Abstract
This study aimed to investigate the correlation of microRNA (miR)-206, vascular endothelial growth factor (VEGF) and miR-206/VEGF axis at different gestational ages with fetal growth retardation (FGR) risk in pregnancies.
Eight hundred twenty pregnancies were consecutively recruited and their plasma samples were collected at early pregnancy (gestational age ≤ 13 weeks), middle pregnancy (gestational age: 14–27 weeks) and late pregnancy (gestational age ≥ 28 weeks), respectively. miR-206 expression and VEGF level in plasma were detected by quantitative polymerase chain reaction and enzyme-linked immunosorbent assay respectively. FGR was diagnosed based on the actual birth weight of fetus.
miR-206 expression was negatively correlated with VEGF expression at early pregnancy, middle pregnancy and late pregnancy. Besides, miR-206 expression and miR-206/VEGF axis were elevated, but VEGF expression was decreased along with the increased gestational age. There were 74 FGR pregnancies and 746 non-FGR pregnancies. And both miR-206 expression and miR-206/VEGF axis were increased, but VEGF expression was reduced in FGR group compared to non-FGR group at early pregnancy, middle pregnancy and late pregnancy. Additionally, miR-206, VEGF and miR-206/VEGF axis at middle pregnancy and late pregnancy all showed good predictive values for FGR risk, and these indexes at late pregnancy exhibited the numerically highest predictive value for FGR risk. Furthermore, compared to miR-206 or VEGF alone, miR-206/VEGF axis presented with numerically higher predictive value for FGR risk.
miR-206 predicts raised FGR risk through the interaction with VEGF in pregnancies, and it may serve as a novel biomarker for FGR prevention.
Keywords: diagnosis, fetal growth retardation, microRNA-206, vascular endothelial growth factor
1. Introduction
Fetal growth retardation (FGR) is defined as a fetus has not reached its intrauterine growth potential, and affects about 5% to 10% of pregnancies, which not only results in perinatal problems (such as perinatal asphyxia and persistent pulmonary hypertension), but also leads to increased risk of postnatal complications such as hypoglycemia and feeding difficulties.[1–5] The current clinical strategy to screen for FGR is according to the estimated fetal weight in ultrasound scans, while sensitivity of universal ultrasound may be not satisfactory enough and misdiagnosis may occur, making FGR still a challenging problem.[5] Hence, exploring more convincing and sensitive biomarkers for assisting fetal surveillance and predicting FGR risk is needed.
microRNAs, the small non-coding RNAs that degrade target genes by targeting the 3′-untranslated region of mRNAs, play important roles in the regulation of gene expression at the post-transcriptional level, which are widely investigated as potential biomarkers for plentiful diseases.[6,7] microRNA-206 (miR-206), located on chromosome 6 in a bicistronic cluster, is well known as a muscle specific microRNA and believed to be a key regulator for myogenic differentiation, which is found to be increased in the serum and placenta of pregnancies who later develops preeclampsia and could predict increased risk of Duchenne muscular dystrophy (DMD) as well as correlates with decreased muscle strength in DMD children, indicating that miR-206 might be involved in the intrauterine growth or postnatal development.[6,8,9] Besides, as a direct target of miR-206,[9–11] vascular endothelial growth factor (VEGF) (that is an important angiogenesis regulator that accelerate angiogenesis and promote endothelial cell division) act as a crucial factor for embryo implantation and placental development.[12,13] Meanwhile, previous data have shown the both serum VEGF level in pregnancies and cord blood plasma VEGF level are positively correlated with birth weight of infants and might predict FGR risk.[14,15]
Based on the above observations, we hypothesized that miR-206 might be involved in the occurrence of FGR through regulating VEGF, and the miR-206/VEGF axis might play a crucial role in predicting FGR risk, while related evidence has not been reported. Thus, this study aimed to investigate the correlation of miR-206, VEGF and miR-206/VEGF axis at different gestational ages with FGR risk in pregnancies.
2. Methods
2.1. Participants
Between January 2015 and June 2018, 820 pregnancies who were gestated within 8 weeks and underwent prenatal examination in People's Hospital of Rizhao were consecutively recruited in this study. The inclusion criteria were:
-
1.
confirmed gestational age ≤ 8 weeks;
-
2.
singleton pregnancy;
-
3.
age between 20 and 35 years old;
-
4.
willingness to participate in this study;
-
5.
able to regularly return hospital for prenatal examinations.
The exclusion criteria were:
-
1.
had pregnancy complications (such as pregnancy hypertension, diabetes, or kidney disease);
-
2.
history of reproductive system surgery or malignancies;
-
3.
severe infections (e.g., human immunodeficiency virus).
This study was approved by the institutional review board of People's Hospital of Rizhao. Written informed consent was collected from all participants before enrollment.
2.2. Data and sample collection
After providing the written informed consents, the participants’ basic characteristics including age, gestational age at delivery, smoking, gestational diabetes mellitus, gestational hypertension, history of FGR, number of gravidities, number of births, and number of abortions were collected. And the peripheral blood samples of all participants were collected at early pregnancy (gestational age ≤ 13 weeks), middle pregnancy (gestational age: 14–27 weeks) and late pregnancy (gestational age ≥ 28 weeks), respectively. After collection, the blood samples were immediately centrifuged at the condition of 1600 g (4°C) for 10 min, then the supernatants were separated and further centrifuged at 16,000 g (4°C) for 10 min. Finally, the plasma was obtained and stored at −80°C until determination.
2.3. Real-time quantitative polymerase chain reaction (qPCR)
The relative expression of miR-206 in plasma was detected by qPCR. First, total RNA was extracted from plasma using TRIzol Reagent (Invitrogen, Waltham, MA). Then, reverse transcription to cDNA was conducted by RT-PCR Quick Master Mix (Toyobo, Osaka, Kansai, Japan). Using QuantiNova SYBR Green PCR Kit (Qiagen, Duesseldorf, Nordrhein-Westfalen, German), qPCR procedure was performed. Besides, U6 was applied as the internal reference, and sequences of primers used in qPCR were as follows: miR-206, forward primer: ACACTCCAGCTGGGTGGAATGTAAGGAAGT, reverse primer: TGTCGTGGAGTCGGCAATTC. U6, forward primer: CTCGCTTCGGCAGCACATATACTA, reverse primer: ACGAATTTGCGTGTCATCCTTGC.
2.4. Enzyme-linked immunosorbent assay (ELISA)
The level of VEGF in plasma was detected by ELISA with the use of a commercial human VEGF ELISA Kit (Abcam, Cambridge, MA). All procedures were conducted according to the manufacturer's protocol. The plasma samples were added to wells, followed by the antibody mixture. After incubation, the wells were washed to remove unbound material. Then tetramethylbenzidine substrate was added in the wells, subsequently, the wells were incubated at room temperature. After stop solution was added, the intensity was measured at 450 nm wavelengths on microplate reader (BioTek, Winosky, VT).
2.5. FGR definition
FGR was diagnosed by the obstetricians and neonatologists with the considering of the actual birth weight of fetus and the medical history and diet of pregnancies. According to the criteria of FGR in Obstetrics and Gynecology (2nd edition, People's medical publishing house), FGR was defined as fetal birth weight less than 2500 g with a gestational age> 37 weeks, or actual fetal weight less than the 10th percentile of the weight of the fetus with same gestational age and sex, or actual fetal weight below 2 standard deviations of the mean weight of the fetus with same gestational age and sex.[16] And according to the diagnosis of FGR, pregnancies were classified into FGR group and non-FGR group.
2.6. Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables were presented as count (percentage). Comparisons of continuous variables between FGR group and non-FGR group were determined by the Student's t test or Wilcoxon rank sum test. Comparisons of categorical variables between FGR group and non-FGR group were determined by Chi-square test. Comparisons of continuous variables among early pregnancy, middle pregnancy and late pregnancy were analysed by Kruskal–Wallis H rank sum test. Correlation of miR-206 relative expression with VEGF expression was determined by Spearman's rank correlation test. The performance of miR-206, VEGF and miR-206/VEGF axis in predicting FGR was evaluated using receiver operating characteristic (ROC) curves and the area under the curve (AUC) with 95% confidence interval (CI). All analyses were performed using SPSS 24.0 software (IBM, Chicago, IL) and figures were made using GraphPad Prism 7.01 software (GraphPad Software, San Diego, CA). P value < .05 was considered significant.
3. Results
3.1. Clinical characteristics of pregnancies
Eight hundred twenty pregnancies with mean age of 28.8 ± 4.5 years were enrolled in this study (Table 1). Besides, 135 (16.5%), 284 (34.6%), 292 (35.6%), 100 (12.2%), and 9 (1.1) pregnancies had 1, 2, 3, 4, and 5 gravidities, respectively. For the number of births, 520 (63.4%), 286 (34.9%), and 14 (1.7%) pregnancies had 1, 2, and 3 births respectively. Additionally, 177 (21.6%), 400 (48.8%), 239 (29.1%), and 4 (0.5%) pregnancies suffered 0, 1, 2, and 3 abortions, respectively. Moreover, pregnancies were classified into FGR group (n = 74) and non-FGR group (n = 746) according to the diagnosis of FGR. Compared to non-FGR group, age (P < .001), number of gravidities (P < .001) and number of abortions (P < .001) were all increased in FGR group, while gestational age at delivery (P < .001) was shorter in FGR group, and no difference of smoking (P = .747), gestational diabetes mellitus (P = .725), gestational hypertension (P = .784), history of FGR (P = .360), number of births (P = .068) was observed between the two groups.
Table 1.
Clinical characteristics of pregnancies.

3.2. Correlation of miR-206 expression and VEGF expression in early, middle, and late pregnancies
miR-206 expression was negatively correlated with VEGF expression in early pregnancies (P < .001, r = −0.384) (Fig. 1A). Additionally, miR-206 expression was also negatively correlated with VEGF expression in middle pregnancies (P < .001, r = −0.426) (Fig. 1B) and late pregnancies (P < .001, r = −0.450) (Fig. 1C).
Figure 1.

miR-206 expression negatively correlated with VEGF expression in early, middle and late pregnancies. Correlation of miR-206 expression with VEGF expression in early pregnancies (A). Correlation of miR-206 expression with VEGF expression in middle pregnancies (B). Correlation of miR-206 expression with VEGF expression in late pregnancies (C). Correlation of miR-206 expression with VEGF expression was determined by Spearman's rank correlation test. miR-206, microRNA-206; VEGF, vascular endothelial growth factor. P < .05 was considered significant.
3.3. Comparison of miR-206, VEGF and miR-206/VEGF axis among early, middle, and late pregnancies
The median miR-206 expression in early, middle and late pregnancies was 1.058 (0.791–1.395), 1.324 (1.019–1.756), and 1.551 (1.224–2.047), respectively, and the miR-206 expression raised along with the increased gestational age (P < .001) (Fig. 2A). Moreover, VEGF expression in early, middle and late pregnancies was 84.8 (66.4–106.9), 62.7 (49.0–78.4), and 49.8 (38.3–60.2), respectively, and VEGF expression decreased along with the increased gestational age (P < .001) (Fig. 2B). Besides, miR-206/VEGF axis in early, middle and late pregnancies was 0.013 (0.008–0.019), 0.022 (0.014–0.033), and 0.032 (0.023–0.048), respectively, and it was elevated along with increased gestational age (P < .001) (Fig. 2C).
Figure 2.
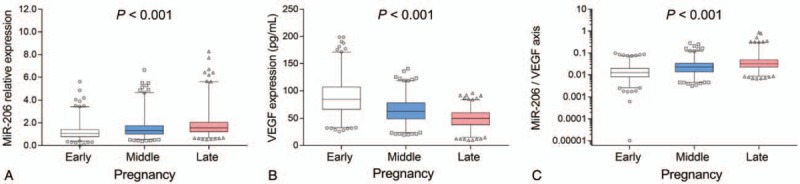
Detection of miR-206, VEGF, and miR-206/VEGF axis in early, middle, and late pregnancies. miR-206 expression in early, middle and late pregnancies (A). VEGF expression in early, middle, and late pregnancies (B). miR-206/VEGF axis in early, middle and late pregnancies (C). Comparison among groups was determined by Kruskal–Wallis H rank sum test. miR-206, microRNA-206; VEGF, vascular endothelial growth factor. P < .05 was considered significant.
3.4. Comparison of miR-206, VEGF and miR-206/VEGF axis between FGR and non-FGR pregnancies
miR-206 expression was increased in FGR group compared to non-FGR group in early pregnancies (P = .022), middle pregnancies (P < .001), and late pregnancies (P < .001) (Fig. 3A). As to VEGF, its expression was reduced in FGR group compared to non-FGR group in early (P = .001), middle (P < .001), and late pregnancies (P < .001) (Fig. 3B). For miR-206/VEGF axis, it was elevated in FGR group compared to non-FGR group in early (P = .002), middle (P < .001), and late pregnancies (P < .001) (Fig. 3C).
Figure 3.
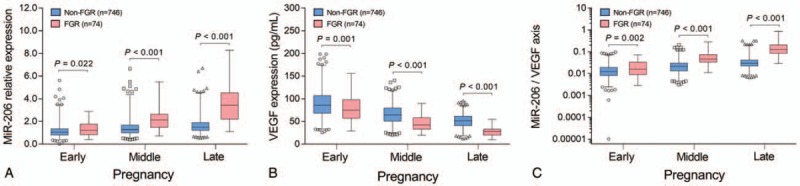
Detection of miR-206, VEGF, and miR-206/VEGF axis in FGR and non-FGR pregnancies. Comparison of miR-206 expression between FGR group and non-FGR group in early, middle, and late pregnancies (A). Comparison of VEGF expression between FGR group and non-FGR group in early, middle, and late pregnancies (B). Comparison of miR-206/VEGF axis between FGR group and non-FGR group in early, middle and late pregnancies (C). Comparison between groups was determined by Wilcoxon rank sum test. miR-206, microRNA-206; VEGF, vascular endothelial growth factor; FGR, fetal growth retardation. P < .05 was considered significant.
3.5. Predictive value of miR-206, VEGF and miR-206/VEGF axis for FGR risk
ROC curves displayed that miR-206 at early pregnancy could predict raised FGR risk, while the predictive value was relatively low (AUC: 0.581, 95% CI: 0.506–0.655). Besides, miR-206 at middle pregnancy (AUC: 0.764, 95%: 0.703–0.825) and late pregnancy (AUC: 0.894, 95% CI: 0.855–0.933) both exhibited good predictive values for increased FGR risk. And miR-206 at late pregnancy showed the numerically highest predictive value for raised FGR risk (Fig. 4A). Moreover, VEGF low expression at early pregnancy presented with a weak predictive value for higher FGR risk (AUC: 0.615, 95% CI: 0.541–0.698), while VEGF low expression at middle pregnancy (AUC: 0.782, 95% CI: 0.730–0.870) as well as late pregnancy (AUC: 0.909, 95% CI: 0.877–0.940) showed good predictive values for increased FGR risk, and this index at late pregnancy exhibited the numerically highest predictive value for FGR risk (Fig. 4B). Furthermore, miR-206/VEGF axis at early pregnancy could predict increased FGR risk (AUC: 0.607, 95% CI: 0.533–0.680), whereas miR-206/VEGF axis at middle pregnancy (AUC: 0.816, 95% CI: 0.763–0.870) and late pregnancy (AUC: 0.928, 95% CI: 0.901–0.956) had stronger predictive values for increased FGR risk, and this index at late pregnancy presented with the numerically highest predictive value for raised FGR risk (Fig. 4C).
Figure 4.
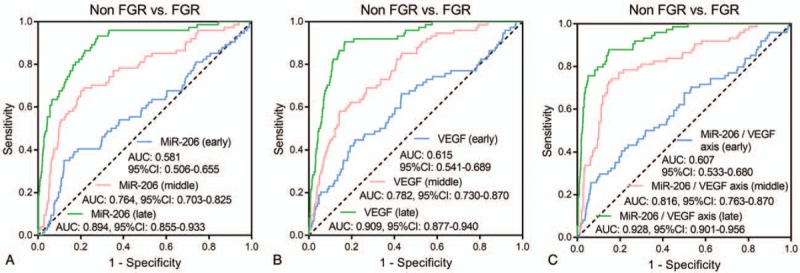
ROC curves. Predictive values of miR-206 at early, middle, and late pregnancy for FGR risk (A). Predictive values of VEGF at early, middle, and late pregnancy for FGR risk (B). Predictive values of miR-206/VEGF axis at early, middle, and late pregnancy for FGR risk (C). Performance of miR-206, VEGF, and miR-206/VEGF axis in predicting FGR was evaluated using ROC curves and AUC with 95% CI. ROC, receiver operating characteristic; miR-206, microRNA-206; VEGF, vascular endothelial growth factor; FGR, fetal growth retardation; AUC, area under the curve; CI, confidence interval.
4. Discussion
miR-206, as a member of the “myomiR” family that regulates myogenesis and striated muscle growth, has been found to be involved in the pathologies wherein muscular disorder is involved.[17] For instance, miR-206 represses osteogenic differentiation of bone marrow mesenchymal stem cells via targeting glutaminase, and represses hypertrophy of myogenic cells through suppressing histone deacetylase 4.[18,19] Notably, an interesting experiment pays attention to the role of miR-206 in pregnant period, which displays that miR-206 inhibits brain-derived neurotrophic factor (BDNF) through targeting 3′-untranslated region (3′-UTR) in pregnant hypothyroid rats, and decreased BDNF is frequently reported to be closely related to reduced birth weight, suggesting that miR-206 may be involved in the pathology of restrained fetal growth.[20,21] Besides, in clinical data, a previous study discloses that miR-206 in found overexpressed in both serum and placenta of 28-week samples of women who later develops preeclampsia, along with decreased expressions of miR-206 target genes.[6] Moreover, a study shows that miR-206 predicts increased DMD risk, and miR-206 high expression is associated with lower muscle strength and muscle function in DMD children.[8] These studies indicate that miR-206 overexpression not only correlates with decreased muscle function in children but also correlates with placental abnormality in pregnancies. Hence, we speculated that miR-206 might played a role in the abnormal fetal growth, especially FGR, while related evidence is seldomly reported. To validate this hypothesis, our study investigated miR-206 expression in pregnancies at different gestational age, and compared the miR-206 expression between FGR pregnancies and non-FGR pregnancies. We observed that miR-206 expression was raised along with the increased gestational age, meanwhile, it was overexpressed in FGR pregnancies and predicted elevated FGR risk in early, middle as well as late pregnancies. Moreover, miR-206 at late pregnancy presented with the numerically highest predictive value for raised FGR risk. These results might be due to:
-
1.
miR-206 might downregulate some growth factors (such as BDNF and transforming growth factor to restrict the normal cell differentiation and epithelial development) which further repressed the fetal growth and increased FGR risk[20,21];
-
2.
miR-206 might downregulate its target factor (such as VEGF as we displayed in our data), thereby inhibited angiogenesis and contributed to placental insufficiency, thus miR-206 predicted higher FGR risk[9–11];
-
3.
under normal circumstance, vascular structure of the placenta and utero-placental blood flow extended more progressively during late pregnancy compared to early pregnancy and middle pregnancy due to the growing needs of the fetus, while miR-206 repressed angiogenesis and led to endothelial damage, which would impede the utero-placental blood flow and the fetal growth, thus the restriction on fetal growth induced by miR-206 might be more remarkable at late pregnancy compared to earlier gestational age, and miR-206 at late pregnancy showed stronger predictive value for FGR risk compared to early pregnancy and middle pregnancy numerically.[22]
VEGF plays an important role in physiological or pathological antiogenesis, and recently, it has been reported to be involved in the placental antiogenesis.[23,24] For example, an experiment shows that VEGF delivery to placental basal plate is able to promote the uterine artery remodeling, which facilitates the fetal growth in the primate.[23] Besides, a study discloses that inhibition of VEGF impairs VEGF signaling and diminishes vascularization in the placenta and fetal organs, which further induces abnormal architecture in the retinal vascular network and causes FGR in pregnant mice.[24] These data emphasize that VEGF may participate in the pathology of FGR. As to the related clinical data, a study displays that serum VEGF level in pregnancy-induced hypertension syndrome patients is positively correlated with neonatal weight and Apgar score of infants.[14] And another study shows that cord blood plasma VEGF level is positively correlated with birth weight and head circumference in premature infants.[15] These clinical data reveal that VEGF expression may positively correlated with the birth weight of infants, while the direct evidence about the association of VEGF with FGR occurrence is limited, especially the evidence about the role of VEGF in FGR pregnancies at different gestational ages. In our study, we detected the VEGF level in pregnancies at their different gestational ages (early, middle, and late), and we found that VEGF expression was decreased along with the increased gestational age, moreover, We observed that VEGF expression was reduced along with the increased gestational age, meanwhile, it was under-expressed in FGR pregnancies and its low expression predicted higher FGR risk in early, middle as well as late pregnancies. Furthermore, VEGF low expression at late pregnancy showed the numerically highest predictive value for raised FGR risk. The possible reasons for these results might be that:
-
1.
VEGF promoted the uterine artery remodeling and enhanced the vascularization in the placenta and fetal organs, thereby facilitated the fetal growth and decreased FGR occurrence, thus the insufficient VEGF might failed to decreased FGR occurrence and predicted higher FGR risk[23,24];
-
2.
VEGF not only maintained the proangiogenic effect but also neutralized the normal antiangiogenic environment at late pregnancy due to the secretion of antiangiogenic factors (like sFlt1), which was not appeared at earlier gestational age, therefore, more VEGF was required at late pregnancy for rapid fetal growth, conversely, the insufficient VEGF at late pregnancy would result in severer defective angiogenesis compared to earlier gestational age, thus the predictive value of VEGF low expression at late pregnancy for raised FGR risk was stronger compared to VEGF low expression at early or middle pregnancy numerically.[25]
According to indications revealed in previous studies, miR-206 may be involved in the intrauterine growth, meanwhile, its direct target, VEGF, is regarded as a crucial factor for placental development and reported to potentially predict FGR risk. Thus, we speculated that miR-206 might participate in the occurrence of FGR via interacting with VEGF, and the miR-206/VEGF axis might facilitate predicting FGR risk, however, the limited evidence had been reported. Hence, we explored the association of miR-206 expression with VEGF expression, and further investigated the correlation of miR-206/VEGF axis with FGR risk in pregnancies at different gestational ages. We observed that miR-206 expression was negatively correlated with VEGF level in early, middle as well as late pregnancies, and the miR-206/VEGF axis was elevated along with the increased gestational age. Moreover, miR-206/VEGF axis presented with good predictive value for FGR risk, which was numerically higher compared to miR-206 or VEGF alone, suggesting that miR-206/VEGF axis might act as a more convincing and sensitive biomarker for diagnosis of FGR. Additionally, miR-206/VEGF axis at late pregnancy had better predictive value for FGR risk compared to miR-206/VEGF axis at early pregnancy and middle pregnancy numerically, which might be on account of that: both overexpressed miR-206 and insufficient VEGF inhibited angiogenesis and contributed to placental insufficiency, while fetal growth during late pregnancy needed normal angiogenesis and placental blood flow more than ever, thus the elevated miR-206/VEGF axis at late pregnancy would lead to severer placental insufficiency and worse restriction on fetal growth compared to earlier gestational age, and the predictive value of miR-206/VEGF axis at late pregnancy for higher FGR risk was stronger compare to early pregnancy and middle pregnancy numerically.
Some limitations still existed in our study:
-
1.
this was a single-center study, which might have some selective bias;
-
2.
although we disclosed the negative correlation of miR-206 and VEGF expressions, the detailed mechanism of miR-206/VEGF axis in FGR was not investigated.
Further multi-center study is needed to verify our results, and molecular mechanisms of miR-206/VEGF axis in FGR needed to be explored.
In conclusion, miR-206 predicts raised FGR risk through interacting with VEGF in pregnancies, and it may serve as a novel biomarker for FGR prevention.
Author contributions
Conceptualization: Jiaqiang Liu.
Data curation: Ying Li.
Formal analysis: Ying Li.
Investigation: Ying Li, Jiaqiang Liu.
Methodology: Ying Li.
Resources: Ying Li, Jiaqiang Liu.
Supervision: Jiaqiang Liu.
Validation: Jiaqiang Liu.
Writing – original draft: Ying Li, Jiaqiang Liu.
Writing – review & editing: Jiaqiang Liu.
Footnotes
Abbreviations: 3′-UTR = 3′-untranslated region, AUC = area under the curve, BDNF = brain-derived neurotrophic factor, CI = confidence interval, DMD = Duchenne muscular dystrophy, ELISA = Enzyme-linked immunosorbent assay, FGR = Fetal growth retardation, miR-206 = microRNA-206, qPCR = quantitative polymerase chain reaction, ROC = receiver operating characteristic, SD = standard deviation, VEGF = vascular endothelial growth factor.
How to cite this article: Li Y, Liu J. MicroRNA-206 predicts raised fetal growth retardation risk through the interaction with vascular endothelial growth factor in pregnancies. Medicine. 2020;99:7(e18897).
The authors have no conflicts of interest to disclose.
References
- [1].Cottrell E, Tropea T, Ormesher L, et al. Dietary interventions for fetal growth restriction—therapeutic potential of dietary nitrate supplementation in pregnancy. J Physiol 2017;595:5095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bamfo JE, Odibo AO. Diagnosis and management of fetal growth restriction. J Pregnancy 2011;2011:640715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kesavan K, Devaskar SU. Intrauterine growth restriction: postnatal monitoring and outcomes. Pediatr Clin North Am 2019;66:403–23. [DOI] [PubMed] [Google Scholar]
- [4].Rosenberg A. The IUGR newborn. Semin Perinatol 2008;32:219–24. [DOI] [PubMed] [Google Scholar]
- [5].Leite DFB, Morillon AC, Melo Junior EF, et al. Metabolomics for predicting fetal growth restriction: protocol for a systematic review and meta-analysis. BMJ Open 2018;8:e022743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Akehurst C, Small HY, Sharafetdinova L, et al. Differential expression of microRNA-206 and its target genes in preeclampsia. J Hypertens 2015;33:2068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fu G, Brkic J, Hayder H, et al. MicroRNAs in human placental development and pregnancy complications. Int J Mol Sci 2013;14:5519–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hu J, Kong M, Ye Y, et al. Serum miR-206 and other muscle-specific microRNAs as non-invasive biomarkers for Duchenne muscular dystrophy. J Neurochem 2014;129:877–83. [DOI] [PubMed] [Google Scholar]
- [9].Wang M, Ji Y, Cai S, et al. MiR-206 suppresses the progression of coronary artery disease by modulating Vascular Endothelial Growth Factor (VEGF) expression. Med Sci Monit 2016;22:5011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang T, Liu M, Wang C, et al. Down-regulation of MiR-206 promotes proliferation and invasion of laryngeal cancer by regulating VEGF expression. Anticancer Res 2011;31:3859–63. [PubMed] [Google Scholar]
- [11].Liang Z, Bian X, Shim H. Downregulation of microRNA-206 promotes invasion and angiogenesis of triple negative breast cancer. Biochem Biophys Res Commun 2016;477:461–6. [DOI] [PubMed] [Google Scholar]
- [12].Su MT, Tsai PY, Tsai HL, et al. miR-346 and miR-582-3p-regulated EG-VEGF expression and trophoblast invasion via matrix metalloproteinases 2 and 9. Biofactors 2017;43:210–9. [DOI] [PubMed] [Google Scholar]
- [13].Murthi P, Brouillet S, Pratt A, et al. An EG-VEGF-dependent decrease in homeobox gene NKX3.1 contributes to cytotrophoblast dysfunction: a possible mechanism in human fetal growth restriction. Mol Med 2015;21:645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tang Y, Ye W, Liu X, et al. VEGF and sFLT-1 in serum of PIH patients and effects on the foetus. Exp Ther Med 2019;17:2123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Voller SB, Chock S, Ernst LM, et al. Cord blood biomarkers of vascular endothelial growth (VEGF and sFlt-1) and postnatal growth: a preterm birth cohort study. Early Hum Dev 2014;90:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xu F, Xu S, Shao X, et al. Deficiency of calcium and microelements predict the risk of fetal growth restriction. Int J Clin Exp Med 2017;10:7491–9. [Google Scholar]
- [17].Falcone G, Perfetti A, Cardinali B, et al. Noncoding RNAs: emerging players in muscular dystrophies. Biomed Res Int 2014;2014:503634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen Y, Yang YR, Fan XL, et al. miR-206 inhibits osteogenic differentiation of bone marrow mesenchymal stem cells by targetting glutaminase. Biosci Rep 2019;39: doi: 10.1042/BSR20181108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Winbanks CE, Beyer C, Hagg A, et al. miR-206 represses hypertrophy of myogenic cells but not muscle fibers via inhibition of HDAC4. PLoS One 2013;8:e73589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xing Q, Shan Z, Gao Y, et al. Differential expression of MicroRNAs and miR-206-mediated downregulation of BDNF expression in the rat fetal brain following maternal hypothyroidism. Horm Metab Res 2018;50:696–703. [DOI] [PubMed] [Google Scholar]
- [21].Christian LM, Mitchell AM, Gillespie SL, et al. Serum brain-derived neurotrophic factor (BDNF) across pregnancy and postpartum: Associations with race, depressive symptoms, and low birth weight. Psychoneuroendocrinology 2016;74:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Valdes G, Erices R, Chacon C, et al. Angiogenic, hyperpermeability and vasodilator network in utero-placental units along pregnancy in the guinea-pig (Cavia porcellus). Reprod Biol Endocrinol 2008;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Babischkin JS, Aberdeen GW, Lindner JR, et al. Vascular endothelial growth factor delivery to placental basal plate promotes uterine artery remodeling in the primate. Endocrinology 2019;160:1492–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Morita A, Nakahara T, Abe N, et al. Treatment of mid-pregnant mice with KRN633, an inhibitor of vascular endothelial growth factor receptor tyrosine kinase, induces abnormal retinal vascular patterning in their newborn pups. Birth Defects Res B Dev Reprod Toxicol 2014;101:293–9. [DOI] [PubMed] [Google Scholar]
- [25].Patten IS, Rana S, Shahul S, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012;485:333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


