Abstract
Objective
In the SOLANA trial, we sought to physiologically characterize benralizumab’s onset of effect and maintenance of that effect for patients with severe eosinophilic asthma.
Methods
SOLANA (NCT02869438) was a multicenter, randomized, double-blind, parallel-group, placebo-controlled, Phase IIIb study conducted at 49 centers in six countries (Chile, Germany, Hungary, the Philippines, South Korea, and the United States). Eligible patients with baseline blood eosinophil counts ≥300 cells/µL were randomized to subcutaneous benralizumab (30 mg) or placebo administered at Days 0, 28, and 56. The primary endpoint was the average change from baseline in prebronchodilator forced expiratory volume in 1 s (pre-BD FEV1) during the Day 28‒Day 84 period for benralizumab vs placebo. Secondary endpoints included patient-reported outcomes (PROs). A subset of patients participated in a whole-body plethysmography substudy. Safety was also assessed.
Results
In total, 233 patients were randomized to benralizumab (n=118) or placebo (n=115). Improvement from baseline in pre-BD FEV1 with benralizumab 30 mg was not statistically significant compared with placebo (least-squares mean change difference [95% confidence interval] 57 mL [−22 to 135]; p=0.16). Compared with placebo, benralizumab demonstrated early (Day 7) nonstatistically significant improvements in whole-body plethysmography assessments of hyperinflation and clinically meaningful improvements in PRO measures (Asthma Control Questionnaire 6 at Day 14 and St. George’s Respiratory Questionnaire at Day 28), which were maintained over the treatment period. Benralizumab’s safety profile was commensurate with previously reported studies.
Conclusion
The observed early changes in lung volume despite relatively small improvements in airflow obstruction suggest that the anti-inflammatory effect of benralizumab may be manifested as deflation over time for patients with hyperinflation, who potentially have a greater degree of airway remodeling. This early effect could partially explain the rapid PRO improvements observed for certain patients.
Keywords: anti–IL-5Rα, benralizumab, interleukin-5, randomized controlled trial, eosinophilic, severe asthma
Plain Language Summary
In Phase III clinical trials, benralizumab reduced the occurrence of asthma attacks and improved lung function and symptom control for patients with severe, uncontrolled asthma. Despite high-dosage inhaled maintenance treatment, asthma had remained uncontrolled for these patients at entry into these clinical trials. Patients also had eosinophilic inflammation in the lungs, a type of inflammation that involves eosinophils, a kind of white blood cell. In the Phase III SOLANA trial, we investigated how quickly improvements with benralizumab therapy occurred and how long they lasted for these patients. In SOLANA, benralizumab substantially depleted eosinophils at Day 3, and this depletion was maintained through Day 84. Based on patients’ reports of their asthma symptoms and health-related quality of life, we found that benralizumab resulted in improvements as early as 7 days after patients began treatment. Patients also experienced early changes in lung function, although they had only relatively small improvements in airflow.
Introduction
Benralizumab is an interleukin-5 receptor α‒directed cytolytic monoclonal antibody that has been demonstrated to reduce asthma exacerbations and improve lung function and disease control for patients with severe, uncontrolled eosinophilic asthma.1,2 Improvements in asthma-related symptoms have also been observed.3 Despite some heterogeneity observed in Phase III studies of benralizumab, some patients with severe, uncontrolled eosinophilic asthma report rapid improvement of symptoms (as early as 3 days) following initiation of benralizumab treatment.3 It is unclear what leads to this improvement, but some of the factors that have been considered are: (a) rapid improvement in airflow obstruction, (b) rapid improvement in hyperinflation indices, and/or (c) a decrease in the pulmonary inflammatory milieu. Whether one or a combination of these factors plays an important role in rapid symptom improvement has yet to be established. Benralizumab induces a direct, rapid, and nearly complete depletion of blood eosinophils (a surrogate marker for airway eosinophils) via enhanced antibody-dependent cell-mediated cytotoxicity.4–6 The maximum effect of benralizumab on blood eosinophils occurs within approximately 24 hrs of administration for patients with asthma.6,7 We hypothesized that the rapid depletion of eosinophils would result in improvements in airflow obstruction and hyperinflation and would ultimately be reflected in patient-reported outcomes (PROs).
We sought to physiologically characterize benralizumab’s onset of effect and maintenance of effect on lung function, blood eosinophil counts, asthma control metrics, and health-related quality of life for patients with severe, uncontrolled eosinophilic asthma in the SOLANA (NCT02869438) trial, a 12-week, Phase IIIb study. A subset of patients also participated in a whole-body plethysmography substudy, in which we investigated the effect of benralizumab on hyperinflation parameters.
Materials and Methods
Study Design and Participants
SOLANA was a multicenter, randomized, double-blind, parallel-group, placebo-controlled, Phase IIIb study conducted at 49 centers in six countries (Chile, Germany, Hungary, the Philippines, South Korea, and the United States).
An independent ethics committee at each trial center or central institutional review boards approved the study protocol. The study was conducted in accordance with the principles of the Declaration of Helsinki, and all patients provided written informed consent to participate.
Following a 5-week screening/run-in period, eligible patients were randomized to a 12-week treatment period and received benralizumab 30 mg or placebo subcutaneously at Day 0, Day 28 (±3 days), and Day 56 (±3 days). An end-of-treatment (EOT) visit was conducted at Day 84 (Week 12), and a follow-up visit was conducted at Day 112 (Week 16; Figure 1).
Figure 1.
SOLANA study design. BD, bronchodilator; FEV1, forced expiratory volume in 1 s; FU, follow-up; SC, subcutaneously; V, Visit.
Included patients were men and women aged 18–75 years who weighed ≥40 kg and who had physician-diagnosed severe asthma requiring treatment with inhaled corticosteroids (ICS)/long-acting β2-agonists (LABA) for ≥30 days before enrollment. Patients were required to have peripheral blood eosinophil counts ≥300 cells/µL assessed by a central laboratory at Visit 1 and at least two documented asthma exacerbations requiring systemic corticosteroid therapy or a temporary increase in maintenance oral corticosteroid (OCS) dosage within 12 months before enrollment.
Additional inclusion criteria included prebronchodilator (pre-BD) forced expiratory volume in 1 s (FEV1) <80% predicted at screening (Visit 2/Visit 3); a documented post-BD FEV1 reversibility ≥12% and ≥200 mL demonstrated at Visit 1, 2, or 3; and an Asthma Control Questionnaire 6 (ACQ-6) score ≥1.5 at enrollment.
Patients with a clinically important pulmonary disease other than asthma, life-threatening asthma (within 12 months before Visit 1), or upper or lower respiratory tract infections requiring antibiotics or antiviral medication (within 30 days before Visit 1) were excluded. Patients were also excluded if they had a ≥20% change in mean pre-BD FEV1 at randomization (Visit 4) from the mean of pre-BD FEV1 values recorded at Visit 2 and Visit 3.
Randomization and Masking
Eligibility criteria were confirmed at Visit 4 (Day 0) prior to randomization. Enrolled patients were randomized in a 1:1 ratio to benralizumab 30 mg or placebo. Patients received benralizumab or placebo subcutaneously via an accessorized prefilled syringe every 4 weeks for three doses, at Day 0, Day 28 (±3 days), and Day 56 (±3 days). Placebo solution was visually matched with the benralizumab solution. Patients were stratified by geographic region and whether they entered the whole-body plethysmography substudy. Randomization codes were assigned sequentially in each stratum as patients became eligible for randomization. All patients and investigators were blinded to treatment allocation.
Study Assessments
Spirometry to ascertain lung function via pre-BD FEV1 and pre-BD forced vital capacity (FVC) was performed by the investigator according to American Thoracic Society/European Respiratory Society guidelines8 at screening and at Days 0 (prior to randomization), 3, 7, 14, 28, 56, 84, and 112. Airway inflammation was evaluated with a standardized, single-breath fractional exhaled nitric oxide (FeNO) test on Days 0, 3, 7, 14, 28, 56, and 84.9 Forced expiratory flow at 25–75% of FVC (FEF25–75%) was also assessed. Vital signs, blood eosinophil counts, pharmacokinetics (assessed pre-dose at dosing visits), use of concomitant medications, and adverse events (AEs) were also assessed at every visit.
PRO assessments included asthma symptom score; ACQ-6 score at enrolment (Visit 1), baseline (Visit 4; Day 0), and Days 14–84 (Visits 7–10); and St. George’s Respiratory Questionnaire (SGRQ) score at baseline (Visit 4; Day 0) and Days 28–84 (Visits 8–10).
Outcomes
The primary endpoint in SOLANA was least-squares (LS) mean difference between benralizumab and placebo for change in pre-BD FEV1 from baseline (Visit 4) based on the average of pre-BD FEV1 values at Day 28 (Visit 8), Day 56 (Visit 9), and Day 84 (Visit 10).
Pre-BD FEV1 was also measured at Days 3, 7, and 14 to characterize the onset of effect of benralizumab. Other secondary endpoints included change from baseline to EOT in blood eosinophil counts and FeNO test results as well as time-point assessments of pre-BD FEV1, pre-BD FVC, ACQ-6 score (Days 0, 14, 28, 56, 84), and SGRQ score (Days 0, 28, 56, 84). FEF25–75% was assessed as a post-hoc analysis. Safety outcomes included AEs, serious adverse events (SAEs), laboratory variables, electrocardiograms, vital signs, and immunogenicity.
Whole-Body Plethysmography Substudy
Additional assessments were performed for a subset of 40 patients at designated sites in Germany (n=19), Hungary (n=12), and the Philippines (n=9) to determine the effect of benralizumab on the change in lung function over time through whole-body plethysmography. Patients entering the substudy were required to have FEV1 reversibility ≥12% and ≥200 mL at Visit 1 or Visit 2 and residual volume (RV) ≥125% predicted at Visit 3.
The primary efficacy variable in the substudy was change in RV from baseline to EOT (Visit 10). Secondary variables were changes from baseline to EOT in lung volume subdivisions, including total lung capacity (TLC), RV/TLC ratio, inspiratory capacity (IC), functional residual capacity (FRC), and vital capacity (VC).
Statistical Analysis
The target sample size was determined based on the benralizumab Phase IIb MI-CP220 study10 and the benralizumab Phase III asthma studies (SIROCCO, CALIMA).1,2 A sample size of approximately 115 patients per treatment arm (230 in total) was expected to provide approximately 90% power for the primary endpoint, pre-BD FEV1, with type I error controlled at a two-sided alpha level of 0.05 if the true average over mean treatment difference (benralizumab–placebo) from baseline over Days 28 (Visit 8), 56 (Visit 9), and 84 (Visit 10) was 138 mL. This sample size would also be sufficient to have approximately 80% power for an average over mean treatment difference of 120 mL. Within-group standard deviation for assumed variability was 375 mL, and within-patient correlation was 0.6.
All efficacy analyses were performed with an intention-to-treat approach based on the full analysis set. This set included all randomized patients who received either benralizumab or placebo and continued study participation regardless of protocol adherence. The safety analysis set included all patients who received at least one dose of study treatment. All analyses were conducted with SAS version 9.4.
Subgroup analyses were performed with factors such as OCS use at baseline, patient characteristics, and prior exacerbations to explore the uniformity of the detected overall treatment effect on the primary efficacy variable and PROs.
The primary endpoint of change from baseline in FEV1 was analyzed via a mixed model for repeated measures (MMRM), adjusted for treatment group, baseline, region, visit, and treatment×visit interaction as fixed effects. The variance–covariance matrix was assumed to be unstructured. A similar modeling approach was used for other continuous variables. FEV1 treatment response was also modeled with an exponential model over time with a Bayesian method as a supportive analysis to further describe the onset of effect.
ACQ-6 and SGRQ responders were defined as patients with ≥0.5- and ≥4-point decreases from baseline in scores, respectively. The percentages of ACQ-6 and SGRQ responders at the post-baseline visit were analyzed with a logistic regression model for repeated measures, fitted with the generalized estimating equations (GEE) method with the same baseline covariates included as in the MMRM model described above. P-values for all analyses are nominal, with no adjustment for multiplicity.
Results
Patients were recruited from November 9, 2016, to March 9, 2018. The study was completed on August 1, 2018. In total, 721 patients were enrolled. Of these, 233 were randomized and 233 (100%) received treatment; 118 (51%) patients received benralizumab 30 mg and 115 (49%) received placebo (Figure S1). A total of 228 (98%) patients completed the study. Two patients discontinued from the benralizumab treatment group after receiving the first dose. Reasons for withdrawal were hypersensitive reaction to benralizumab and malaise with palpitations.
Demographics and baseline patient characteristics were mostly similar between treatment groups (Table 1). However, the percentage of patients who were morbidly obese (defined as body mass index >35 kg/m2) was lesser in the benralizumab group than in the placebo group (4% [n=5] vs 17% [n=19], respectively). Baseline lung function, as well as key respiratory and other disease characteristics, were similar between groups (Table 1). Most patients were female (63% [n=74] and 72% [n=83] of the benralizumab and placebo groups, respectively), and the average age was 51.4 years.
Table 1.
Demographics and Baseline Clinical Characteristics
| Benralizumab 30 mg (n=118) | Placebo (n=115) | |
|---|---|---|
| Mean age, years (SD) | 51.9 (13.62) | 50.9 (12.34) |
| Sex, n (%) | ||
| Male | 44 (37) | 32 (28) |
| Female | 74 (63) | 83 (72) |
| Race, n (%) | ||
| White | 69 (59) | 67 (58) |
| Black or African-American | 3 (3) | 4 (3) |
| Asian | 39 (33) | 40 (35) |
| Othera | 7 (6) | 4 (3) |
| Ethnic group, n (%) | ||
| Hispanic or Latino | 31 (26) | 31 (27) |
| Not Hispanic or Latino | 87 (74) | 84 (73) |
| Mean weight, kg (SD) | 72.7 (16.00) | 75.9 (18.80) |
| Mean BMI, kg/m2 (SD) | 26.70 (4.56) | 28.65 (6.43) |
| >35 kg/m2, n (%) | 5 (4.2) | 19 (16.5) |
| Mean blood eosinophil count, cells/µL (SD) | 434.3 (233.44) | 441.9 (258.29) |
| Mean pre-BD FEV1, mL (SD) | 1740 (636) | 1655 (556) |
| Mean pre-BD FEV1, % PN (SD) | 60.4 (14.6) | 59.6 (14.4) |
| Mean pre-BD FEV1/FVC, % (SD) | 64 (12) | 64 (12) |
| Mean reversibility, % (SD) | 30.7 (17.0) | 32.6 (21.2) |
| Number of patients with asthma diagnosis, n (%) | 118 (100) | 115 (100) |
| Number of exacerbations in the last 12 months, mean (SD) | 2.5 (1.27) | 2.4 (0.83) |
| Number of exacerbations in the last 12 months, n (%) | ||
| 2 | 93 (79) | 86 (75) |
| 3 | 14 (12) | 18 (16) |
| 4 | 5 (4) | 7 (6) |
| ≥5 | 6 (5) | 4 (3) |
| Number of exacerbations resulting in hospitalization in the last 12 months, mean (SD) | 0.1 (0.35) | 0.1 (0.37) |
| Mean ACQ-6 score (SD) | 2.65 (0.88) | 2.61 (0.89) |
Notes: Baseline was defined as the last nonmissing value before the first dose of study treatment. Reversibility (%)=([baseline post-BD FEV1−baseline pre-BD FEV1]×100)/baseline pre-BD FEV1. aIncluded in the electronic case report forms race categories “Native Hawaiian or other Pacific Islander,” “American Indian or Alaska Native,” and “Other.”
Abbreviations: ACQ-6, Asthma Control Questionnaire 6; BD, bronchodilator; BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PN, predicted normal; SD, standard deviation.
Median baseline blood eosinophil count was 400 cells/µL. Although all enrolled patients had eosinophil counts ≥300 cells/µL at screening, 70 patients (35 in each group) had eosinophil counts <300 cells/µL at randomization.
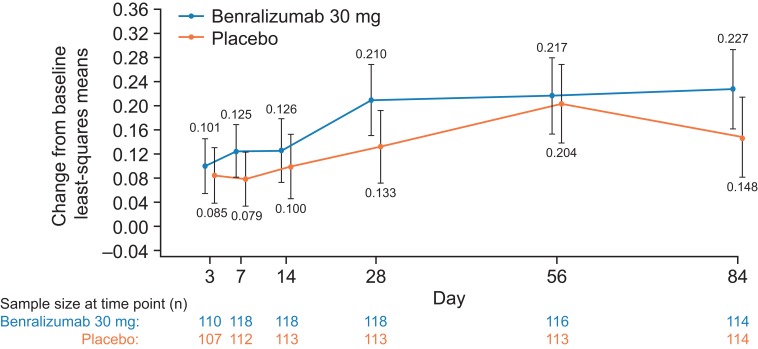
The primary efficacy outcome was the mean difference between benralizumab and placebo for average change from baseline in pre-BD FEV1 at Days 28, 56, and 84. There was a numerical improvement in LS mean change from baseline through Day 84 (EOT) in pre-BD FEV1 for benralizumab and placebo groups (Figure 2). Treatment with benralizumab 30 mg resulted in an improvement from baseline in pre-BD FEV1 compared with placebo (LS mean change difference [95% confidence interval {CI}] 57 mL [−22 to 135]; p=0.16). A large placebo effect was observed at Day 56, which may have influenced the average treatment effect over Days 28–84 (the primary endpoint).
Figure 2.
Change from Baseline in Prebronchodilator FEV1 (L) by Time Point (Full Analysis Set). Error bars represent 95% confidence intervals. P-values were from repeated measure analysis. FEV1, forced expiratory volume in 1 s; n, number of patients with data at the specified visit.
Figure S2 provides the exponential model fit of pre-BD FEV1 change from baseline estimated. The model estimated that the median time to reach a change of 150 mL was shorter for patients receiving benralizumab than for those receiving placebo (13 vs 49 days, respectively). Similarly, median estimated time to reach a change of 200 mL was shorter for the benralizumab group than for the placebo group (36 vs >84 days, respectively). The percentage of pre-BD FEV1 responders (defined as patients who had improvements in FEV1 at the following cutoffs: 50, 100, 150, 200, 250, and 300 mL) with benralizumab was numerically greater compared with placebo at all time points and cutoff values, with the exception of Day 3 at cutoff values of 50 mL and 300 mL (range for improvements with benralizumab compared with placebo: −2% to 15%).
Improvements in pre-BD FVC were observed at all time points for the benralizumab-treated group, although no improvements reached nominal significance compared with placebo (Table S1). Over the treatment period (Days 28, 56, and 84), average LS mean change from baseline difference in pre-BD FVC with benralizumab compared with placebo was 63 mL (95% CI −20 to 147; nominal p=0.14).
Small increments in FeNO change from baseline were observed for patients treated with benralizumab (Table S1). Over the treatment period (Days 28, 56, and 84), average LS mean change from baseline in FeNO with benralizumab compared with placebo was 8.1 ppb (95% CI 0.1–16.1; nominal p=0.048; Table S1).
Improvements in FEF25–75% were also observed at all time points for the benralizumab-treated group. However, no improvements reached nominal significance compared with placebo (Table S1). During the treatment period (Days 28, 56, and 84), average LS mean change from baseline difference in FEF25–75% with benralizumab compared with placebo was 69 mL (95% CI −38 to 177; nominal p=0.20).
Treatment with benralizumab 30 mg demonstrated clinically meaningful improvements that reached nominal statistical significance in asthma control and health-related quality of life, assessed by ACQ-6 and SGRQ scores, respectively. These improvements occurred early, at the first post-treatment assessment (Day 14 and Day 28 for ACQ-6 and SGRQ scores, respectively), remained consistent over time, and remained nominally significant compared with placebo over the treatment period (Table 2).
Table 2.
Change from Baseline in Health-Related Quality of Life Endpoints (Full Analysis Set)
| Benralizumab 30 mg (n=118) | Placebo (n=115) | |
|---|---|---|
| ACQ-6 score | ||
| Baseline | n=118 | n=115 |
| Mean (SD) | 2.647 (0.877) | 2.613 (0.893) |
| Change from baseline at Day 14 | n=118 | n=113 |
| Mean (SD) | −0.989 (0.901) | −0.665 (0.837) |
| LSM difference (95% CI) | −0.293 (−0.481 to −0.105) | |
| P-value | 0.0024 | |
| Change from baseline at Day 28 | n=118 | n=113 |
| Mean (SD) | −1.126 (0.947) | −0.693 (0.869) |
| LSM difference (95% CI) | −0.402 (−0.609 to −0.195) | |
| P-value | 0.0002 | |
| Change from baseline at Day 56 | n=115 | n=113 |
| Mean (SD) | −1.164 (1.132) | −0.827 (1.023) |
| LSM difference (95% CI) | −0.312 (−0.554 to −0.070) | |
| P-value | 0.012 | |
| Change from baseline at Day 84 | n=114 | n=113 |
| Mean (SD) | −1.355 (1.146) | −0.867 (1.114) |
| LSM difference (95% CI) | −0.472 (−0.731 to −0.213) | |
| P-value | 0.0004 | |
| Average change from baseline (Days 28, 56, and 84) | n=118 | n=113 |
| LSM difference (95% CI) | −0.395 (−0.603 to −0.188) | |
| P-value | 0.0002 | |
| SGRQ total score | ||
| Baseline | n=118 | n=115 |
| Mean (SD) | 51.327 (18.221) | 51.218 (19.305) |
| Change from baseline at Day 28 | n=118 | n=113 |
| Mean (SD) | −16.956 (15.510) | −9.444 (14.136) |
| LSM difference (95% CI) | −7.229 (−10.832 to −3.626) | |
| P-value | 0.0001 | |
| Change from baseline at Day 56 | n=115 | n=113 |
| Mean (SD) | −19.941 (21.528) | −13.802 (16.705) |
| LSM difference (95% CI) | −5.942 (−10.538 to −1.346) | |
| P-value | 0.012 | |
| Change from baseline at Day 84 | n=114 | n=113 |
| Mean (SD) | −23.343 (20.302) | −14.385 (18.836) |
| LSM difference (95% CI) | −8.599 (−13.300 to −3.898) | |
| P-value | 0.0004 | |
| Average change from baseline (Days 28, 56, and 84) | n=118 | n=113 |
| LSM difference (95% CI) | −7.257 (−11.133 to −3.380) | |
| P-value | 0.0003 | |
Notes: LSM, least-squares means difference between benralizumab vs placebo based on repeated-measures analyses. Estimate of the mean change from baseline at each day in the active treatment group was compared with the placebo group via a repeated-measures analysis. Estimates were least-squares means. The model was: change from baseline in ACQ-6 score/SGRQ total score=treatment+baseline ACQ-6 score/SGRQ total score+region+visit+treatment×visit. ACQ-6 score was defined as the average of the first six items of the ACQ questionnaire on symptoms, activity limitations, and rescue medication. Scores range from 0 (totally controlled) to 6 (severely uncontrolled). SGRQ scores were expressed as a percentage of overall impairment; 100 represented the worst possible health status and 0 indicated the best possible health status. Baseline was defined as the last nonmissing value prior to randomization.
Abbreviations: ACQ-6, Asthma Control Questionnaire 6; LS, least-squares; SGRQ, St. George’s Respiratory Questionnaire.
Patient-reported asthma control improved with benralizumab compared with placebo, as measured by average change from baseline in ACQ-6 score over Days 28, 56, and 84 (LS mean difference for benralizumab vs placebo [95% CI] −0.395 [−0.603 to −0.188]; nominal p=0.0002; Table 2). This improvement was consistent over the study period, with improvements in LS mean change from baseline in ACQ-6 score observed at every assessment time (Days 14, 28, 56, and 84) compared with placebo (all nominal p≤0.012; Table 2). The percentage of ACQ-6 responders was greater in the benralizumab group than in the placebo group at Day 28 (90 [76%] vs 70 [61%]; odds ratio [OR] 2.20; 95% CI 1.18–4.09; nominal p=0.013) and Day 84 (95 [81%] vs 73 [63%]; OR 2.54; 95% CI 1.35–4.76; nominal p=0.0038). Post-hoc analyses found that the percentage of patients with well-controlled asthma (ACQ-6 score ≤0.75) was consistently greater in the benralizumab group compared with the placebo group from Day 14 (OR [95% CI]: 2.57 [1.12–5.89]; nominal p=0.025) through to Day 84 (OR [95% CI]: 2.78 [1.46–5.30]; nominal p=0.0019; Table S2). Results were similar for the percentage of patients who had either well- or partially controlled asthma (ACQ-6 score ≤1.5) at Day 14 (OR [95% CI]: 1.98 [1.14–3.43]; nominal p=0.015) and Day 84 (OR [95% CI]: 2.21 [1.24–3.94]; nominal p=0.0071).
The impact of disease on overall patient health, as measured by LS mean change from baseline in SGRQ total score, was consistently improved with benralizumab compared with placebo over the treatment period (all nominal p≤0.012; Table 2). The LS mean difference for benralizumab compared with placebo, in average change from baseline over Days 28, 56, and 84, was −7.257 (95% CI −11.133 to −3.380; nominal p=0.0003). Of patients receiving benralizumab, ≥75% were SGRQ responders over Days 28–84. Patients who received benralizumab were 2.41 times more likely to respond, as assessed by SGRQ, at Day 28 than patients who received placebo (93 [79%] vs 71 [62%]; 95% CI 1.33–4.37; nominal p=0.0039). The response rate for the benralizumab group remained high at Days 56 and 84 but was not nominally significant compared with placebo possibly because of the greater response rate for the placebo group at these time points (Table 2).
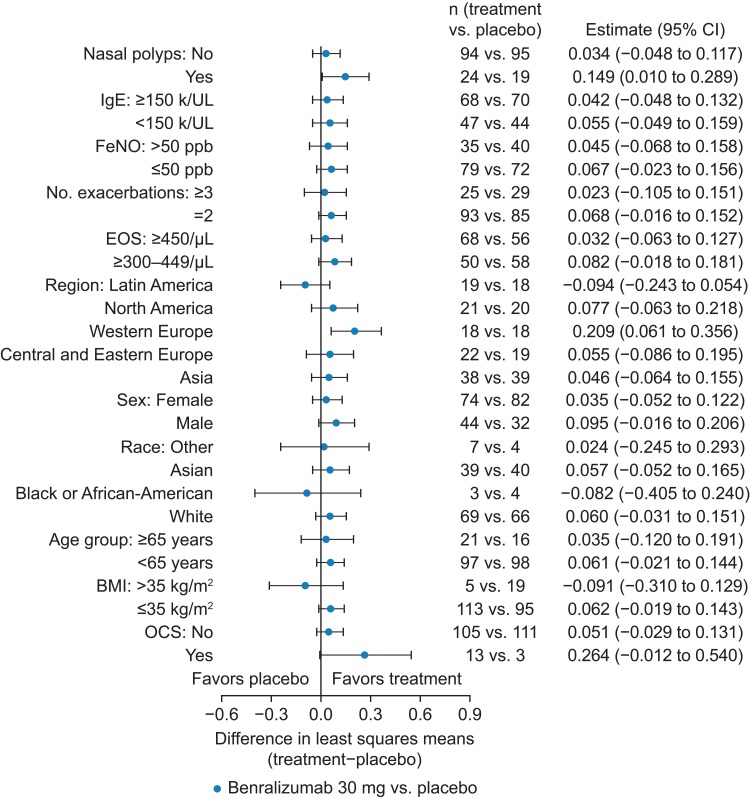
Exploratory subgroup analysis was performed for the primary efficacy variable (not powered to assess efficacy within any predefined subgroup). Change from baseline in pre-BD FEV1 by subgroup exhibited an enhanced response for patients with nasal polyps (149 mL; 95% CI 10–289) and for those receiving OCS (264 mL; 95% CI −12 to 540) (Figure 3). However, the number of patients receiving OCS was greater in the benralizumab group than in the placebo group (n=13 vs n=3, respectively). Benralizumab treatment response varied between geographic regions (Figure 3). Patients in western Europe had the greatest treatment response, whereas there was a large placebo effect for patients in Asia and Latin America. Post-hoc subgroup analysis of ACQ-6 and SGRQ results indicated that the point estimates for the improvements in ACQ-6 and SGRQ scores demonstrated a consistent treatment effect favoring benralizumab over placebo for all subgroups (Figures S3, and S4), although some subgroups were small, thereby limiting definitive conclusions. An enhanced SGRQ response was observed for patients with nasal polyps, as supported by a nominally significant treatment×nasal polyps interaction test p-value of 0.036.
Figure 3.
Change from Baseline in Prebronchodilator FEV1 (L) by Subgroup (Full Analysis Set). Average change from baseline over Days 28, 56, and 84. BMI, body mass index; CI, confidence interval; EOS, eosinophil; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; IgE, immunoglobulin E; OCS, oral corticosteroids.
Median pharmacokinetic steady state for the benralizumab group was achieved by Day 56; values for Cmax and AUC(0–28 days) were consistent with previous studies. Blood eosinophil depletion was observed for the benralizumab group at Day 3 (median change from baseline: −94%) and was maintained through Day 84 (median change from baseline: −92%). Minimal change in blood eosinophil counts was observed for the placebo group throughout the study. At all time points (all nominal p<0.0001), greater reductions in blood eosinophil counts (LS mean percentage change from baseline) were observed for the benralizumab group than for the placebo group (Table S3), with no evidence of eosinophils returning at Day 112 (12 weeks after last dose).
Additional assessments to determine the effect of benralizumab on lung function, as measured by whole-body plethysmography, were performed for a subset of 40 patients (benralizumab n=18; placebo n=22). The size of this substudy was not powered to detect statistical significance. The MMRM model did not provide a good fit to the data; therefore, results are reported as descriptive summaries (Table 3). Patient characteristics, baseline lung function, respiratory disease characteristics, and baseline PROs were similar to those of the overall population. However, a lesser percentage of patients in the benralizumab group than in the placebo group had a body mass index >25 kg/m2 (39% [7] vs 68% [15], respectively), and the percentage predicted normal pre-BD FEV1 also was smaller (54.9% vs 63.5%, respectively). Mean reversibility was similar between treatment groups (33% vs 32% for benralizumab and placebo, respectively). Improvements in lung function for patients who participated in the whole-body plethysmography substudy were consistent with those observed in the main study (Table S4). However, in contrast to the main study, improvements in PROs as assessed by ACQ-6 and SGRQ scores were comparable between benralizumab and placebo groups (Table S4). The small sample size of the substudy may contribute to this difference.
Table 3.
Results of the Whole-Body Plethysmography Substudy
| Lung Volume Subdivision | Day | n, Benralizumab vs Placebo | Change From Baseline | |
|---|---|---|---|---|
| Benralizumab 30 mg | Placebo | |||
| RV [mL], mean (SD) | 7 | 17 vs 20 | −266 (405) | −17 (365) |
| 14 | 18 vs 18 | −276 (327) | −66 (451) | |
| 84 | 18 vs 21 | −415 (609) | −208 (528) | |
| FRC [mL], mean (SD) | 7 | 17 vs 20 | −319 (522) | 22 (318) |
| 14 | 18 vs 18 | −202 (478) | 102 (280) | |
| 84 | 18 vs 21 | −394 (783) | 93 (466) | |
| IC [mL], mean (SD) | 7 | 17 vs 20 | 124 (454) | −167 (571) |
| 14 | 18 vs 18 | 77 (771) | 94 (922) | |
| 84 | 18 vs 21 | 119 (447) | −268 (603) | |
| VC [mL], mean (SD) | 7 | 17 vs 20 | 68 (404) | −131 (587) |
| 14 | 18 vs 18 | 149 (629) | 262 (955) | |
| 84 | 18 vs 21 | 139 (245) | 33 (676) | |
| TLC [mL], mean (SD) | 7 | 17 vs 20 | −198 (604) | −144 (375) |
| 14 | 18 vs 18 | −127 (559) | 196 (868) | |
| 84 | 18 vs 21 | −276 (677) | −175 (418) | |
| RV/TLC | 7 | 17 vs 20 | −0.032 (0.056) | 0.004 (0.066) |
| 14 | 18 vs 18 | −0.043 (0.082) | −0.030 (0.090) | |
| 84 | 18 vs 21 | −0.050 (0.056) | −0.026 (0.087) |
Notes: Baseline was defined as the last nonmissing value prior to randomization.
Abbreviations: FRC, functional residual capacity; IC, inspiratory capacity; RV, residual volume; SD, standard deviation; TLC, total lung capacity; VC, vital capacity.
In the whole-body plethysmography substudy, benralizumab treatment resulted in a greater and earlier decrease in RV compared with placebo (Table 3), with treatment difference favoring benralizumab at Day 7 (mean baseline change −266 mL benralizumab vs −17 mL placebo) and all time points through to Day 84 (mean baseline change −415 mL benralizumab vs −208 mL placebo). For the benralizumab group, mean change from baseline to Day 84 in IC was 119 mL compared with −268 mL for the placebo group. The magnitude and direction of the treatment effect on RV are in line with a clinically meaningful reduction of hyperinflation. The combination of this decrease and the corresponding increase in IC supports a potential reduction of hyperinflation and improvement in patients’ breathing patterns following benralizumab treatment initiation.
Treatment differences for TLC over the treatment period were comparable between benralizumab and placebo, with small and not clinically meaningful decreases observed for both groups (mean change from baseline in TLC ranged from −109 mL to −276 mL and −184 mL to 196 mL, respectively). In addition, minimal changes were observed from baseline in RV/TLC (Table 3).
At Day 84, VC increased by 139 mL for patients receiving benralizumab compared with a lesser increase of 33 mL for patients receiving placebo. FRC decreased by 394 mL for the benralizumab group and increased by 93 mL for the placebo group.
The safety profile reported in this study is similar to that observed in previous benralizumab studies.1,2 A total of 115 patients (49%) experienced an on-study AE. Incidence of AEs was smaller for the benralizumab group than for the placebo group (n=56 [47%] vs n=59 [51%], respectively; Table 4). No deaths were reported during the study. The most common AEs were asthma (n=30 [13%]), nasopharyngitis (n=14 [6%]), and upper respiratory tract infection (n=12 [5%]). The majority of on-study AEs were mild or moderate.
Table 4.
Summary of Adverse Events (Safety Analysis Set)
| Benralizumab 30 mg (n=118) | Placebo (n=115) | Total (n=233) | |
|---|---|---|---|
| Any AE | 56 (47) | 59 (51) | 115 (49) |
| Any AE with outcome of death | 0 | 0 | 0 |
| Any SAE (including events with outcome of death) | 1 (1) | 7 (6) | 8 (3) |
| Any AE leading to discontinuation of benralizumab | 2 (2) | 0 | 2 (1) |
| Most common AEs (frequency of ≥3% in any treatment group) | |||
| Asthma | 11 (9) | 19 (17) | 30 (13) |
| Nasopharyngitis | 8 (7) | 6 (5) | 14 (6) |
| Upper respiratory tract infection | 6 (5) | 6 (5) | 12 (5) |
| Bronchitis | 6 (5) | 3 (3) | 9 (4) |
| Headache | 5 (4) | 2 (2) | 7 (3) |
| Sinusitis | 1 (1) | 4 (3) | 5 (2) |
Note: Data are n (%).
Abbreviations: AE, adverse event; SAE, serious adverse event.
Fewer on-study SAEs were reported by patients receiving benralizumab than by patients receiving placebo (n=1 [1%] vs n=7 [6%], respectively). However, two patients receiving benralizumab discontinued treatment because of AEs, and none in the placebo group discontinued. Overall, a positive anti-drug antibody response was observed for ≤6% of patients (7/117 and 2/114 for benralizumab and placebo, respectively); this had no clear effect on safety.
Discussion
In the SOLANA study, treatment with benralizumab 30 mg resulted in numerically greater improvements in lung function, as measured by pre-BD FEV1 (improvement up to 79 mL), at all time points compared with placebo. There was also a greater percentage of pre-BD FEV1 responders in the benralizumab group than in the placebo group. Furthermore, treatment with benralizumab favored increases compared with placebo in pre-BD FVC, with improvements of up to 92 mL. However, for the primary endpoint, benralizumab did not demonstrate a statistically significant improvement in pre-BD FEV1 LS mean change from baseline over the treatment period (average of Days 28, 56, and 84) compared with placebo. This result is not consistent with results reported for two large Phase III trials of benralizumab, SIROCCO (48 weeks) and CALIMA (56 weeks). In these studies over a longer treatment period, benralizumab was associated with a significant improvement in pre-BD FEV1 compared with placebo for patients with severe, uncontrolled eosinophilic asthma.1,2 In SIROCCO, LS mean difference in pre-BD FEV1 change from baseline at Week 48 vs placebo was 106 mL and 159 mL (p<0.05) for patients receiving benralizumab 30 mg every 4 weeks (Q4W) and every 8 weeks (Q8W; first three doses Q4W), respectively.1 The corresponding values for CALIMA were 125 mL and 116 mL (p<0.05) for benralizumab 30-mg Q4W and Q8W, respectively.2 Differences in inclusion criteria between this study and the SIROCCO and CALIMA studies may have contributed to the decreased magnitude of the observed effect on FEV1. In SOLANA, inclusion criteria included FEV1 stability (≥20% change from run-in period to randomization in pre-BD FEV1), and patients in the whole-body plethysmography substudy were required to have hyperinflation (RV ≥125%). This may have resulted in the selection of patients who were less responsive to changes in airway obstruction than patients in the previous trials because of more extensive airway remodeling and greater hyperinflation. Other aspects that may have contributed to the more modest effect on FEV1 in SOLANA than in previous studies include the fact that 70 patients had a decrease in peripheral blood eosinophil count, from ≥300 cells/µL at screening to <300 cells/µL at randomization.
Despite the observed lesser effect on FEV1 than in previous studies, benralizumab treatment was consistently associated with improvement in PROs and disease symptoms across subgroups. These improvements were observed from Day 14 and remained consistent and nominally significant over all time points evaluated. This result is in agreement with an analysis of pooled PROs data for patients with severe, uncontrolled eosinophilic asthma from the SIROCCO/CALIMA Phase III trials.3 This analysis reported a reduction in rescue medication use by as early as 3 days following benralizumab treatment initiation and decreased activity impairment by Week 2.3 The whole-body plethysmography substudy found that benralizumab treatment was also associated with early improvements in hyperinflation indices. From as early as Day 7, mean change from baseline in RV was −266 mL for the benralizumab group compared with −17 mL for the placebo group. This early deflation effect was also evidenced by decrements in FRC and TLC, which were numerically greater for the benralizumab group compared with placebo. Improvements in IC and VC were also concomitantly observed.
The relatively small number of patients in the SOLANA whole-body plethysmography substudy precludes strong conclusions based on these results. However, the early deflation despite relatively small improvements in FEV1 provides some insights into the effect of benralizumab on lung function. For patients with greater hyperinflation and who potentially have a greater degree of airway remodeling, the anti-inflammatory effect of benralizumab may be manifested as deflation over time. This early effect could partially explain improvements in ACQ-6 and SGRQ scores despite only modest improvements observed in airway obstruction as measured by FEV1. Further studies in a larger patient population are required to confirm these results.
Benralizumab treatment was consistently associated with improvements in PROs throughout the SOLANA trial. Patients receiving benralizumab had odds of achieving well-controlled asthma 2.6 to 5.1 times greater than patients receiving placebo at Day 14 through Day 84 (all nominal p≤0.025), assessed by ACQ-6 score. In addition, of patients receiving benralizumab, ≥75% were SGRQ responders (≥4-point decrease in score from baseline) over Days 28–84. Patients receiving benralizumab had odds of responding 2.4 times greater than patients receiving placebo at Day 28. These PRO results demonstrate that patients derived benefit from benralizumab treatment in this study that was not directly linked with an improvement in FEV1. It should also be noted that PROs incorporate a period of recall (2 weeks for ACQ-6, 4 weeks for SGRQ) and therefore encompass patient experience over a period of time, rather than assessment at a specific time point. These PRO results are consistent with previous studies that have suggested no direct correlation between FEV1 responses and PRO results for patients with asthma.11
Subgroup analysis in this study supports a substantial difference in pre-BD FEV1 from baseline and enhanced improvement in SGRQ score for patients with nasal polyps receiving benralizumab compared with placebo. An enhanced treatment effect for patients with nasal polyps receiving benralizumab has been previously reported. In the SIROCCO and CALIMA trials, benralizumab-treated patients with nasal polyps demonstrated greater improvements in lung function and ACQ-6 than patients without nasal polyps.12
Benralizumab treatment was associated with small increments in FeNO change from baseline at each time point through Day 28 and over the maintenance period. These small increases were not considered clinically meaningful and did not correspond with PRO changes. These results are consistent with previously published studies that have found no association between decreased FeNO and the depletion or reduction of peripheral blood eosinophils resulting from treatment with mepolizumab or corticosteroids.13,14 Therefore, FeNO does not appear to be a useful tool for selecting patients who may benefit from anti-eosinophil treatments or for monitoring the efficacy of these treatments.
In this study, benralizumab treatment resulted in the depletion of eosinophils at Day 3 that was maintained through Day 84. Minimal changes in blood eosinophil counts were observed for the placebo group over the course of the study. Safety results were consistent with those from previous benralizumab studies. Benralizumab was well-tolerated with no new safety concerns observed.
Conclusion
In conclusion, treatment with benralizumab 30 mg resulted in a nonstatistically significant improvement from baseline in lung function over the maintenance period, as assessed by pre-BD FEV1, FVC, and whole-body plethysmography, for patients with severe eosinophilic asthma and previous asthma exacerbations. Improvements in PROs were observed early and across all post-baseline time points for patients receiving benralizumab vs placebo, with all treatment differences reaching nominal significance. Improvements in hyperinflation indices and IC were numerically greater for patients receiving benralizumab vs placebo. These changes occurred early after treatment initiation, and deflation may explain some of the early improvements observed in PROs.
Acknowledgments
The authors thank the investigators, health care providers, research staff, patients, and caregivers who participated in the SOLANA study (Appendix 1). We also thank Nick Adye (AstraZeneca, Cambridge, UK) and Anna Gawrysiak (AstraZeneca, Warsaw, Poland) for their clinical operations leadership in this study. Writing and editing assistance, including preparation of a draft manuscript under the direction and guidance of the authors, incorporating author feedback, and manuscript submission, was provided by Debra Scates (JK Associates, Inc., Conshohocken, PA, USA) and Michael A. Nissen (AstraZeneca, Gaithersburg, MD, USA). This support was funded by AstraZeneca.
Funding Statement
Funding for this study was provided by AstraZeneca. The funder of the study collaborated in the study design, data collection, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Abbreviations
ACQ-6, Asthma Control Questionnaire 6; AE, adverse event; CI, confidence interval; EOT, end of treatment; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; FRC, functional residual capacity; FVC, forced vital capacity; GEE, generalized estimating equations; IC, inspiratory capacity; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; LS, least-squares; MMRM, mixed model for repeated measures; OCS, oral corticosteroids; OR, odds ratio; pre-BD, prebronchodilator; PRO, patient-reported outcome; RV, residual volume; SAE, serious adverse event; SGRQ, St. George’s Respiratory Questionnaire; TLC, total lung capacity; VC, vital capacity.
Data-sharing statement
Data underlying the findings described in this manuscript may be requested in accordance with AstraZeneca’s data-sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home.
Author Contributions
Reynold Panettieri, Peter Barker, Richard F. Olsson, Ubaldo J. Martin, and the funder of this study participated in the study design. Reynold Panettieri, Tobias Welte, Kartik Shenoy, Stephanie Korn, Margret Jandl, Edward Kerwin, and Rosa Feijoo participated in the study and data collection. All authors substantially analyzed and interpreted the data, drafted or provided critical review of the manuscript, approved submission of the final version of the manuscript for publication, and are accountable for the accuracy and integrity of the work.
Disclosure
Reynold A Panettieri reports grants and/or personal fees from AstraZeneca, Theravance, Avillion, Sanofi/Regeneron, MedImmune, RIFM, Equillium, Genentech, and Oncoarendi, during the conduct of the study. Reynold A Panettieri also receives grants and personal fees from AstraZeneca, Equillium, MedImmune, and Sanofi/Regeneron; personal fees from Avillion, Genentech, Novartis, Teva, Theravance, and Boehringer Ingelheim; and grants from Oncoarendi, Theratrophix, Amgen, RFIM, Vertex, Bristol Myers Squibb, Genentech, Gilead, and Boston Scientific, outside of the submitted work. Tobias Welte has received honoraria for lectures and advisory boards from AstraZeneca, Berlin Chemie, Chiesi, GlaxoSmithKline, Sanofi Aventis, and Novartis, and his institution has received research grants from AstraZeneca, Chiesi, GlaxoSmithKline, and Novartis. Kartik V Shenoy has participated in consulting, advisory boards, or received travel reimbursement from AstraZeneca and GlaxoSmithKline. Stephanie Korn has received honoraria for lectures and advisory board attendance from AstraZeneca, GlaxoSmithKline, Novartis, Sanofi, and Teva. Margret Jandl has received honoraria for lectures from AstraZeneca. Edward M Kerwin has participated in consulting, advisory boards, speaker panels, or received travel reimbursement from Amphastar, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Mylan, Novartis, Oriel, Pearl, Sunovion, Teva, and Theravance. He has also conducted multicenter clinical research trials for approximately 40 pharmaceutical companies. Rosa Feijoo reports personal fees from AstraZeneca, during the conduct of the study. Peter Barker, Richard F Olsson, and Ubaldo J Martin are employees of AstraZeneca. The authors report no other conflicts of interest in this work.
References
- 1.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled Phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi: 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 2.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi: 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- 3.Quinn S, Xu X, Hirsch I. Daily patient-reported health status assessment improvements with benralizumab for patients with severe, uncontrolled eosinophilic asthma. J Asthma Allergy. 2019;12:21–33. doi: 10.2147/JAA.S190221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125(6):1344–1353. doi: 10.1016/j.jaci.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 5.Pham TH, Damera G, Newbold P, Ranade K. Reductions in eosinophil biomarkers by benralizumab in patients with asthma. Respir Med. 2016;111:21–29. doi: 10.1016/j.rmed.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 6.Busse WW, Katial R, Gossage D, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a Phase I study of subjects with mild asthma. J Allergy Clin Immunol. 2010;125(6):1237–1244. doi: 10.1016/j.jaci.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 7.Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132(5):1086–1096. doi: 10.1016/j.jaci.2013.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–161. doi: 10.1183/09031936.05.00034505 [DOI] [PubMed] [Google Scholar]
- 9.American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med. 2005;171(8):912–930. doi: 10.1164/rccm.200406-710ST [DOI] [PubMed] [Google Scholar]
- 10.Castro M, Wenzel SE, Bleecker ER, et al. Benralizumab, an anti-interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med. 2014;2(11):879–890. doi: 10.1016/S2213-2600(14)70201-2 [DOI] [PubMed] [Google Scholar]
- 11.Werner CU, Linde K, Schäffner J, Storr C, Schneider A. Weekly self-measurement of FEV1 and PEF and its impact on ACQ (asthma control questionnaire)-scores: 12-week observational study with 76 patients. NPJ Prim Care Respir Med. 2017;27:64. doi: 10.1038/s41533-017-0064-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FitzGerald JM, Bleecker ER, Menzies-Gow A, et al. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med. 2018;6(1):51–64. doi: 10.1016/S2213-2600(17)30344-2 [DOI] [PubMed] [Google Scholar]
- 13.Sweeney J, Brightling CE, Menzies-Gow A, Niven R, Patterson CC, Heaney LG. Clinical management and outcome of refractory asthma in the UK from the British thoracic society difficult asthma registry. Thorax. 2012;67(8):754–756. doi: 10.1136/thoraxjnl-2012-201869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X [DOI] [PubMed] [Google Scholar]