Abstract
Despite many clinical trials on cervical epidural steroid injections, the indications for and long-standing outcomes of this treatment remain controversial. We evaluated the outcomes and indications for transforaminal cervical epidural steroid injection (TCESI) in patients with moderate to severe disability.
We prospectively gathered data from patients with 1 or 2-level cervical degenerative disease (herniated disc, foraminal stenosis) with moderate to severe disability (3.5 < initial visual analog scale < 6.5, 15 < Neck Disability Index < 35) and greater than 12 weeks of pain, despite conservative treatment. Patients with persistent disability and those who desired surgical intervention underwent decompression surgery. The clinical and demographic characteristics were compared between groups.
Of the 309 patients who underwent TCESI, 221 (72%) did not receive surgical treatment during the 1-year follow-up period. The remaining 88 patients (28%) underwent surgery at a mean of 4.1 months after initial TCESI. Patients who underwent injection alone showed a significant decrease in disability and pain that persisted until the 1-year follow-up visit (P < .05). In patients who underwent surgery, the mean disability and pain scores after injection did not decrease for several months, although the scores significantly decreased up to 1 year after surgery (P < .05).
The TCESI significantly decreased pain and disability in the moderate to severe disability group up to 1 year after injection. We recommend cervical TCESI as an initial treatment with moderate to severe disability patients.
Keywords: cervical spinal disease, spinal surgery, transforaminal cervical epidural steroid injection
1. Introduction
Cervical epidural steroid injection is a common interventional procedure for managing radiculopathy and axial neck pain from spinal degenerative disease.[1–3] In some patients, steroid injections may improve symptoms and are a good alternative treatment to surgery.[4,5] Several studies compared steroid injections to placebos with favorable outcomes, but there have been few randomized controlled trials (RCTs).[6–11] Despite many clinical trials on transforaminal cervical epidural steroid injection (TCESI), the indications of this treatment remain controversial. We hypothesized that the benefits of TCESI could be maximized if we determined reasonable indications for TCESI with or without surgery. In this study, we compared the mid-term outcomes between TCESI-only and TCESI plus surgery groups to evaluate the outcomes in a moderate-to-severe disability group with TCESI.
2. Methods
2.1. Subjects
We prospectively gathered data from 423 consecutive patients who had 1 or 2-level cervical degenerative disease (herniated disc, foraminal stenosis) with moderate or severe disability (3.5 < visual analog scale [VAS] < 6.5, 15 < Neck Disability Index [NDI] < 35) and with greater than 12 weeks of pain (radicular symptoms), despite conservative treatment (physical therapy, medication, chiropractic, etc) between 2014 and 2016 at university hospital. We excluded patients with axial neck pain without radicular symptoms and those who received oral steroids (>1 M) or acupuncture as a conservative treatment before injection. Of the 423 patients, we excluded 86 who had more than severe disability or neurologic deficit (eg, serial increase in pain and disability [VAS >6.5, NDI >35], myelopathy, progressive motor weakness), and required surgery rather than conservative treatment. All remaining 337 patients received fluoroscopy-guided transforaminal cervical epidural steroid injections (lidocaine 0.5% 2 mL + dexamethasone 5 mg/level) targeting the affected nerve roots (eg, C4/5 disc with C4 and C5 root injection on symptomatic side). Mean age of the subjects were 52.1, with 159 males and 178 females. The institutional review board approved this study (IRB 2013AS0556).
2.2. Transforaminal cervical epidural steroid injection technique
The TCESI was performed with the patient lying in the lateral decubitus position. After confirming the correct oblique view of the target foramen, a needle was passed into the neck through a point overlying the posterior half of the target foramen. Under direct, real-time, fluoroscopy view, a small volume of contrast medium was injected. When the target nerve had been correctly outlined, a small volume of local anesthetic and corticosteroid were injected. The patients were subsequently evaluated at a 1-year follow-up visit. If the patient experienced improvement of radicular symptoms (50%> of reduction persisted up to 2 weeks) with each set of TCESI, we performed serial TCESI up to 3 times, within a 2-week interval. We defined the TCESI-only group, if patients showed significant reduction in VAS or NDI after 3 consecutive injections. If the symptoms persisted after any injections, the patient was then placed into the surgical group. If pain recurred within 1 month after last injection, we transfer the patients to surgery group regardless of patients’ preference for conservative or surgical treatment.
2.3. Outcome assessment
We collected patient data on sex, age, duration of symptoms, medical, psychiatric, and social history. For outcome measurements, VAS and NDI data were collected at 3, 6, 9, and 12 months after initial injection regardless of whether the patient later underwent surgery. We excluded patients who were lost to follow-up at any point up to 1 year. To minimize bias, an independent reviewer collected the data, including side effects, when patients visited the hospital for follow-up. Decisions regarding surgical treatment were confirmed by 2 independent, experienced surgeons (J.Y.H., J.S.P.). Patients placed in the surgical group underwent decompressive surgery of the affected nerve root with or without fusion of the spinal segment, and all surgeries were performed by a single surgeon (J.Y.H.). VAS and NDI data were also collected in the surgical group at every follow-up points.
2.4. Statistical analysis
Statistical analyses (Student t test and chi-square test) were performed to determine significant differences and correlations between groups, and per-protocol analysis was performed to minimize bias. We used SPSS version 20.0 (SPSS, Chicago, IL) for all statistical analyses.
3. Results
Among the 337 patients, we excluded 28 who failed to report for follow-up evaluation. The final number of patients that underwent TCESI and was included in this study was 309, 221 (72%) of whom did not undergo surgery during the 1-year follow-up period. However, 88 patients (28%) underwent surgery a mean of 4.1 months after initial TCESI (Fig. 1).
Figure 1.
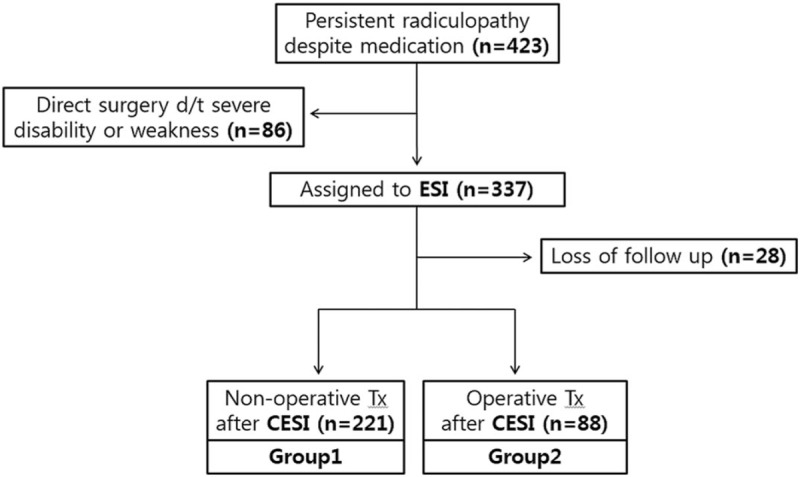
Study subjects.
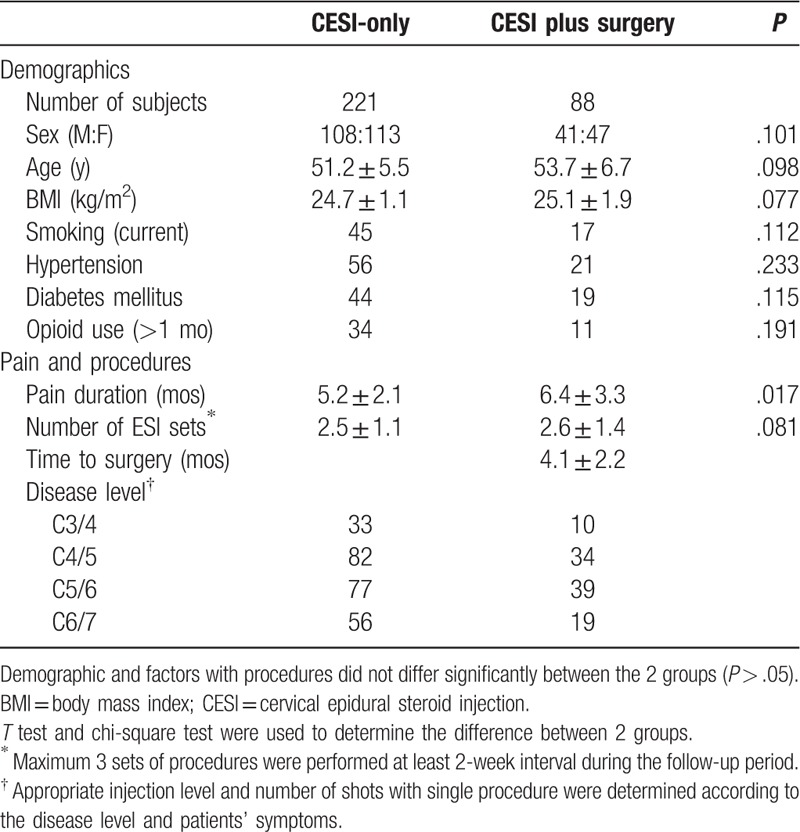
The TCESI-only group and the TCESI plus surgery group had similar characteristics with regard sex, age, duration of symptoms, medical, psychiatric and social history, duration of symptoms, number of injections, and level of disease (Table 1). We did not find any significant differences upon analysis of risk factors between the TCESI-only and the TCESI plus surgery groups (P > .05).
Table 1.
Comparison of parameters between groups.
The initial NDI and VAS were similar in the 2 groups (25.2, 5.9 vs 26.1, 5.8, respectively; P > .05). However, there were differences in outcomes between the 2 groups after TCESI. The TCESI plus surgery group did not have decreased pain and disability or a rebound of these scores within a short time period (P > .05). The mean disability and pain scores were not decreased for several months after the injection in the TCESI plus surgery group, although the scores were decreased significantly after surgery for up to 1 year (P < .05; Table 2, Figs. 2 and 3). There were no significant changes in 6 and 9 months. On the contrary, the TCESI-only group showed significant improvement in disability scores within 3 months, and the improvement lasted up to 1 year.
Table 2.
Comparisons of outcomes.
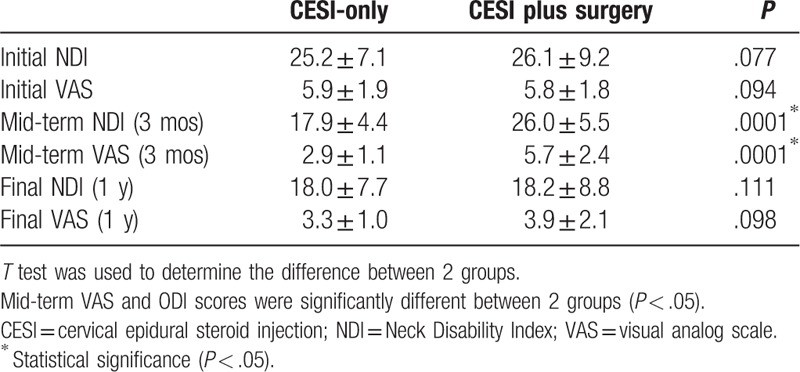
Figure 2.
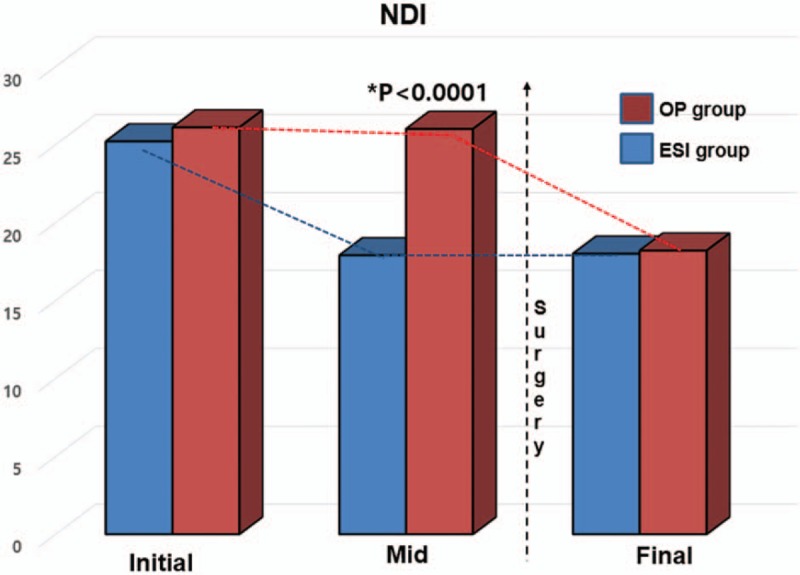
Serial comparison of Neck Disability Index (NDI) scores for up to 1 year between TCESI-only and TCESI plus surgery groups. TCESI = transforaminal cervical epidural steroid injection.
Figure 3.
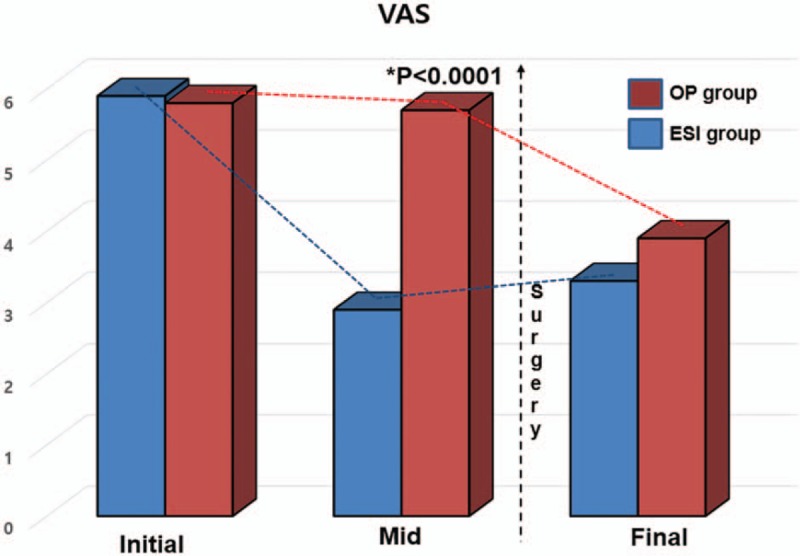
Serial comparison of visual analog scale (VAS) scores for up to 1 year between TCESI-only and TCESI plus surgery groups. TCESI = transforaminal cervical epidural steroid injection.
4. Discussion
Several studies support epidural steroid injections in the treatment of cervical radiculopathy.[4–6,9,12] However, due to the lack of large well-designed studies, the effectiveness of cervical epidural steroid injection remains controversial. A recent multicenter prospective study assigned 169 consecutive patients with cervical radiculopathy to 1 of 3 groups: cervical epidural injection only, physical therapy plus pharmacotherapy, or combination injection, physical therapy, and pharmacotherapy. All 3 groups experienced slight improvement in symptoms at 3 months, but there was no significant difference among the treatment groups at 6 months. However, this study was open-label, and the treatment regimens were nonstandardized, which draws robust conclusions on comparative effectiveness.[13,14] Physicians can adopt different indications and techniques that may influence patient outcomes, and this influence should be addressed to determine the effectiveness of cervical epidural steroid injection.[15–22] In addition, the indications for epidural steroid injection vary from mild axial neck pain to significant disability. And, injection route, and treatment regimen and technique can change patient outcomes. We thought that standardization of the study procedure and indications may be crucial, although, we could not randomize the patients with relatively small numbers of participants comparing other large-scale studies. In this study, we prospectively analyzed single-center data to minimize bias, which was derived from different techniques and regimens in other studies. In addition, we strictly included only patients with a minimum 1-year of follow-up to determine the mid-term efficacy of TCESI. We intended to further elucidate the specific indications for TCESI and to identify the specific characteristics of patients who respond to TCESI. We intended to determine the efficacy of TFESI in potential candidates for surgical treatment.
Abdi et al[5] reviewed the literature and found moderate evidence to support the use of epidural steroid injection in patients with chronic cervical radiculopathy with long-term relief. Similarly, an RCT showed significant benefits in pain and function in patients receiving injections for at least 1 year who had not experienced improvement with physical therapy or nonsteroidal anti-inflammatory drugs.[8] In addition, several cohort studies showed significant improvement in recalcitrant radicular pain by administration of cervical epidural steroid injections for, in most cases, at least 1 year.[6,7,9–11,16,17,23] However, they did not analyze the proportion of patients who eventually required surgery, despite having received epidural steroid injection. The main benefits of TCESI are the simplicity of the procedure and the ease of conversion to surgery in cases with poor outcomes. To determine the effectiveness of TCESI in patients with disability, we collected data on patients with moderate to severe disability from 1 or 2-segment cervical spinal disease for up to 1 year. In our study, 72% of subjects did not undergo surgery during the 1-year follow-up period, and we found that TCESI may be helpful for patients with moderate to severe disability for up to a 1-year period. Compression of the cervical nerve root by a herniated disc produces symptoms with persistent and transient sensitivity. Spinal neuropeptide expression may have a significant role in pain symptoms.[24,25] We substantiate that the chemical basis of neuropathic pain may resolve over time for the majority of patients, and TCESI may be a powerful conservative tool to avoid unnecessary surgery in the early stages of disease.[24,25] This study helps further clarify that patients with an NDI value between 15 and 35 are good candidates for the procedure.
Although we found positive results in patients with a moderate to severe degree of disability, there are significant risks associated with cervical transforaminal or interlaminar steroid injections, including neurological deficits, epidural hematomas, and vascular infarction.[26] We gave particular attention to 1 article that provided information on the injection technique (precautionary checks like radiographic confirmation of position, aspiration, injection of contrast medium under digital subtraction angiography, neurography, and administration of a test dose of local anesthetic), and whether particulate or nonparticulate steroids were injected.[27]
There were several limitations to this study. It was an observational study without randomization or blinding, although we prospectively gathered the patients’ data. In addition, sample size of the study was relatively small to compare the efficacy of TCESI. To identify that TCESI could be substitute for surgery, different study designs can be adopted. We can compare the group between TCESI and surgery in group of the patients who did not show clinical improvement after physical therapy or medication to identify it. In addition, we only included the moderate pain group in this study; we cannot determine which pain severity group shows better result with TCESI. Lastly, several factors can influence the outcome of the study.
5. Conclusions
Cervical epidural steroid injection significantly decreased pain and disability in the moderate to severe disability group for up to 1 year after injection. We recommend cervical TCESI as an initial treatment with moderate to severe disability patients.
Author contributions
Conceptualization: Jae-Young Hong, Jin-Sung Park.
Data curation: Jae-Young Hong, Jin-Sung Park, Seung-Woo Suh.
Formal analysis: Jae-Young Hong, Seung-Woo Suh, Si-Young Park.
Funding acquisition: Jae-Hyuk Yang, Si-Young Park.
Investigation: Jae-Hyuk Yang.
Footnotes
Abbreviations: NDI = Neck Disability Index, RCT = randomized controlled trial, TCESI = transforaminal cervical epidural steroid injection, VAS = visual analog scale.
How to cite this article: Hong JY, Park JS, Suh SW, Yang JH, Park SY, Kim BT. Transforaminal epidural steroid injections in cervical spinal disease with moderate to severe disability: Comparative study in patients with or without surgery. Medicine. 2020;99:7(e19266).
Funding: This research was supported by the Korea University Future Research Grant.
The authors have no conflicts of interest to disclose.
References
- [1].Woods BI, Hilibrand AS. Cervical radiculopathy: epidemiology, etiology, diagnosis, and treatment. J Spinal Disord Tech 2015;28:E251–9. [DOI] [PubMed] [Google Scholar]
- [2].Eubanks JD. Cervical radiculopathy: nonoperative management of neck pain and radicular symptoms. Am Fam Physician 2010;81:33–40. [PubMed] [Google Scholar]
- [3].Manchikanti L, Nampiaparampil DE, Candido KD, et al. Do cervical epidural injections provide long-term relief in neck and upper extremity pain? A systematic review. Pain Physician 2015;18:39–60. [PubMed] [Google Scholar]
- [4].Abdi S, Datta S, Lucas LF. Role of epidural steroids in the management of chronic spinal pain: a systematic review of effectiveness and complications. Pain Physician 2005;8:127–43. [PubMed] [Google Scholar]
- [5].Abdi S, Datta S, Trescot AM, et al. Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician 2007;10:185–212. [PubMed] [Google Scholar]
- [6].Kwon JW, Lee JW, Kim SH, et al. Cervical interlaminar epidural steroid injection for neck pain and cervical radiculopathy: effect and prognostic factors. Skeletal Radiol 2007;36:431–6. [DOI] [PubMed] [Google Scholar]
- [7].Diwan S, Manchikanti L, Benyamin RM, et al. Effectiveness of cervical epidural injections in the management of chronic neck and upper extremity pain. Pain Physician 2012;15:E405–34. [PubMed] [Google Scholar]
- [8].Stav A, Ovadia L, Sternberg A, et al. Cervical epidural steroid injection for cervicobrachialgia. Acta Anaesthesiol Scand 1993;37:562–6. [DOI] [PubMed] [Google Scholar]
- [9].Bush K, Hillier S. Outcome of cervical radiculopathy treated with periradicular/epidural corticosteroid injections: a prospective study with independent clinical review. Eur Spine J 1996;5:319–25. [DOI] [PubMed] [Google Scholar]
- [10].Castagnera L, Maurette P, Pointillart V, et al. Long term results of cervical epidural steroid injection with and without morphine in chronic cervical radicular pain. Pain 1994;58:239–43. [DOI] [PubMed] [Google Scholar]
- [11].Cicala RS, Thoni K, Angel JJ. Long-term results of cervical epidural steroid injections. Clin J Pain 1989;5:143–5. [DOI] [PubMed] [Google Scholar]
- [12].Rathmell JP, Aprill C, Bogduk N. Cervical transforaminal injection of steroids. Anesthesiology 2004;100:1595–600. [DOI] [PubMed] [Google Scholar]
- [13].Woods BI, Hilibrand AS. Cervical radiculopathy epidemiology, etiology, diagnosis, and treatment. J Spinal Disord Tech 2015;28:E251–9. [DOI] [PubMed] [Google Scholar]
- [14].Cohen SP, Hayek S, Semenov Y, et al. Epidural steroid injections, conservative treatment, or combination treatment for cervical radicular pain: a multicenter, randomized, comparative-effectiveness study. Anesthesiology 2014;121:1045–55. [DOI] [PubMed] [Google Scholar]
- [15].Vallée J, Feydy A, Carlier R, et al. Chronic cervical radiculopathy: lateral-approach periradicular corticosteroid injection. Radiology 2001;218:886–92. [DOI] [PubMed] [Google Scholar]
- [16].Kolstad F, Leivseth G, Nygaard O. Transforaminal steroid injections in the treatment of cervical radiculopathy. A prospective outcome study. Acta Neurochir (Wien) 2005;147:1067–70. [DOI] [PubMed] [Google Scholar]
- [17].Lin E, Lieu V, Halevi L, et al. Cervical epidural steroid injections for symptomatic disc herniations. J Spinal Disord Tech 2006;19:183–6. [DOI] [PubMed] [Google Scholar]
- [18].Razzaq A, O’Brien D, Mathew B, et al. Efficacy and durability of fluoroscopically guided cervical nerve root block. Br J Neurosurg 2007;21:365–9. [DOI] [PubMed] [Google Scholar]
- [19].Persson L, Anderberg L. Repetitive transforaminal steroid injections in cervical radiculopathy: a prospective outcome study including 140 patients. Evid Based Spine Care J 2012;3:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dreyfuss P, Baker R, Bogduk N. Comparative effectiveness of cervical transforaminal injections with particulate and nonparticulate corticosteroid preparations for cervical radicular pain. Pain Med 2006;7:237–42. [DOI] [PubMed] [Google Scholar]
- [21].Lee J, Lee S-H. Comparison of clinical effectiveness of cervical transforaminal steroid injection according to different radiological guidances (C-arm fluoroscopy vs. computed tomography fluoroscopy). Spine J 2011;11:416–23. [DOI] [PubMed] [Google Scholar]
- [22].Schellhas KP, Pollei SR, Johnson BA, et al. Selective cervical nerve root blockade: experience with a safe and reliable technique using an anterolateral approach for needle placement. AJNR Am J Neuroradiol 2007;28:1909–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kumar N, Gowda V. Cervical foraminal selective nerve root block: a “two-needle technique” with results. Eur Spine J 2008;17:576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rothman SM, Kreider RA, Winkelstein BA. Spinal neuropeptide responses in persistent and transient pain following cervical nerve root injury. Spine (Phila Pa 1976) 2005;30:2491–6. [DOI] [PubMed] [Google Scholar]
- [25].Rothman SM, Winkelstein BA. Chemical and mechanical nerve root insults induce differential behavioral sensitivity and glial activation that are enhanced in combination. Brain Res 2007;1181:30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Scanlon GC, Moeller-Bertram T, Romanowsky SM, et al. Cervical transforaminal epidural steroid injections: more dangerous than we think? Spine 2007;32:1249–56. [DOI] [PubMed] [Google Scholar]
- [27].Hodler J, Boos N, Schubert M. Must we discontinue selective cervical nerve root blocks? Report of two cases and review of the literature. Eur Spine J 2013;22:S466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


