Abstract
Acute conjunctivitis is inflammation of conjunctiva of less than 3 to 4 weeks duration, characterized by cellular infiltration and exudation. It may also result into corneal, lid or orbital involvement which may lead to various complications.
A hospital based prospective study was conducted in Assam Medical College and Hospital with 110 culture proven acute bacterial conjunctivitis cases. Primary objective was to evaluate the bacteriological pattern and secondary objectives were to evaluate seasonal variation, association of different organisms with various complications and antibiotic sensitivity pattern of the isolates.
Maximum frequency of bacterial conjunctivitis observed from May to September. SA was the predominant organism isolated throughout the year (32.1%). Commonest single organism isolates were SE (26.1%) and SA (21.6%). True membrane formation was significantly associated with CD (P < .05), whereas pseudo-membrane formation was associated with SA and STBH isolation (P < .05). Isolation of SE, SA, and PA was associated with corneal involvement (P < .05). Lid involvement was seen with SA and Diphtheroid, whereas SP isolation was associated with concomitant dacryocystitis (P < .05). All the major organisms were (SE, SA, D, STBH, SP) highly sensitive to amino-glycosides, cephalosporins, chloromphenicol, vancomycin and linezolid, whereas high level of resistance was seen towards fluroquinolones (ciprofloxacin and moxifloxacin).
All acute bacterial conjunctivitis cases don’t require antibiotic therapy. In case if required, periodical culture and sensitivity may guide initial pre-emptive antibiotic therapy. Further choice of antibiotic should be govern by culture and sensitivity status.
Keywords: acute bacterial conjunctivitis, antimicrobial sensitivity, bacterial profile, complications, seasonal variation
1. Introduction
Acute conjunctivitis is defined as the inflammation of conjunctiva of less than 3 to 4 weeks duration, characterized by cellular infiltration and exudation.[1,2] Commonest presentation is foreign body sensation, redness and blurring of vision with associated purulent or mucopurulent discharge.[1,3] The route of spread is mainly reported to be contagious or from own conjunctival flora.[4] In severe cases, corneal, lid or orbital involvement may be seen resulting into various complications.[5]
Prevalence and etiology of acute bacterial conjunctivitis varies from place to place, even within the same country owing to geographical, cultural and socioeconomic variation.[6,7] Till now no data is available regarding pattern of bacteriological flora of acute bacterial conjunctivitis in north-east Indian population. Studies evaluating association of different organisms and complications of acute bacterial conjunctivitis is not reported till date. The north-east Indian region needs a separate investigation as this area is very humid, rains heavily, its typical geographic location, wide temperature variation, predominance of low and middle socioeconomic class of people, ethnic and socio-cultural variation as compared to mainstream India. Again antibiotic sensitivity pattern of the organisms (conjunctival swab culture) in this region is also unknown. Here comes the need of the study. The primary objective of our study was to evaluate the bacteriological pattern in culture positive cases of acute bacterial conjunctivitis. The secondary objectives were evaluation of seasonal variation of organism profile, evaluation of the association between different organisms involved and complications of acute bacterial conjunctivitis and antibiotic sensitivity profile of different organisms.
2. Material and method
A hospital based cross sectional study was conducted in the department of ophthalmology, Assam Medical College and Hospital, Assam (duration of study: 1 year).
All culture positive cases of acute bacterial conjunctivitis were included (both gender, any age group). Patients who had pre-existing ocular surface disorders, foreign body or trauma, patients already on antibiotic therapy, patient on steroid therapy at the time of first contact were excluded.
Conjunctival swab culture was done in all patients with clinical symptoms of acute bacterial conjunctivitis willing to get enrolled into the study. Culture positive patients were recruited and were further evaluated for any complications. Culture positive cases were further subjected to antibacterial sensitivity screen.
2.1. Clinical evaluation and complication assessment
After taking detailed patient history, a through local and systematic evaluation was done. Snellen chart was used for visual aquity evaluation. Patients were examined under first by torch light examination followed by detailed evaluation under slit lamp to detect associated complications. Patients were evaluated in details for occurrence of different complications of acute bacterial conjunctivitis e.g. occurrence of true and pseudo-membrane, different form of keratitis (marginal keratitis, punctate epithelial keratitis, peripheral ulcerative keratitis etc.), corneal erosion, corneal thinning, corneal ulcer, corneal opacity and lid and adnexa involvement.
For the study, acute bacterial conjunctivitis was defined as, presence of conjunctival congestion, chemosis, purulent or mucopurulent discharge, matted eyelash with less than 4 week duration.[3]
2.2. Collection and processing of conjunctival swab
Purulent material from conjunctival sac and inner canthus (with special precaution not to touch the eyelid) were collected with 2 sterile cotton swabs. Both the swabs were sent immediately to the Department of Microbiology. Gram staining and direct microscopy was done in one part and the other part was used for bacterial culture. Culture media used were blood agar enriched with 5% sheep blood, Mac Conkey agar, and chocolate agar (incubated at 37°C for 24–48 hours). In case no organism was grown, it was reported as ‘No Growth.’ Positive culture was considered if growth was observed at least any of the two media.[8,9] Various biochemical tests and identification methods were utilized to identify the growth identified.[10]
2.3. Antibiotic susceptibility test
Kirby-Bauer disc diffusion method was used for antibiotic susceptibility testing on Mueller Hinton agar according to CLSI recommendation.[11] Zone of inhibition were measured and the antibiotic sensitivity was reported as sensitive, intermediate or resistant to the specific antibiotic tested according to manufacturer guideline (HiMedia, Mumbai, India).[12]
2.4. Sample size calculation
From our clinical practice, assuming a prevalence of 7% of acute bacterial conjunctivitis amongst all the patients attending ophthalmological OPD, with 5% precision, 95% confidence interval, with infinite population, a sample size of 101 was calculated. Taking a 10% drop out rate a total of 111 sample size was calculated.
2.5. Ethical considerations
Ethical approval for the study was obtained from the institutional Ethics committee of Assam Medical College and Hospital (ethics permission number AMC/EC/PG: 7269 dated 31.5.2014). Informed consent was taken from all participants and from legally acceptable representatives in case of children before enrolling into the study.
2.6. Statistical analysis
Continuous data was presented as mean ± SD or Median (Range), whereas categorical data was represented as frequency (%). Logistic regression model was used for evaluation of predictors of complications. SPSS version 22 was used in analysis of data. P value < .05 was taken as criteria for statistical significance.
3. Result
The study is reported as per STROBE guidelines.[13] Participant flow chart is showed in Figure 1. We have screened 172 patients showing clinical signs and symptoms of acute bacterial conjunctivitis. Out of which, 110 patients came out to be culture positive and were included in the study.
Figure 1.
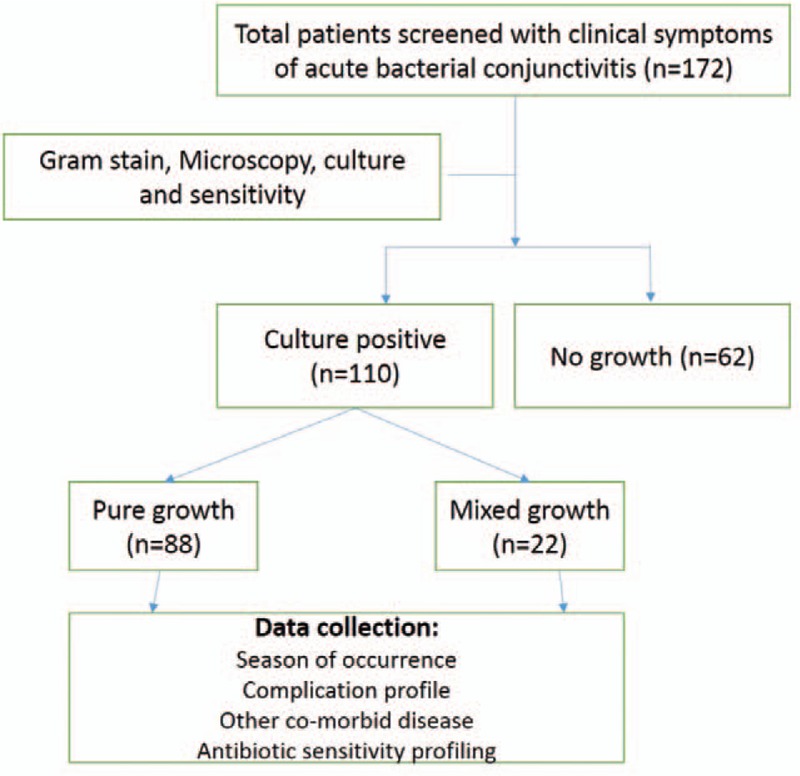
Participant flow chart.
3.1. Demographic characteristics
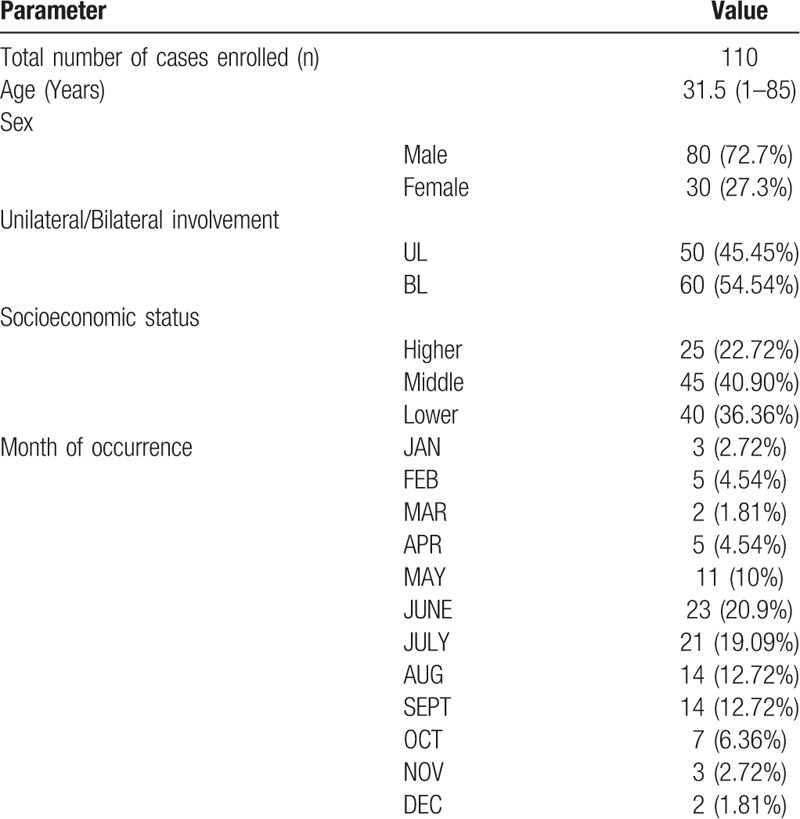
Data is showed in Table 1. Total no of culture positive cases in our study were 110 with median age of 31.5 year (Range 1 year to 85 year). Single eye involvement were seen in 45.45% patients (N = 50) and bilateral involvement was seen in 54.54% (N = 60). Middle class socio economic group has the maximum no of cases 40.90% (N = 45) followed by lower socioeconomic group 36.36% (N = 40). Higher socioeconomic group was less frequently involved which consisted of 22.72% (N = 25).
Table 1.
Demographic characteristics of acute bacterial conjunctivitis.
3.2. Seasonal variation
Maximum no of cases was seen in the month of June which consisted of 20.9% of total cases. Increased frequency were seen in the months of May (10%), June (20.9%), July (19.09%), August (12.72%) and September (12.72%). Overall, highest number of cases were found in the season of May to September (n = 83, 75%). In the month of October to April, the no of bacterial conjunctivitis cases were less (25%). Month wise frequency of bacterial pathogen and seasonal variation is shown in Table 1.
3.3. Clinical presentation of acute bacterial conjunctivitis
All the patients complained of blurring of vision, photophobia and foreign body sensation, while only 80% cases complained pain. On examination, conjunctival congestion was seen in all the cases (100%). Mucopurulent and purulent discharge was seen in 58.18% and 41.8% cases. Lid involvement was seen in 20% patients and corneal involvement was seen in 42.72% patients. Lymphadenopathy was seen in 10% of total cases. Data showed in Table 2.
Table 2.
Clinical presentation of acute bacterial conjunctivitis.

3.4. Microbiological profile
SA (32.1%) was the predominant organism isolated throughout the year [MRSA (2.7%)] followed by SE (29.1%), SP (14.5%), D (12.8%) and STBH (8.4%). MC (4.5%), HI (4.5%), Klebsiella (3.6%), PA (3.6%),EC (1.8%), NG (1.8%), Enterobacter (0.9%)and CD (0.9%) were isolated less frequently in our study population. [Data showed in Table 3].
Table 3.
Organism, frequency month wise and total occurrence.
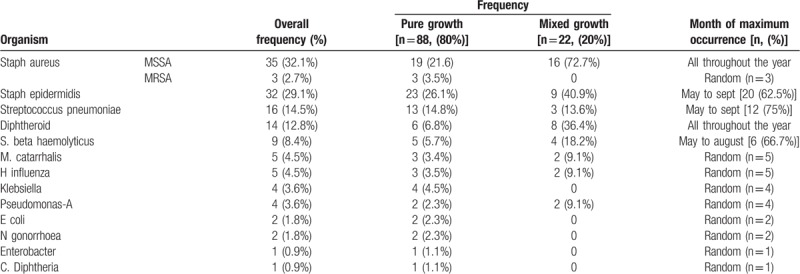
Maximum number of SE cases isolated was from May to September (62.5% cases). Similarly occurrence of maximum no of cases of STBH and SP were in the month of May to August (66.7%) and May to September (75%). SA and Diphtheroid were isolated all throughout the year. Minor constituents of bacterial profile namely MC, HI, K, PA, MRSA, EC, NG, E and catarrhalis were seen randomly in the year with no predominance in any season. Frequency of occurrence of organism and maximum occurrence in the year showed in Table 3.
3.5. Complications of conjunctivitis and associated organisms
3.5.1. True membrane
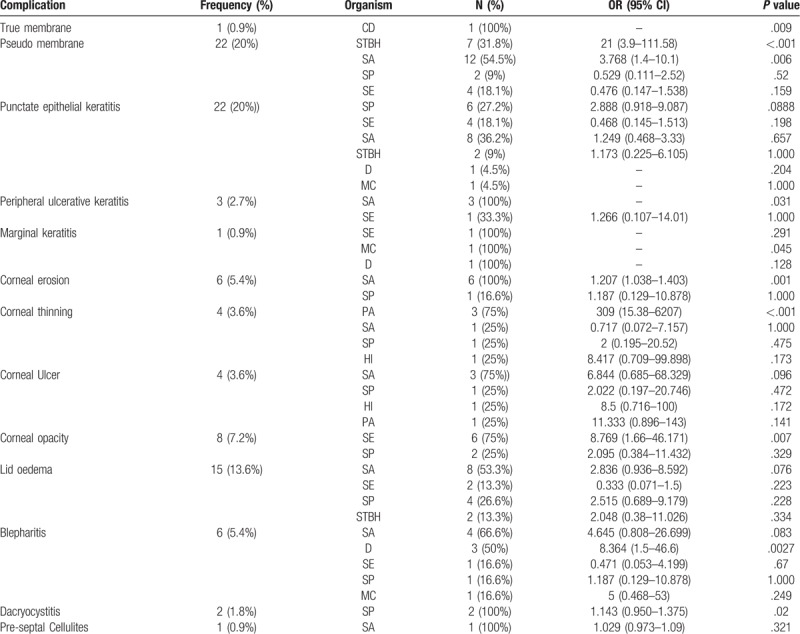
True membrane was seen in 0.9% of all cases of culture positive acute bacterial conjunctivitis. Isolation of C Diphtheria was significantly associated with true membrane formation (n = 1, P = .009).
3.5.2. Pseudo membrane
Pseudo membrane was seen in 20% cases. The organisms isolated STBH (31.8%), SA (54.5%), SP (9%) and SE (18.1%). However, only STBH and SA isolation were significantly associated with occurrence of pseudo membrane (P < .001and P = .006 respectively).
3.5.3. Punctate epithelial keratitis (PEK)
PEK was seen in 20% of all cases. Maximum no of isolation associated with occurrence of PEK were seen in SA (36.2%) and SP (27.2%) isolation in culture. But no statistical significance association was seen with any of the isolates.
3.5.4. Marginal keratitis
Marginal keratitis was seen in a single case (0.9%), where mixed growth was seen (components were SE, MC, and D). However none of the isolation in culture was significantly associated with occurrence of marginal keratitis.
3.5.5. Peripheral ulcerative keratitis (PUK)
PUK was seen in 2.7% cases. Organisms isolated were SA (100%) and SE (33.3%). Significant association was found between SA isolation and occurrence of PUK (P = .03).
3.5.6. Corneal erosion
Corneal erosion was seen in 5.4% cases. Organisms isolated were SA and SP, however, only isolation of SA were significantly associated with occurrence of corneal erosion (P = .001).
3.5.7. Corneal thinning
Corneal thinning was seen in 3.6% cases. Organisms isolated were PA (75%), SA (25%), SP (25%) and HI (25%). However, only PA isolation was found to be significantly associated with corneal thinning (P < .001).
3.5.8. Corneal ulcer
Corneal ulcer was seen in 3.6% cases. Organisms isolated were SA (75%), SP (25%), HI (25%) and PA (25%). None of the isolates were significantly associated with occurrence of corneal ulcer.
3.5.9. Corneal opacity
Corneal opacity was seen in 7.2% cases. Organisms isolated were SE (75%) and SP (25%). Only STBH isolation (75%) in culture was significantly associated with occurrence of corneal opacity. (P = .007).
3.5.10. Lid edema
Lid edema was seen in 13.6% cases. Organisms isolated were SA (53.3%), SE (13.3%), SP (26.6%) and STBH (13.3%).
3.5.11. Blepharitis
Blepharitis was seen in 5.4% cases. Organisms isolated were SA (66.6%), D (50%), SE (16.6%), SP (16.6%) and MC (16.6%). Isolation of Diphtheroid in culture were significantly associated with occurrence of blepharitis (P = .0027) and near significance was seen in case of SA (P = .083).
3.5.12. Dacryocystitis
Dacryocystitis was seen in 1.8% cases and organisms isolated were SP as single isolate. Significant association was seen between of SP isolation and occurrence of concomitant dacryocystitis (P = .02).
3.5.13. Pre-septal cellulites
Pre-septal cellulitis was seen in 0.9% cases. SA was the single organism isolated. [Organism associated with specific complication showed in Table 4]
Table 4.
Complications of conjunctivitis and associated organisms.
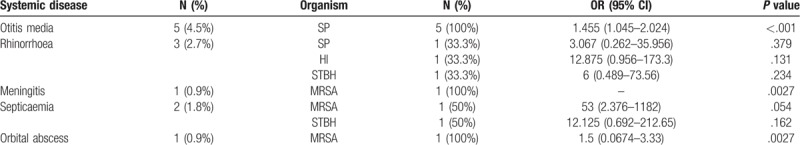
3.6. Conjunctivitis as a part of other systemic disease
3.6.1. Otitis media
Concomitant otitis media was seen in 4.5% of cases and SP was isolated from all the cases. Significant association was seen between SP isolation in culture and occurrence of concomitant otitis media (P ≤ .001).
3.6.2. Rrihonorrhoea
Rhinorrhea was seen in 2.7% cases. No significant association was seen in any of the isolates (SP, HI, and STBH) with concurrent rhinorrhea.
3.6.3. Meningitis
Conjunctivitis associated with meningitis was seen in 1 case in which MRSA was isolated in culture.
3.6.4. Septicemia
In 2 cases, concomitant septicemia was there and organisms isolated were MRSA (50%) and STBH (50%).
3.6.5. Orbital abscess
In one case concurrent orbital abscess was seen and MRSA growth was found in culture. [Conjunctivitis as a part of other systemic disease has been showed in Table 5]
Table 5.
Conjunctivitis as a part of other systemic disease.
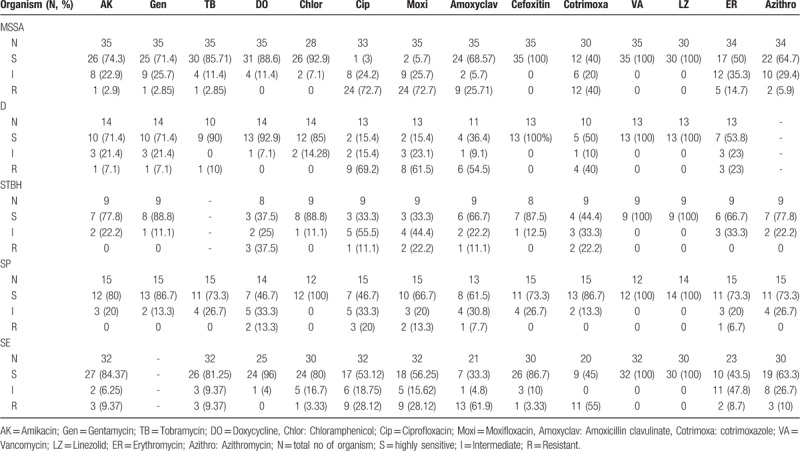
3.7. Antibiotic sensitivity pattern of individual organism
More than 70% isolates of MSSA were sensitive to aminoglycosides (amikacin, gentamycin, tobramycin), doxycycline, chloramphenicol, cephalosporins, vancomycin, and linezolid, whereas highest number of isolates were resistant to ciprofloxacin and moxifloxacin (>70% isolates).
More than 70% Diphtheroid isolates were sensitive to aminoglycosides (amikacin, gentamycin and tobramycin), doxycycline, chloramphenicol and cephalosporins, whereas more than 50% isolates were resistant to fluroquinolones (ciprofloxacin, moxifloxacin) and amoxicillin-clavulinic acid.
More than 70% STBH isolates were sensitive to aminoglycosides (amikacin and gentamycin), chloramphenicol, cephalosporins and macrolides (Azithromycin) whereas more than 50% isolates showed intermediate or high level resistance to doxycycline and fluroquinolones (ciprofloxacin and moxifloxacin).
More than 70% of SP isolates were sensitive to aminoglycosides (amikacin, gentamycin and tobramycin), chloramphenicol, cephalosporins, co-trimoxazole and macrolides (erythromycin and azithromycin). The 5% to 20% isolates were resistant to doxycycline, fluroquinolones, amoxicillin-clavulinic acid and erythromycin.
More than 80% of SE isolates were sensitive to aminoglycosides (amikacin and tobramycin), doxycycline, chloramphenicol and cephalosporins. More than 20% isolates were resistant to fluroquinolones (ciprofloxacin and moxifloxacin).
All the major organisms (SA, SE, D, STBH, and SP) were sensitive to vancomycin and linezolid. [Antibiotic sensitivity pattern of major organisms (>5 isolates) has been shown in Table 6].
Table 6.
Antibiotic sensitivity pattern of various organisms.
4. Discussion
4.1. Demographic profile
In our study, out of 172 patients screened with signs and symptoms of acute bacterial conjunctivitis, 110 (63.95%) showed culture positivity. Median age of presentation was 31.5 year (Range 1–85 year). Bilateral eye involvement was seen in 54.54% cases.
4.2. Seasonal variation
World-wide, both the frequency and the etiology of bacterial conjunctivitis vary according to climate, socioeconomic status and hygienic conditions.[2,14,15] In our study population, prevalence of acute bacterial conjunctivitis was more in middle and lower socioeconomic group. Maximum number of cases was seen in the month of May to September (75%). In the month of November to January, there was a declining trend of culture positive bacterial conjunctivitis cases were seen. Similar finding was reported by Aggarwal et al, where it was found that the frequency of bacterial conjunctivitis follows a step ladder pattern.[6] During the month of March to September (summer) its frequency was increased, sudden decreases in frequency was seen in autumn and remained low during winter season (November).[6] SA and Diphtheroid were isolated throughout the year, whereas SE and SP were mainly isolated during the season “May to September”, and STBH cases were isolated from “May to August” (data showed in Table 3). Results of our study is supported by findings by Aggarwal et al.[6]
4.3. Clinical presentation
Redness and conjunctival congestion were the most common clinical presentation (seen in 100% cases) in the first contact. All the patients complained of blurring of vision, photophobia and foreign body sensation, while only 80% cases complained of pain. None of the patients complained diminished vision. Mucopurulent and purulent discharge was seen in 58.18% and 41.8% cases. Lid involvement was seen in 20% and corneal involvement was seen in 42.72% patients. Lymphadenopathy was seen in 10% among all our study participants. Our findings are in accordance with previous literatures.[14,15]
4.4. Culture profile: Mixed growth versus single organism growth
In our study mixed growth was seen in 20% cases whereas 80% cases showed single organism growth. Mixed growth is commonly reported in conjunctival swab culture. Aggarwal et al reported 39.4% single organism growth, 30.5% mixed infection and no growth in 30% cases.[6] On the other hand, Perkins et al, reported 84.7% mixed growth in their study.[6,16] In a study by Hashish et al mixed growth of 2 organisms were 38% whereas mixed growth of three or more organisms were reported to be 9.8% among all samples.[17]
4.5. Microbial profile
Overall, SA (32.1%) was the predominant organism [MRSA (2.7%)] followed by SE (29.1%), SP (14.5%), D (12.8%) and STBH (8.4%) in our study. MC (4.5%), HI (4.5%), K (3.6%), PA (3.6%), EC (1.8%), NG (1.8%), E (0.9%) and CD (0.9%) were isolated less frequently in our study population.
SA was the most common isolate (Overall) and second most common single isolate in our study population. Our finding is in accordance with Seal et al and Mahajan et al, where SA is reported to be the most frequent single isolate in bacterial conjunctivitis cases.[2,18–20] Ramesh et al, Boralkar et al, and Stenson et al also reported SA to be the predominant single isolate from in eyelids and conjunctival infections.[21–23] mong the Staph aureus infection, 3% is estimated to be methicillin-resistant S aureus (MRSA) conjunctivitis. [4] In our study population, MRSA was seen in 2.7% of all the positive culture cases and the cases were seen randomly in the study period.
SE was the second most common isolate (29.1%) and commonest single organism isolates (26.1%).Our finding is in accordance with Aggarwal et al[6] and Perkin et al.[16]
SP was the third most common single organism isolate in our study population (14.5%). Rao and Rao observed SP as the second most prevalent organism (Mysore, India)[6] while Perkin et al reported SP to be most common isolate in acute bacterial conjunctivitis cases.[16] Martin et al reported uncapsulated strain of SP to be the most predominant organism in Florida and non typable SP as single or as component of mixed growth in New York State as the predominant organism in epidemic bacterial conjunctivitis.[2,24]
STBH was isolated in 8.4% of cases and it was component of both mixed and single growth. In a study by Aggarwal et al STBH was isolated in 1.1% cases (0.4% was single growth and 0.7% was mixed growth).[6] Similarly, Rajbanshi et al reported hemolytic streptococci to be 6.8% of total cases of bacterial conjunctivitis.[25]
In our study, Diphtheroid was present in 12.8% of total cases. Although Diphtheroid is commonly considered as commensel and contaminant in clinical samples, Rajbanshi et al reported Diphtheroid to be associated with bacterial conjunctivitis.[25–28] Similarly Watkins et al, Rubinfield et al, Joussen et al, Chace and Locatcher also considers Diphtheroid as causative agent of bacterial conjunctivitis.[29–32]
MC was isolated in 4.5% culture positive cases which was the most frequent gram negative isolate which was seen both as single growth (3.4%) and as a part of mixed growth (9.1%) in our study population. Although many authors consider MC as a commensel of conjunctiva and upper respiratory tract, however, Verduine et al reported it to be an emerging pathogen, both in healthy and immune compromised patients. [33]
Other micro-organisms that were isolated from the bacterial conjunctivitis cases were MC, HI, K, PA, MRSA, EC, NG, Enterobacter and CD. [Culture plate and microscopic picture of organisms isolated has been shown in Fig. 2]
Figure 2.
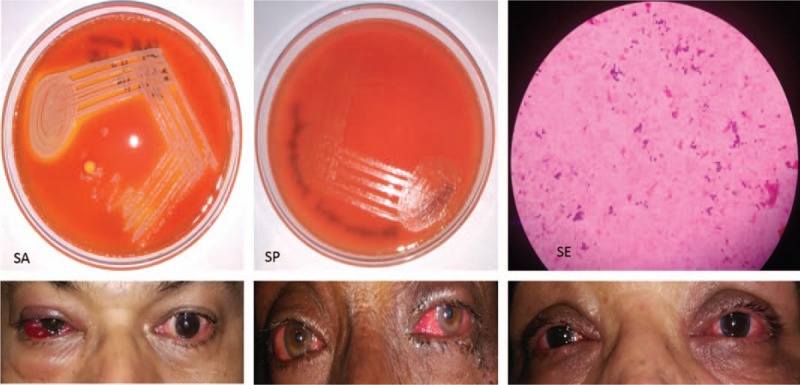
Culture plate and microscopic picture of organism.
5. Complication
5.1. Membrane formation
Both true and pseudo membrane formation is reported as complication of bacterial conjunctivitis is reported in literature.[34] In our study, true membrane were seen in 0.9% of all cases and association with C Diphtheria was statistically significant. Pseudo membrane was seen in 20% cases and organisms isolated were STBH, SA, SP and SE, however, STBH and SA isolation were significantly associated with occurrence of pseudo membrane.
C diphtheria is known for its association with occurrence true membrane but due to mass vaccination its prevalence is decreased except in population of lower socioeconomic status due to crowded environment.[34] Pseudo membranous conjunctivitis has been reported by various authors.[35,36] Bernaeuer et al reported S. beta hemolyticus and C. diphtheria as bacterial etiology for true and pseudo membrane formation.[34] Beta hemolytic Streptococcus is an invasive microorganism producing exotoxin causing severe purulent conjunctivitis with both type of membrane formation with cornea involvement.[34] Pseudo membranous conjunctivitis by Staph aureus, pneumococci, meningococci and Klebsiella has been reported in previous literature.[35–37]
5.2. Cornea involvement
In our study, corneal involvement in different forms of corneal involvement was seen [punctate epithelial keratitis (in 20% cases), marginal keratitis (0.9% cases), peripheral ulcerative keratitis (2.7%), corneal erosion (5.4%), corneal ulcer (3.6%) and corneal opacity (7.2%)] and organisms significantly isolated with different forms of conjunctival involvement were SP, SA, MC, PA, and SE.
Overall, risk of cornea involvement is around 60% amongst all acute bacterial conjunctivitis cases.[2] Dry eye, abnormal blinking pattern and meibomian gland dysfunction are risk factors in development of keratitis in conjunctivitis patients.[2,38,39] Punctate epithelial keratitis and marginal keratitis are often associated with bacterial conjunctivitis by Staph. aureus specially in patients with dry eye[2] and staphylococcal exotoxin is hypothesized to be the culprit.[2,38] Regarding other organisms, there are reports of Moraxella and Corynebacterium species causing keratitis.[21] In a study in New Zealand, coagulase negative staphylococci was the commonest gram positive organism whereas Moraxella was the predominant gram negative organism responsible for bacterial keratitis cases.[20] Punctate epithelial keratitis is also associated with Streptococcus group.[38] Maske et al[40] reported Staph epidermidis as a causative agent of corneal ulcer and Mahajan et al[18]as a pathogen for kerato-conjunctivitis supports the tendency of this organism to opacity formation. This supports our finding of corneal opacity formation in keratitis by S epidermidis.[18,40] In our study, 60% cases all the corneal opacity developed were not visually significant and only complaints were glare and blurring of vision. It takes an average of 5 months for the symptoms to disappear.
5.3. Lid and adnexa involvement
Lid edema was seen in 13.6% cases and SA was isolated in maximum (53.3%). Blepharitis was seen in 5.4% cases [SA (66.6%) and Diphtheroid (50%)]. Isolation of Diphtheroid was significantly associated with occurrence of blepharitis. Our finding is in accordance with that of previous literature.[16,38,41,42] Associated dacryocystitis was seen in 1.8% cases and SP was isolated in all the cases. Association between dacryocystitis and S Pneumonia isolation in culture is previously reported[21,43].The blockage of the lacrimal duct system results in accumulation of tears and creates a fertile environment for secondary bacterial infection.[2] Also infection can spread from nasopharynx via the nasolacrimal duct.[2]Pre-septal cellulitis was seen in 0.9% cases. Staph aureus was the single organism isolated. SA and SP are reported to be associated with preseptal cellulitis.[44,45]
5.4. Rare complications
In our study, 4 cases of corneal thinning were seen and the commonest organism isolated was PA (in 75% cases). Corneal involvement is reported with PA and emergency treatment is recommended.[46]
5.5. Conjunctivitis as part of other systemic disease
In our study, conjunctivitis was seen to be occurring as part of other systemic diseases e.g. URTI presenting as rhinorrhea (2.7%), otitis media (5.4%), meningitis (0.9%), septicemia (1.8%) and orbital abscess (0.9%) and organisms isolated were SP, HI, STBH and MRSA. Concurrent occurrence of otitis media and upper respiratory tract infection (URTI) with acute bacterial conjunctivitis by H. Influenza and S. pneumonia is reported in literatures and examining ears and lymph node is recommended with special emphasis on pediatric population.[1,2,47,48]Streptococcus species are also reported to be rare associates.[14,48,49] In a case series by McKinley et al, Staphylococcus was the commonest organism isolated in cases of orbital abscess; among them 36% were methicillin resistant Staph. aureus followed by Streptococcus.[44,45]
5.6. Antibiotic sensitivity pattern
Although, in around 60% cases of acute bacterial conjunctivitis resolves within 1 to 2 weeks of presentation, topical antibiotics reduce the duration of disease.[1,4] In case of culture positive conjunctivitis cases, topical antibiotics seem to be more effective in achieving clinical and microbiological cure. [1,4]
In our study, most of the major isolates were sensitive to aminoglycosides, chloramphenicol, cephalosporins, macrolides (Azithromycin) and were also sensitive to important reserved antibiotic like vancomycin and linezolid.
One important finding of our study is high level of fluroquinolones resistance amongst Staph aureus (>70% isolates), Diphtheroid (>60% isolates), S. beta hemolyticus (50% cases are intermediate level and 10–20% cases resistant), S. pneumonia (13–20% isolates resistant) and staph. epidermidis (>28% isolates). Although, moxifloxacin is a fourth generation fluroquinolones with high activity towards both gram negative and positive organisms, and is taken as an agent of choice for empirical therapy,[50] the emerging pattern of resistance is a matter of concern and highlights its irrational use, wide marketing practices. Higher antibiotic prescription practices (upto 44% of all prescriptions) and fluroquinolones being most common of them, both at community level and at tertiary case level, definitely carries risk of high level of resistance.[51]
One of the factors that underlie the irrational use of antibiotics is that, many a time differentiating bacterial conjunctivitis from viral conjunctivitis is tough. Non-specificity of signs and symptoms is a reason of the same[4] and is a cause of irrational antibiotic use. We need a good scale study to distinguish acute bacterial conjunctivitis from acute viral conjunctivitis highlighting differentiating different bacterial and viral subtypes or some good biomarker and also need personalized antibiotic therapy in those who needs it.[52]
6. Summary and conclusion
In our study, most of the cases occurred in May to September and SA was the predominant organism isolated throughout the year (overall data) followed by SE, SP, D and STBH. Amongst single organism cultures also, most common organism were SE, followed by SA, SP, D and STBH.
One important finding of our study is isolation of SE, D and MC as single organism culture from acute bacterial conjunctivitis cases.
Membrane formation was seen in association with C. diphtheria (true membrane), STBH and SA (pseudo membrane). Cornea involvement was seen in association with SP, SA, MC, PA and SE. Lid and adnexa involvement was seen in association with SA and D. Although Diphtheroid is commonly considered as contaminant, its frequent isolation as single organism growth and existing literature reports highlights its growing importance.
Most of the isolates were sensitive to aminoglycosides, chloramphenicol, cephalosporins, macrolides (Azithromycin) and were also sensitive to important reserved antibiotic like vancomycin and linezolid, however high level of resistance was seen towards fluroquinolones.
6.1. Recommendations and conclusion
-
1.
Detailed ophthalmic evaluation with special reference to occurrence of any complications to be carried out.
-
2.
Systemic evaluation to see whether conjunctivitis is a local disease or part of a systemic disease.
-
3.
Presumptive identification of organism involved from complications caused and other systemic clinical features and organism specific features.[53]
-
4.
Initial choice of therapy should be based on presumptive organism involved and its regional sensitivity pattern.
-
5.
Season of occurrence and contact exposure can guide to anti-bacterial therapy
-
6.
Once culture and sensitivity report is available choice of antibiotic should be govern by culture and sensitivity.
-
7.
In case of presumptive antibiotic therapy, the local organism profile periodic culture and sensitivity reports can guide to antibiotic therapy.
-
8.
In case of limited resource settings, simple gram staining and microscopy can guide initial empirical antibiotic therapy.
6.2. Strengths and limitations of the study
This is the first study addressing all these issues from North-east India. Another issue is most of the existing literature evaluated the association between different organisms and complications using simple frequency approach. In our study, we have used measures of association to measure the strengths of the relations and their significance (odds ratio and regression analysis) highlighting a strong methodology of our study.
Acknowledgments
The authors acknowledge Dr. JJ Kuli and Dr.Bhanu Devi, Department of Ophthalmology, Assam Medical college for their administrative support, Dr Bikash Medhi, professor department of pharmacology, PGIMER Chandigarh, Dr.ReemaNath, Dept. of Microbiology, Assam Medical College, Dibrugarh and Dr.Charu Singh, Dept. of Microbiology, PGIMER, Chandigarh for their expert opinion in the microbiology section of the study.
Author contributions
Conceptualization: Phulen Sarma.
Data curation: Anusuya Bhattacharyya, Phulen Sarma, Bhaswati Sarma, Subodh Kumar, Hardeep Kaur, Manisha Prajapat.
Formal analysis: Anusuya Bhattacharyya, Phulen Sarma, Subodh Kumar, Hardeep Kaur, Manisha Prajapat.
Investigation: Anusuya Bhattacharyya, Tapan Gogoi, Bhaswati Sarma.
Methodology: Anusuya Bhattacharyya, Phulen Sarma, Bhaswati Sarma.
Project administration: Anusuya Bhattacharyya.
Resources: Tapan Gogoi.
Supervision: Phulen Sarma.
Writing – original draft: Anusuya Bhattacharyya, Phulen Sarma, Tapan Gogoi, Subodh Kumar.
Writing – review & editing: Anusuya Bhattacharyya, Phulen Sarma, Tapan Gogoi, Bhaswati Sarma, Subodh Kumar. Hardeep Kaur, Manisha Prajapat.
Footnotes
Abbreviations: CD = corynebacterium diphtheriae, D = diphtheroid, E = enterobacter, EC = Escherichia coli, HI = hemophilus influenza, K = klebsiella, MC = Moraxella catarrhalis, MRSA = methicillin resistant staph aureus, NG = neisseria gonorrhoea, PA = pseudomonas aeruginosa, SA = staph aureus, SE = staphylococcus epidermidis, SP = streptococcus pneumonia, STBH = streptococcus beta hemolyticus.
How to cite this article: Bhattacharyya A, Sarma P, Sarma B, Kumar S, Gogoi T, Kaur H, prajapat M. Bacteriological pattern and their correlation with complications in culture positive cases of acute bacterial conjunctivitis in a tertiary care hospital of upper Assam: A cross sectional study. Medicine. 2020;99:7(e18570).
AB and PS have contributed equally to this work. So both are designated as first author.
Institutional Ethics Committee approval number: Permission number AMC/EC/PG: 7269 dated 31.5.2014.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Epling J. Bacterial conjunctivitis. BMJ Clin Evid 2012;2012.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3635545/https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3635545/. [access date February 20, 2019]. [PMC free article] [PubMed] [Google Scholar]
- [2].Høvding G. Acute bacterial conjunctivitis. Acta Ophthalmologica 2008;86:5–17. [DOI] [PubMed] [Google Scholar]
- [3]. Acute bacterial conjunctivitis – antibiotic susceptibility and resistance to commercially available topical antibiotics in Nepal. Research Gate. [DOI] [PubMed] [Google Scholar]
- [4].Azari AA, Barney NP. Conjunctivitis. JAMA 2013;310:1721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Silvester A, Neal T, Czanner G, et al. Adult bacterial conjunctivitis: resistance patterns over 12 years in patients attending a large primary eye care centre in the UK. BMJ Open Ophthalmol 2016;1:e000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Agarwala HS, Raichaudhury M, Munsi NK. Bacterial flora in acute conjunctivitis. J All India Ophthalmol Soc 1967;15:58–66. [PubMed] [Google Scholar]
- [7].Bharathi MJ, Ramakrishnan R, Shivakumar C, et al. Etiology and antibacterial susceptibility pattern of community-acquired bacterial ocular infections in a tertiary eye care hospital in south India. Indian J Ophthalmol 2010;58:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Srinivasan M, Gonzales C, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol 1997;81:965–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sharma S. Diagnosis of infectious diseases of the eye. Eye (Lond) 2012;26:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boyle VJ, Fancher ME, Ross RW. Rapid, modified kirby-bauer susceptibility test with single, high-concentration antimicrobial disks. Antimicrob Agents Chemother 1973;3:418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. wayne P. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Eleventh Edition. Vol. 32. 11th ed. [Google Scholar]
- [12]. HTM002.pdf. http://himedialabs.com/TD/HTM002.pdf. [access date March 26, 2019]. [Google Scholar]
- [13]. STROBE Statement: Home. https://www.strobe-statement.org/index.php?id=strobe-home. [accessed date April 17, 2019]. [Google Scholar]
- [14].Weiss A, Brinser JH, Nazar-Stewart V. Acute conjunctivitis in childhood. J Pediatr 1993;122:10–4. [DOI] [PubMed] [Google Scholar]
- [15].Weiss A. Acute conjunctivitis in childhood. Curr Probl Pediatr 1994;24:4–11. [DOI] [PubMed] [Google Scholar]
- [16].Perkins RE, Kundsin RB, Pratt MV, et al. Bacteriology of normal and infected conjunctiva. J Clin Microbiol 1975;1:147–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hashish AA, Elbakary MA, Allam WA. Resistant infantile bacterial conjunctivitis in Egypt: a microbiology study. J Pediatr Ophthalmol Strabismus 2018;55:135–9. [DOI] [PubMed] [Google Scholar]
- [18].Mahajan VM. Acute bacterial infections of the eye: their aetiology and treatment. Br J Ophthalmol 1983;67:191–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Seal DV, Barrett SP, McGill JI. Aetiology and treatment of acute bacterial infection of the external eye. Br J Ophthalmol 1982;66:357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hall RC, McKellar MJ. Bacterial keratitis in Christchurch, New Zealand. Clin Experiment Ophthalmol 2004;32:478–81. [DOI] [PubMed] [Google Scholar]
- [21].Ramesh S, Ramakrishnan R, Bharathi MJ, et al. Prevalence of bacterial pathogens causing ocular infections in South India. Indian J Pathol Microbiol 2010;53:281–6. [DOI] [PubMed] [Google Scholar]
- [22].Boralkar AN, Dindore PR, Fule RP, et al. Microbiological studies in conjunctivitis. Indian J Ophthalmol 1989;37:94–5. [PubMed] [Google Scholar]
- [23].Stenson S, Newman R, Fedukowicz H. Laboratory studies in acute conjunctivitis. Arch Ophthalmol 1982;100:1275–7. [DOI] [PubMed] [Google Scholar]
- [24].Martin M, Turco JH, Zegans ME, et al. An outbreak of conjunctivitis due to atypical streptococcus pneumoniae. N Engl J Med 2003;348:1112–21. [DOI] [PubMed] [Google Scholar]
- [25].Rajvanshi VS. Bacterial flora of the conjunctiva. J All India Ophthalmol Soc 1968;16:24–8. [PubMed] [Google Scholar]
- [26].Chandran R, Puthukkichal DR, Suman E, et al. Diphtheroids-Important Nosocomial Pathogens. J Clin Diagn Res 2016;10:DC28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nagassar RP, Nicholson AM, Williams W, et al. Diphtheroids as a cause of endocarditis in a haemodialysis patient. Case Reports 2012;2012:bcr1020114894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Leal SM, Jones M, Gilligan PH. Clinical significance of commensal gram-positive rods routinely isolated from patient samples. J Clin Microbiol 2016;54:2928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Watkins DA, Chahine A, Creger RJ, et al. Corynebacterium striatum: a diphtheroid with pathogenic potential. Clin Infect Dis 1993;17:21–5. [DOI] [PubMed] [Google Scholar]
- [30].Chace RR, Locatcher-Khorazo D. Keratoconjunctivitis due to a diphtheroid-like organism; report of a case. Arch Ophthal 1947;37:497–503. [DOI] [PubMed] [Google Scholar]
- [31].Rubinfeld RS, Cohen EJ, Arentsen JJ, et al. Diphtheroids as ocular pathogens. Am J Ophthalmol 1989;108:251–4. [DOI] [PubMed] [Google Scholar]
- [32].Joussen AM, Funke G, Joussen F, et al. Corynebacterium macginleyi: a conjunctiva specific pathogen. Br J Ophthalmol 2000;84:1420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Verduin CM, Hol C, Fleer A, et al. Moraxella catarrhalis: from emerging to established pathogen. Clin Microbiol Rev 2002;15:125–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bernauer W, Dart JKG, Elder MJ. Cicatrising Conjunctivitis. Karger Medical and Scientific Publishers 1997;28:1–0. [Google Scholar]
- [35]. Pseudomembranous conjunctivitis following bone marrow transplantation: Immunopathological and ultrastructural study of one case - ScienceDirect. https://www.sciencedirect.com/science/article/pii/S004681779690074X. [access date March 23, 2019] [DOI] [PubMed] [Google Scholar]
- [36].Doddaiah V, Padmini HR, Seenivasen S, et al. Pseudomembranous conjunctivitis caused by Staphylococcus aureus. J Acad Clin Microbiol 2014;16:104. [Google Scholar]
- [37].Hogan MJ. Conjunctivitis with Membrane Formation. Am J Ophthalmol 1947;30:1495–513. [DOI] [PubMed] [Google Scholar]
- [38].Rajesh S, Divya B, Aruna V. Microbiological Profile of External Ocular Infections in a Tertiary Care Hospital in South India. Int J Curr Microbiol App Sci 2017;6:4343–52. [Google Scholar]
- [39]. Classification of Chronic Blepharitis - ScienceDirect. https://www.sciencedirect.com/science/article/pii/S0161642082346692. [access date March 2, 2019]. [Google Scholar]
- [40].Maske R, Hill JC, Oliver SP. Management of bacterial corneal ulcers. Br J Ophthalmol 1986;70:199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fahmy JA, Møller S, Bentzon MW. Bacterial flora of the normal conjunctiva I. Topographical distribution. Acta Ophthalmologica 1974;52:786–800. [DOI] [PubMed] [Google Scholar]
- [42].Ratnumnoi R, Keorochana N, Sontisombat C. Normal flora of conjunctiva and lid margin, as well as its antibiotic sensitivity, in patients undergoing cataract surgery at Phramongkutklao Hospital. Clin Ophthalmol 2017;11:237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Weiser J, Henke HA, Hector N, et al. Sub-inhibitory tigecycline concentrations induce extracellular matrix binding protein Embp dependent Staphylococcus epidermidis biofilm formation and immune evasion. Int J Med Microbiol 2016;306:471–8. [DOI] [PubMed] [Google Scholar]
- [44].Lee S, Yen MT. Management of preseptal and orbital cellulitis. Saudi J Ophthalmol 2011;25:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].McKinley SH, Yen MT, Miller AM, et al. Microbiology of pediatric orbital cellulitis. Am J Ophthalmol 2007;144:497–501. [DOI] [PubMed] [Google Scholar]
- [46].Watson S, Cabrera-Aguas M, Khoo P. Common eye infections. Aust Prescr 2018;41:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chawla R, Kellner JD, Astle WF. Acute infectious conjunctivitis in childhood. Paediatr Child Health 2001;6:329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bodor FF, Marchant CD, Shurin PA, et al. Bacterial etiology of conjunctivitis-otitis media syndrome. Pediatrics 1985;76:26–8. [PubMed] [Google Scholar]
- [49].Block SL, Hedrick J, Tyler R, et al. Increasing bacterial resistance in pediatric acute conjunctivitis. Antimicrob Agents Chemother 2000;44:1650–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Abdullah FE, Khan MI, Waheed S. Current pattern of antibiotic resistance of clinical isolates among conjunctival swabs. Pak J Med Sci 2012;29:81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Jadhav PR, Moghe VV, Deshmukh YA. Drug Utilization Study in Ophthalmology Outpatients at a Tertiary Care Teaching Hospital. International Scholarly Research Notices. [Google Scholar]
- [52]. Duration of antibiotic therapy: Fixed duration versus personalization/Individualization of antibiotic therapy duration: which one to be preferred? 2019. https://www.bmj.com/content/358/bmj.j3418/rr-36. [access date June 1, 2019] [Google Scholar]
- [53]. Conjunctivitis Preferred Practice Pattern. https://www.aao.org/Assets/455bd16b-af54-4551-9a91-9ab126c56b06/636777136764030000/conjunctivitis-preferred-practice-pattern-2018-pdf-pdf. [access date April 13, 2019] [Google Scholar]


