We identified a small molecule that, at picomolar concentrations, rescues mutant CFTR chloride channel from protein degradation.
Abstract
F508del, the most frequent mutation causing cystic fibrosis (CF), results in mistrafficking and premature degradation of the CFTR chloride channel. Small molecules named correctors may rescue F508del-CFTR and therefore represent promising drugs to target the basic defect in CF. We screened a carefully designed chemical library to find F508del-CFTR correctors. The initial active compound resulting from the primary screening underwent extensive chemical optimization. The final compound, ARN23765, showed an extremely high potency in bronchial epithelial cells from F508del homozygous patients, with an EC50 of 38 picomolar, which is more than 5000-fold lower compared to presently available corrector drugs. ARN23765 also showed high efficacy, synergy with other types of correctors, and compatibility with chronic VX-770 potentiator. Besides being a promising drug, particularly suited for drug combinations, ARN23765 represents a high-affinity probe for CFTR structure-function studies.
INTRODUCTION
The F508del mutation is the most frequent among patients with cystic fibrosis (CF) (1). F508del severely impairs the folding and stability of the CF transmembrane conductance regulator (CFTR) protein, a chloride channel expressed in the apical membrane of various epithelia in the respiratory, gastrointestinal, and reproductive systems (1–2). As a consequence, F508del-CFTR protein trafficking is stopped at the endoplasmic reticulum where it is largely degraded through the ubiquitin-proteasome pathway (3, 4). Peripheral quality control systems are also involved in F508del-CFTR degradation if the protein reaches the plasma membrane (5). F508del also causes a defect in CFTR channel gating that is evident as a reduced time spent in the open (ion-conducting) state (6). F508del mutation, in the homozygous condition or in combination with other types of severe mutations, is associated with a classical form of CF, characterized by progressive loss of respiratory function, pancreatic insufficiency, and infertility (1). These problems are due to defective epithelial secretion of chloride and bicarbonate, which results in the production of dense mucus secretions that clog the airways and exocrine gland ducts. An additional characteristic of patients with severe CF mutations is the high NaCl concentration (>100 mM) in sweat, a defect caused by impairment of chloride reabsorption by the sweat gland duct (1).
The trafficking defect caused by F508del can be rescued by small molecules called correctors (7). Various high-throughput screening campaigns have led to the identification of correctors that rescue F508del-CFTR activity (8–12). The mechanism of action of these compounds has not yet been defined, but they may act by directly binding to F508del-CFTR or by modulating other proteins involved in the cell quality control system or the protein trafficking machinery. Only a few molecules have progressed to clinical trial stage. Most of the other correctors lack adequate efficacy, specificity, and consistent activity in different cell contexts. Two drugs have been approved so far for the rescue of F508del-CFTR, i.e., Orkambi and Symdeko, both developed by Vertex Pharmaceuticals. Orkambi consists of a combination of the corrector VX-809 (10) and the potentiator VX-770 (13). The potentiator is included to address the gating defect and, hence, to maximize the rescue of F508del-CFTR function. However, Orkambi treatment is associated with a relatively modest clinical benefit in patients homozygous for F508del (14) and no effect at all, at least on pulmonary function, on individuals with a single copy of the mutation (15). These results contrast with the high clinical benefit obtained by the potentiator VX-770 alone on patients having mutations (e.g., G551D) that only cause a gating defect (16). The insufficient efficacy of corrector therapy in vivo has been explained with the particular resistance of F508del trafficking defect to treatment with a single agent since the mutation not only causes multiple defects to protein stability and folding (17–20) but also may be due to specific liabilities of VX-809 when administered in vivo. Symdeko was recently approved by the U.S. Food and Drug Administration and consists of the combination of the corrector VX-661 (tezacaftor) and the potentiator VX-770. As for Orkambi, the efficacy of Symdeko in F508del homozygous patients was smaller than that of VX-770 in patients with the G551D mutation (21). Another possible reason for the limited efficacy of corrector-potentiator combinations tested so far is the “anti-corrector” effect that VX-770 seems to cause when administered chronically in vitro (22, 23). More recently, better clinical results have been obtained with triple combinations including the potentiator VX-770 and two correctors having complementary mechanisms of action, i.e., VX-661 together with VX-445 or VX-659 (24, 25). The triple combinations were designed to include VX-661 and not VX-809 since the latter one was reported to have a relatively high frequency of adverse events (26).
In this study, we report the discovery and biological characterization of novel and potent F508del correctors. Starting from a hit identified by high-throughput screening of a carefully selected compound library, an iterative process of chemical synthesis and functional/biochemical evaluation of hit analogs in multiple cell systems, including bronchial epithelial cells from patients with CF, allowed us to obtain a lead compound that rescued F508del-CFTR at low nanomolar concentrations. Further elaboration of the chemical structure of the lead compound led to the discovery of ARN23765, a corrector with picomolar potency and high efficacy over a 3–log unit concentration range.
RESULTS
For the primary screening, we used a carefully selected collection of compounds, which was assembled starting from an initial set of ca. 300,000 commercially available molecules. A series of progressively more stringent filters were applied to discard compounds with suboptimal drug-like properties and with core chemical structures known to bind to multiple target proteins or that are extensively exploited in medicinal chemistry. A subsequent stepwise clustering protocol based on an unweighted pair group method with arithmetic mean hierarchical agglomerative algorithm (27) allowed us to select 11,334 maximally diverse compounds.
The whole chemical library was screened in parallel on two cell lines, FRT and CFBE41o−, both stably expressing F508del-CFTR (28, 29). Cells also expressed the halide-sensitive yellow fluorescent protein (HS-YFP), which allows evaluation of F508del-CFTR activity in the cell membrane by calculating the fluorescence quenching rate resulting from iodide influx (30). The search for correctors was done by treating the cells with each compound at 5 μM (CFBE41o−) or 10 μM (FRT) for 24 hours. The screening on each cell line was run in duplicate using two separate cell preparations. Each microplate also contained corr-4a (5 μM) and VX-809 (1 μM) as positive controls. The Z factor (31) was satisfactory for all screenings: 0.5 for FRT cells and 0.65 for CFBE41o− cells. Figure 1A summarizes the results obtained for both cell types. It can be easily appreciated that FRT cells showed a much larger number of putatively active compounds, a feature that is consistent with a more relaxed cell quality control system. We considered as hits all compounds whose activity, calculated from the two rounds of the screening, was higher than a threshold resulting from the average plus 4 SDs of activity measured in vehicle-treated cells. This threshold corresponds to 125% (FRT) or 135% (CFBE41o−) of vehicle-treated cells. In this way, we selected 117 hits in FRT and 11 hits in CFBE41o− cells. Two compounds appeared as hits in both cell types. All 126 compounds were retested on FRT and CFBE41o− at different concentrations to confirm activity and obtain information on potency and efficacy. A large fraction of correctors identified in the FRT screening was confirmed when tested in the same cells. However, in agreement with the primary screening results, most FRT hits were inactive in CFBE41o− cells. The two hits that were found in both FRT and CFBE41o− screenings were confirmed when retested with the HS-YFP assay, and three additional compounds, which were initially missed in the CFBE41o− screening, were found to be active in both cell types. Figure 1B shows dose-response relationships for the five active compounds, tested as correctors on FRT and CFBE41o− cells. Incubation with these compounds at micromolar concentrations modestly increased the function of F508del-CFTR. These compounds were subsequently evaluated on primary CF bronchial epithelial cells homozygous for the F508del mutation (Fig. 1C). Cells differentiated under air-liquid condition on porous membranes were treated by including correctors (5 to 10 μM) or vehicle alone in the basolateral medium for 24 hours. After treatment, the epithelia were mounted in a perfusion chamber to measure short-circuit current. After blocking the epithelial sodium channel ENaC with amiloride (10 μM), cells were stimulated with chlorophenylthio–adenosine 3′,5′-cyclic monophosphate (CPT-cAMP) (100 μM) plus VX-770 (1 μM) to maximally activate F508del-CFTR. The resulting current was then inhibited with the potent CFTR inhibitor-172 (inh-172; 10 μM). The drop in transepithelial current elicited by inh-172 was taken as the parameter reflecting F508del-CFTR function in the apical membrane. Only one compound, ARN5562, was effective, leading to a rescue of F508del-CFTR equivalent to nearly 60% of that achieved with VX-809 (Fig. 1C). ARN5562 was also evaluated in Western blot experiments. In these experiments, the electrophoretic mobility of F508del-CFTR reports the extent of maturation and trafficking. Mutant CFTR migrates as “band B” (at approximately 150 kDa) that corresponds to the core-glycosylated immature form of the protein. Correctors improve the trafficking of the protein from the endoplasmic reticulum to the Golgi, thus allowing full glycosylation and appearance of “band C” (at approximately 170 kDa), the prevalent form in cells expressing wild-type CFTR. Figure 1D shows that ARN5562 is effective in improving F508del-CFTR maturation, although less than VX-809.
Fig. 1. Search of F508del-CFTR correctors by high-throughput screening.
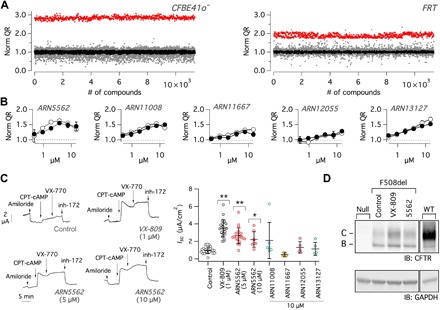
(A) Summary of results obtained by screening the entire chemical library on CFBE41o− and FRT cells expressing F508del-CFTR and the HS-YFP. The graphs report the normalized HS-YFP quenching rate (QR), reflecting CFTR-dependent iodide influx, for cells treated with test compounds (gray dots), VX-809 (red dots), and vehicle [dimethyl sulfoxide (DMSO); black dots]. (B) Dose-response relationships for compounds that, when retested, showed activity on both CFBE41o− (open circles) and FRT (filled circles) cells. (C) Representative traces and summary of results obtained from short-circuit current recordings on F508del/F508del bronchial epithelial cells. Data reported in the scatter dot plot are the amplitude of the current blocked by inh-172 (10 μM) in epithelia treated with the indicated compounds. *P < 0.05 and **P < 0.01 versus control [analysis of variance (ANOVA) with Dunnett’s post hoc test]. (D) Analysis of F508del-CFTR maturation by immunoblot (IB). FRT cells were treated with vehicle (DMSO), VX-809 (1 μM), or ARN5562 (5 μM). Lysates from null FRT or FRT cells expressing wild-type (WT) CFTR were also included in the analysis. The images are representative of n = 3 similar experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
The promising results obtained with ARN5562 prompted us to start a medicinal chemistry campaign with the goal to improve potency and efficacy. Changes were introduced into the structure of ARN5562 to explore the structure-activity relationships (SARs) in the rescue of F508del-CFTR activity for this class of compounds. At each round of synthesis, all compounds were first tested at multiple concentrations on FRT and CFBE41o− with the HS-YFP assay. Then, the active compounds were evaluated with the transepithelial electrical conductance (TEEC) assay on FRT cells. Last, the best compounds resulting from HS-YFP and TEEC assays were tested on CF bronchial epithelial cells and in Western blot experiments.
Figure 2A shows the structure of hit ARN5562 and of two key compounds that were discovered during the optimization process: the advanced hit ARN21586, and the lead compound ARN22081. The chemical evolution of hit ARN5562 focused primarily on its right end part, i.e., the benzodioxane ring, and left end part, i.e., the 3,5-dimethyl-4-chloropyrazol-1-yl moiety. The key compounds identified in the SAR study and the data obtained in the HS-YFP assay in F508del-FRT cells are reported in Table 1. The full SAR study will be reported elsewhere. Replacement of the benzodioxane with a benzodioxole ring on the right end part of ARN5562 and introduction of a trifluoromethyl group at position 3 of the pyrazolyl moiety led to ARN21586, an analog with improved efficacy and potency. The extensive exploration of substituents on the pyrazolyl ring resulted in the identification of the 4-methoxybenzoic acid at position 5 as a group imparting good potency and efficacy. Combining this group with a gem-difluoro-benzodioxole ring on the right end part of the ARN21586 scaffold yielded the lead compound ARN22081.
Fig. 2. Hit to lead optimization.
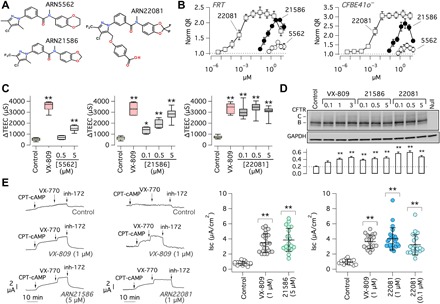
(A) Chemical structures of the initial hit ARN5562, the advanced hit ARN21586, and the lead compound ARN22081. (B) Dose-response relationships for the three compounds determined with the HS-YFP assay in FRT and CFBE41o− cells. Data are presented as means ± SD (n = 4 to 6). (C) Evaluation of correctors in FRT cells by TEEC. The box plot graphs report the ∆TEEC value, i.e., the change in TEEC caused by the CFTR inhibitor (PPQ102) following maximal stimulation of CFTR with forskolin plus genistein. *P < 0.05 and **P < 0.01 (ANOVA with Dunnett’s post hoc test; n = 7 to 10). (D) Analysis of F508del-CFTR maturation with Western blot experiments. CFBE41o− cells expressing F508del-CFTR were treated with vehicle or correctors at the indicated concentrations (in μM). The bar graph (bottom) reports the densitometric analysis (means ± SD, n = 3) of band C normalized to GAPDH. **P < 0.01 versus control (ANOVA with Dunnett’s post hoc test). (E) Representative traces and summary of results obtained from short-circuit current recordings on F508del/F508del bronchial epithelial cells. **P < 0.01 versus control (ANOVA with Dunnett’s post hoc test).
Table 1. Chemical structure, maximal efficacy (Emax), and potency data of ARN5562 and selected analogs in F508del-CFTR FRT cells (HS-YFP assay).
The Emax values are normalized to the activity with DMSO (negative control).
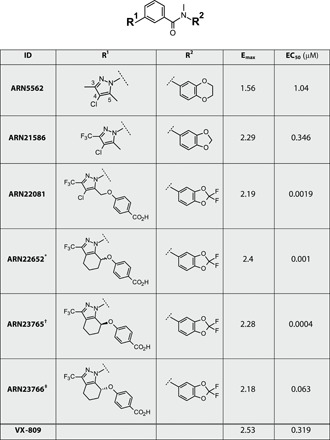 | ||||
Figure 2B shows dose-response relationships obtained for the three key compounds with the HS-YFP assay. It can be seen that a marked improvement in efficacy and potency was achieved. While the initial hit ARN5562 was only modestly effective at micromolar concentrations, ARN22081 markedly rescued F508del-CFTR with single-digit nanomolar potency (Fig. 2B). This extent of activity was confirmed with the TEEC assay (Fig. 2C). This assay exploits the ability of FRT cells on porous membranes to form epithelia with very high electrical resistance. Rescue of F508del-CFTR to the plasma membrane generates a pathway for transepithelial flux of chloride ions that results in an increase in CFTR-dependent TEEC, a parameter that can be measured with chopstick electrodes. A detailed description of this method is reported elsewhere (32). Figure 2C shows that ARN5562 has a limited ability to correct F508del-CFTR, compared to VX-809, which is only achieved at 5 μM. A progressive improvement in potency and efficacy is observed with ARN21586 and ARN22081. With the latter compound, a correction similar to that of VX-809 is obtained with submicromolar concentrations. These results were confirmed at the protein level with Western blot experiments (Fig. 2D) and at the functional level with short-circuit current experiments done on CF primary bronchial epithelial cells (Fig. 2E). In these experiments, it can be appreciated that ARN22081 rescues mutant CFTR maturation and function to an extent that is comparable to that obtained with VX-809.
Further medicinal chemistry efforts on ARN22081 led to the discovery of the tetrahydroindazole analog ARN22652, a racemate retaining the maximal efficacy and potency of the parent compound. Separation of ARN22652 components by chiral column chromatography yielded the two enantiomers, ARN23765 and ARN23766 (Fig. 3A). Notably, using the HS-YFP assay (Fig. 3B), the eutomer, ARN23765, showed efficacy comparable to ARN22652 but subnanomolar potency [median effective concentration (EC50) = 0.4 nM], whereas the dystomer, ARN23766, displayed a ca. 150-fold drop in potency (EC50 = 63 nM). The rescue of F508del-CFTR protein by ARN23765 at low concentrations was also observed using Western blot experiments (Fig. 3C). The high potency and efficacy of ARN23765, compared to ARN23766 and the racemate, was confirmed with the TEEC assay on FRT cells (Fig. 3D) and, particularly, with short-circuit current experiments on bronchial epithelial cells from a patient with F508del/F508del (Fig. 3E). Note the marked difference in potency of ARN23765 with respect to VX-809 in bronchial epithelial cells. While VX-809 had an EC50 of nearly 200 nM, ARN23765 showed a value of 38 pM, thus indicating a more than 5000-fold improvement (Fig. 3E). Rescue activity by ARN23765 decreased at 1 μM. However, a rescue close to maximal effect was maintained in the range of concentrations between 0.1 and 100 nM. Besides VX-809, we also tested other correctors including VX-661, FDL-169 (developed by Flatley Discovery Lab), and GLPG2222 (developed by Galapagos). ARN23765 was consistently more potent than the other correctors, with GLPG2222 (EC50 ~ 2 nM) being the one that had the closest properties to our compound (Fig. 3E). Unexpectedly, VX-661 appeared to have a modest corrector activity, 40% of VX-809, only observed at micromolar concentrations.
Fig. 3. Evaluation of ARN23765 as F508del-CFTR corrector.
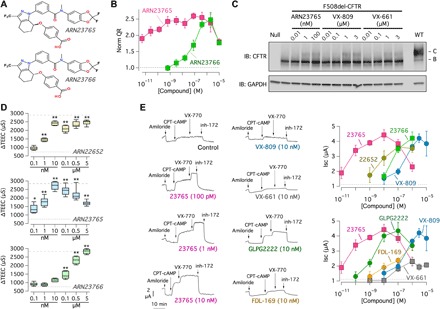
(A) Chemical structures of the two enantiomers ARN23765 and ARN23766. (B) Dose-response relationships for the two compounds (24 hours of treatment) determined with the HS-YFP assay in CFBE41o− cells. Each symbol is the mean ± SD of n = 3 experiments. (C) Western blot analysis of corrector effects at the indicated concentrations (in nM or μM; 24 hours of treatment). The image is representative of n = 3 similar experiments. (D) Evaluation of correctors (0.1 nM to 5 μM; 24 hours) in FRT cells by TEEC (n = 7 to 11). The dashed lines indicate the values for vehicle and VX-809 (1 μM; 24 hours). *P < 0.05 and **P < 0.01 versus vehicle (ANOVA with Dunnett’s post hoc test; n = 6 to 11). (E) Representative traces and summary of data obtained from short-circuit current recordings on F508del/F508del bronchial epithelial cells. The graphs report the means ± SD of amplitude of current drop elicited by inh-172 in epithelia treated with the indicated correctors (24 hours of treatment) and concentrations (n = 10 to 27).
To confirm that ARN23765 activity is not affected by genetic background, we used cells from separate patients homozygous for F508del mutation. For this purpose, we modified the TEEC assay used for FRT cells. In this way, we had a better throughput compared to conventional short-circuit recordings. Besides acquiring TEEC values, we also measured the transepithelial electrical potential difference (PD) at each step. All values were taken at resting, after addition of apical amiloride, after maximal stimulation of F508del-CFTR activity (with forskolin plus genistein), and after block with PPQ102. From TEEC and PD, we calculated the equivalent short-circuit current (Ieq). The results from a typical experiment are shown in Fig. 4A. Treatment with VX-809 (1 μM) or with ARN23765 (10 nM) elicited an increase in the response to the activating cocktail and, correspondingly, to the CFTR blocker with respect to cells treated with the vehicle alone. For each epithelium, we measured the difference in Ieq before and after block with PPQ102 (∆Ieq). These values are reported in Fig. 4B for the different conditions. Tests on cells from four separate patients with F508del/F508del confirmed the results obtained with the conventional short-circuit current technique: Incubation with ARN23765 (10 nM) markedly increased F508del-CFTR function, with results comparable to those of VX-809 (1 μM). We also evaluated epithelia from two patients with a single copy of F508del and a premature stop codon (R553X and G542X) on the other allele (Fig. 4B). ARN23765 was also notably effective on these cells. As expected from the halved amount of F508del-CFTR produced, absolute values of ∆Ieq were smaller than those obtained for F508del/F508del epithelia. We also carried out TEEC/PD measurements on non-CF epithelia (Fig. 4B). In agreement with the expression of wild-type CFTR protein, ∆Ieq values in vehicle-treated cells were more than 10-fold larger compared to those measured in CF epithelia under the same conditions. Treatment of non-CF epithelia with ARN23765, but not with VX-809, elicited a significant increase of wild-type CFTR function (Fig. 4B).
Fig. 4. Characterization of ARN23765 in bronchial epithelial cells.
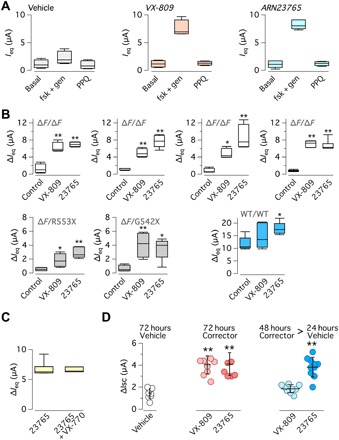
(A) Examples of data obtained with the TEEC/PD technique. Graphs show the values of short-circuit current equivalent (Ieq) calculated from TEEC and PD under basal conditions (already containing amiloride), with forskolin (fsk; 20 μM) plus genistein (gen; 50 μM), and after block with PPQ102 (PPQ; 30 μM). Before measurements, cells were treated for 24 hours with vehicle, VX-809 (1 μM), or ARN23765 (10 nM). Data are from four to six experiments. (B) TEEC/PD data obtained with vehicle, VX-809 (1 μM), and ARN23765 (10 nM) on cells from four different patients with F508del/F508del, two patients with F508del/other mutation, and a non-CF individual. The box plot graphs report ∆Ieq, i.e., the amplitude of the effect of PPQ102. *P < 0.05 and **P < 0.01 (ANOVA with Dunnett’s post hoc test; n = 5 to 6). (C) Chronic treatment of F508del/F508del bronchial epithelial cells with VX-770 (1 μM) plus ARN23765 (10 nM) for 24 hours. For comparison, cells were treated with ARN23765 alone. After treatment, cells were acutely stimulated with forskolin plus genistein as done for the others TEEC/PD experiments. F508del-CFTR activity, measured as ∆Ieq with the TEEC/PD technique, showed no differences between the two conditions (n = 6). (D) Persistence of ARN23765 corrector effect. F508del/F508del bronchial epithelial cells were treated with VX-809 (1 μM) or ARN23765 (1 nM), for 72 hours or for 48 hours followed by 24 hours without compound. F508del-CFTR activity was determined in short-circuit current recordings. **P < 0.01 (ANOVA with Dunnett’s post hoc test).
It has been reported that long-term treatment of cells with corrector-potentiator combinations leads to a reduced rescue of F508del-CFTR (22, 23). This effect has been attributed to an impairment of F508del-CFTR stability caused by VX-770 and other potentiators. To verify whether this effect was observed also with our corrector, we treated F508del/F508del epithelia with ARN23765 (10 nM) plus VX-770 (0.5 μM) for 24 hours. Then, these compounds were removed, and cells were acutely stimulated with forskolin and genistein for the other TEEC/PD experiments. As shown in Fig. 4C, the function of F508del-CFTR (∆Ieq) in cells rescued with ARN23765 alone was the same as that of cells also treated with VX-770 for 24 hours.
Next, we carried out short-circuit current recordings on F508del/F508del epithelia treated with VX-809 (1 μM) or ARN23765 (1 nM) for 72 hours (Fig. 4D). The extent of rescue was comparable to that of epithelia treated with these correctors for only 24 hours (compare with Fig. 3E), indicating that prolonged exposure to ARN23765 did not negatively affect the epithelia. In this set of experiments, we also removed the correctors in the last 24 hours of treatment. Epithelia that had been treated with ARN23765 showed a persistent level of F508del-CFTR function, while rescue was lost in epithelia previously treated with VX-809 (Fig. 4D and fig. S1A). To further confirm these data, we repeated the experiments for a longer time, treating the cells for 120 hours with VX-809 (1 μM) or ARN23765 (1 nM). F508del-CFTR rescue was still maintained for both compounds compared to 72 hours (fig. S1, A and B). In this set of experiments, correctors were also removed in the last 36 hours. F508del-CFTR function was partially maintained in cells treated with ARN23765 but returned to control levels in cells treated with VX-809 (fig. S1B).
To further characterize the corrector activity of ARN23765 on mutant CFTR, we evaluated the stability of F508del-CFTR in CFBE41o− cells following 24 hours of treatment with ARN23765 or with VX-809, followed by addition of cycloheximide to the medium to block protein synthesis. We then lysed cells at different time points, and cell lysates were subjected to SDS–polyacrylamide gel electrophoresis (SDS-PAGE), followed by immunoblotting to evaluate CFTR expression. As shown in Fig. 5 (A and B), the expression of mutant CFTR (both bands B and C) decreases over time. However, treatment with ARN23765 (1 nM) significantly increased, by threefold, the half-life of mature CFTR (band C). Upon treatment with VX-809 (1 μM), the stability of band C was increased by twofold.
Fig. 5. Biochemical evaluation of ARN23765 effects.
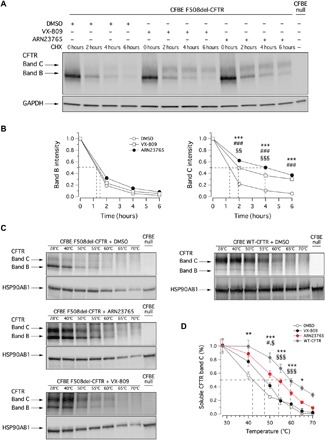
(A) Immunoblot detection of mutant CFTR in whole lysates derived from CFBE41o− cells treated with vehicle alone (DMSO), VX-809 (1 μM), or ARN23765 (1 nM) and at different time points following cycloheximide (CHX)–induced block of protein synthesis. (B) Quantification of mutant CFTR (band B and band C) half-life (n = 3). (C) Conformational stability of mutant CFTR evaluated as thermoaggregation propensity, determined in cell lysates of CFBE41o− cells treated with vehicle alone (DMSO), VX-809 (1 μM), or ARN23765 (1 nM), in comparison to WT-CFTR. (D) Quantification of soluble CFTR band C by densitometry, normalized by HSP90AB1 expression (n = 3). Symbols (B and D) represent statistical significance of ARN23765 versus DMSO (*), VX-809 versus DMSO (#), or ARN23765 versus VX-809 (§). One symbol, P < 0.05; two symbols, P < 0.01; and three symbols, P < 0.001 (ANOVA with Dunnett’s post hoc test).
The conformational stability of mutant CFTR protein under resting conditions and following rescue with correctors was evaluated by determining the denaturation temperature, which induces conversion of detergent-solubilized CFTR into SDS-resistant aggregates (33). Accordingly, F508del-CFTR CFBE41o− cells were treated for 24 hours with ARN23765 or VX-809 and then lysed. In parallel, cell lysates were generated from CFBE41o− cells stably expressing wild-type CFTR. Cell lysates were heat-denatured at 28° to 70°C, and the aggregation-resistant CFTR protein was quantified by SDS-PAGE, followed by Western blotting (Fig. 5, C and D). Treatment with ARN23765 (1 nM) and, to a lower extent, with VX-809 (1 μM) significantly increased the resistance to thermoaggregation of mature CFTR, suggesting that ARN23765 promotes the accumulation of conformationally stabilized complex-glycosylated F508del-CFTR (Fig. 5, C and D).
Recently, Lukacs and collaborators (11) have reported the identification of novel correctors targeting distinct structural defects of CFTR, which synergistically rescue mutant expression and function at the plasma membrane. We were able to retrieve from a commercial source (Life Chemicals) one of the compounds, referred to as 4172, that was classified as a type 3 corrector, according to its mechanism of action (11, 17). Thus, we tested 4172 alone and in combination with ARN23765 or VX-809 on FRT and CFBE41o− cells using the HS-YFP assay (Fig. 6, A and B). The combinations between 4172 and ARN23765 or VX-809 were significantly more effective in increasing CFTR activity than ARN23765 or VX-809 alone, respectively, in both cell models. These results were confirmed using short-circuit current recordings on F508del/F508del bronchial epithelia. Cells were treated for 24 hours with 4172 (10 μM), VX-809 (1 μM), or ARN23765 (10 nM) alone and in combination (Fig. 6, C and D). The combinations between 4172 and ARN23765 or 4172 and VX-809 resulted in a similar extent of rescue, although the concentration used for ARN2765 was 100 times lower than the one used for VX-809 (Fig. 6, C and D). These results suggested that ARN2765 and VX-809 belong to the same class of correctors, i.e., class 1 (11). In agreement with this interpretation, we found no additive effect when the two compounds were combined together (Fig. 6E).
Fig. 6. Analysis of corrector combinations.

(A and B) F508del-CFTR activity determined in CFBE41o− cells (A) or FRT cells (B) with the HS-YFP. Cells were treated with 4172 (10 μM), ARN23765 (10 nM), or VX-809 (1 μM), as single agents or as combinations. ***P < 0.001 (ANOVA with Tukey’s post hoc test). (C and D) Effect of single agents and combinations in F508del/F508del bronchial epithelial cells with the short-circuit current technique. *P < 0.05, **P < 0.01, and ***P < 0.001 (ANOVA with Tukey’s post hoc test). (E) Evaluation of ARN23765/VX-809 combination. The graphs report F508del-CFTR activity (QR) in CFBE41o− cells treated with VX-809 (1 μM), ARN23765 (10 nM), or both. There was no additive effect elicited by the corrector combination. **P < 0.01 versus vehicle (ANOVA with Dunnett’s post hoc test). (F) Evaluation of F508del-CFTR rescue on apical fluid pH. Experiments were done on F508del/F508del cultured bronchial epithelia treated for 24 hours with ARN23765 (10 nM), 4172 (10 μM), or both compounds together. *P < 0.05, **P < 0.01, and ***P < 0.001 (ANOVA with Tukey’s post hoc test).
We evaluated the effect of single correctors and corrector combinations on the pH of the apical fluid of cultured CF bronchial epithelia as a relevant pathophysiological parameter. Both ARN23765 (10 nM) and 4172 (10 μM) elicited a significant increase in pH with respect to control-treated cells (Fig. 6F). The combination of the two correctors further increased pH to a level significantly higher than that measured with each corrector alone (Fig. 6F).
To further characterize ARN23765, we carried out a series of additional experiments. First, we asked whether ARN23765 improves the response of F508del cells to a receptor-mediated physiological stimulus. Therefore, we stimulated the cells with isoprotenerol, a β-adrenergic agonist that, by increasing cytosolic cAMP, should lead to CFTR activation. The response to isoprotenerol, as well as that resulting from subsequent addition of VX-770, was very small in control-treated cells (fig. S2A). In contrast, cells treated with VX-809 (1 μM) or ARN23765 (10 nM) showed relatively large responses to both agents, thus indicating that correctors improve the amount of F508del-CFTR responding to the β-adrenergic stimulus and that the VX-770 potentiator further amplifies this type of response. In agreement with the involvement of CFTR, the addition of inh-172 at the end of recordings caused a current drop that was significantly larger in corrector-treated versus control-treated cells (fig. S2A). Cells treated with correctors were also investigated to assess the extent of Ca2+-activated Cl− secretion and ENaC-dependent Na+ absorption (fig. S2, B and C). The former process was elicited with 100 μM uridine 5′-triphosphate (UTP). The peak of UTP was not modified by treatment with correctors (fig. S2B). ENaC function was estimated from the response to apical amiloride (10 μM). The amplitude was modestly reduced by both VX-809 and ARN23765 (fig. S2C).
As a second type of experiment, we evaluated the possibility that ARN23765 has an additional activity as CFTR potentiator. Cells were treated for 24 hours at low temperature (32°C) or with correctors, VX-809 or ARN23765, to correct the trafficking defect. Then, ARN23765 was acutely added to the cells at various concentrations. There was no increase in activity in contrast to real potentiators genistein and VX-770 (fig. S3, A to C).
We also tested ARN23765 on other types of mutations, N1303K and P574H. N1303K is characterized by severe trafficking and gating defects. In particular, in a previous study, it was found that rescue of N1303K-CFTR function requires the combination of a potentiator plus the co-potentiator ASP-11 (34). We cotransfected human embryonic kidney (HEK) 293 cells with N1303K-CFTR and HS-YFP plasmids. We found that N1303K-CFTR function was significantly stimulated by VX-770 and ASP-11 and that this effect was further amplified by previous incubation with correctors ARN23765 or VX-809 (fig. S4, A and B). To study P574H, we used FRT cells with stable coexpression of this CFTR mutant and HS-YFP. The assay showed that ARN23765 improves the function of P574H-CFTR, particularly in combination with low temperature (fig. S5).
Last, we used a panel of assays to assess the effect of ARN23765 on cell viability. In contrast to positive controls, i.e., celastrol or cytochalasin D, ARN23765 did not impair cell proliferation nor caused cell toxicity (fig. S6, A to C). In addition, to detect potential off-target effects, we outsourced to contract research organizations (Eurofins Cerep and SOLVO Biotechnology) the test of ARN23765 on a panel of relevant biological targets including receptors, monoamine transporters, adenosine 5′-triphosphate–binding cassette (ABC) proteins, and hERG channel. There were no alerting effects of the compound on these targets at a concentration (10 μM) well above the EC50 of ARN23765 as CFTR corrector. As for ABC transporters, at 10 μM, ARN23765 inhibited ABC-B1 (multidrug resistance mutation 1 or P-glycoprotein) and ABC-C2 (multidrug resistance-associated protein 2) by 13 and 27%, respectively.
DISCUSSION
Identification of novel pharmacological correctors of the mistrafficking caused by the F508del mutation is highly needed to develop effective treatments for a large number of patients with CF. These treatments require the combination of small molecules having a complementary mechanism of action since F508del causes multiple defects (11, 17). It is generally accepted that a single agent is not enough to rescue the mutant protein to high levels and to generate a significant clinical benefit.
Here, we used the screening of a maximally diverse chemical library to find novel correctors. We previously showed that corrector activity is often cell type–dependent (35), i.e., correctors identified by screening in a single cell line often fail to be confirmed in other cell lines. Instead, compounds with activity on more than one cell line have a higher probability to be also effective in native airway epithelial cells from patients with CF. For this reason, to maximize the probability of finding real correctors, we decided to perform a double screening in parallel on two cell lines expressing F508del-CFTR, i.e., FRT and CFBE41o−. As expected from previous studies, we found a higher number of active compounds in the FRT cell system compared to CFBE41o−. The small number of hits detected in the bronchial cell line may arise from a more stringent cell quality control system. By retesting all hits in the two cell lines, we found five compounds with activity on both cell systems. One of them, ARN5562, was active in native bronchial epithelial cells. Because of this very promising profile, we concentrated our efforts to improve the efficacy and potency of this molecule.
After several rounds of chemical synthesis and functional/biochemical evaluation with multiple assays, we obtained ARN23765, an extremely potent and highly effective corrector for the F508del mutant protein. Regarding potency, ARN23765 appears as the compound with the lowest EC50 developed so far. Compared to correctors already used in patients with CF, namely, VX-809 and VX-661, it is effective at concentrations that are more than 5000 times lower. Another interesting property of ARN23765 is the lack of interference by VX-770, previously shown to be a critical negative aspect of the latter compound (22, 23). We found no decrease in activity rescue when the cells were chronically treated with both compounds together. Furthermore, ARN23765 elicits a long-term correction that can be observed up to 36 hours after removal of the compound. This behavior was not observed for VX-809 despite the use of a concentration that was 1000 times higher. At the moment, the mechanism explaining the lasting correction produced by ARN23765 is not clear. It may derive from a persistence of the compound in intracellular compartments or from a long-term effect of the compound on mutant CFTR. Future studies are required to elucidate this issue.
Regarding the mechanism of action, the lack of additivity with VX-809 suggests that ARN23765 is a class 1 corrector (17). Comparison of the chemical structures of ARN23765 and the Vertex corrector shows that the two compounds share a common moiety, i.e., the gem-difluorobenzodioxole residue. However, the connectivity of the latter group with the rest of the molecule in ANR23765 is different from that in VX-809. Overall, ARN23765 appears to have less conformational freedom than VX-809. We speculate that, if the two compounds share the same binding site, the higher potency of ARN23765 may derive from (i) a lower energy to achieve the productive binding conformation, (ii) more favorable interactions with critical amino acid side chains at the binding pocket, or a combination of the two. Further studies are required to explain the unexpectedly higher potency of ARN23765 compared with VX-809 and other correctors.
To assess the value of ARN23765 as a possible drug candidate in the CF field, we carried out a series of experiments to determine its effects on other epithelial functions, other CFTR mutants, and cell viability. Treatment of bronchial epithelial with ARN23765 caused an increase in apical fluid pH that was further enhanced when combined with 4172. It has been shown that the apical surface of CF airway epithelia is acidic because of defective CFTR-dependent bicarbonate secretion (36, 37). This abnormality may lead to defective mucociliary clearance and impaired antibacterial defense. Therefore, the change in pH elicited by correctors appears as an effect that may be beneficial on important epithelial functions. We also found that the response to isoprotenerol, a stimulus that mimics physiological activation of CFTR, was significantly improved by ARN23765. We tested ARN23765 and VX-809 on Ca2+-activated Cl− secretion and ENaC-dependent Na+ absorption. While the former process was unaffected, the latter was partially inhibited by both compounds. In the original study on VX-809, it was found that the corrector enhances the amount of fluid on the apical surface, possibly as a result of enhanced fluid secretion and/or decreased ENaC-dependent Na+ absorption (10). Future studies will need to address whether the decrease in ENaC function is a consequence of CFTR rescue. Furthermore, we evaluated the efficacy of ARN23765 on other CF mutations. We found a significant increase in CFTR-dependent function, particularly in combination with other agents. These results suggest that ARN23765 may be used as an adjuvant agent for mutations other than F508del. Last, we performed an initial assessment of ARN23765 safety in vitro. There was no significant effect on cell viability using different assays. Furthermore, no safety alert emerged from a screening on a panel of receptors, transporters, and ion channels. Despite the promising pharmacological profile of ARN23765 in vitro, it needs to be stressed that further studies are needed to rule out potential liabilities (systemic/organ toxicity and/or unsatisfactory pharmacokinetics and pharmacodynamics) that could hinder its use in vivo.
In conclusion, we discovered a small molecule that rescues mutant CFTR with very high potency. This characteristic may be crucial in the future development of therapies requiring combinations of up to three CFTR modulators. In this respect, the use of drugs that work at very low concentrations, thus decreasing the probability of unwanted side effects, would be highly desirable. In addition to potency, ARN23765 appears to have very good efficacy, markedly higher than that of VX-661, which is the class 1 corrector presently included in triple combinations.
The high potency of ARN23765 is also appealing for its use as a research tool. There is evidence suggesting that class 1 correctors directly bind to CFTR protein (11, 38, 39), possibly with the first transmembrane domain, but a definite proof is lacking. ARN23765 may represent a high-affinity probe to identify the corresponding binding site using the strategy that was recently used for potentiators (40). The high affinity of VX-770 and GLPG1837 was exploited to determine the cryo–electron microscopy structures of potentiator-CFTR complexes. Studies are ongoing to determine the binding site of ARN23765 to CFTR.
MATERIALS AND METHODS
Cell models and cell culture procedures
We generated CFBE41o− and FRT cells stably expressing either mutant F508del or wild-type CFTR and the HS-YFP YFP-H148Q/I152L, as previously described (28–30). CFBE41o− cells were cultured using minimum essential medium (MEM), while for FRT cells, we used the Coon’s modification of Ham’s F12 medium. Both media were supplemented with 10% fetal calf serum, 2 mM l-glutamine, penicillin (100 U/ml), and streptomycin (100 mg/ml). For HS-YFP assays of CFTR activity, CFBE41o− or FRT cells were plated (50,000 cells per well) on clear-bottom 96-well black microplates (Corning Life Sciences, Acton, MA). We also generated FRT cells with stable expression of P574H-CFTR and HS-YFP.
The isolation, culture, and differentiation methods of primary bronchial epithelial cells have previously been described in detail (41). Briefly, epithelial cells were obtained from mainstem bronchi of CF individuals undergoing lung transplant. For this study, cells were obtained from four patients with CF (homozygous for F508del mutation), from two patients with CF (compound heterozygous for F508del mutation), and from one non-CF patient. Bronchi were set overnight at 4°C in a solution containing protease XIV to detach cells. Epithelial cells were then cultured in a serum-free medium (LHC9 mixed with RPMI 1640, 1:1) supplemented with hormones and supplements that favor cell number amplification. For cells deriving from patients with CF, the culture medium contained a complex mixture of antibiotics (usually colistin, piperacillin, and tazobactam) to eradicate bacteria in the first days.
To obtain differentiated epithelia, cells were seeded at high density on porous membranes [500,000 cells for 1-cm2 Snapwell inserts (Corning, code 3801), for Ussing chamber studies; 200,000 cells for 0.33-cm2 mini-Transwell inserts (Corning, code 3379), for transepithelial electrical resistance (TEER)/PD measurements]. After 24 hours, the serum-free medium was replaced with Dulbecco’s modified Eagle’s medium/Ham’s F12 containing 2% fetal bovine serum plus hormones and supplements. Differentiation of cells into a tight epithelium was monitored by measuring TEER and PD with an epithelial voltohmmeter (EVOM1; World Precision Instruments). The medium was replaced daily on both sides of the permeable supports up to 8 to 10 days (liquid-liquid culture). Subsequently, the apical medium was totally removed, and the cells received nutrients only from the basolateral side [air-liquid culture (ALC)]. This condition favored a further differentiation of the epithelium. Cells were maintained under ALC for 2 to 3 weeks before experiments.
To test putative correctors, compounds were added to the basolateral medium 24 hours before the experiments to achieve the required concentration. Control epithelia were treated with vehicle alone [dimethyl sulfoxide (DMSO)].
HS-YFP assay
At the time of the assay, cells were washed with phosphate-buffered saline (PBS) containing 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, 1 mM CaCl2, and 0.5 mM MgCl2. Cells were then incubated for 25 min with 60 μl of PBS plus forskolin (20 μM) and VX-770 (1 μM) to maximally stimulate F508del-CFTR. Cells were then transferred to microplate readers (FLUOstar OPTIMA; BMG LABTECH, Offenburg, Germany) for CFTR activity determination. The plate readers were equipped with high-quality excitation (HQ500/20x: 500 ± 10 nm) and emission (HQ535/30m: 535 ± 15 nm) filters for YFP (Chroma Technology). For the primary screening, the assay consisted of a continuous 12-s fluorescence reading, 1 s before and 11 s after injection of 165 μl of an iodide-containing solution (PBS with Cl− replaced by I−; final I− concentration, 100 mM). Data were normalized to the initial background-subtracted fluorescence. To determine I− influx rate, the final 10 s of the data for each well were fitted with a linear function to extrapolate initial slope (dF/dt). For the secondary evaluation of compounds, the assay had a duration of 14 s, with continuous fluorescence reading, 2 s before and 12 s after the injection of the iodide-containing solution. I− influx rate was determined by fitting the final 11 s of the data for each well with an exponential function. This assay was used for CFBE41o− cells expressing F508del-CFTR, FRT cells expressing F508del-CFTR or P574H-CFTR, and HEK-293 cells transiently transfected with N1303K-CFTR.
TEER and PD difference measurements
Both primary bronchial and FRT cells were treated with compounds included in the appropriate culture medium at the indicated concentrations for 24 hours at 37°C and 5% CO2, before measuring the TEER and/or PD. In all experiments, TEER and PD were evaluated with an epithelial voltohmmeter (EVOM1; World Precision Instruments).
For primary bronchial cells, the electrical measurements were done in Coon’s modified Ham’s F12 medium where NaHCO3 was replaced with 20 mM Na-Hepes (pH 7.3). After equilibration for 1 hour, TEER and PD were measured in each well under basal conditions, after ENaC inhibition with apical amiloride (10 μM), after CFTR stimulation with forskolin (10 μM) and genistein (50 μM) on both sides, and after CFTR inhibition with apical PPQ102 (30 μM). After each treatment, we waited 10 min before recording electrical parameters. The TEER and PD values for each well were converted into short-circuit current equivalent by Ohm’s law.
For FRT cells, experiments were performed in a solution containing 130 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 1 mM CaCl2, 0.5 mM MgCl2, 10 mM Na-Hepes (pH 7.3), and 10 mM glucose. We measured TEER under basal conditions with the same solution on both sides of the permeable supports. Then, we added 20 μM forskolin and 50 μM genistein to activate CFTR channels. Last, we added 30 μM PPQ102 to block CFTR. As done for bronchial epithelial cells, we waited 10 min after each treatment before taking the measurement. The TEER values were converted into TEEC.
Short-circuit current recordings
Snapwell inserts carrying differentiated bronchial epithelia were mounted in a vertical diffusion chamber, resembling an Ussing chamber with internal fluid circulation. Both apical and basolateral hemichambers were filled with 5 ml of a solution containing 126 mM NaCl, 0.38 mM KH2PO4, 2.13 mM K2HPO4, 1 mM MgSO4, 1 mM CaCl2, 24 mM NaHCO3, and 10 mM glucose. Both sides were continuously bubbled with a gas mixture containing 5% CO2:95% air, and the temperature of the solution was kept at 37°C. The transepithelial voltage was short-circuited with a voltage clamp (DVC-1000; World Precision Instruments) connected to the apical and basolateral chambers via Ag/AgCl electrodes and agar bridges (1 M KCl in 1% agar). The offset between voltage electrodes and the fluid electrical resistance were cancelled before each set of experiments. The short-circuit current was recorded with a PowerLab 4/25 (ADInstruments) analog-to-digital converter connected to a PC.
Antibodies
The following antibodies were used: mouse monoclonal anti-CFTR (570 and 596), provided by J. R. Riordan through a program of the Cystic Fibrosis Foundation (42); mouse monoclonal anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (clone 6C5, Santa Cruz Biotechnology); horseradish peroxidase (HRP)–conjugated anti-mouse immunoglobulin G (IgG) (Abcam); or HRP-conjugated anti-rabbit IgG (Dako).
Western blot
CFBE41o− cells treated with vehicle alone (DMSO), with VX-809, or with test compounds (at the desired concentrations) were grown to confluence on 60-mm-diameter dishes and lysed in radioimmunoprecipitation assay (RIPA) buffer containing a complete protease inhibitor (Roche). Cell lysates were subjected to centrifugation at 15,300g at 4°C for 10 min.
Supernatant protein concentration was calculated using bicinchoninic acid assay (EuroClone) following the manufacturer’s instructions. Equal amounts of protein (10 μg to detect CFTR and GAPDH) were resolved in gradient (4 to 15% or 4 to 20%, depending on target protein molecular weight) Criterion TGX precast gels, transferred to nitrocellulose membranes with a Trans-Blot Turbo system (Bio-Rad), and analyzed by Western blotting. Proteins were detected using the antibodies described above and subsequently visualized by chemiluminescence using the SuperSignal West Femto Substrate (Thermo Fisher Scientific). Chemiluminescence was monitored using the Molecular Imager ChemiDoc XRS System. Images were analyzed with ImageJ software (National Institutes of Health). Bands were analyzed as region of interest, normalized against the GAPDH loading control or (for thermoaggregation assays) against HSP90AB1.
To evaluate F508del-CFTR half-life, the day after plating, CFBE41o− cells were treated with indicated compounds or with vehicle alone (DMSO) for 24 hours, after which protein synthesis was blocked by adding cycloheximide (150 μg/ml) (Sigma-Aldrich) to the medium. At different time points (0, 2, 4, and 6 hours), the cells were then lysed and subjected to SDS-PAGE, as previously described.
Thermoaggregation assay
Thermoaggregation assays were performed as previously described (43). Briefly, cells were grown to confluence and treated with cycloheximide (150 μg/ml) for 2 hours before lysis. Lysis was performed in RIPA buffer on ice, and the lysates were cleared by centrifugation at 15,000g for 10 min at 4°C. To evaluate the aggregation tendency of wild-type and mutant F508del-CFTR, equal amounts of lysates (50 μg) were exposed to 28°, 40°, 50°, 55°, 60°, 65°, and 70°C for 15 min using polymerase chain reaction thermocyclers (Finnzymes). Macromolecular aggregates were eliminated by centrifugation at 15,000g for 15 min at 4°C. The remaining soluble wild-type and F508del CFTR, HSP90AB1 (as loading control), in the supernatant were measured by quantitative immunoblotting.
Apical fluid pH measurement
Differentiated CF bronchial epithelial cells under air-liquid interface condition were treated for 24 hours with vehicle alone (DMSO) or with ARN23765 (10 nM), 4172 (10 μM), or with the combination of ARN23765 plus 4172. At the end of the treatment, cells were incubated (37°C, 5% CO2 atmosphere) with 75 μl of a modified PBS solution with low buffer capacity on the apical side (37). The modified PBS solution had the following composition: 145 mM NaCl, 2.7 mM KCl, 0.81 mM Na2HPO4, 0.15 mM KH2PO4, 1 mM CaCl2, 0.5 mM MgCl2, 100 μM CPT-cAMP, and 1 μM VX-770 (pH 7.35). After 3 hours, the apical fluid was recovered in a single step, and 50 μl of each sample was mixed with the pH-sensitive fluorescent probe SNARF-1 (D3304, Thermo Fisher Scientific; final concentration, 0.1 mg/ml) in a 96-well microplate. The fluorescence was measured in a FLUOstar Omega plate reader (BMG LABTECH) using single excitation (544 nm)/double emission (590 to 640 nm). The ratio of fluorescence emitted at 590 and 640 nm was then converted to pH values using a calibration curve.
Cell proliferation
CFBE41o− cells stably expressing F508del-CFTR and the HS-YFP were plated at low density (5000 cell per well) on 96-well plates suitable for high-content imaging. After 24 hours, cells were treated with different concentrations of test compounds or vehicle alone (DMSO). Cell proliferation was monitored for 24 hours using the Opera Phenix (PerkinElmer) high-content screening system.
MTT cell viability assay
CFBE41o− cells were plated (50,000 cell per well) on 96-well plates. After 24 hours, cells were treated with different concentrations of test compounds or vehicle alone (DMSO). The following day, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) substrate was added to the medium at a final concentration of 0.5 mg/ml and incubated for 3 hours at 37°C. Formazan production (proportional to the number of viable cells) was then quantified by recording changes in absorbance at 560 nm using a plate reader equipped for absorbance measurements (VICTOR3; PerkinElmer).
Cell death analysis
CFBE41o− cells were plated at high density (50,000 cells per well) on 96-well plates suitable for high-content imaging. After 24 hours, cells were treated with test compounds or vehicle alone (DMSO). After 24 hours, cells were counterstained with Hoechst 33342 and propidium iodide to visualize total cells and dead/dying cells, respectively, and imaged using the Opera Phenix (PerkinElmer) high-content screening system. Wells were imaged using a ×20 air objective. Hoechst 33342 signal was laser-excited at 405 nm, and the emission was collected between 435 and 480 nm. The propidium iodide signal was laser-excited at 560 nm, and the emission was measured between 570 and 630 nm.
Statistics
Statistical significance of the effect of single treatments on CFTR activity or expression was tested by parametric one-way analysis of variance (ANOVA), followed by Dunnett’s multiple comparisons test (all groups against the control group) as post hoc test. In the case of combination of treatments, statistical significance was verified by ANOVA, followed by Tukey’s test (for multiple comparisons) as post hoc test. Differences were considered statistically significant when P was less than 0.05.
Study approval
The collection of bronchial epithelial cells and their study to investigate the mechanisms of transepithelial ion transport and the response to CFTR modulators were specifically approved by the Ethics Committee of the Istituto Giannina Gaslini following the guidelines of the Italian Ministry of Health (updated registration number: ANTECER, 042-09/07/2018). Each patient provided informed consent to the study using a form that was also approved by the Ethics Committee.
Supplementary Material
Acknowledgments
We thank G. Bottegoni for the computational work for the selection of the compounds of the library, S. Venzano for the preparation of the plates with the compounds’ solution for the screening, S. M. Bertozzi for analytical ULPC/MS analyses and for separation of the enantiomers by chiral HPLC, and L. Goldoni for NMR analyses of final compounds. We also thank A. S. Verkman for providing us with the ASP-11 co-potentiator. Funding: This work was supported by Fondazione Italiana per la Ricerca sulla Fibrosi Cistica through a strategic project named “Task Force for Cystic Fibrosis.” Author contributions: N.P., F. Berto., E.C., F.S., P.D.F., V.T., L.F., A.R.-G., F. Berti, E.P., E.S., A.G., and P.S. did the experimental work. N.P., F. Berto., T.B., and L.J.V.G. designed the study and analyzed data. N.P., T.B., and L.J.V.G. wrote the manuscript. Competing interests: T.B., F. Berto., P.D.F., A.R.-G., F.S., F. Berti, E.C., L.F., N.P., and L.J.V.G. are inventors on a patent/patent application related to this work filed by Fondazione Istituto Italiano di Tecnologia, Istituto Giannina Gaslini, and Fondazione per la Ricerca sulla Fibrosi Cistica–Onlus (PCT international publication no. WO 2018167690 A1; international publication date, 20 September 2018). T.B., F. Berto., P.D.F., F.S., E.C., L.F., N.P., and L.J.V.G. are inventors on a patent/patent application related to this work filed by Fondazione Istituto Italiano di Tecnologia, Istituto Giannina Gaslini, and Fondazione per la Ricerca sulla Fibrosi Cistica–Onlus (PCT international publication no. WO 2018167695 A1; international publication date, 20 September 2018). The authors declare no other competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/8/eaay9669/DC1
Supplementary Materials and Methods
Fig. S1. Persistence of corrector effect.
Fig. S2. Evaluation of ARN23765 on epithelial ion transport systems.
Fig. S3. Evaluation of ARN23765 as a potentiator.
Fig. S4. Rescue of N1303K-CFTR mutant.
Fig. S5. Rescue of P574H-CFTR mutant.
Fig. S6. Evaluation of ARN23765 on cell viability.
File S1. Uncropped immunoblot images.
References and Notes
- 1.Elborn J. S., Cystic fibrosis. Lancet 388, 2519–2531 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Riordan J. R., CFTR function and prospects for therapy. Annu. Rev. Biochem. 77, 701–726 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Kim S. J., Skach W. R., Mechanisms of CFTR folding at the endoplasmic reticulum. Front. Pharmacol. 3, 201 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younger J. M., Chen L., Ren H. Y., Rosser M. F., Turnbull E. L., Fan C. Y., Patterson C., Cyr D. M., Sequential quality-control checkpoints triage misfolded cystic fibrosis transmembrane conductance regulator. Cell 126, 571–582 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Okiyoneda T., Barrière H., Bagdány M., Rabeh W. M., Du K., Höhfeld J., Young J. C., Lukacs G. L., Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science 329, 805–810 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F., Zeltwanger S., Hu S., Hwang T.-C., Deletion of phenylalanine 508 causes attenuated phosphorylation-dependent activation of CFTR chloride channels. J. Physiol. 524, 637–648 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Boeck K., Davies J. C., Where are we with transformational therapies for patients with cystic fibrosis? Curr. Opin. Pharmacol. 34, 70–75 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Pedemonte N., Lukacs G. L., Du K., Caci E., Zegarra-Moran O., Galietta L. J. V., Verkman A. S., Small-molecule correctors of defective ∆F508-CFTR cellular processing identified by high-throughput screening. J. Clin. Invest. 115, 2564–2571 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Goor F., Straley K. S., Cao D., González J., Hadida S., Hazlewood A., Joubran J., Knapp T., Makings L. R., Miller M., Neuberger T., Olson E., Panchenko V., Rader J., Singh A., Stack J. H., Tung R., Grootenhuis P. D. J., Negulescu P., Rescue of ∆F508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am. J. Physiol. 290, L1117–L1130 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Van Goor F., Hadida S., Grootenhuis P. D., Burton B., Stack J. H., Straley K. S., Decker C. J., Miller M., McCartney J., Olson E. R., Wine J. J., Frizzell R. A., Ashlock M., Negulescu P. A., Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. U.S.A. 108, 18843–18848 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veit G., Xu H., Dreano E., Avramescu R. G., Bagdany M., Beitel L. K., Roldan A., Hancock M. A., Lay C., Li W., Morin K., Gao S., Mak P. A., Ainscow E., Orth A. P., McNamara P., Edelman A., Frenkiel S., Matouk E., Sermet-Gaudelus I., Barnes W. G., Lukacs G. L., Structure-guided combination therapy to potently improve the function of mutant CFTRs. Nat. Med. 24, 1732–1742 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sondo E., Falchi F., Caci E., Ferrera L., Giacomini E., Pesce E., Tomati V., Mandrup Bertozzi S., Goldoni L., Armirotti A., Ravazzolo R., Cavalli A., Pedemonte N., Pharmacological inhibition of the ubiquitin ligase RNF5 rescues F508del-CFTR in cystic fibrosis airway epithelia. Cell Chem. Biol. 25, 891–905.e8 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Van Goor F., Hadida S., Grootenhuis P. D. J., Burton B., Cao D., Neuberger T., Turnbull A., Singh A., Joubran J., Hazlewood A., Zhou J., McCartney J., Arumugam V., Decker C., Yang J., Young C., Olson E. R., Wine J. J., Frizzell R. A., Ashlock M., Negulescu P., Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. U.S.A. 106, 18825–18830 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wainwright C. E., Elborn J. S., Ramsey B. W., Marigowda G., Huang X., Cipolli M., Colombo C., Davies J. C., De Boeck K., Flume P. A., Konstan M. W., McColley S. A., McCoy K., McKone E. F., Munck A., Ratjen F., Rowe S. M., Waltz D., Boyle M. P.; TRAFFIC Study Group; TRANSPORT Study Group , Lumacaftor–ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N. Engl. J. Med. 373, 220–231 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe S. M., McColley S. A., Rietschel E., Li X., Bell S. C., Konstan M. W., Marigowda G., Waltz D., Boyle M. P.; VX09-809-102 Study Group , Lumacaftor/Ivacaftor treatment of patients with cystic fibrosis heterozygous for F508del-CFTR. Ann. Am. Thorac. Soc. 14, 213–219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramsey B. W., Davies J., McElvaney N. G., Tullis E., Bell S. C., Dřevínek P., Griese M., McKone E. F., Wainwright C. E., Konstan M. W., Moss R., Ratjen F., Sermet-Gaudelus I., Rowe S. M., Dong Q., Rodriguez S., Yen K., Ordoñez C., Elborn J. S.; VX08-770-102 Study Group , A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 365, 1663–1672 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okiyoneda T., Veit G., Dekkers J. F., Bagdany M., Soya N., Xu H., Roldan A., Verkman A. S., Kurth M., Simon A., Hegedus T., Beekman J. M., Lukacs G. L., Mechanism-based corrector combination restores ΔF508-CFTR folding and function. Nat. Chem. Biol. 9, 444–454 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farinha C. M., King-Underwood J., Sousa M., Correia A. R., Henriques B. J., Roxo-Rosa M., Da Paula A. C., Williams J., Hirst S., Gomes C. M., Amaral M. D., Revertants, low temperature, and correctors reveal the mechanism of F508del-CFTR rescue by VX-809 and suggest multiple agents for full correction. Chem. Biol. 20, 943–955 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Rabeh W. M., Bossard F., Xu H., Okiyoneda T., Bagdany M., Mulvihill C. M., Du K., di Bernardo S., Liu Y., Konermann L., Roldan A., Lukacs G. L., Correction of both NBD1 energetics and domain interface is required to restore ΔF508 CFTR folding and function. Cell 148, 150–163 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendoza J. L., Schmidt A., Li Q., Nuvaga E., Barrett T., Bridges R. J., Feranchak A. P., Brautigam C. A., Thomas P. J., Requirements for efficient correction of ΔF508 CFTR revealed by analyses of evolved sequences. Cell 148, 164–174 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor-Cousar J. L., Munck A., McKone E. F., van der Ent C. K., Moeller A., Simard C., Wang L. T., Ingenito E. P., McKee C., Lu Y., Lekstrom-Himes J., Elborn J. S., Tezacaftor–ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N. Engl. J. Med. 377, 2013–2023 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Veit G., Avramescu R. G., Perdomo D., Phuan P.-W., Bagdany M., Apaja P. M., Borot F., Szollosi D., Wu Y.-S., Finkbeiner W. E., Hegedus T., Verkman A. S., Lukacs G. L., Some gating potentiators, including VX-770, diminish ΔF508-CFTR functional expression. Sci. Transl. Med. 6, 246ra97 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cholon D. M., Quinney N. L., Fulcher M. L., Esther Jr. C. R., Das J., Dokholyan N. V., Randell S. H., Boucher R. C., Gentzsch M., Potentiator ivacaftor abrogates pharmacological correction of ΔF508 CFTR in cystic fibrosis. Sci. Transl. Med. 6, 246ra96 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies J. C., Moskowitz S. M., Brown C., Horsley A., Mall M. A., McKone E. F., Plant B. J., Prais D., Ramsey B. W., Taylor-Cousar J. L., Tullis E., Uluer A., McKee C. M., Robertson S., Shilling R. A., Simard C., Van Goor F., Waltz D., Xuan F., Young T., Rowe S. M.; VX16-659-101 Study Group , VX-659–tezacaftor–ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N. Engl. J. Med. 379, 1599–1611 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keating D., Marigowda G., Burr L., Daines C., Mall M. A., McKone E. F., Ramsey B. W., Rowe S. M., Sass L. A., Tullis E., McKee C. M., Moskowitz S. M., Robertson S., Savage J., Simard C., Van Goor F., Waltz D., Xuan F., Young T., Taylor-Cousar J. L.; VX16-445-001 Study Group , VX-445–tezacaftor–ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N. Engl. J. Med. 379, 1612–1620 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Habib A. R., Kajbafzadeh M., Desai S., Yang C. L., Skolnik K., Quon B. S., A systematic review of the clinical efficacy and safety of CFTR modulators in cystic fibrosis. Sci. Rep. 9, 7234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.A. R. Leach, V. J. Gillet, An Introduction to Chemoinformatics (Springer, 2007). [Google Scholar]
- 28.Sondo E., Tomati V., Caci E., Esposito A. I., Pfeffer U., Pedemonte N., Galietta L. J. V., Rescue of the mutant CFTR chloride channel by pharmacological correctors and low temperature analyzed by gene expression profiling. Am. J. Physiol. Cell Physiol. 301, C872–C885 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang H., Shelat A. A., Guy R. K., Gopinath V. S., Ma T., Du K., Lukacs G. L., Taddei A., Folli C., Pedemonte N., Galietta L. J., Verkman A. S., Nanomolar affinity small molecule correctors of defective ∆F508-CFTR chloride channel gating. J. Biol. Chem. 278, 35079–35085 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Galietta L. J. V., Haggie P. M., Verkman A. S., Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 499, 220–224 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Zhang J.-H., Chung T. D. Y., Oldenburg K. R., A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4, 67–73 (1999). [DOI] [PubMed] [Google Scholar]
- 32.Pesce E., Sondo E., Ferrera L., Tomati V., Caci E., Scudieri P., Musante I., Renda M., Baatallah N., Servel N., Hinzpeter A., di Bernardo D., Pedemonte N., Galietta L. J. V., The autophagy inhibitor spautin-1 antagonizes rescue of mutant CFTR through an autophagy-independent and USP13-mediated mechanism. Front. Pharmacol. 9, 1464 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma M., Benharouga M., Hu W., Lukacs G. L., Conformational and temperature-sensitive stability defects of the ∆F508 cystic fibrosis transmembrane conductance regulator in post-endoplasmic reticulum compartments. J. Biol. Chem. 276, 8942–8950 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Phuan P.-W., Son J.-H., Tan J.-A., Li C., Musante I., Zlock L., Nielson D. W., Finkbeiner W. E., Kurth M. J., Galietta L. J., Haggie P. M., Verkman A. S., Combination potentiator (‘co-potentiator’) therapy for CF caused by CFTR mutants, including N1303K, that are poorly responsive to single potentiators. J. Cyst. Fibros. 17, 595–606 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedemonte N., Tomati V., Sondo E., Galietta L. J. V., Influence of cell background on pharmacological rescue of mutant CFTR. Am. J. Physiol. Cell Physiol. 298, C866–C874 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Tang X. X., Ostedgaard L. S., Hoegger M. J., Moninger T. O., Karp P. H., McMenimen J. D., Choudhury B., Varki A., Stoltz D. A., Welsh M. J., Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J. Clin. Invest. 126, 879–891 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scudieri P., Musante I., Caci E., Venturini A., Morelli P., Walter C., Tosi D., Palleschi A., Martin-Vasallo P., Sermet-Gaudelus I., Planelles G., Crambert G., Galietta L. J. V., Increased expression of ATP12A proton pump in cystic fibrosis airways. JCI Insight 3, e123616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loo T. W., Bartlett M. C., Clarke D. M., Corrector VX-809 stabilizes the first transmembrane domain of CFTR. Biochem. Pharmacol. 86, 612–619 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Laselva O., Molinski S., Casavola V., Bear C. E., Correctors of the major cystic fibrosis mutant interact through membrane-spanning domains. Mol. Pharmacol. 93, 612–618 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Liu F., Zhang Z., Levit A., Levring J., Touhara K. K., Shoichet B. K., Chen J., Structural identification of a hotspot on CFTR for potentiation. Science 364, 1184–1188 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scudieri P., Caci E., Bruno S., Ferrera L., Schiavon M., Sondo E., Tomati V., Gianotti A., Zegarra-Moran O., Pedemonte N., Rea F., Ravazzolo R., Galietta L. J. V., Association of TMEM16A chloride channel overexpression with airway goblet cell metaplasia. J. Physiol. 590, 6141–6155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui L., Aleksandrov L., Chang X. B., Hou Y. X., He L., Hegedus T., Gentzsch M., Aleksandrov A., Balch W. E., Riordan J. R., Domain interdependence in the biosynthetic assembly of CFTR. J. Mol. Biol. 365, 981–994 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Veit G., Oliver K., Apaja P. M., Perdomo D., Bidaud-Meynard A., Lin S.-T., Guo J., Icyuz M., Sorscher E. J., Hartman J. L. IV, Lukacs G. L., Ribosomal stalk protein silencing partially corrects the ΔF508-CFTR functional expression defect. PLOS Biol. 14, e1002462 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/8/eaay9669/DC1
Supplementary Materials and Methods
Fig. S1. Persistence of corrector effect.
Fig. S2. Evaluation of ARN23765 on epithelial ion transport systems.
Fig. S3. Evaluation of ARN23765 as a potentiator.
Fig. S4. Rescue of N1303K-CFTR mutant.
Fig. S5. Rescue of P574H-CFTR mutant.
Fig. S6. Evaluation of ARN23765 on cell viability.
File S1. Uncropped immunoblot images.


