Abstract
Background:
Huangqi Guizhi Wuwu Decoction (HGWD) is a common prescription for the treatment of cervical radiculopathy (CR). And the effectiveness and safety of HGWD for CR were assessed in this study.
Methods:
Seven databases were searched. Randomized controlled trials involving HGWD alone or HGWD combined with conventional treatment were enrolled. The authors in pairs independently assessed the risk of bias and extracted the data.
Results:
Eight studies involving 783 participants with CR were included. Meta-analysis revealed that the efficacy of HGWD for CR was significantly superior compared with control treatment (risk ratio = 1.12, 95% confidence interval [CI]:1.06–1.19, Z = 3.71; P = .0002). Compare with control group, there is an increase in visual analog scale (mean difference [MD] = 0.99; 95% CI: 0.83–1.14; Z = 12.57; P < .00001). There was also an improvement of neck disability index (MD = 9.2; 95% CI: 8.28–10.11; Z = 19.75; P < .00001). Adverse events were not mentioned in the 8 trials.
Conclusion:
HGWD alone or HGWD plus other treatment may be helpful to patients with CR. However, the methodological quality of the randomized controlled trials was generally low. Larger and better-designed randomized controlled trials are recommended.
Keywords: cervical radiculopathy, efficacy, Huangqi Guizhi Wuwu Decoction, meta-analysis, neck disability index, visual analog scale
1. Introduction
Cervical radiculopathy (CR), which is most often stems from degenerative disease in the cervical spine, has increasingly become a common and frequently occurring disease in clinic due to the popularity of electronic products, such as computes and cell phones. Significant functional limitations and disabilities are common presenting complaints for people suffering from CR.[1,2] In general, they seek clinical assistance owing to neck and shoulder pain.[3] Also, along with the extension of the course of disease, some other symptoms will also appear, such as stiffness and fatigue in the neck, disturbances of sensory and motor functions, and even weakness tendon reflexes in the arm.[4] Usually, the most common cause of the disease are cervical disc herniation and spondylosis.[5] The annual age-adjusted incidence of CR is 83 per 100,000 persons, and it has been reported to 107.3 per 100,000 for men and 63.5 per 100,000 for women.[3,4,6] Typically, a peak incidence is seen in middle age.[7] In addition, cigarette smoking, cervical axial load, and prior lumbar radiculopathy may predispose patients to CR.[8] At present, treatment for CR includes surgical and nonsurgical approaches. According to statistics, surgical treatment, which chiefly is decompress the spinal nerve with a posterior foraminotomy, has a positive relieving symptoms.[9,10] However, the surgical treatment of CR is still controversial as a result of many complications after operation involving adjacent segment degeneration, loss in intervertebral disc height, and so on.[11] So, conservative measures are often used. And therapeutic strategies mainly include immobilization, traction, pharmacy therapy, steroid injections, physical therapy, and manipulation.[12] Little high-quality evidence supports the use of traditional Chinese medicine in countries other than China. Nevertheless, traditional Chinese medicine may hold some promise for CR.
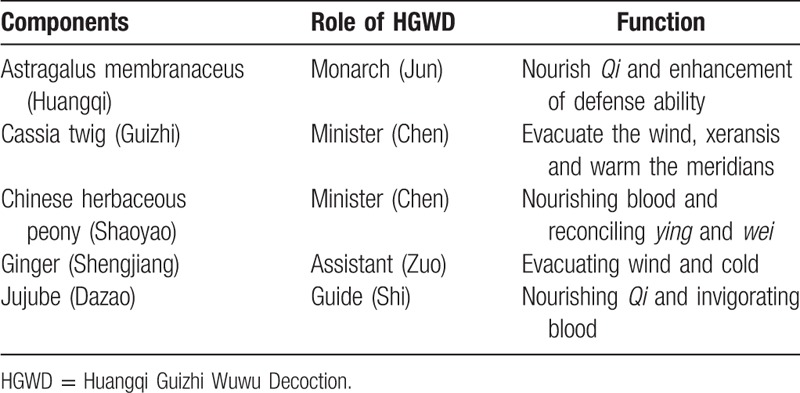
CR may fall under the traditional Chinese medicine categories of “arthromyodynia,” which is wind, cold, dampness and other pathogenic factors invade the human body, resulting in obstruction of meridians, stagnation of Ying and Wei, and stagnation of Qi and blood in Zang Fu organs.[13] Huangqi Guizhi Wuwu Decoction (HGWD) is a classical prescription described in Jingui Yaolue, written by Zhang zhongjing during the Han dynasty. As shown in Table 1, HGWD is composed of astragalus membranaceus (Huangqi), cassia twig (Guizhi), Chinese herbaceous peony (Shaoyao), jujube (Dazao), all of which are reported to nourishing Qi, Removing pathogenic factors such as wind cold and damp, warming and smoothing meridian, invigorating the blood to promote coronary circulation.[14] In general, HGWD is used to treat “blood impended” characterized by a feeling of pain and numbness, muscle discomfort, muscular atrophy, consistent with the features of CR. Some new research has shown that the mechanisms of action of HGWD in rats include relieving pain by raising the pain threshold,[15] playing anti-inflammatory effects by reducing inflammatory factors such as interleukin-4 (IL-4), inducible nitric oxide synthase (iNOS),[16] which plays an important role in the development of symptoms of CR.[17] Moreover, HGWD exerts multiple therapeutic effects by acting on multiple targets.[18] Although previous studies estimated that HGWD is therapeutically effective for CR, a systematic review of these studies as regards HGWD treating for CR is required to more accurately assess the efficacy and safety.
Table 1.
Composition and function of HGWD.
2. Methods
All pooled analyses are based on previously published studies, and thus no ethical approval and patient consent are required. The protocol of systematic review was published in the
International Prospective Register of Systematic Reviews (PROSPERO registration no.CRD42018105000, which was available on https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=105000).
2.1. Literature research
As with the original review, we used the search strategies recommended by the Cochrane Back Review Group for the identification of randomized controlled trials (RCTs).[19] The literature was retrieved using multiple online databases including Pubmed, Web of Science, Embase, the Cochrane Library, Chinese National Knowledge Infrastructure Database, Wanfang database, and VIP database, for all years up to August, 2018. There were no limits on study dates or any language, publication type, and status restrictions. The key terms used in these searches were “cervical radiculopathy,” “cervical spondylotic radiculopathy,” “nerve-root type cervical spondylosis,” “neck and arm pain,” “neck pain with radiculopathy,” “neck disorder with radiculopathy,” “Huangqi Guizhi Wuwu Tang,” “Huangqi Guizhi Wuwu Decoction,” “Huangqi Guizhi Wuwu.” Different search strategies were used for Chinese and foreign language databases. In addition, the reference lists of previously published systematic reviews on the subject of HGWD for the treatment of CR were manually examined for pertinent studies.
2.2. Inclusion criteria
The retrieved literature was screened by 2 independent investigators to evaluate eligibility, and any discrepancies were settled by discussion and consensus. First, the titles and abstracts of searched studies were screened. Then, full papers were reviewed to examine whether each study met the following criteria:
-
(1)
RCT;
-
(2)
types of participants must be patients suffering from CR;
-
(3)
experimental studies using HGWD or modified HGWD (without restriction for control group).
When multiple time points were reported either in 1 particular report of a study or over the course of several articles from the same study, the longest follow-up period on treatment was considered in our article. If overlapping subject populations were enrolled in different reports, the one of higher quality or with a larger sample size was selected for inclusion. Full texts of all references were available.
2.3. Exclusion criteria
The excluded studies were excluded due to the following reasons:
-
(1)
studies does not conform to the above criteria;
-
(2)
both the treatment group and the control group included HGWD;
-
(3)
studies were in the form of letters, abstracts, reviews, or comments;
-
(4)
studies were impossible to extract relevant data;
-
(5)
the CR patients were treated with surgery.
2.4. Data extraction
The following data were independently extracted by 2 authors: the name of first author, year of publication, study type, country, number of patients under HGWD treatment and control group, sample size, age, gender of patients, disease course, the details of control interventions, Follow-up duration, outcome, and intervention period. When relevant data had not been reported, we contacted the authors by email or in other ways to attempt to obtain the missing information.
2.5. Quality assessment
We assessed the risk of bias of RCTs in this review using the Cochrane Collaboration Risk of Bias Tool. And risk of bias was assessed according to the Cochrane Handbook.[19] For each included study, each type of bias was rated as high, low, or unclear and entered into the risk of bias table. Four review authors, 2 with methodological expertise and 2 with content expertise, independently assessed the risk of bias of the included studies. The review authors resolved any disagreements by discussion, including input from a third independent review author if required.
2.6. Outcome measures
Clinical efficacy was the main outcome, and the secondary outcomes were visual analog scale (VAS), neck disability index (NDI).
2.7. Data synthesis and statistical analysis
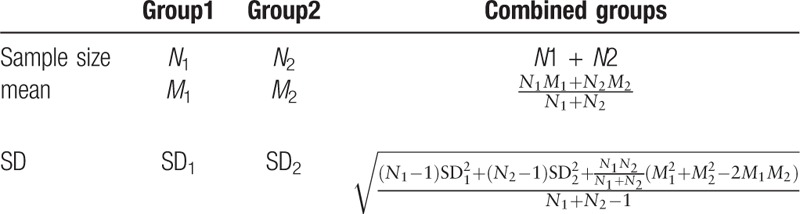
The outcomes of interest include dichotomous data and continuous variables, Dichotomous data were expressed as the risk ratio (RR) and mean difference (MD) was used to assess the difference in the continuous outcomes between the groups. Also, Standardized mean difference (SMD) was chosen if clinical outcome was the same but measured using different methods in the different trials. Its corresponding 95% confidence interval (CI) for each parameter was computed in HGWD-treated versus control group. Statistical heterogeneity across included studies was examined by the Cochrane Q test. An I2 > 50% signified the possibility of statistical heterogeneity, and the random-effects model was chosen for the computation of MD or SMD with its corresponding 95% CI. Otherwise, no obvious heterogeneity (I2 < 50%) was considered to have occurred in the included studies, and the fixed-effects model was selected to generate the MD or SMD with its corresponding 95% CI. The forest plot for each parameter was constructed to illustrate the weight ratio of each incorporated study. In order to evaluate the sensibility of the meta-analysis, articles were excluded one by one and the differences of the combing effect before and after exclusion were compared. If the pooled outcomes reversed after exclusion, the outcomes may be unstable. All statistical analyses were carried out using the RevMan5.3 software, and the significance threshold was a 2-sided P < .05. According to Cochrane Handbook 5.3, if Inclusion studies include a study with multiple intervention groups, the recommended method in most situations is to combine all relevant experimental intervention groups of the study into a single group and to combine all relevant control intervention groups into a single control group. And the formulates of continuous variables for combining groups are displayed in Table 2. For dichotomous outcomes, both the sample sizes and the numbers of people with events can be summed across groups. Furthermore, the within-subject change standard deviation, which was calculated based on means and standard deviations at baseline and follow-up provided in articles, was required for the meta-analysis. Thus weighted by the sample size of each trial:
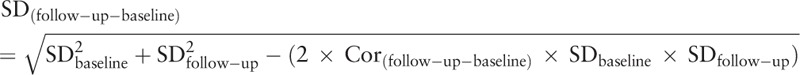 |
Table 2.
Formulate of continuous variables for combining groups.
Moreover, the main outcome is analyzed by trial sequential analysis (TSA), the TSA program V.0.9 beta software (http://www.ctu.dk/tsa) was used. In this study, The type I error (α) and power (1-β) were set as 0.05 and 0.80, respectively. And a relative risk reduction of 20% in incidence of CR. The control event rates were calculated from the control group.
2.8. Grading of recommendations assessment, development, and evaluation (GRADE)
The GRADE method was used to assess the quality of the evidence for each outcome of meta-analysis. Levels of quality of evidence recommended by the GRADE Working Group were defined as high (++++), moderate (+++), low (++), and very low (+). The judgments were based on risk of bias, inconsistency, indirectness, imprecision, and publication bias. We operated on this web page: https://gradepro.org/.
3. Results
3.1. Literature search and study sample characteristics
The search results are displayed in Figure 1. The primary searches indentified a total of 306 references using the outlined literature search strategy. Of these, 138 references were repeated literature in different databases and were excluded. According to the inclusion and exclusion criteria, 121 articles were excluded after reading the title and summaries. Then, after a detailed evaluation of full text, an additional 39 references were excluded. Among these, 1 trial was excluded because it use similar data with other trial. Nine studies were excluded because of incomplete data or unable to extract data. Five studies claimed that the trials were RCTs, but actually quasi-RCTs. 6 trials lack to a control group. Four duplicates of same article were excluded. Six studies were excluded because they used another traditional Chinese medicine in the treatment group. Eight trials were not in according with the inclusion criteria or compliance with exclusion criteria. Finally, 8 RCTs[20–27] were included in the systematic review.
Figure 1.
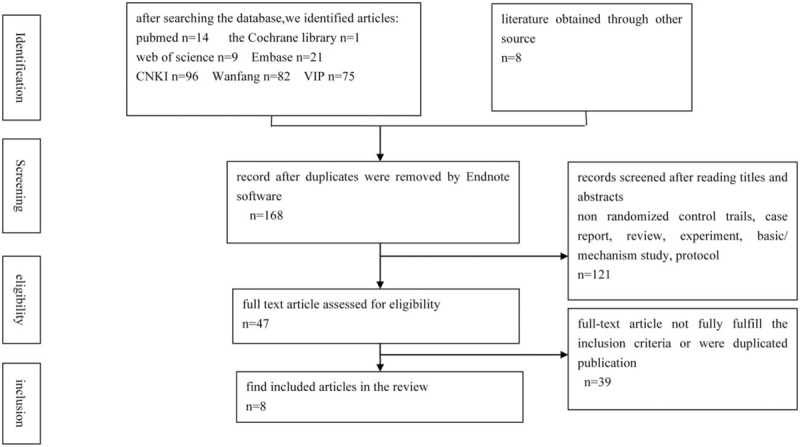
Flow diagram of literature search.
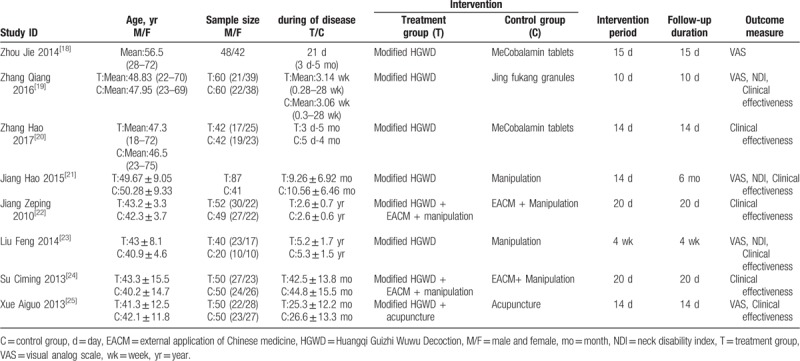
The characteristics of the included trials are summarized in Table 3. All trials were published in Chinese. Two studies[23,25] were RCTs with 3 parallel arms and the rest were trials with 2 parallel arms. In this meta-analysis, a total of 783 participants with CR were involved (429 and 354 in the treatment and control group, respectively). The main intervention strategies were categorized as “Modified HGWD,”[20–23,25] “Modified HGWD, external application of Chinese medicine plus manipulation,”[24,26] “Modified HGWD plus acupuncture.”[27] The trial sample size ranged from 60 to 128 participants. The intervention period is reported between 10 days and 4 weeks. Baseline imbalance was not found in the demographic characteristics or the outcomes between the study groups. No trial reported details of sample size calculations, and none of 8 trials were placebo-controlled trials.
Table 3.
Basic characteristics of the included trials.
3.2. Risk of bias
Figure 2 showed the graph of methodological quality. In the included studies, 6 trials described methods of randomization using a random mumble table[20,21,23,24,27] or computer random method.[25] The remaining 2 trials[22,26] indicated “randomly allocating,” the method used to generate the randomization sequence was not described. Only 1 study showed allocation concealment using “envelope method.”[27] And the other studies did not report it clearly. All trials did not mention whether or not to use the blind method, No trial reported participant losses, so attrition bias was low risk. Selective reporting was difficult to assess, and trial protocols were unavailable. All trials were generally assessed low in quality and contained risk of bias, suggesting future studies might influence the results in this meta-analysis and require revising the conclusion.
Figure 2.
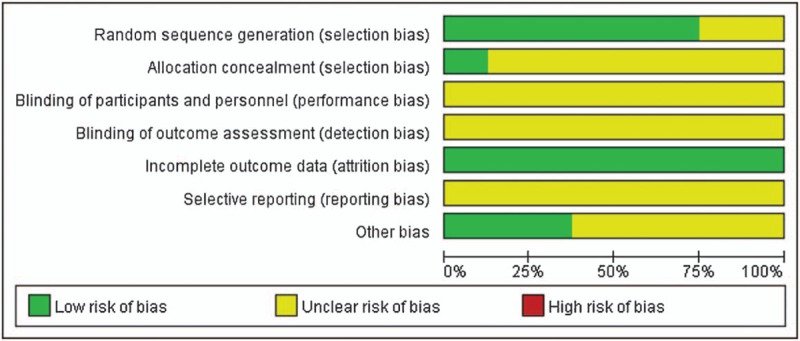
Risk of bias graph.
3.3. Meta-analysis results
3.3.1. Efficacy
The clinical efficacy is shown in Figure 3, the results of the 7 trials[21–27]were included in the meta-analysis, and demonstrated a significant difference in clinical efficacy between the treatment group and the control group. A fixed-effects model was used for statistical analysis according to the insignificant heterogeneity (I2 = 12%). Results from the pooled analysis indicated that there were significant differences in improving clinical efficacy in favor of the HGWD treatment (n = 693; RR = 1.12; 95%CI: 1.06–1.19; Z = 3.71; P = .0002). In addition, TSA showed that the cumulative Z curves crossed the conventional and the trial sequential monitoring boundaries, reached the significant area, which led us to draw a conclusion. Thus, it is unlikely that further trials will change the conclusion and are not necessary (Fig. 4).
Figure 3.
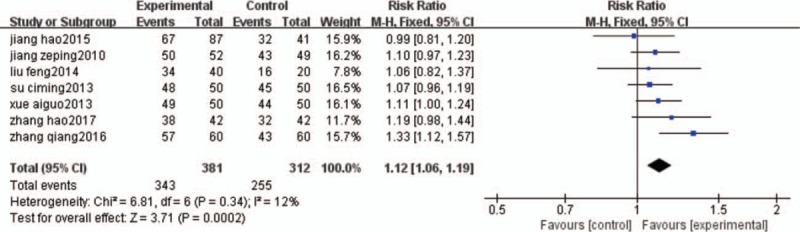
clinical efficacy comparison.
Figure 4.
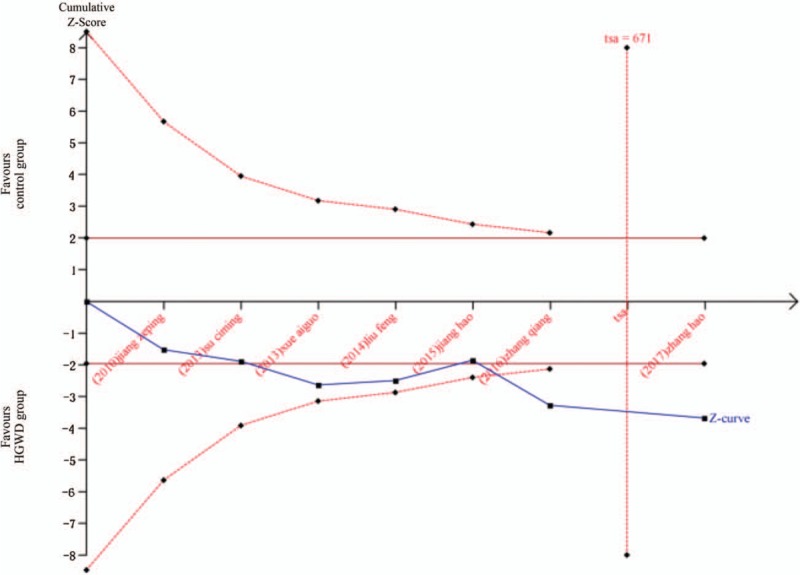
Trial sequential analysis with clinical efficacy.
3.3.2. VAS
Five trials,[20,21,23,25,27] involving a total of 469 patients, reported VAS as an outcome in the groups, and these trials exhibited significant heterogeneity (I2 = 86%), as shown in Figure 5. And accordingly, a random-effects model was used for statistical analysis. The meta-analysis of 5 trials revealed that patients treated by the HGWD had a statistically significant decrease in VAS (MD = 0.99; 95% CI: 0.83–1.14; Z = 12.57; P < .00001).
Figure 5.
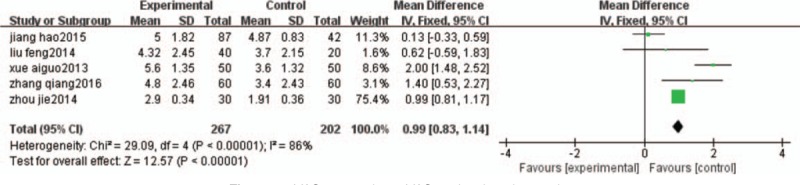
VAS comparison. VAS = visual analog scale.
3.3.3. NDI
NDI was reported in 3 studies.[21,23,25] The significant heterogeneity between trials was observed (I2 = 96%), and; therefore, a random-effects model was used for statistical analysis (Fig. 6). The meta-analysis from the 3 independent trials demonstrated that participants treated with HGWD therapy improving more significantly than participants treated with other therapy (MD = 9.2; 95% CI: 8.28–10.11; Z = 19.75; P < .00001).
Figure 6.
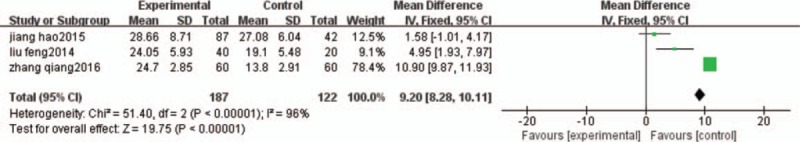
NDI comparison. NDI = neck disability index.
3.3.4. Adverse effects
As shown in Table 1, none of 8 studies reported adverse effects. No trial paid attention to the side effects from treatment or adverse events for the participants.
3.3.5. Publication bias
For the same intervention and outcome, the number of included trials (less than 10) was too small to conduct any sufficient additional analysis of publication bias.
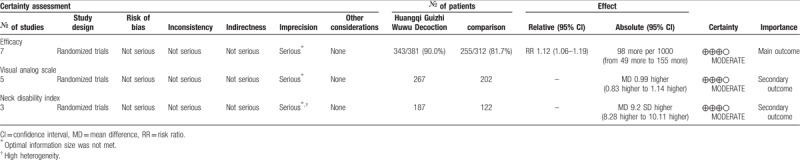
3.3.6. Grade
The GRADE level of evidence is moderate for each outcome. Table 4 shows the GRADE evidence profiles. The main reasons for a deceasing level were inconsistency and optimal information size was not met.
Table 4.
Summary of the evidence for each outcome.
4. Discussion
The clinical manifestations of CR are broad and may include pain, sensory deficits, motor deficits, diminished reflexes, or any combination of the above resulting from compression of cervical nerve roots by obstructions such as intervertebral, osteophyte, and so on.[28] The past decades have witnessed an unprecedented expansion in the fields of drugs for the treatment of CR discovery and development. However, treatment by western medicine is of limited clinical efficacy, safer and more effective therapies are urgently needed. Additionally, it has been increasingly acknowledged that traditional Chinese medicine has substantial therapeutic potential for treating CR.[29] HGWD, as a long-term traditional Chinese medicine prescription, is widely used to treat discomfort of the limbs in clinical practice.[18,29] And many studies displayed positive results with this decoction for treating CR.[20–27] Therefore, the effectiveness and safety of the herbal medicine for treating CR should be assessed by using mete-analysis of currently published trials.
In this finding, 8 trials involving 783 participants with CR were included. The results revealed that the efficacy of HGWD for CR was significantly superior to that of the treatments for the control group. Additionally, HGWD also were associated with significantly decreased VAS and NDI. Recently, a similar meta-analysis regards HGWD treating for CR was published by Chao et al.[30] The data showed that HGWD was more useful for increasing clinical efficacy (Western medicine-control: RR = 1.23; 95% CI: 1.14–1.33; Z = 5.38; P < .00001; Manipulation-control: RR = 1.13; 95% CI: 1.03–1.24; Z = 2.49; P = .01) and reducing VAS (Western medicine-control: MD = −1.24; 95% CI: −1.52 to −0.96; Z = 8.63; P < .00001; Manipulation-control: RR = 1.19; 95% CI: −1.73 to −0.66; Z = 4.37; P < .00001). However, although 10 studies were included in Chao's study,[30] not all studies were RCTs and several RCTs were not brought into. Besides, a outcome, NDI, a high-grade evaluation indicator recommended in the guideline for CR by the North American Spinal Association, is not yet analyzed. A strength of the current review is that we collected more appropriate literature, which included only RCTs. Also, more outcome indicators can be analyzed. As the latest and most comprehensively updated meta-analysis, the present study further reinforces the earlier results of previous meta-analysis. In addition, we registered the protocol of this study on PROSPERO. The transparency and quality of meta-analysis may be increased by registering a protocol. Moreover, the GRADE approach was performed to give the level of evidence. Thus, the conclusions of this study can be clinically used and easily transferred to guidelines. Finally, TSA was further applied to increase the robustness of this meta-analysis, estimating the effect more conservatively. It is unlikely that further trials will change the conclusion and are not necessary as for the main outcome, efficacy.
Several limitations are worthy to be mentioned. First, only 8 RCTs were included in this meta-analysis; therefore, a more definitive results of HGWD for treating CR requires larger and better-designed RCTs. Also, subgroup analysis cannot be carried out due to a small sample size. Second, some heterogeneity was detected, the origin of which may be the herb ingredients, dosage and treatment period varied among the trials. And thus, it might have negatively impacted the reliability of the outcomes. Thirdly. Long-term effectiveness of HGWD for treating people with CR is not known, and data concerning cost-effectiveness of HGWD was unavailable in this meta-analysis.
5. Conclusion
The available evidence suggests that HGWD could be considered as a potential treatment of CR, it can significantly reducing the VAS and NDI, which means that he can relieve the pain of CR and improve its symptoms, so as to achieve the purpose of treating this disease. Despite the apparently positive results, it is premature to conclude the effectiveness of HGWD for treating CR in virtue of the high heterogeneity and low methodological quality of the included studies. Hence, well-designed, large-scale, high-quality, long-term RCTs are required to provide more reliably assessments.
Author contributions
Conceptualization: Long Liang, Liguo Zhu.
Data curation: Gongbo Yang, Xunlu Yin, Shuaiqi Zhou, Kaiming Li.
Investigation: Minshan Feng, Shuaiqi Zhou, Mao Yang, Xingyu Wang.
Methodology: Xu Wei, Jie Yu, Gongbo Yang, Xunlu Yin.
Resources: Minshan Feng.
Software: Xu Wei, Gongbo Yang, Xunlu Yin, Kaiming Li, Mao Yang, Xingyu Wang.
Supervision: Minshan Feng, Liguo Zhu, Jie Yu.
Writing – original draft: Long Liang, Xu Wei.
Writing – review and editing: Long Liang, Liguo Zhu, Jie Yu.
Long Liang orcid: 0000-0001-7193-8547.
Footnotes
Abbreviations: CI = confidence intervals, CR = cervical radiculopathy, GRADE = the grading of recommendations assessment, development, and evaluation, HGWD = Huangqi Guizhi Wuwu Decoction, MD = mean difference, NDI = neck disability index, PROSPERO = international prospective register of systematic reviews, RCT = randomized controlled trials, RR = risk ratio, TSA = trial sequential analysis, VAS = visual analog scale.
How to cite this article: Liang L, Wei X, Feng M, Zhu L, Yu J, Yang G, Yin X, Zhou S, Li K, Yang M, Wang X. Huangqi Guizhi Wuwu Decoction for treating cervical radiculopathy: A systematic review and meta-analysis of randomized controlled trials. Medicine. 2020;99:7(e19137).
The project was supported by the National Administration of Traditional Chinese Medicine (GZY-FJS-2018-218, GZY-YGJ-2018-032, awarded to Liguo Zhu); Key Project of China Academy of Chinese Medical Sciences (ZZ10-022, awarded to Liguo Zhu); China Scholarship Council (201908110307, awarded to Long Liang); China Academy of Chinese Medical Sciences (CX201905, awarded to Long Liang).
The authors have no conflicts of interest to disclose.
References
- [1].Carette S, Fehlings MG. Clinical practice. Cervical radiculopathy. N Engl J Med 2005;353:392–9. [DOI] [PubMed] [Google Scholar]
- [2].Kim DG, Chung SH, Jung HB. The effects of neural mobilization on cervical radiculopathy patients’ pain, disability, ROM, and deep flexor endurance. J Back Musculoskelet Rehabil 2017;30:951–9. [DOI] [PubMed] [Google Scholar]
- [3].Corey DL, Comeau D. Cervical radiculopathy. Med Clin North Am 2014;98:791–9. [DOI] [PubMed] [Google Scholar]
- [4].Onks CA, Billy G. Evaluation and treatment of cervical radiculopathy. Prim Care 2013;40:837–48. [DOI] [PubMed] [Google Scholar]
- [5].Dedering A, Halvorsen M, Cleland J, et al. Neck-specific training with a cognitive behavioural approach compared with prescribed physical activity in patients with cervical radiculopathy: a protocol of a prospective randomised clinical trial. BMC musculoskelet Disord 2014;15:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Eubanks JD. Cervical radiculopathy: nonoperative management of neck pain and radicular symptoms. Am Fam Phys 2010;81:33–40. [PubMed] [Google Scholar]
- [7].Hogg-Johnson S, van der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the bone and joint decade 2000-2010 task force on neck pain and its associated disorders. Spine 2008;33:S39–51. [DOI] [PubMed] [Google Scholar]
- [8].Roth D, Mukai A, Thomas P, et al. Cervical radiculopathy. Dis Mon 2009;55:737–56. [DOI] [PubMed] [Google Scholar]
- [9].Caridi JM, Pumberger M, Hughes AP. Cervical radiculopathy: a review. HSS J 2011;7:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].van Geest S, Kuijper B, Oterdoom M, et al. CASINO: surgical or nonsurgical treatment for cervical radiculopathy, a randomised controlled trial. BMC Musculoskelet Disord 2014;15:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gutman G, Rosenzweig DH, Golan JD. Surgical treatment of cervical radiculopathy: meta-analysis of randomized controlled trials. Spine 2018;43:E365–72. [DOI] [PubMed] [Google Scholar]
- [12].Cui XJ, Yao M, Ye XL, et al. Shi-style cervical manipulations for cervical radiculopathy: a multicenter randomized-controlled clinical trial. Medicine 2017;96:e7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang NN. Jin Jie's experience in treating cervical radiculopathy with Huangqi Guizhi Wuwu Decoction. China's Naturopathy 2016;24:13–4. [Google Scholar]
- [14].Chen B, Yuan PW, Kang WL, et al. Application of Huangqi Guizhi Wuwu Decoction in orthopedics. Chin J Tradit Med Traumatol Orthoped 2015;23:71–4. [Google Scholar]
- [15].Xiong DQ. Protective effect of Huangqi Guizhi Wuwu Decoction on diabetic peripheral neuropathic pain. Strait Pharm J 2017;29:38–40. [Google Scholar]
- [16].Zhao L, Li YY, Wang YH, et al. Effect of Huangqi Guizhi Wuwu Tang on immune related cytokines in rats with Yang Deficiency and Cold Coagulation Type Osteoarthritis. Chin J Exp Tradit Med Formul 2017;23:161–6. [Google Scholar]
- [17].Scuderi GJ, Brusovanik GV, Anderson DG, et al. Cytokine assay of the epidural space lavage in patients with lumbar intervertebral disk herniation and radiculopathy. J Spinal Disord Tech 2006;19:266–9. [DOI] [PubMed] [Google Scholar]
- [18].Pang B, Zhao TY, Zhao LH, et al. Huangqi Guizhi Wuwu Decoction for treating diabetic peripheral neuropathy: a meta-analysis of 16 randomized controlled trials. Neural Regen Res 2016;11:1347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Furlan AD, Malmivaara A, Chou R, et al. 2015 Updated method guideline for systematic reviews in the Cochrane back and neck group. Spine 2015;40:1660–73. [DOI] [PubMed] [Google Scholar]
- [20].Zhou J, Jiang RJ, Lei M, et al. Huangqi Guizhi Wuwu Decoction plus scorpion and centipede for treatment of 90 cases of cervical radiculopathy. J Emerg Tradit Chin Med 2014;23:725–6. [Google Scholar]
- [21].Zhang Q, Zhang Y, Zhang FX. Clinical observation on treating cervical radiculopathy with modified Huangqi Guizhi Wuwu Decoction. J Pract Tradit Chin Med 2016;32:535–6. [Google Scholar]
- [22].Zhang H. Clinical observation on Nong Ben Fang granule Huangqi Guizhi Wuwu Decoction in treating limb numbness of cervical radiculopathy. Hubei J Tradit Chin Med 2017;39:49–50. [Google Scholar]
- [23].Jiang H, Xu HT, Liu LB, et al. Treatment of cervical spondylotic radiculopathy with fixed-axis rotation manipulation and combined with Huangqi Guizhi Wuwu Decoction. Chin Manipul Rehabil Med 2015;6:3–5. (in Chine. [Google Scholar]
- [24].Jiang ZP. Cervical traction and external application of traditional Chinese medicine combined with Huangqi Guizhi Wuwu Decoction in the treatment of 52 cases of cervical spondylotic radiculopathy. J N Chin Med 2010;42:35–6. [Google Scholar]
- [25].Liu F. Modified Huangqi Guizhi Wuwu Decoction combined with muscle tendon relax reduction manipulation in the treatment of cervical radiculopathy. Nei Mongol J Tradit Chin Med 2015;33:12–3. [Google Scholar]
- [26].Su CM, Huang JJ, Lei LM. Treatment of 50 cases of cervical spondylotic radiculopathy with traditional Chinese medicine. Hunan J Tradit Chin Med 2013;29:81–2. [Google Scholar]
- [27].Xue AG, Ruan BY, Li HE. Observation on the therapeutic effect of acupuncture combined with Astragalus and cinnamou twig five ingredients decoction for cervical spondylotic radiculopathy. China Med Pharm 2013;3:94–5. [Google Scholar]
- [28].Iyer S, Kim HJ. Cervical radiculopathy. Curr Rev Musculoskelet Med 2016;9:272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Luo J, Zhao GD, Gao JH, et al. Analysis of Huangqi Guizhi Wuwu Decoction in treatment of cervical spondylotic radiculopathy with numbness. China J Tradit Chin Med Pharm 2010;65:127–35. [Google Scholar]
- [30].Chao CY, Pei CH, Bin C, et al. Systematic evaluation of Huangqi Guizhi Wuwutang in the treatment of cervical spondylotic radiculopathy. Rheumatism Sarthritis 2018;7:37–42. [Google Scholar]


