Abstract
Hemodynamic stability is one of the most critical aspects of adrenal surgery for pheochromocytoma. Few articles have evaluated the hemodynamic status of patients undergoing posterior retroperitoneal adrenalectomy (PRA) for pheochromocytoma. The aim of this study is to compare the intraoperative hemodynamic parameters between lateral transperitoneal adrenalectomy (TPA) and PRA in this groups of patients.
This report describes a retrospective study of 53 pheochromocytoma patients who underwent endoscopic adrenalectomy via transperitoneal (22 patients) or posterior retroperitoneal (31 patients) approaches from January 2008 to March 2015. Data from these patients were compared to investigate the differences in hemodynamic parameters between the 2 approaches.
Clinical parameters at presentation were similar between the 2 groups, except for tumor size, which was larger in the TPA group. The PRA group is associated with reduced operative time, blood loss, and length of hospital stay compared to TPA even after adjusting for the tumor size. There was greater BP fluctuations and higher maximum systolic and diastolic blood pressure (BP) within the TPA group compared to PRA during univariate analysis. This was however not significant after adjusting for tumor size. There was no difference in the intraoperative inotropic support requirement between the 2 groups.
PRA is associated with stable intraoperative hemodynamic status, as well as favorable perioperative outcomes compared to TPA in patients with small pheochromocytomas.
Keywords: hemodynamic stability, pheochromocytoma, posterior retroperitoneal adrenalectomy
1. Introduction
Pheochromocytoma is often regarded as a confounding tumor because of physiological issues and technical challenges encountered before, and during surgery. Since the 1990s, laparoscopic adrenalectomy (LA) has become the “gold standard” for the treatment of small- to moderate-sized adrenal tumors. Multiple studies have demonstrated the safety and efficacy of LA for the management of pheochromocytoma compared with open adrenalectomy.[1–3] Among the various approaches of LA, lateral transperitoneal adrenalectomy (TPA) was traditionally preferred by surgeons due to increased familiarity with the anatomy and operating view. Nevertheless, posterior retroperitoneoscopic adrenalectomy (PRA) has been gaining popularity with several benefits over traditional approaches. PRA can provide direct and rapid access to the adrenal gland without mobilizing intraperitoneal organs or lysing adhesions from prior abdominal operations, as well as the ability to perform bilateral adrenalectomy without repositioning the patient.[4–10] However, there is still a paucity of data regarding the intraoperative hemodynamic status of PRA in pheochromocytoma patients. The author recently started conducting PRA, as described by Dr. Walz, for various adrenal pathologies including pheochromocytoma. PRA is the preferred approach in our institution in most cases, and we have reported excellent surgical outcomes.[4–10] The aim of this study is to compare the outcomes of patients with pheochromocytoma using the TPA and PRA approaches, specifically focusing on the intraoperative hemodynamic changes.
2. Materials and methods
2.1. Patients
The study included a total of 362 consecutive patients who underwent LA from January 2008 to March 2015 for various adrenal pathologies in the Department of Endocrine Surgery, Yonsei University College of Medicine, Seoul, Korea. Among these, 14 (19.7%) and 57 (80.3%) patients with pheochromocytoma underwent open and endoscopic adrenalectomies, respectively. The records of 57 patients who underwent endoscopic adrenalectomy due to pheochromocytoma were retrospectively reviewed. Four cases of bilateral adrenalectomies and combined cases were excluded, leaving a total of 53 patients enrolled in this study. The surgical outcomes and intraoperative hemodynamic parameters of both patient groups were compared. All patients exhibited preoperative hypertension (systolic blood pressure (BP) >140 mm Hg; diastolic BP > 100 mm Hg) and were thus prepared for surgery by administration of an alpha-blocker (phenoxybenzamine) for 2 weeks to control their BP and block catecholamine surges during the operation. Additionally, patients exhibiting tachycardia were treated with beta-blockers. To prevent postoperative hypotension, adequate hydration was applied to all patients preoperatively. Pheochromocytoma diagnosis was based on preoperative manifestation of excess catecholamine and postoperative pathologic confirmation. Central venous and arterial catheters were used for all patients.
This study was approved by the Institutional Review Board of Yonsei University College of Medicine (IRB 4-2018–0578). Individual patient consent was waived because of the retrospective nature of the study.
3. Methods of operation
3.1. TPA
With the patient positioned in a lateral decubitus position, the operative bed was flexed just above the level of the iliac crest, and the lumbar bridge was elevated to maximally widen the space between the iliac crest and the costal margin. We generally used 3 ports for left adrenalectomies and 4 trocars for the right side. All trocars were inserted 2 cm caudally from the subcostal margin to ensure adequate space between instruments. For left sided operations, the spleen and pancreatic tail were mobilized and retracted anteromedially to expose the left adrenal gland. For the right side, the right lobe of the liver was mobilized and retracted medially to expose the adrenal gland and inferior vena cava (IVC). Once exposed, the adrenal gland was detached from the kidney and retroperitoneal soft tissue. All feeding vessels were ligated using a Harmonic Scalpel (Ethicon Endo-Surgery, Cincinnati, OH), and the central vein of the adrenal gland was clipped and divided.
3.2. PRA
The patient is placed in the prone jackknife position with the hip joint at right angle to maximize exposure of the posterior retroperitoneal space from the subcostal margin to the iliac crest. A 1.5 cm transverse skin incision is made just below the lowest tip of the 12th rib. A 10-mm trocar is then inserted through a second incision located 2 to 3 cm medially from the first incision. A third skin incision for another 5-mm trocar is made along the lowest margin of the 11th rib approximately 4 to 5 cm laterally from the first incision. A 12-mm blunt trocar with a ballooning tip is inserted through the first incision, and CO2 is insufflated up to 18 mm Hg pressure for pneumoretroperitoneum creation. After creating the retroperitoneal working space, renal hilar dissection and mobilization of the kidney upper pole are initially performed to expose the lowest part of the adrenal tumor. We start the tumor dissection with lower margin detachment from the upper pole of kidney in a medial to lateral direction. After isolation and ligation of the adrenal central vein, the adrenal gland is circumferentially dissected away from the surrounding adipose tissue.
3.3. Statistical analysis
Comparison between TPA and PRA was made by applying Fisher exact test for the categorical variables and Student t test for the continuous variables. Variables that did not meet the normality assumption were log-transformed and noted accordingly in the tables. The linear regression model was used to analyze the relationship between each parameter and surgical approaches, with and without the confounding factor being adjusted. The significance level was set as α = 0.05. All analysis was carried out using R version 3.2.2 (The R Foundation for Statistical Computing, Vienna, Austria).
4. Results
The patients clinicopathologic characteristics are summarized in Table 1. The mean tumor size was 4.80 ± 2.51 cm. There was no need for blood transfusion or conversion to laparotomy, and no mortalities or serious complications were observed in either group.
Table 1.
Clinicopathological characteristics of transperitoneal adrenalectomy vs posterior retroperitoneal adrenalectomy.
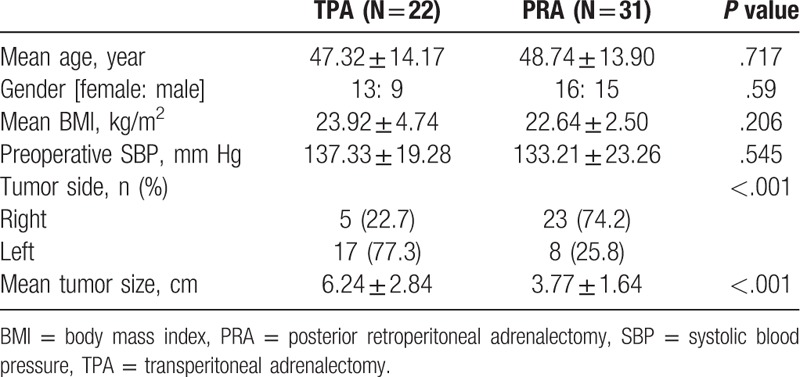
TPA was performed in 22 patients (41.5%) and PRA in 31 patients (58.5%), respectively. There were no significant differences in patient age, gender, body mass index, and preoperative systolic BP between those 2 groups. There was however a significant difference in the tumor size (TPA: 6.24 ± 2.84 vs PRA: 3.77 ± 1.64 cm, P < .001) (Table 1).
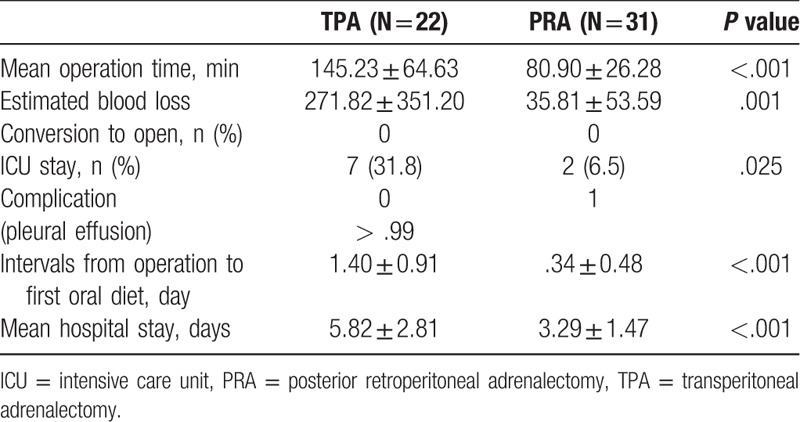
The surgical outcomes for patients who underwent TPA vs PRA are shown in Table 2. The PRA group demonstrated a shorter mean operative time (TPA: 145.23 ± 64.63 vs PRA: 80.90 ± 26.28 minutes, P < .001) and lower estimated blood loss (EBL) during surgery than the TPA group (TPA: 271.82 vs PRA: 35.81 ml, P = .001). The PRA group also had less ICU stay (TPA: 7 cases vs PRA: 2 cases, P = .025) and interval to first oral intake after operation (TPA: 1.40 ± 0.91 vs PRA: 0.34 ± 0.48 days, P < .001). Additionally, the mean postoperative length of hospital stay (LOS) was shorter in the PRA group (TPA: 5.82 ± 2.81 vs PRA: 3.29 ± 1.47 days, P < .001). Pleural effusion occurred in 1 patient in the PRA group but resolved with conservative management in a week.
Table 2.
Operative outcomes of transperitoneal adrenalectomy vs posterior retroperitoneal adrenalectomy.
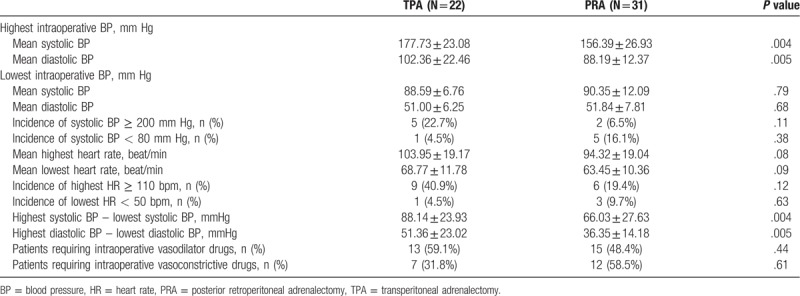
The intraoperative hemodynamic parameters of these 2 groups are described in Table 3. There were no significant differences between the groups in the incidence of extreme hypertensive episodes (systolic BP ≥ 200 mm Hg), extreme hypotensive episodes (systolic BP ≤ 80 mm Hg) and the highest and lowest heart rates. However, significant differences were observed in the mean highest intraoperative systolic BP (TPA: 177.73 ± 23.08 vs PRA: 156.39 ± 26.93 mm Hg, P = .004) and diastolic BP (TPA: 102.36 ± 22.46 vs PRA: 88.19 ± 12.37 mm Hg, P = .005). The differences between the highest and lowest systolic and diastolic BP, which reflect intraoperative BP fluctuation, was significantly wider in the TPA group than that in the PRA group (maximal difference of systolic BP [TPA: 88.14 ± 23.93 vs PRA vs 66.03 ± 27.63 mm Hg, P = .004], maximal difference of diastolic BP [TPA vs PRA 51.36 ± 23.02 vs 36.35 ± 14.18, P = .005], respectively). There was no significant difference in the intraoperative use of vasoconstrictive or vasodilatory drugs between the 2 groups.
Table 3.
Intraoperative hemodynamic parameters of transperitoneal adrenalectomy vs posterior retroperitoneal adrenalectomy.
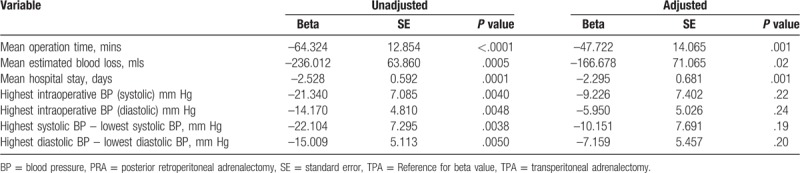
To evaluate the detailed relationship between each significant parameter and the 2 different surgical approaches, the linear regression model was used, with and without confounding factor being adjusted (Table 4). All parameters in the unadjusted model showed significant differences between the 2 groups. The beta indicates the different mean values in the parameters between the 2 groups (TPA vs PRA). For example, the beta in the operation time is −64.32, which indicates that the mean operating time in PRA was 64.3 minutes less than that in TPA; this difference was statistically significant (P < .001). To overcome the bias that can be caused by different tumor sizes between the 2 groups, the linear regression model was adjusted by the tumor size as the confounding factor, and the size-adjusted model was used. In the adjusted model, the average operation time, EBL, and LOS were significantly less in the PRA group than in the TPA group. Other parameters, which reflect the hemodynamic stability during the operation, showed a decreasing trend in the PRA group; however, they were not significantly different.
Table 4.
Linear regression model according to outcome comparing transperitoneal adrenalectomy and posterior retroperitoneal adrenalectomy; Adjusted by tumor size.
5. Discussion
Pheochromocytoma is a rare tumor arising from chromaffin cells of the adrenal medulla. Since Charles Mayo and Cesar Roux first reported the successful surgical removal of pheochromocytoma in 1926,[11] the progress in understanding the pathophysiology of pheochromocytoma, improvement in medical and anesthetic care, and advances in surgical techniques have resulted in excellent outcomes for patients with these tumors. Since the introduction of LA in the early 1990s,[12] several groups have demonstrated that LA affords multiple advantages over conventional open adrenalectomy.[13,14] Furthermore, LA has been demonstrated to be a feasible treatment option for patients with pheochromocytoma.[1–3,15–18]
TPA and PRA are the most commonly performed techniques for conducting LA. Currently, most surgeons prefer TPA because of the large working space, great visibility, increased familiarity with the anatomy, and the ability to conduct another operation simultaneously if required. However, because TPA involves the intraperitoneal space, injury or irritation of intraperitoneal organs can sometimes occur, which is the most common cause of postoperative ileus.[19]
Recently, PRA has been demonstrated to be a safe and effective procedure for various adrenal lesions with excellent surgical outcomes. PRA permits direct access to the adrenal gland, without any intraperitoneal visceral contact. In addition, PRA does not require adhesiolysis in patients with previous abdominal operations to expose the adrenal gland. Multiple reports have demonstrated the advantages of PRA in terms of early postoperative mobilization, reduced LOS, minimal blood loss, and reduced postoperative pain. In addition, this approach offers the option of bilateral adrenalectomy without repositioning the patient, which markedly reduces operation time.[9,10,20,21]
To our knowledge, there has only been 1 published article comparing the intraoperative hemodynamic status in patients undergoing PRA and TPA for pheochromocytoma.[22] The article by Vorselaars et al was a multi-institutional retrospective analysis of 341 patients across 6 medical centers in Europe, the United States, and Canada. It reviewed the hemodynamic instability during adrenalectomy using the transperitoneal vs retroperitoneal approaches for pheochromocytoma. The authors concluded that the retroperitoneal approach carries a greater risk of significant hypotension during surgery. However, the overall cardiovascular morbidity rates were similar between the 2 groups, and the occurrence of hemodynamic instability was influenced by the medical center where the operations were performed, which is one of the limitations of the study. Our study in contrast, is a single institution study, whereby all the surgeries were performed by a single surgeon, thus eliminating the different operating techniques and preoperative preparation and intraoperative patient management as confounding factors. Based on our results, PRA for pheochromocytoma provided favorable intraoperative hemodynamic stability compared with TPA. In our study, the number of extreme hypertensive or hypotensive episodes were similar between the 2 groups; however, the mean maximal systolic and diastolic BP was significantly higher in TPA during the operation. Furthermore, the difference between maximal and minimal systolic and diastolic BP were significantly greater with TPA than that for PRA, suggesting that BP fluctuation during PRA was more controllable than TPA.
Several reports have highlighted the importance of early ligation of the adrenal central vein in pheochromocytoma patients. This reduces the risk of excessive catecholamine secretion,[23–25] thus preventing fluctuation of intraoperative BP in these patients. In PRA, direct handling of the tumor before ligation of central vein is not frequent; therefore, the risk of catecholamine surge is relatively low.
In left sided PRA, the first step is exposing the renal hilum without touching the tumor. The central vein which drains directly into the renal vein can then be exposed and clipped relatively quickly before the tumor is dissected. On the right side, the vein is reached in an early phase of the procedure. After dissecting the renal hilum, the inferior adrenal vessels and middle adrenal arteries are exposed just above the IVC and ligated. Further dissection of the posterior aspect of IVC and the inferior margin of the adrenal gland facilitates exposure of the central vein, which can be ligated without significant handling of the tumor. Thus, the anatomical benefits in PRA enables minimum manipulation of the tumor and early identification and ligation of the central vein.
The significant reduction in blood loss observed in patients undergoing PRA is possibly due to the relatively less dissection required to expose the adrenal gland. In addition, high gas insufflation pressure of more than 18 mm Hg during PRA (as opposed to 12–15 mm Hg of pneumoperitoneum during TPA) tends to induce tamponade of minor bleeding from small vessels. Furthermore, in PRA, venous returns from the adrenal gland are usually reduced as the IVC or renal vein are compressed by the high retroperitoneal insufflation pressure; thereby, decreasing the risk of hemodynamic instability by reducing catecholamine release during the procedure.
A review of the literature concerning the size limits of tumors suitable for PRA suggests that tumors between 5 to 7 cm as good candidates for PRA.[10,26–28] Walz, et al additionally suggested that hormonally active 7 cm tumors could be safely resected using PRA.[29] This method utilizes a smaller working space and closer laparoscopic ports than TPA, which can lead to manipulation problems when tumors are large.[9] Based on these studies, the authors excluded patients with tumors >8 cm in size, clear signs of malignancy, a concomitant intra-abdominal pathology, or severe obesity for PRA.[27]
The major limitation of this study was the small sample size and non-controlled retrospective nature of the study. Specifically, the mean tumor size in PRA was smaller than that in TPA. The single surgeon who performed the operations only performed TPA prior to 2009. From 2009 onwards, the surgeon started using both techniques, and assigned the patients to either TPA or PRA based on tumor size. Tumors greater than 6 cm were generally removed using the TPA method early on, but the size criteria gradually increased to up to 8 cm in recent years. Despite including cases performed during the learning curve period, outcomes between the 2 techniques were still comparable, if not more favorable in the PRA group.
In pheochromocytoma, tumor size is considered to be directly related to catecholamine surge and BP fluctuation during surgery. Observations in this study show that the intraoperative hemodynamics of PRA was comparable to TPA, particularly for smaller sized pheochromocytomas, acknowledging that the data was biased towards PRA given the smaller tumors in that group. After adjusting for tumor size, however, the mean operating time, EBL and LOS were still less in the PRA group compared with the TPA group.
6. Conclusion
The PRA approach produces favorable intraoperative hemodynamic outcome compared to TPA for smaller sized pheochromocytomas. Future larger, randomized studies will be required to confirm the hemodynamic superiority of PRA in patients with larger pheochromocytomas.
Author contributions
Conceptualization: Sang-Wook Kang.
Data curation: Eun Jeong Ban, Cho Rok Lee.
Funding acquisition: Kee-Hyun Nam, Woong Youn Chung.
Investigation: Cho Rok Lee.
Methodology: Sang-Wook Kang.
Project administration: Jandee Lee, Jong Ju Jeong.
Supervision: Sang-Wook Kang, Jandee Lee, Jong Ju Jeong, Kee-Hyun Nam, Woong Youn Chung.
Writing – original draft: Eun Jeong Ban, Zeng Yap.
Writing – review & editing: Zeng Yap, Emad Kandil.
Zeng Yap orcid: 0000-0001-5827-933X.
Footnotes
Abbreviations: BP = blood pressure, EBL = estimated blood loss, IVC = inferior vena cava, LA = laparoscopic adrenalectomy, LOS = length of stay, PRA = posterior retroperitoneal adrenalectomy, TPA = transperitoneal adrenalectomy.
How to cite this article: Ban EJ, Yap Z, Kandil E, Lee CR, Kang SW, Lee J, Jeong JJ, Nam KH, Chung WY. Hemodynamic stability during adrenalectomy for pheochromocytoma: A case control study of posterior retroperitoneal versus lateral transperitoneal approaches. Medicine. 2020;99:7(e19104).
EJB and ZY equally contributed to this article as the first authors.
This study was supported by a faculty research grant from Yonsei University College of Medicine (6-2017-0163). Statistical support was provided by the Department of Statistics, Yonsei University College of Medicine.
The authors declare that they have no competing interests, financial or otherwise.
References
- [1].Toniato A, Boschin IM, Opocher G, et al. Is the laparoscopic adrenalectomy for pheochromocytoma the best treatment? Surgery 2007;141:723–7. [DOI] [PubMed] [Google Scholar]
- [2].Humphrey R, Gray D, Pautler S, et al. Laparoscopic compared with open adrenalectomy for resection of pheochromocytoma: a review of 47 cases. Can J Surg 2008;51:276–80. [PMC free article] [PubMed] [Google Scholar]
- [3].Tiberio GA, Baiocchi GL, Arru L, et al. Prospective randomized comparison of laparoscopic versus open adrenalectomy for sporadic pheochromocytoma. Surg Endosc 2008;22:1435–9. [DOI] [PubMed] [Google Scholar]
- [4].Park JH, Kim SY, Lee CR, et al. Robot-assisted posterior retroperitoneoscopic adrenalectomy using single-port access: technical feasibility and preliminary results. Ann Surg Onco 2013;20:2741–5. [DOI] [PubMed] [Google Scholar]
- [5].Lee CR, Walz MK, Park S, et al. A comparative study of the transperitoneal and posterior retroperitoneal approaches for laparoscopic adrenalectomy for adrenal tumors. Ann Surg Oncol 2012;19:2629–34. [DOI] [PubMed] [Google Scholar]
- [6].Giger U, Vonlanthen R, Michel JM, et al. Trans- and retroperitoneal endoscopic adrenalectomy: experience in 26 consecutive adrenalectomies. Dig Surg 2004;21:28–32. [DOI] [PubMed] [Google Scholar]
- [7].Lezoche E, Guerrieri M, Feliciotti F, et al. Anterior, lateral, and posterior retroperitoneal approaches in endoscopic adrenalectomy. Surg Endosc 2002;16:96–9. [DOI] [PubMed] [Google Scholar]
- [8].Naya Y, Nagata M, Ichikawa T, et al. Laparoscopic adrenalectomy: comparison of transperitoneal and retroperitoneal approaches. BJU Int 2002;90:199–204. [DOI] [PubMed] [Google Scholar]
- [9].Rubinstein M, Gill IS, Aron M, et al. Prospective, randomized comparison of transperitoneal versus retroperitoneal laparoscopic adrenalectomy. J Urol 2005;174:442–5. discussion 445. [DOI] [PubMed] [Google Scholar]
- [10].Suzuki K, Kageyama S, Hirano Y, et al. Comparison of 3 surgical approaches to laparoscopic adrenalectomy: a nonrandomized, background matched analysis. J Urol 2001;166:437–43. [PubMed] [Google Scholar]
- [11].Welbourn RB. Early surgical history of phaeochromocytoma. Br J Surg 1987;74:594–6. [DOI] [PubMed] [Google Scholar]
- [12].Gagner M, Lacroix A, Bolte E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med 1992;327:1033. [DOI] [PubMed] [Google Scholar]
- [13].Gill IS. Needlescopic urology: current status. Urol Clin North Am 2001;28:71–83. [DOI] [PubMed] [Google Scholar]
- [14].Shen WT, Kebebew E, Clark OH, et al. Reasons for conversion from laparoscopic to open or hand-assisted adrenalectomy: review of 261 laparoscopic adrenalectomies from 1993 to 2003. World J Surg 2004;28:1176–9. [DOI] [PubMed] [Google Scholar]
- [15].Cheah WK, Clark OH, Horn JK, et al. Laparoscopic adrenalectomy for pheochromocytoma. World J Surg 2002;26:1048–51. [DOI] [PubMed] [Google Scholar]
- [16].Jaroszewski DE, Tessier DJ, Schlinkert RT, et al. Laparoscopic adrenalectomy for pheochromocytoma. Mayo Clin Proc 2003;78:1501–4. [DOI] [PubMed] [Google Scholar]
- [17].Kercher KW, Park A, Matthews BD, et al. Laparoscopic adrenalectomy for pheochromocytoma. Surg Endosc 2002;16:100–2. [DOI] [PubMed] [Google Scholar]
- [18].Nau P, Demyttenaere S, Muscarella P, et al. Pheochromocytoma does not increase risk in laparoscopic adrenalectomy. Surg Endosc 2010;24:2760–4. [DOI] [PubMed] [Google Scholar]
- [19].Zhang XP, Wei JX, Zhang WX, et al. [Transperitoneal laparoscopic adrenalectomy for adrenal neoplasm: a report of 371 cases]. Ai Zheng 2009;28:730–3. [DOI] [PubMed] [Google Scholar]
- [20].Whittle DE, Schroeder D, Purchas SH, et al. Laparoscopic retroperitoneal left adrenalectomy in a patient with Cushing's syndrome. Aust N Z J Surg 1994;64:375–6. [DOI] [PubMed] [Google Scholar]
- [21].Mazzaglia PJ, Vezeridis MP. Laparoscopic adrenalectomy: balancing the operative indications with the technical advances. J Surg Oncol 2010;101:739–44. [DOI] [PubMed] [Google Scholar]
- [22].Vorselaars WMCM, Postma EL, Mirallie E, et al. Hemodynamic instability during surgery for pheochromocytoma: comparing the transperitoneal and retroperitoneal approach in a multicenter analysis of 341 patients. Surgery 2018;163:176–82. [DOI] [PubMed] [Google Scholar]
- [23].Janetschek G, Neumann HP. Laparoscopic surgery for pheochromocytoma. Urol Clin N Am 2001;28:97–105. [DOI] [PubMed] [Google Scholar]
- [24].Salomon L, Rabii R, Soulie M, et al. Experience with retroperitoneal laparoscopic adrenalectomy for pheochromocytoma. J Urol 2001;165(6 Pt 1):1871–4. [DOI] [PubMed] [Google Scholar]
- [25].Fernandez-Cruz L, Taura P, Saenz A, et al. Laparoscopic approach to pheochromocytoma: hemodynamic changes and catecholamine secretion. World J Surg 1996;20:762–8. discussion 768. [DOI] [PubMed] [Google Scholar]
- [26].Li QY, Li F. Laparoscopic adrenalectomy in pheochromocytoma: retroperitoneal approach versus transperitoneal approach. J Endourol 2010;24:1441–5. [DOI] [PubMed] [Google Scholar]
- [27].Walz MK, Alesina PF, Wenger FA, et al. Posterior retroperitoneoscopic adrenalectomy--results of 560 procedures in 520 patients. Surgery 2006;140:943–8. discussion 948-950. [DOI] [PubMed] [Google Scholar]
- [28].Berber E, Tellioglu G, Harvey A, et al. Comparison of laparoscopic transabdominal lateral versus posterior retroperitoneal adrenalectomy. Surgery 2009;146:621–5. discussion 625-626. [DOI] [PubMed] [Google Scholar]
- [29].Walz MK, Peitgen K, Walz MV, et al. Posterior retroperitoneoscopic adrenalectomy: lessons learned within five years. World J Surg 2001;25:728–34. [DOI] [PubMed] [Google Scholar]


