 2018;doi: 10.1021/acs.analchem.7b04424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman ALP, Mocatta TJ, Shiva S, Seidel A, Chen B, Khalilova I, Paumann-Page ME, Jameson GNL, Winterbourn CC, Kettle AJ. Ceruloplasmin is an endogenous inhibitor of myeloperoxidase. J Biol Chem 2013;288:6465–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res 2015;43:W251–W257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wickham H ggplot2: Elegant Graphics for Data Analysis, 1st ed. New York, NY: Springer New York; 2009. doi: 10.1007/978-0-387-98141-3. [DOI] [Google Scholar]
- 17.Salinas DB, Sosnay PR, Azen C, Young S, Raraigh KS, Keens TG, Kharrazi M. Benign and Deleterious Cystic Fibrosis Transmembrane Conductance Regulator Mutations Identified by Sequencing in Positive Cystic Fibrosis Newborn Screen Children from California. PLoS ONE 2016;11:e0155624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pattison DI, Davies MJ. Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem Res Toxicol 2001;14:1453–1464. [DOI] [PubMed] [Google Scholar]
- 19.Taylor-Cousar JL, Kessel Von KA, Young R, Nichols DP. Potential of anti-inflammatory treatment for cystic fibrosis lung disease. J Inflamm Res 2010;3:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grasemann H, Ratjen F. Early lung disease in cystic fibrosis. Lancet Respir Med 2013;1:148–157. [DOI] [PubMed] [Google Scholar]
- 21.Thomson E, Brennan S, Senthilmohan R, Gangell CL, Chapman ALP, Sly PD, Kettle AJ. Identifying peroxidases and their oxidants in the early pathology of cystic fibrosis. Free Radic Biol Med 2010;49:1354–1360. [DOI] [PubMed] [Google Scholar]
- 22.Chandler JD, Day BJ. Biochemical mechanisms and therapeutic potential of pseudohalide thiocyanate in human health. Free Radic Res 2015;49:695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnhold J, Monzani E, Furtmüller PG, Zederbauer M, Casella L, Obinger C. Kinetics and Thermodynamics of Halide and Nitrite Oxidation by Mammalian Heme Peroxidases. Eur J Inorg Chem 2006;2006:3801–3811. [Google Scholar]
- 24.Skaff O, Pattison DI, Davies MJ. Hypothiocyanous acid reactivity with low-molecular-mass and protein thiols: absolute rate constants and assessment of biological relevance. Biochem J 2009;422:111–117. [DOI] [PubMed] [Google Scholar]
- 25.Winterbourn CC. The biological chemistry of hydrogen peroxide. Methods Enzymol 2013;528:3–25. [DOI] [PubMed] [Google Scholar]
- 26.Go Y-M, Chandler JD, Jones DP. The cysteine proteome. Free Radic Biol Med 2015;84:227–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn WB, Erban A, Weber RJM, Creek DJ, Brown M, Breitling R, Hankemeier T, Goodacre R, Neumann S, Kopka J, Viant MR. Mass appeal: metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics 2013;9:44–66. [Google Scholar]
- 28.Wang B, Shi Z, Weber GF, Kennedy MA. Introduction of a new critical p value correction method for statistical significance analysis of metabonomics data. Anal Bioanal Chem 2013;405:8419–8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zang X, Monge ME, McCarty NA, Stecenko AA, Fernández FM. Feasibility of Early Detection of Cystic Fibrosis Acute Pulmonary Exacerbations by Exhaled Breath Condensate Metabolomics: A Pilot Study. J Proteome Res 2017;16:550–558. [DOI] [PubMed] [Google Scholar]
- 30.Kettle AJ, Turner R, Gangell CL, Harwood DT, Khalilova IS, Chapman AL, Winterbourn CC, Sly PD, on behalf of AREST CF. Oxidation contributes to low glutathione in the airways of children with cystic fibrosis. Eur Respir J 2014;erj01702–2013.doi: 10.1183/09031936.00170213. [DOI] [PubMed] [Google Scholar]
- 31.Day BJ, van Heeckeren AM, Min E, Velsor LW. Role for Cystic Fibrosis Transmembrane Conductance Regulator Protein in a Glutathione Response to Bronchopulmonary. Infect Immun 2004;72:2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kemp M, Go Y-M, Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: A perspective on redox systems biology. Free Radic Biol Med 2008;44:921–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harwood DT, Kettle AJ, Winterbourn CC. Production of glutathione sulfonamide and dehydroglutathione from GSH by myeloperoxidase-derived oxidants and detection using a novel LC–MS/MS method. Biochem J 2006;399:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuschillo S, De Felice A, Balzano G. Mucosal inflammation in idiopathic bronchiectasis: cellular and molecular mechanisms. Eur Respir J 2008;31:396–406. [DOI] [PubMed] [Google Scholar]
- 35.Polineni D, Dang H, Gallins PJ, Jones LC, Pace RG, Stonebraker JR, Commander LA, Krenicky JE, Zhou Y-H, Corvol H, Cutting GR, Drumm ML, Strug LJ, Boyle MP, Durie PR, Chmiel JF, Zou F, Wright FA, O’Neal WK, Knowles MR. Airway Mucosal Host Defense Is Key to Genomic Regulation of Cystic Fibrosis Lung Disease Severity. Am J Respir Crit Care Med 2018;197:79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corvol H, Blackman SM, lle P-YBE, Gallins PJ, Pace RG, Stonebraker JR, Accurso FJ, Clement A, Collaco JM, Dang H, Dang AT, Franca A, Gong J, Guillot L, Keenan K, Li W, Lin F, Patrone MV, Raraigh KS, Sun L, Zhou Y-H, Neal WKOR, Sontag MK, Levy H, Durie PR, Rommens JM, Drumm ML, Wright FA, Strug LJ, et al. Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nature Commun 2015;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dang H, Gallins PJ, Pace RG, Guo X-L, Stonebraker JR, Corvol H, Cutting GR, Drumm ML, Strug LJ, Knowles MR, O’Neal WK. Novel variation at chr11p13 associated with cystic fibrosis lung disease severity. Hum Genome Var 2016;3:16020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee BC, Lee HM, Kim S, Avanesov AS, Lee A, Chun B-H, Vorbruggen G, Gladyshev VN. Expression of the methionine sulfoxide reductase lost during evolution extends Drosophila lifespan in a methionine-dependent manner. Sci Rep 2018;8:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee BC, Le DT, Gladyshev VN. Mammals reduce methionine-S-sulfoxide with MsrA and are unable to reduce methionine-R-sulfoxide, and this function can be restored with a yeast reductase. J Biol Chem 2008;283:28361–28369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konstan MW, VanDevanter DR, Sawicki GS, Pasta DJ, Foreman AJ, Neiman EA, Morgan WJ. Association of High-Dose Ibuprofen Use, Lung Function Decline, and Long-Term Survival in Children with Cystic Fibrosis. Annals ATS 2018;doi: 10.1513/AnnalsATS.201706-486OC. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen VG, Webster RO. Inhibition of human polymorphonuclear leukocyte functions by ibuprofen. Immunopharmacology 1987;13:61–71. [DOI] [PubMed] [Google Scholar]
- 42.Koelsch M, Mallak R, Graham GG, Kajer T, Milligan MK, Nguyen LQ, Newsham DW, Keh JS, Kettle AJ, Scott KF, Ziegler JB, Pattison DI, Fu S, Hawkins CL, Rees MD, Davies MJ. Acetaminophen (paracetamol) inhibits myeloperoxidase-catalyzed oxidant production and biological damage at therapeutically achievable concentrations. Biochem Pharmacol 2010;79:1156–1164. [DOI] [PubMed] [Google Scholar]
- 43.Tidén A-K, Sjögren T, Svensson M, Bernlind A, Senthilmohan R, Auchère F, Norman H, Markgren P-O, Gustavsson S, Schmidt S, Lundquist S, Forbes LV, Magon NJ, Paton LN, Jameson GNL, Eriksson H, Kettle AJ. 2-thioxanthines are mechanism-based inactivators of myeloperoxidase that block oxidative stress during inflammation. J Biol Chem 2011;286:37578–37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casaril AM, Ignasiak MT, Chuang CY, Vieira B, Padilha NB, Carroll L, Lenardão EJ, Savegnago L, Davies MJ. Selenium-containing indolyl compounds: Kinetics of reaction with inflammation-associated oxidants and protective effect against oxidation of extracellular matrix proteins. Free Radic Biol Med 2017;113:395–405. [DOI] [PubMed] [Google Scholar]
- 45.Conrad C, Lymp J, Thompson V, Dunn C, Davies Z, Chatfield B, Nichols D, Clancy J, Vender R, Egan ME, Quittell L, Michelson P, Antony V, Spahr J, Rubenstein RC, Moss RB, Herzenberg LA, Goss CH, Tirouvanziam R. Long-term treatment with oral N-acetylcysteine: affects lung function but not sputum inflammation in cystic fibrosis subjects. A phase II randomized placebo-controlled trial. J Cyst Fibros 2015;14:219–227. [DOI] [PubMed] [Google Scholar]
- 46.Griese M, Kappler M, Rietschel E, Hartl D, Hector A. Inhalation Treatment with Glutathione in Patients with Cystic Fibrosis. A Randomized Clinical Trial. Am J Respir Crit Care Med 2013;188:83–89. [DOI] [PubMed] [Google Scholar]
- 47.Chandler JD, Min E, Huang J, McElroy CS, Dickerhof N, Mocatta T, Fletcher AA, Evans CM, Liang L, Patel M, Kettle AJ, Nichols DP, Day BJ. Antiinflammatory and Antimicrobial Effects of Thiocyanate in a Cystic Fibrosis Mouse Model. Am J Respir Cell Mol Biol 2015;53:193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


