Abstract
This study investigated the systemic inflammatory response and mechanism of pulmonary lesions induced by Crotalus durissus cascavella venom in murine in the state of Bahia. In order to investigate T helper Th1, Th2 and Th17 lymphocyte profiles, we measured interleukin (IL) -2, IL-4, IL-6, IL-10, IL-17, tumor necrosis factor (TNF) and interferon gamma (IFN-γ) levels in the peritoneal fluid and macerated lungs of mice and histopathological alterations at the specific time windows of 1h, 3h, 6h, 12h, 24h and 48h after inoculation with Crotalus durissus cascavella venom. The data demonstrated an increase of acute-phase cytokines (IL-6 and TNF) in the first hours after inoculation, with a subsequent increase in IL-10 and IL-4, suggesting immune response modulation for the Th2 profile. The histopathological analysis showed significant morphological alterations, compatible with acute pulmonary lesions, with polymorphonuclear leukocyte (PMN) infiltration, intra-alveolar edema, congestion, hemorrhage and atelectasis. These findings advance our understanding of the dynamics of envenomation and contribute to improve clinical management and antiophidic therapy for individuals exposed to venom.
Introduction
Accidents involving snakes have been considered a neglected disease by the World Health Organization (WHO) since 2007. It has been recognized as a public health issue with approximately 2.5 million envenomation accidents worldwide, leading to 125,000 deaths and many victims with serious permanent sequelae [1]. Although the physiopathology of these accidents is a complex event, it is known that inflammatory mediators play an important role in the envenomation, dynamics, and this has been demonstrated in diverse studies in experimental models and humans [2–6].
In Brazil, the Crotalus durissus species was responsible for 23.264 accidents over a 10-year period (2007–2017), and represent the most common cause of ophidian accident in the Northeast region [7]. These accidents are characterized by local pain and swelling [8–10], with can be associated with neuromuscular block, acute respiratory distress [9,11], coagulation disturbances [11–13] and acute renal failure (ARF) [12,14,15]. Crotoxin, convulxin, gyroxin and crotamine toxins may be associated with most of these clinical manifestations [16–19].
Generally, there is an imbalance in the recruitment of immune response cells after envenomation, including the T helper (Th) lymphocyte, that can be subdivided into subpopulations (Th1, Th2 or Th17) with different immune response functions and cytokine profiles [20–23]. The Th1 lymphocytes are characterized by the production of IFN-γ, TNF, IL-2 and IL-12, affecting the activation of phagocytes, recruitment and lymphocyte TCD8+ activation. Th2 profile produces IL-4, IL-5, IL-10 and IL-13, all involved in the activation of eosinophils, mastocytes, and B-lymphocytes, as well as producing class IgE antibodies. The Th17 profile lymphocytes produce IL-17 and IL-22, which appear to be related to acute and neutrophil inflammatory responses [24,25].
Cytokine production in response to envenomation is an area increasingly under study. Evidence suggests that bothropic and crotalic venom induce elevated levels of IL-1β, IL-6, IL-10, TNF and IFN, contributing to a leukocyte influx [2,4,5,26,27]. This compromises different organs, including the lungs, leading to respiratory failure, septic shock and multiple organ and systems failure [2,13,28,29].
Pulmonary alterations coupled with the action of C. durissus venom, such as leukocyte recruitment, congestion, hemorrhage, atelectasis and emphysema have been previously reported [3,30,31]. However, the inflammatory mediators that may be involved in pulmonary damage provoked by venom are still not fully understood.
The present study evaluated the systemic inflammatory response and pulmonary lesions induced by Crotalus durissus cascavella venom in mice, by quantifying TNF, IL-6, IL-4, IL-10, IFNγ, IL-17A and IL-2 cytokines and evaluating histopathologic alterations in the pulmonary parenchyma.
Materials and methods
Crotalus durissus cascavella venom from the state of Bahia
Crotalus durissus cascavella venom from a Caatinga ecosystem of Bahia, Brazil, was collected individually of specimens kept in the Scientific Breeding of Venomous Animals of the State University of Feira de Santana, geographic coordinates Latitude 12° 16'00” S Longitude 38° 58'00” W. The bioterium is homologated by the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA), registration of Federal Registration Number 480922 and by SisGen (Sistema Nacional do Patrimônio Genético e do Conhecimento Tradicional Associado (protocol numbers ABC319C). After extraction, the venom was vacuum-dried and stored at -20˚ C until analyses. Venom protein concentration was determined using bovine serum albumin (Sigma, Chemical Company) as the protein standard [32].
Animals
Male Swiss mice weighing from 18-22g were supplied by Central Rodent Bioterium of Breeding and Experimentation at State University of Feira de Santana and kept in a controlled environment in a 12/12 hour light-dark cycle. Animals had access to an ad libitum supply of food and water. Expression of species-specific behaviors were favored by adequate housing. Throughout the experiment, careful manipulation of the animals was performed only when necessary in a noise free environment. Concerned parties ensured the highest level of comfort possible and animals welfare, minimal suffering and euthanasia with brevity. We have avoided analgesics and anesthetics as they may interfere both in cytokines production and costimulatory signals required for antigen presentation and T lymphocyte differentiation. The research team, under the permanent supervision of the veterinarian, monitored animals throughout the entire experiment. Observations were conducted every hour to evaluate clinical behavior, level of activity, posture, temperament, locomotion, water and food intake. Signs of respiratory distress, pain or abnormal behavior have been evaluated and recorded. Animals were euthanized immediately at protocol predeterminated times (1h, 3h, 6h, 12h, 24h and 48 hours) with an overdose with ketamine (100mg/Kg) and xylazine (10mg/kg) with immediate extraction of peritoneal fluid and lung tissue. No animals were found dead during the experiment period and all mice were euthanized at the predetermined times.
Ethics statement
The study was conducted in accordance with the ethical principles for animal research adopted by the Brazilian Society of Animal Science and the National Brazilian Legislation n°.11.794/08 and was approved by the State University of Feira de Santana Animal Ethics Committee (CEUA-UEFS—protocol number 006/2018). The research team received obligatory training in animal welfare, handling and sacrifice technics.
Inflammatory response in mice induced by Crotalus durissus cascavella venom
The experiments were performed a total of 72 mice subdivided into control group (n = 5) and experimental group (n = 7) for each determined time. The experimental group received the venom challenge dose of 50μg/kg [3], by the intraperitoneal (i.p.) route, diluted in 500μL of sterile saline solution (NaCl 0.9%) [3]. The control group received 500μL of sterile saline solution (NaCl 0.9%). Both groups were monitored during the entire experiment (1, 3, 6, 12, 24 and 48 hours). The animals were euthanized with an overdose with ketamine (100mg/Kg) and xylazine (10mg/kg) to obtain the peritoneal fluid and lung.
Obtaining the peritoneal fluid
In both groups, 2mL of phosphate saline solution (Phosphate Buffered Saline—PBS) was injected into the peritoneal cavity to obtain the peritoneal fluid. Then, the animals had their abdomens massaged to wash the entire cavity. The peritoneal fluid was extracted using a syringe, centrifuged at 3000rpm at 4°C, for 20 minutes [33] and the supernatant separated and stored at -20°C for subsequent cytokine dosages.
Lung tissue disintegration and histopathologic analysis
The inferior lobe of the right lung was removed and stored in 1mL of PBS solution, then, macerated and centrifuged at 3000 rpm at 4°C, for 20 minutes [33]. Supernatant was separated and stored at 20°C for subsequent cytokine dosage. The left lung and the superior lobe of the right lung were initially fixed in 10% buffered formalin for a maximum of 48 hours. Fragments underwent ethanol and xylene dehydration and were diaphanized and cut to a width of 4μm and subsequently stained in a hematoxylin-eosin solution (HE) and Masson’s trichrome. The cuts were examined, and images captured by Olympus BX 51 microscope with a coupled digital camera (DP25) and digitalized on cellSens software. Morphometric analysis was performed on five randomly selected microscopic fields on lung parenchymal slides in Swiss mice at different exposure times (1h, 3h, 6h, 12h, 24h, 48h). The number of inflammatory cells was counted in mm2 with a 20x eyepiece and a 20x objective, in an area of 60 mm2. Collagen deposition was quantified by counting the areas marked by Masson's trichrome in mm2 in five randomly selected microscopic fields on the lung parenchyma slides at 6h, 12h, 24h exposure times. All data obtained were analyzed using ImageJ software (USA). Statistical results were evaluated by the nonparametric Mann-Whitney Test with significance of p<0,05. The graphs were generated by GraphPad Prism 5.0 (GraphPad, San Diego, CA, USA).
Cytokine measurements using Cytometric Bead Array (CBA)
Cytokine concentrations in the peritoneal fluid supernatant and macerated lungs were determined using a BD™ CBA Mouse Th1/Th2/Th17 Cytokine Kit (BD Biosciences, USA) with a FACSCalibur flow cytometer (San Francisco, BD Biosciences). Cytometric Bead Array analysis allowed the simultaneous detection of cytokines, TNF, IL-6, IL-4, IL-10, IFNγ, IL-17A and IL-2, and was performed in accordance with the manufacturer´s instructions. In brief, the Th1/Th2/Th17 cytokine standards were prepared using a vial of lyophilized Mouse and Assay Diluent using the serial dilutions technique. Capture beads were added into each tube containing samples and standards and then incubated for 2 hours at room temperature in the absence of light. The samples were washed with 1 mL buffer at 200g for 5 minutes and resuspended in 150μL wash buffer. Data acquisition was performed using FCAP Array v2.0 software (Soft Flow, Hungary).
Statistical analysis
The data distribution was evaluated using the Kolmogorov-Smirnov test. To compare the time intervals evaluated, normal distribution data were analyzed using one-way analysis of variance (ANOVA) and Tukey´s test. The Kruskall-Wallis test followed by Dunn's Multiple Comparison Test was used in case of skewed distribution. The T student test was employed to compare experiment and control groups, and non-parametric distributions through the Mann-Whitney test. Results were expressed as mean (standard deviation—SD) and median (interquartile range) and values of p<0.05 were considered to be statistically significant. Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad, San Diego, CA, EUA).
Results
Clinical manifestations induced by Crotalus durissus cascavella venom in mice
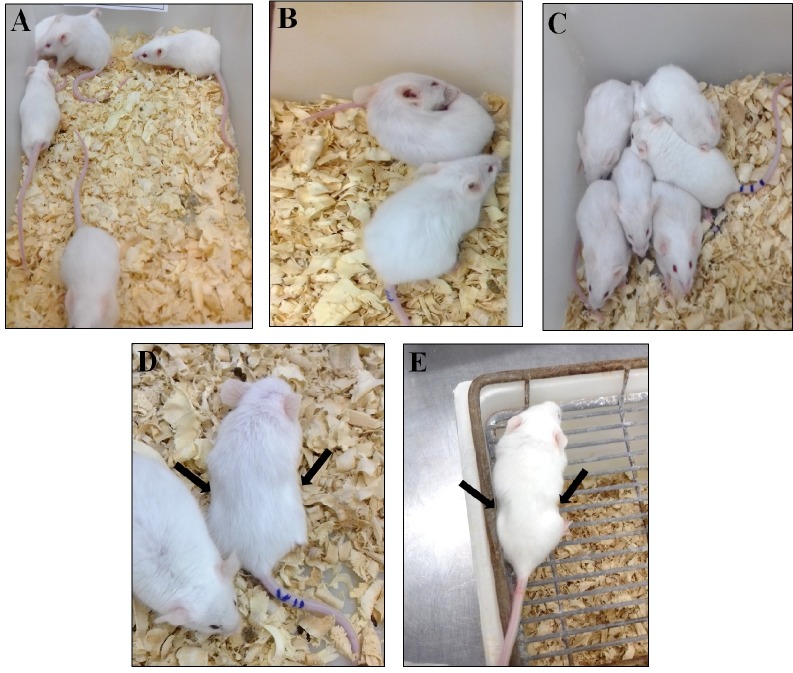
Experimental animals presented agitated behavior, pruritus and wound licking. One hour after inoculation, subjects showed patterns of nesting behavior, prostration, progressive lethargy and tremors (Fig 1A, 1B and 1C). Peak respiratory discomfort occurs between 6 and 48 hours, characterized by tachypnea with intense abdominal contractions and thoracic effort (Fig 1D and 1E). Animals in the control group showed no clinical deterioration throughout the experimental period.
Fig 1. Mice and their clinical manifestations.
Control group was inoculated (i.p.) with 500μL saline solution (A). Experimental group inoculated (i.p.) with 50μg/kg Crotalus durissus cascavella venom diluted in 500μL saline solution (B-D): Initial clinical symptoms observed were pruritus, wound licking (B) and nesting behavior (C); after three hours the animals presented lethargy and respiratory distress (D); 24 hours after inoculation, there were intense abdominal contractions and thoracic effort (E).
Determining Th1/Th2/Th17 cytokine levels in the peritoneal fluid and macerated lung
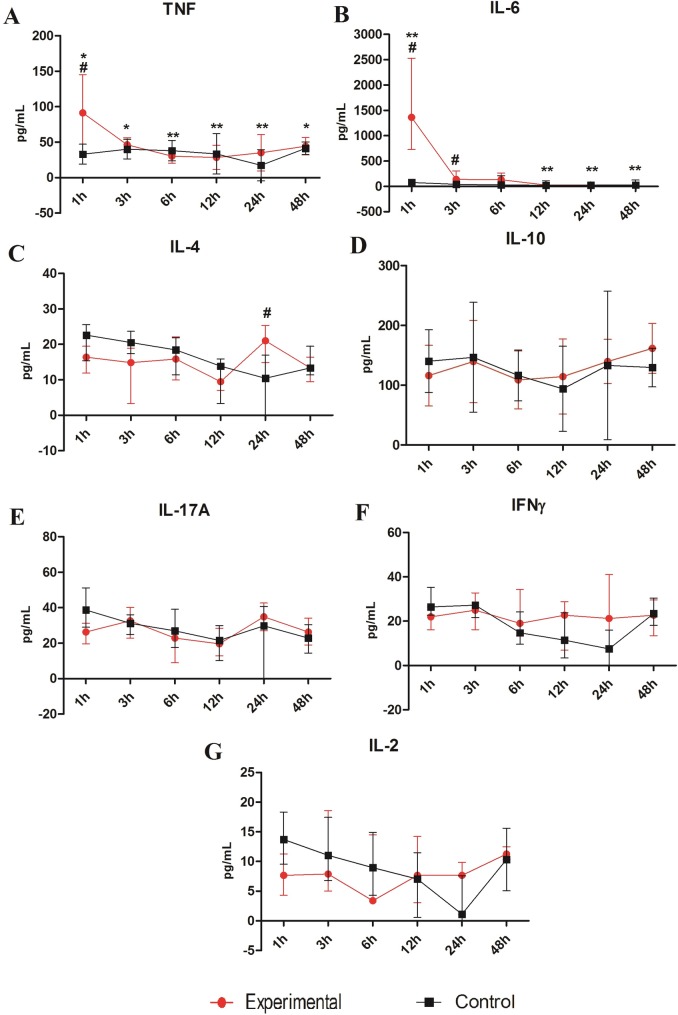
Analysis of the peritoneal fluid and macerated lung indicated that Crotalus durissus cascavella venom induced varied levels of Th1/Th2/Th17 cytokines and regulatory response at all intervals analyzed. Significant differences between the experimental and control groups were found in TNF (Fig 2A), IL-6 (Fig 2B) and IL-4 (Fig 2C) concentrations in the peritoneal fluid. The experimental group presented significantly higher TNF (#p<0.05) and IL-6 (#p<0.05) 1 hour after inoculation. IL-6 levels of the experimental group continued to be higher than the control group at the 3-hour temporal window (#p<0.05). There was an increase in IL-4 concentration at the 24-hour time (#p<0.05).
Fig 2.
Cytokine levels TNF (A), IL-6 (B), IL-10 (C), IL-4 (D), IL-17A (E), IFN-γ (F) and IL-2 (G) measured in peritoneal fluid of the Swiss mice at different time 1, 3, 6, 12, 24 and 48 hours. The mice were inoculated via i.p with 50μg/kg Crotalus durissus cascavella venom diluted in 500μL saline solution. The control group animals were inoculated with 500μL sterile saline solution. Each point represents the mean–SD (A and C) and median—IQR (B, D, E, F and G) of animals per group. *p< 0.05 and **p<0.01 in relation to the venom treatment times and #p<0.05 when compared to the control group.
Despite not statistically significant, IL-10 (Fig 2D) levels were detected after 12 hours, peaking at 48 hours. IL-17A (Fig 2E) and IFN-γ (Fig 2F) had increased levels at 24 hours with subsequent decline 48 hours after exposure, while IL-2 (Fig 2G) levels remained constant.
Analyzing the kinetics of cytokine production in the peritoneal fluid of the experimental group, TNF reaches highest levels over the first hour with subsequent gradual decline (*p<0.05 e **p<0.01). IL-6 also presented a statistically significant difference in the experimental group (*p<0.05 e **p<0.01), with a peak in the first hour and a subsequent progressive decline over the other time windows. IL-4 production remained similar in the first time windows (1, 3 and 6h), dropping off at 12 hours, with elevated late levels at 24 hours (#p<0.05). Levels of IFNγ, IL-17A and IL-2 have not presented a statistically significant difference.
Among cytokines in the macerated lungs (Fig 3), both IL-4, at 3 hour and IL-10, at the 3 and 12 hour times, demonstrated significant difference between the experimental and the control groups (#p<0.05). The remaining analyzed cytokines have not showed significant differences. During follow up period, only IFN-γ presented a statistically significant profile between the 3 and 48 hour time windows (*p<0.05), peaking at 3 hours and declining to its lowest level at 48 hours.
Fig 3.
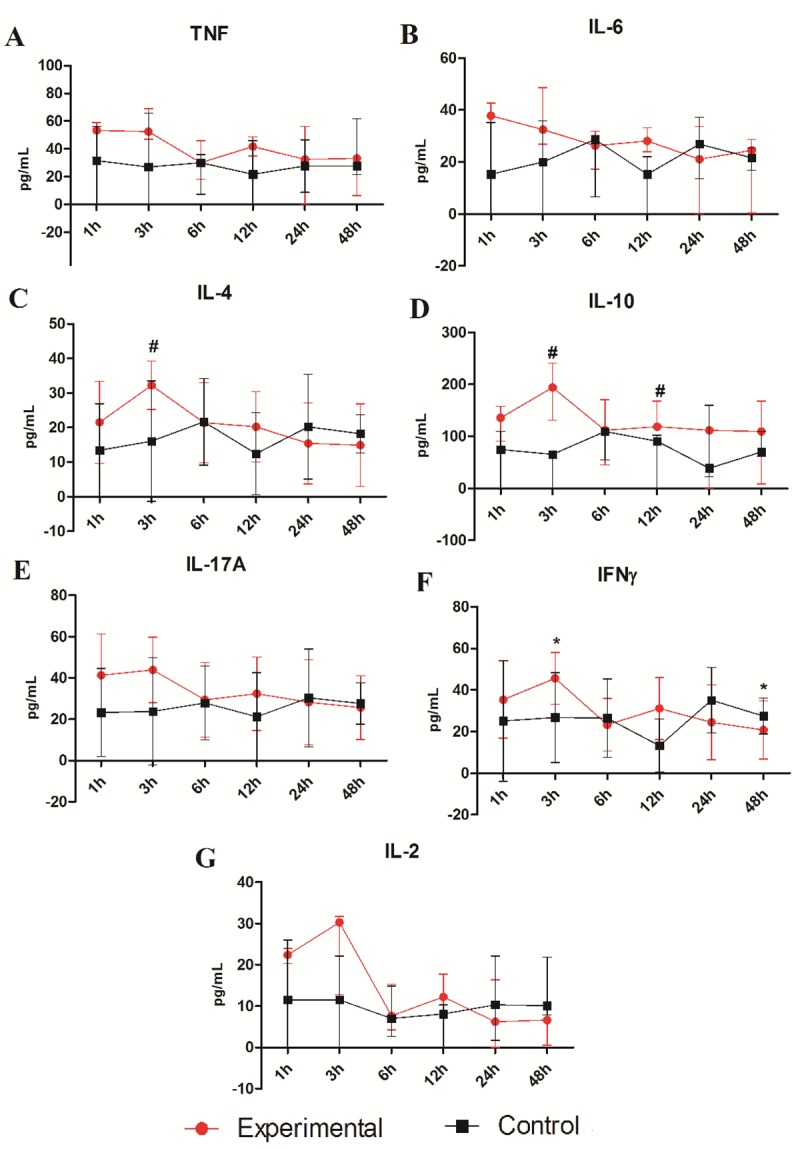
Cytokine levels TNF (A), IL-6 (B), IL-4 (C), IL-10 (D), IL-17A (E), IFN-γ (F) and IL-2 (G) measured in the macerated lungs of the Swiss mice at different time 1, 3, 6, 12, 24 and 48 hours. The mice were inoculated via i.p with 50μg/kg Crotalus durissus cascavella venom diluted in 500μL saline solution. The control group animals were inoculated with 500μL sterile saline solution. Each point represents the mean–SD (C, E and F) and median–IQR (A, B, D and G) of animals per group. *p< 0.05 in relation to the venom treatment times and #p<0.05 when compared to the control group.
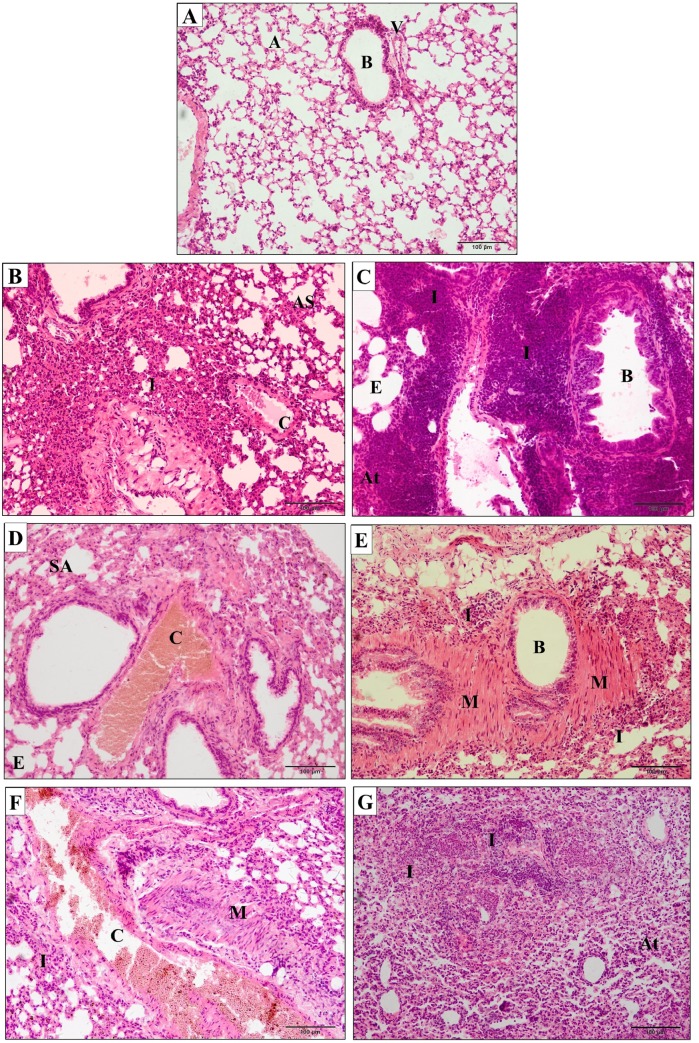
Histopathology of lung damage induced by Crotalus durissus cascavella venom
There was no physiopathological alteration of the pulmonary parenchyma in control group (Fig 4A). Nevertheless, Crotalus durissus cascavella venom induced early recruitment of inflammatory cells, thickening of alveolar septa and vascular congestion in the initial phase (1 hour) (Fig 4B). At 3 hours, there was a significant increase of peribronchial inflammatory infiltrates, emphysema and focal atelectasis indicative of severe pulmonary inflammation (Fig 4C).
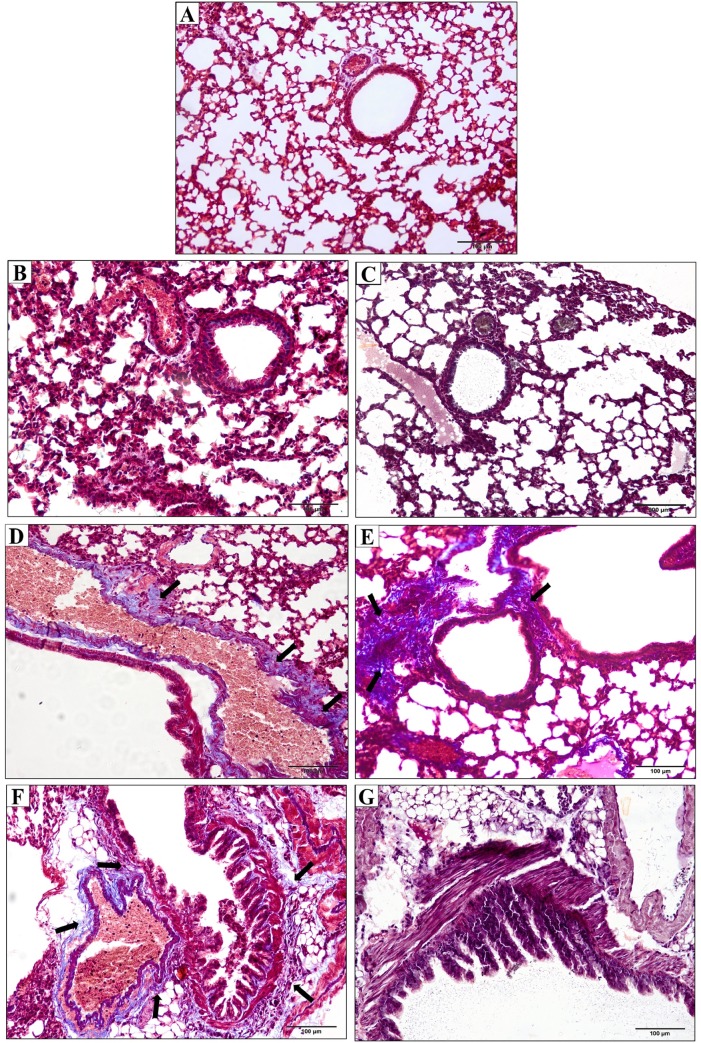
Fig 4. Photomicrographs of pulmonary parenchyma of mice.
(A) Control group inoculated with 500μL sterile saline solution presented preserved pulmonary architecture (A–alveolus; B–bronchial; V–vessels). (B–G) Experimental groups inoculated with 50μg/kg Crotalus durissus cascavella venom at different observation time: (B) 1h; (C) 3h; (D) 6h; (E) 12h; (F) 24h; (G) 48h. (I–Inflammatory infiltrates; AS–alveolar septum thickening; C–vascular congestion; E–emphysema; At–atelectasis; M—bronchial muscle distension).
At six hours, a decrease in the activity of inflammatory response is observed, despite an increase in vascular congestion, alveolar septa thickening and emphysematous areas (Fig 4D). Between 12 and 48 hours, there were perceptible inflammatory infiltrates in the pulmonary parenchyma, hemorrhagic focuses, vascular congestion and bronchial muscle distension (Fig 4E).
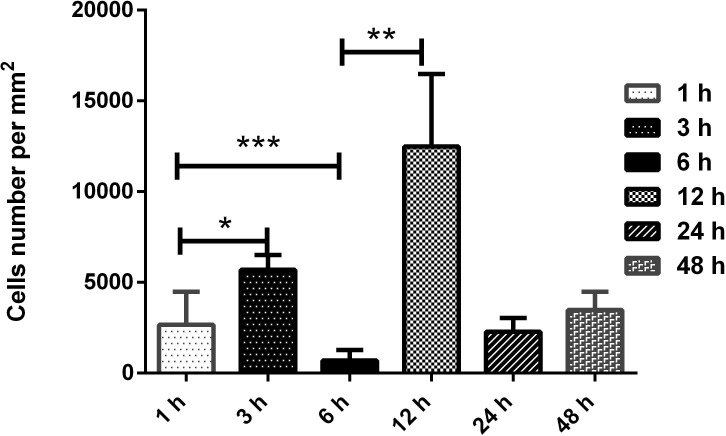
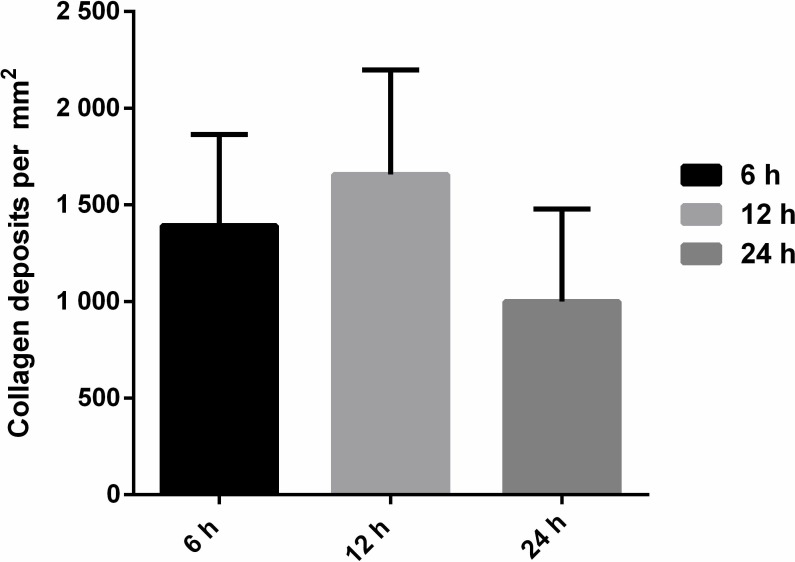
An intense chronic inflammatory infiltrate was observed between 24 and 48 hours, consisting mainly of neutrophils and eosinophils, compressed bronchiole, atelectasis and bronchial muscle distension (Fig 4F e 4G). The morphometric quantification of polymorphonuclear cells in inflammatory infiltrates in pulmonary parenchyma showed significant difference between the times of 1 and 3 hours (p = 0,00159); 1 and 6 hours (p = 0,00317); the times 3, 6, 12 and 24 hours (p = 0,0079); 3 and 48 hours (p = 0,00317), 6 and 12 hours (p = 0,0079), 6 and 24 hours (p = 0,0159); 6 and 48 hours (p = 0,0079), 12 and 24 and between 12 and 48 hours (p = 0,0079). No statistical difference was showed between concentrations at 24 and 48 hours (Fig 5). Masson's Trichrome staining revealed matrix changes with collagen deposition at 6, 12 and 24 hours (Fig 6), although without statistically significant difference, as confirmed by quantification (Fig 7).
Fig 5. Morphometric analysis of the frequency of polymorphonuclear cells in inflammatory infiltrates in pulmonary parenchyma of Swiss mice.
Experimental groups inoculated with 50μg/kg Crotalus durissus cascavella venom at different observation time showed significant differences with p = 0,00159 (1 and 3 hours); p = 0,00317 (1 and 6 hours); p = 0,0079 (3, 6, 12 and 24 hours); p = 0,00317 (3 and 48 hours); p = 0,0079 (6 and 12 horas); p = 0,0159 (6 and 24 hours); p = 0,0079 (6 and 48 hours); p = 0,0079 (12 and 24/ 12 and 48 hours). No statistical difference was showed between concentrations at 24 and 48 hours. Statistical analysis was performed by the bilateral Mann—Whitney Test. *p<0,05 were considered statistically significant.
Fig 6. Photomicrographs of pulmonary parenchyma of mice stained with Masson’s trichrome.
(A) Control group inoculated with 500μL sterile saline. (B-G). Experimental groups inoculated with 50μg/kg Crotalus durissus cascavella venom at different observation time: (B) 1h; (C) 3h; (D) 6h; (E) 12h; (F) 24h; (G) 48h. The arrows indicate collagen deposits (stained in blue) in the peribronchial and perivascular regions (Masson’s trichrome staining).
Fig 7. Quantification of collagen deposits in Swiss mice inoculated with 50μg/kg Crotalus durissus cascavella venom at different observation time.
Evaluation of collagen deposits by Masson's trichrome staining revealed no significant differences at any of the correlated points. Statistical analysis performed by the bilateral Mann-Whitney test. p<0,05 was considered statistically significant.
Discussion
Physiopathogenic mechanisms that lead to pulmonary damage induced by Crotalus durissus venom are still not fully understood, especially inflammatory response and cytokine production [2,28,30,31]. The present study observed a systemic inflammatory response induced by Crotalus durissus cascavella venom associated with aggressive acute pulmonary injury characterized by peribronchial inflammatory infiltrate and altered vascular permeability with patchy hemorrhagic foci.
In response to acute antigenic stimulus induced by toxins, a modulated Th1, Th2 or Th17 immune response occurs [24,25]. The systemic action of toxins triggers an inflammatory response and enhance production of several immunological mediators that activates recruitment, proliferation and differentiation of leukocytes [29,34].
An early increase of IL-6 and TNF levels in the peritoneal fluid and macerated lungs suggests that pro-inflammatory cytokines play a key role in acute inflammation [29]. Previous studies have reported a similar inflammatory profile in the first hours after inoculation of C. d. terrificus venom with subsequent late immunomodulation. These characteristics can be related to crotoxin activity in inhibiting neutrophil chemotaxis and modulating adaptive immune response [17,21,35–37].
Snakebite envenoming induces high levels of IL-6, TNF and IL-1β cytokines and may provoke fever, lethargic, vasodilation and a variety of other symptoms resulting from edema induction, T and B cell activation, and leukocyte recruitment [2,38,39]. It is plausible that high levels of these cytokines are directly related to aggressive clinical manifestations, such as prostration, lethargy and huddling.
Increased cytokines levels during the acute stage have been described by several authors in accidents involving venomous animals [4,33,40–42]. Studies involving scorpion venom demonstrated a positive physiopathological correlation between cytokine concentrations and severity of clinical manifestations [43–46]
Systemic toxicity is markedly affected by cytokines release. Increase IL-10 levels in peritoneal fluid at 12 hour and at 3h and 12h in macerated lungs may play an important role in immune response modulation, favoring a shift from a Th1 towards a Th2 cytokine. The increase in IL-4 in macerated lungs at 3h and at 24h in peritoneal fluid strengthened this hypothesis. Similar data were observed with C. d. terrificus venom [29]. It was also shown that Tityus serrulatus scorpion venom induced an increase in IL-10 and a decrease in IL-6 and TNF [46].
Cardoso and Sampaio et al. [21,47] also reported that inhibitory effects induced by crotoxin on chemotaxis and macrophage phagocytosis may increase IL-10. Furthermore, phospholipase A2 (PLA2), a subunit of crotoxin, could trigger IL-4 production through mastocyte activation [38].
Anaphylaxis is a severe acute allergic manifestation with potentially fatal clinical repercussions, usually prompted by IgE mediated hypersensitivity. The levels of IL-4 become particularly elevated and induce an increase susceptibility to vasoactive mediators [48]. Previous studies observed anaphylactic response to snake venom characterized by rising serum IgE levels. These observations suggest that increase levels of IL-4 and IL-6 may be related to the anaphylactic response in the experimental group. Further research is required to provide evidence of this anaphylactic pathway due to their potential implications for the clinical management of patients bitten by Crotalus durissus cascavella.
The low levels of IL-2 found in the macerated lungs and peritoneal fluid indicated an inhibitory effect on lymphoproliferation, as observed in studies in vitro with C. d. terrificus and C. d. collilineatus venom, and with C. d. terrificus crotoxin [17,49]. However, short half-life and low circulation of IL-2 [50] may reduce detection levels of this cytokine.
Decline of IFNγ in macerated lungs and the absence of significant levels of IL-17 may be related to IL-4 production. This cytokine plays a pivotal role decreasing the production of Th1 and Th17 cells. However, the increase in IFNγ in the first hours (3h) in macerated lungs, despite the presence of IL-4 and IL-10, may lead to systemic disorders such disseminated vascular coagulopathy [3]. Furthermore, metalloproteinases can activate prothrombin and coagulation factor X, which inhibit platelet aggregation, and lead to apoptotic activity and hemostatic changes [51].
Despite its severity, few studies have examined pulmonary alterations in snakebite accidents [28,52–54]. In crotalic accidents, crotoxin blocks the neuromuscular transmission that contributes to the development of paralysis, muscular respiratory insufficiency and acute respiratory distress [11,28]. The respiratory abnormalities related in this study are similar to the severe cases of respiratory paralysis reported in accidents caused by snakes in the Elapidae family [55,56], and Vipera palaestine [57].
Hemorrhage focuses, congestion, atelectasis and emphysema in the pulmonary parenchyma within 3 hours of exposure demonstrate an early physiopathological pathway of pulmonary damage and impaired respiratory mechanics, leading to acute lung injury (ALI). Similar alterations were observed with Crotalus durissus cascavella and Tityus e Androctonus venom [31,58–61]. Respiratory damage have also been attributed to Bothrops jararaca venom, either by hemorrhage due metalloproteinases or by the action of PLA2 which can lead to pulmonary inflammation [62]. These data emphasize that snakebite victims presenting respiratory alterations require intensive monitoring.
Massive PMN cells proliferation in the pulmonary parenchyma, present up to the 48 hour time window, is probably linked to an increase in IL-17 levels [30,31]. Collagen deposition in the pulmonary parenchyma observed at 6, 12 and 24 hours can be related to mechanisms of repair and remodeling following an aggressive inflammatory response [63,64].
In summary, these findings advance our understanding of the pathophysiologic events and acute lung injury (ALI) induced by Crotalus durissus cascavella venom. High levels of acute phase cytokines detected in the peritoneal fluid and macerated lungs induced a systemic inflammatory response. There is a positive correlation between severity of clinical manifestations and pro-inflammatory cytokines levels (IL-6, TNF). Nevertheless, enhanced IL-4 production indicates a dynamic switch from Th1 to Th2 response and may be related to anaphylaxis triggered by venom components. Public policies should to be designed to prevent and improve patient outcomes in snake accidents.
Acknowledgments
The authors are grateful to Comissão de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Laboratory of Venomous Animals and Herpetology team, especially M. Nolasco, I. Cabral, D. Andrade and E. Dias for their assistance in the experimental work and Dr. L. Gusmão of the State University of Feira de Santana—Mycology Laboratory for making the microscope available for image acquisition.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received financial support from Comissão de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
- 1.World Health Organization. Rabies and envenomings. A neglected public health issue: report of a consultative meeting. [Internet]. World Health Organization, editor. Geneva; 2007. 32 p. Available from: https://apps.who.int/iris/handle/10665/43858
- 2.Barravieira B, Lomonte B, Tarkowski A, Hanson LÃ, Meira DA. Acute-phase reactions, including cytokines, in patients bitten by Bothrops and Crotalus snakes in Brazil. J Venom Anim Toxins [Internet]. 1995;1:11–22. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-79301995000100003&nrm=iso [Google Scholar]
- 3.Biondi I. Caracterização biológica e bioquímica da peçonha de Crotalus durissus cascavella no Estado da Bahia. State University of Feira de Santana; 2009. [Google Scholar]
- 4.Petricevich VL, Teixeira CFP, Tambourgi D V, Gutiérrez JM. Increments in serum cytokine and nitric oxide levels in mice injected with Bothrops asper and Bothrops jararaca snake venoms. Toxicon [Internet]. 2000;38(9):1253–66. Available from: http://www.sciencedirect.com/science/article/pii/S0041010199002275 10.1016/s0041-0101(99)00227-5 [DOI] [PubMed] [Google Scholar]
- 5.Luna K, Melo C, Pascoal VPM, Martins-Filho OA, Pereira VRA. Bothrops erythromelas snake venom induces a proinflammatory response in mice splenocytes. Int J Interf Cytokine Mediat Res. 2011. January 1;3:9–18. [Google Scholar]
- 6.Burin SM, Menaldo DL, Sampaio S V, Frantz FG, Castro FA. An overview of the immune modulating effects of enzymatic toxins from snake venoms. Int J Biol Macromol [Internet]. 2018;109:664–71. Available from: http://www.sciencedirect.com/science/article/pii/S0141813017345956 10.1016/j.ijbiomac.2017.12.101 [DOI] [PubMed] [Google Scholar]
- 7.Ministério da Saúde. Datasus, Tecnologia da Informação a Serviço do SUS. 2019.
- 8.Jorge MT, Ribeiro LA. Epidemiologia e quadro clínico do acidente por cascavel sul-americana (Crotalus durissus). Rev do Inst Med Trop SÃ\poundso Paulo [Internet]. 1992;34:347–54. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0036-46651992000400013&nrm=iso [PubMed] [Google Scholar]
- 9.Ministério da Saúde. Manual de diagnóstico e tratamento de acidentes por animais peçonhentos. [Internet]. 2nd ed. Fundação Nacional de Saúde(FUNASA), editor. Brasília; 2001. 120 p. Available from: https://www.icict.fiocruz.br/sites/www.icict.fiocruz.br/files/Manual-de-Diagnostico-e-Tratamento-de-Acidentes-por-Animais-Pe—onhentos.pdf
- 10.Pinho FMO, Pereira ID. Ofidismo. Rev Assoc Med Bras [Internet]. 2001;47:24–9. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-42302001000100026&nrm=iso 10.1590/s0104-42302001000100026 [DOI] [PubMed] [Google Scholar]
- 11.Cupo P, Azevedo-Marques MM de, Hering SE. Acidentes por animais peçonhentos: escorpiões e aranhas TT—Envenomation caused by poisonous animals: scorpions and spiders. Med (Ribeiräo Preto) [Internet]. 2003;36(2/4):490–7. Available from: http://pesquisa.bvsalud.org/portal/resource/pt/lil-400408 [Google Scholar]
- 12.Amaral CFS, Rezende NA, Pedrosa TMG, Silva OA da, Pedroso ERP. Afibrinogenemia secundária a acidente ofídico crotálico (Crotalus durissus terrificus). Rev do Inst Med Trop SÃ\poundso Paulo [Internet]. 1988;30:288–92. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0036-46651988000400007&nrm=iso [DOI] [PubMed] [Google Scholar]
- 13.Tomy SC, Campolina D, Dias MB, Castro SCBDE, Amaral CFS, Fisiopatologia D, et al. Coagulopathy following lethal and non-lethal envenoming of humans by the South American rattlesnake (Crotalus durissus) in Brazil. 2001;551–9. [DOI] [PubMed] [Google Scholar]
- 14.Magalhães RA, Ribeiro MMF, Rezende NA de, Amaral CFS. Rabdomiólise secundária a acidente ofídico crotálico (Crotalus durissus terrificus). Rev Inst Med Trop Sao Paulo [Internet]. 1986;28:228–33. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0036-46651986000400004&nrm=iso 10.1590/s0036-46651986000400004 [DOI] [PubMed] [Google Scholar]
- 15.Martins AMC, Toyama MH, Havt A, Novello JC, Marangoni S, Fonteles MC, et al. Determination of Crotalus durissus cascavella venom components that induce renal toxicity in isolated rat kidneys. Toxicon [Internet]. 2002;40(8):1165–71. Available from: http://www.sciencedirect.com/science/article/pii/S0041010102001198 10.1016/s0041-0101(02)00119-8 [DOI] [PubMed] [Google Scholar]
- 16.Francischetti IMB, Saliou B, Leduc M, R. Carlini C, Hatmi M, Randon J, et al. COnvulxin, a potent platelet-aggregating protein from Crotalus durissus terrificus venom, specifically binds to platelets. Toxicon [Internet]. 1997;35(8):1217–28. Available from: http://www.sciencedirect.com/science/article/pii/S0041010197000214 10.1016/s0041-0101(97)00021-4 [DOI] [PubMed] [Google Scholar]
- 17.Rangel-Santos A, Lima C, Lopes-Ferreira M, Cardoso DF. Immunosuppresive role of principal toxin (crotoxin) of Crotalus durissus terrificus venom. Toxicon. 2004. December 1;44:609–16. 10.1016/j.toxicon.2004.07.004 [DOI] [PubMed] [Google Scholar]
- 18.Fonseca F, Antunes E, Morganti RP, S.A. Monteiro H, Martins A, Toyama D, et al. Characterization of a New Platelet Aggregating Factor from Crotoxin Crotalus durissus cascavella Venom. Protein J. 2006. May 1;25:183–92. 10.1007/s10930-006-9001-z [DOI] [PubMed] [Google Scholar]
- 19.Boldrini-França J, Corrêa-Netto C, Silva MMS, Rodrigues RS, De La Torre P, Pérez A, et al. Snake venomics and antivenomics of Crotalus durissus subspecies from Brazil: Assessment of geographic variation and its implication on snakebite management. J Proteomics [Internet]. 2010;73(9):1758–76. Available from: http://www.sciencedirect.com/science/article/pii/S1874391910001776 10.1016/j.jprot.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 20.Mosmann TR, Coffman RL. Heterogeneity of Cytokine Secretion Patterns and Functions of Helper T Cells In: Dixon FJBT-A in I, editor. Advances in Immunology [Internet]. Academic Press; 1989. p. 111–47. Available from: http://www.sciencedirect.com/science/article/pii/S0065277608606525 10.1016/s0065-2776(08)60652-5 [DOI] [PubMed] [Google Scholar]
- 21.Cardoso DF, Lopes-Ferreira M, Faquim-Mauro EL, Macedo MS, Farsky SH. Role of crotoxin, a phospholipase A2 isolated from Crotalus durissus terrificus snake venom, on inflammatory and immune reactions. Mediators Inflamm [Internet]. 2001. June;10(3):125–33. Available from: https://www.ncbi.nlm.nih.gov/pubmed/11545249 10.1080/09629350124986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zambelli VO, Sampaio SC, Sudo-Hayashi LS, Greco K, Britto LRG, Alves AS, et al. Crotoxin alters lymphocyte distribution in rats: Involvement of adhesion molecules and lipoxygenase-derived mediators. Toxicon. 2008;51(8):1357–67. 10.1016/j.toxicon.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 23.Hernandez A, Navarro L, Mendonça R, Petricevich V. Inflammatory Mediators Release in Urine from Mice Injected with Crotalus durissus terrificus Venom. Mediators Inflamm. 2011. November 29;2011:103193 10.1155/2011/103193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feili-Hariri M, Falkner DH, Morel PA. Polarization of naive T cells into Th1 or Th2 by distinct cytokine-driven murine dendritic cell populations: implications for immunotherapy. J Leukoc Biol [Internet]. 2005. September 1;78(3):656–64. Available from: 10.1189/jlb.1104631 [DOI] [PubMed] [Google Scholar]
- 25.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol [Internet]. 2006;6(4):329–34. Available from: 10.1038/nri1807 [DOI] [PubMed] [Google Scholar]
- 26.Zamuner SR, Gutiérrez JM, Muscará MN, Teixeira SA, Teixeira CFP. Bothrops asper and Bothrops jararaca snake venoms trigger microbicidal functions of peritoneal leukocytes in vivo. Toxicon [Internet]. 2001;39(10):1505–13. Available from: http://www.sciencedirect.com/science/article/pii/S0041010101001234 10.1016/s0041-0101(01)00123-4 [DOI] [PubMed] [Google Scholar]
- 27.Zamuner S, Zuliani J, Fernandes C, Gutiérrez J, Teixeira C. Inflammation induced by Bothrops asper venom: Release of proinflammatory cytokines and eicosanoids, and role of adhesion molecules in leukocyte infiltration. Toxicon. 2006. January 1;46:806–13. [DOI] [PubMed] [Google Scholar]
- 28.Amaral CFS, Magalhães RA, Rezende NA de. Comprometimento respiratório secundário a acidente ofídico crotálico (Crotalus durissus). Rev do Inst Med Trop SÃ\poundso Paulo [Internet]. 1991;33:251–5. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0036-46651991000400002&nrm=iso [PubMed] [Google Scholar]
- 29.Hernandez A, Sara G, Mendonça R, Petricevich V. Pro- and Anti-Inflammatory Cytokines Release in Mice Injected with Crotalus durissus terrificus Venom. Mediators Inflamm. 2008. February 1;2008:874962 10.1155/2008/874962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nonaka PN, Amorim CF, Paneque Peres AC, e Silva CAM, Zamuner SR, Ribeiro W, et al. Pulmonary mechanic and lung histology injury induced by Crotalus durissus terrificus snake venom. Toxicon [Internet]. 2008;51(7):1158–66. Available from: http://www.sciencedirect.com/science/article/pii/S0041010108000457 10.1016/j.toxicon.2008.02.006 [DOI] [PubMed] [Google Scholar]
- 31.de Oliveira Neto J, Alison de Moraes Silveira J, Serra D, de Araújo Viana D, Borges-Nojosa D, Maria Souza Sampaio C, et al. Pulmonary mechanic and lung histology induced by Crotalus durissus cascavella snake venom. Toxicon. 2017. July 1;137:144–9. 10.1016/j.toxicon.2017.07.023 [DOI] [PubMed] [Google Scholar]
- 32.LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem [Internet]. 1951. November;193(1):265–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14907713 [PubMed] [Google Scholar]
- 33.Lima C, Clissa P, Amélia Piran-Soares A, Tancioni I, M Moura-da-Silva A, Lopes-Ferreira M. Characterisation of local inflammatory response induced by Thalassophryne nattereri fish venom in a mouse model of tissue injury. Toxicon. 2003. November 1;42:499–507. 10.1016/s0041-0101(03)00228-9 [DOI] [PubMed] [Google Scholar]
- 34.Petricevich VL. Cytokine and nitric oxide production following severe envenomation. Vol. 3, Current Drug Targets: Inflammation and Allergy. 2004. p. 325–32. 10.2174/1568010043343642 [DOI] [PubMed] [Google Scholar]
- 35.Cardoso DF, Mota I. Effect of Crotalus venom on the humoral and cellular immune response. Toxicon [Internet]. 1997;35(4):607–12. Available from: http://www.sciencedirect.com/science/article/pii/S0041010196001341 10.1016/s0041-0101(96)00134-1 [DOI] [PubMed] [Google Scholar]
- 36.Favoretto BC, Ricardi R, Silva SR, Jacysyn JF, Fernandes I, Takehara HA, et al. Immunomodulatory effects of crotoxin isolated from Crotalus durissus terrificus venom in mice immunised with human serum albumin. Toxicon [Internet]. 2011;57(4):600–7. Available from: http://www.sciencedirect.com/science/article/pii/S0041010111000067 10.1016/j.toxicon.2010.12.023 [DOI] [PubMed] [Google Scholar]
- 37.de Souza Almeida C, Andrade-Oliveira V, Olsen Saraiva Câmara N, F Jacysyn J, L Faquim-Mauro E. Crotoxin from Crotalus durissus terrificus Is Able to Down-Modulate the Acute Intestinal Inflammation in Mice. PLoS One. 2015. April 8;10:e0121427 10.1371/journal.pone.0121427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voronov E, Apte RN, Sofer S. The systemic inflammatory response syndrome related to the release of cytokines following severe envenomation. J Venom Anim Toxins [Internet]. 1999;5:5–33. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-79301999000100002&nrm=iso [Google Scholar]
- 39.Petricevich V. Cytokine and Nitric Oxide Production Following Severe Envenomation. Curr Drug Targets Inflamm Allergy. 2004. October 1;3:325–32. 10.2174/1568010043343642 [DOI] [PubMed] [Google Scholar]
- 40.F Barros S, Friedlanskaia I, Petricevich V, L Kipnis T. Local inflammation, lethality and cytokine release in mice injected with Bothrops atrox venom. Mediators Inflamm. 1998. January 1;7:339–46. 10.1080/09629359890866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carneiro AS, Ribeiro OG, Cabrera WHK, Vorraro F, De Franco M, Ibañez OM, et al. Bothrops jararaca venom (BjV) induces differential leukocyte accumulation in mice genetically selected for acute inflammatory reaction: The role of host genetic background on expression of adhesion molecules and release of endogenous mediators. Toxicon [Internet]. 2008;52(5):619–27. Available from: http://www.sciencedirect.com/science/article/pii/S0041010108004522 10.1016/j.toxicon.2008.07.012 [DOI] [PubMed] [Google Scholar]
- 42.Menezes TN, Carnielli JBT, Gomes HL, Pereira FEL, Lemos EM, Bissoli NS, et al. Local inflammatory response induced by scorpionfish Scorpaena plumieri venom in mice. Toxicon [Internet]. 2012;60(1):4–11. Available from: http://www.sciencedirect.com/science/article/pii/S0041010112000700 10.1016/j.toxicon.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 43.D M Fukuhara Y, Reis M, Joviliano R, Cunha F, Donadi E. Increased plasma levels of IL-1beta, IL-6, IL-8, IL-10 and TNF-alpha in patients moderately or severely envenomed by Tityus serrulatus scorpion sting. Toxicon. 2003. February 1;41:49–55. 10.1016/s0041-0101(02)00208-8 [DOI] [PubMed] [Google Scholar]
- 44.Pessini AC, de Souza AM, Faccioli LH, Gregório ZMO, Arantes EC. Time course of acute-phase response induced by Tityus serrulatus venom and TsTX-I in mice. Int Immunopharmacol [Internet]. 2003;3(5):765–74. Available from: http://www.sciencedirect.com/science/article/pii/S156757690300078X 10.1016/S1567-5769(03)00078-X [DOI] [PubMed] [Google Scholar]
- 45.Abdel-Haleem AHA, Meki ARMA, Noaman HA, Mohamed ZT. Serum levels of IL-6 and its soluble receptor, TNF-α and chemokine RANTES in scorpion envenomed children: Their relation to scorpion envenomation outcome. Toxicon [Internet]. 2006. March 15 [cited 2019 Aug 27];47(4):437–44. Available from: https://www.sciencedirect.com/science/article/pii/S0041010105004253#! 10.1016/j.toxicon.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 46.Fialho EMS, Maciel MCG, Silva ACB, Reis AS, Assunção AKM, Fortes TS, et al. Immune cells recruitment and activation by Tityus serrulatus scorpion venom. Toxicon. 2011. November;58(6–7):480–5. 10.1016/j.toxicon.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 47.Sampaio SC, Brigatte P, Sousa-e-Silva MCC, dos-Santos EC, Rangel-Santos AC, Curi R, et al. Contribution of crotoxin for the inhibitory effect of Crotalus durissus terrificus snake venom on macrophage function. Toxicon [Internet]. 2003;41(7):899–907. Available from: http://www.sciencedirect.com/science/article/pii/S0041010103000692 10.1016/s0041-0101(03)00069-2 [DOI] [PubMed] [Google Scholar]
- 48.Ogawa Y, Grant JA. Mediators of Anaphylaxis. Immunol Allergy Clin North Am [Internet]. 2007;27(2):249–60. Available from: http://www.sciencedirect.com/science/article/pii/S0889856107000355 10.1016/j.iac.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 49.Bastos Ribeiro C, Cristina dos Santos J, Medeiros J, Henrique Silva de Godoi P, Magalhães M, Spadafora Ferreira M, et al. Crotalus durissus collilineatus Venom Induces TNF-α and IL-10 Production in Human Peripheral Blood Mononuclear Cells. ISRN Inflamm. 2014. January 19;2014:563628 10.1155/2014/563628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery [Internet]. 2000;127(2):117–26. Available from: http://www.sciencedirect.com/science/article/pii/S0039606000584438 10.1067/msy.2000.101584 [DOI] [PubMed] [Google Scholar]
- 51.Markland FS, Swenson S. Snake venom metalloproteinases. Toxicon [Internet]. 2013;62:3–18. Available from: http://www.sciencedirect.com/science/article/pii/S0041010112007477 10.1016/j.toxicon.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 52.Benvenuti LA, França FOS, Barbaro KC, Nunes JR, Cardoso JLC. Pulmonary haemorrhage causing rapid death after Bothrops jararacussu snakebite: a case report. Toxicon [Internet]. 2003;42(3):331–4. Available from: http://www.sciencedirect.com/science/article/pii/S0041010103001673 10.1016/s0041-0101(03)00167-3 [DOI] [PubMed] [Google Scholar]
- 53.Gnanathasan A, Rodrigo C. Pulmonary Effects and Complications of Snakebites. Chest [Internet]. 2014;146(5):1403–12. Available from: http://www.sciencedirect.com/science/article/pii/S0012369215524094 10.1378/chest.13-2674 [DOI] [PubMed] [Google Scholar]
- 54.Bart G, Pineau S, Biron C, Connault J, Artifoni M. Bilateral Pulmonary Embolism Following a Viper Envenomation in France: A Case Report and Review. Medicine (Baltimore). 2016. May 1;95:e2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bucaretchi F, De Capitani E, José Vieira R, K Rodrigues C, Zannin M, Silva N, et al. Coral snake bites (Micrurus spp.) in Brazil: A review of literature reports. Clin Toxicol (Phila). 2016. January 25;54:1–13. [DOI] [PubMed] [Google Scholar]
- 56.Manock S, Suarez G, Graham D, Avila-Aguero M, A Warrell D. Neurotoxic envenoming by South American coral snake (Micrurus lemniscatus helleri): case report from eastern Ecuador and review. Trans R Soc Trop Med Hyg. 2008. June 1;102:1127–32. 10.1016/j.trstmh.2008.03.026 [DOI] [PubMed] [Google Scholar]
- 57.Paret G, Ben-Abraham R, Ezra D, Shrem D, Eshel G, Vardi A, et al. Vipera palaestinae snake envenomations: experience in children. Hum Exp Toxicol [Internet]. 1997. November 1;16(11):683–7. Available from: 10.1177/096032719701601110 [DOI] [PubMed] [Google Scholar]
- 58.D’Suze G, Comellas A, Pesce L, Sevcik C, Sanchez-de-León R. Tityus discrepans venom produces a respiratory distress syndrome in rabbits through an indirect mechanism. Toxicon [Internet]. 1999;37(1):173–80. Available from: http://www.sciencedirect.com/science/article/pii/S0041010198001809 10.1016/s0041-0101(98)00180-9 [DOI] [PubMed] [Google Scholar]
- 59.D’Suze G, Salazar V, Díaz P, Sevcik C, Azpurua H, Bracho N. Histophatological changes and inflammatory response induced by Tityus discrepans scorpion venom in rams. Toxicon. 2004;44(8):851–60. 10.1016/j.toxicon.2004.08.021 [DOI] [PubMed] [Google Scholar]
- 60.Paneque Peres AC, Nonaka PN, de Tarso Camillo de Carvalho P, Toyama MH, Melo e Silva CA, de Paula Vieira R, et al. Effects of Tityus serrulatus scorpion venom on lung mechanics and inflammation in mice. Toxicon [Internet]. 2009;53(7):779–85. Available from: http://www.sciencedirect.com/science/article/pii/S0041010109000889 [DOI] [PubMed] [Google Scholar]
- 61.Saidi H, Bérubé J, Laraba-Djebari F, Hammoudi-Triki D. Involvement of Alveolar Macrophages and Neutrophils in Acute Lung Injury After Scorpion Envenomation: New Pharmacological Targets. Inflammation. 2018;41(3):773–83. 10.1007/s10753-018-0731-9 [DOI] [PubMed] [Google Scholar]
- 62.Silveira KSO, Boechem NT, do Nascimento SM, Murakami YLB, Barboza APB, Melo PA, et al. Pulmonary mechanics and lung histology in acute lung injury induced by Bothrops jararaca venom. Respir Physiol Neurobiol. 2004. January;139(2):167–77. 10.1016/j.resp.2003.10.002 [DOI] [PubMed] [Google Scholar]
- 63.Ward PA, Hunninghake GW. Lung Inflammation and Fibrosis. Am J Respir Crit Care Med [Internet]. 1998. April 1;157(4):S123–9. Available from: 10.1164/ajrccm.157.4.nhlbi-10 [DOI] [PubMed] [Google Scholar]
- 64.Todd NW, Luzina IG, Atamas SP. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair [Internet]. 2012. July 23;5(1):11 Available from: https://www.ncbi.nlm.nih.gov/pubmed/22824096 10.1186/1755-1536-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]