Abstract
Background:
Traditional Chinese medicine (TCM) has been used to treat diabetic complications including diabetic retinopathy for many years.
Objectives:
This review was performed to systematically assess the efficacy and safety of TCM for treating non- proliferative diabetic retinopathy (NPDR).
Methods:
Retrieval from 7 electronic databases was conducted to determine eligible trials published until March 1, 2018. Randomized controlled trials of NPDR that comparing compound Chinese medicine containing the therapeutic method of activating blood and remove stasis versus controls were included for analysis. Primary outcomes were progression of retinopathy. Secondary outcomes included visual acuity, mean defect of visual field, micro-aneurysms, hemorrhage areas, exudates, capillary nonperfusion areas, hemorheological indicators, oscillatory potentials (Ops), glycated haemoglobin (HbA1c), and adverse events. Data extraction and quality assessment were performed. Results expressing as risk ratios (RRs) or mean differences (MD) were analyzed with a fixed- or random- effect model. I2 statistics were used to assess heterogeneity.
Results:
A total of 33 trials and 3373 participants were included. Findings revealed that no included studies reported the progression of retinopathy. Compared with conventional medicine, TCM was significantly better at improving visual acuity (MD, −0.10; 95% confidence interval [CI] −0.16 to −0.05) and Ops (MD, −4.68, 95% CI −8.51 to −0.85), and reducing the mean defect of visual field (MD, −1.43; 95%CI, −2.17 to −0.68), micro-aneurysms (MD, −4.51; 95% CI, −6.23 to −2.79), hemorrhage areas (MD, −0.62; 95% CI, −1.06 to −0.19), plasma viscosity (MD, −0.10; 95% CI, −0.20 to 0.00), and HbA1c (MD, −0.22; 95% CI, −0.42 to −0.03). Compared with placebo, TCM was also associated with a decline in the number of microaneurysms (MD, −4.35; 95% CI, −6.25 to −2.45), exudates (MD, −0.17; 95% CI −0.31 to −0.03), capillary nonperfusion areas (MD, −0.18; 95% CI, −0.31 to −0.04), and HbA1c (MD, −0.88; 95% CI, −1.44 to −0.32). Compared with blank groups, TCM was superior at decreasing the mean defect of visual field (MD, −0.87; 95% CI −0.95 to −0.79) and the numbers of micro-aneurysms (MD, −3.35; 95% CI, −4.73 to −1.97). Adverse events were also assessed.
Conclusion:
Activating blood compound Chinese herbal medicine could help to improve visual acuity, micro-aneurysms and HbA1c. Further trials are needed to provide more reliable evidence.
Keywords: diabetic retinopathy, randomized controlled trials, systematic review, traditional chinese medicine
1. Introduction
The dramatic increase in the incidence of diabetes mellitus (DM) is becoming a major public health issue. Parallel with the growing DM pandemic, the occurrence of diabetic retinopathy (DR) is also increasing. DR is the most common cause of preventable blindness in working-aged adults (20–74 years).[1] Epidemiological data from rural China suggested that the incidence was 43% for any retinopathy and 6.3% for vision-threatening retinopathy.[2] Another study of mostly urban Chinese individuals indicated that the prevalence of DR was 8.1% among patients with DM.[3] Vision-threatening retinopathy is serious and irreversible, dramatically affecting the quality of life of diabetic patients. Moreover, the expenses of diabetic vascular complications accounted for 80% of the total direct medical expenses, resulting in a large economic burden for society.[4] Therefore, early prevention and treatment are necessary. However, conventional treatment options are limited and mainly include glucose control, blood pressure and lipid control, aspirin, and lifestyle modifications. No approaches have been developed specifically to prevent and treat DR. More and more other effective measures have been given attention.[5]
Recently, traditional Chinese medicine (TCM) has become more popular and drawn more attention due to its positive clinical efficacy.[6–7] Recent clinical and experimental studies have proven that TCM is effective in the prevention and treatment of DR.[7–10] Evidences from the clinical trials has suggested that herbal medicine possibly promotes blood microcirculation, improves vascular endothelial function, protects the blood retinal barrier, and inhibits the oxidation and inflammation state, and so on.[7,9–10] The main basis of treatment in TCM is syndrome differentiation. According to syndrome differentiation, TCM has different treatment principles for DR, such as boosting qi and nourishing yin, enriching the liver and kidney, invigorating the spleen and removing dampness, and activating blood and removing stasis thus unblocking the collaterals. The use of herbs also differs according to these principles.[11–12] In recent years, there have been many studies that use activating blood herbs for the treatment of DR. According to the TCM theory, blood stasis is 1 of the most important factors in the pathogenesis of DR, and thus activating blood and unblocking the collaterals principle is considered to be the key treatment principle.[13–14] Although there have been some systematic reviews and meta analysis to assess the efficacy and safety of TCM for DR, these studies did not differentiate the categories of the herbs used.[7,15–16] Systematic evidence that summarizing the activating blood compound for DR has been lacking. Therefore, we conducted a systematic review to assess the efficacy and safety of the method of activating blood and removing stasis method for the treatment of DR while taking into account the treatment principles. Our findings should serve as a reference for clinicians seeking effective treatment.
2. Method
This review was performed based on the PRISMA statement for reporting of systematic reviews and meta-analysis of health care interventions.[17] The trial registration number is as follows: PROSPERO registration no. CRD42016039367.
2.1. Search strategy
We searched the following electronic databases to identify eligible trials published from inception to March 1, 2018: including Cochrane Library, PubMed, EMBASE, Chinese Biomedical Literature Database, Chinese National Knowledge Infrastructure Database, Chinese Science and Technique Journals Database, and the Wanfang Database. Conference abstracts were searched manually. The search terms were as follows: (“diabetic retinopathy” OR “retinal disorders” OR “diabetic eye disease”; “retinal disease” OR “proliferative diabetic retinopathy” OR “diabetic macular edema” OR “diabetic maculopathy” OR “vision loss”) AND (“Chinese herbal medicine” OR “herb” OR “herbal medicine” OR “Chinese herb” OR “traditional Chinese medicine”) AND (“randomized controlled trial” OR “controlled clinical trial” ” OR “clinical trial” OR “clinical research” OR “random” OR “randomly” OR “randomized” OR “control”). Different search strategies were applied for Chinese and foreign language databases. If necessary, we contacted the author of the article for additional data.
2.2. Study selection
The inclusion criteria were as follows:
-
(1)
The study included non-proliferative diabetic retinopathy (NPDR) patients who were clearly diagnosed by domestically and internationally recognized criteria;
-
(2)
The study included a randomized controlled trial (RCT);
-
(3)
We assessed use of compound Chinese medicine containing the therapeutic method of activating blood and removing stasis as the treatment group, without restriction for the control group, whether using conventional medicine (CM) (such as Calcium dobesilate, vitamins, etc), placebo, or blank. Basic treatment (glucose control, blood pressure control, and blood lipid regulation) accompanied with both of the groups;
-
(4)
We merely included trials whose treatment duration lasted for 12 weeks or more and whose sample size was more than 30 cases; and
-
(5)
The progression of retinopathy was considered the primary outcome.
The progression of retinopathy refers to the proportion of participants who showed improved progression, or it was not calculated.[7] The secondary outcomes included visual acuity, mean defect of visual field, micro-aneurysm, hemorrhage area, exudate, capillary nonperfusion area, hemorheological indicators (mainly plasma viscosity and high shear blood viscosity), oscillatory potentials (OPs), glycated hemoglobin (HbA1c), as well as adverse events.
The exclusion criteria included the following:
-
(1)
Studies describing interventions combined with other TCM therapies (compound Chinese medicine, traditional Chinese patent medicine, acupuncture or acupoint injection) were excluded;
-
(2)
Non-randomized trials were excluded;
-
(3)
Studies with a treatment duration of less than 12 weeks and/or a sample size of less than 30 cases were excluded.
2.3. Data extraction
The details of included trials were extracted independently by 2 authors (Ya-li Qin and Shuo Feng) using a standard data extraction form, which included the following items: general information (title, authors, year published); participant characteristics (sample size, age, gender, duration of DM, and diagnostic criteria); interventions (ingredients and dosage of herbal medicine, details of the control interventions, and duration of treatment); and outcome measures (primary outcome and secondary outcomes). Discrepancies were resolved by consensus or with the involvement of a third party (Qing Ni).
2.4. Quality assessment
Two authors (Guang-tong Dong and Jia Wang) assessed the risk of bias in the included studies according to the Cochrane Handbook for Systematic Reviews of Interventions,[18–19] based on 6 items: random sequence generation (selection bias); allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); and selective reporting (reporting bias) and other sources of bias. We judged each item from 3 levels: “high risk”, “low risk” and “unclear”, and then we assessed the trials as having a low risk of bias if all items were in the low risk of bias group; a high risk of bias if at least 1 item was in the high risk of bias group; unclear risk of bias if at least 1 item was in unclear. Discrepancies was resolved by consensus or with the involvement of a third party (Xiao-lin Tong).
2.5. Statistical analysis
Data regarding outcomes in the eligible trials were combined in the meta-analysis using the Rev Man 5.3 software (Cochrane Collaboration, Oxford). Dichotomous outcomes were indicated as risk ratios (RRs) using the method of Mantel-Haenszel, and continuous variables were indicated as mean differences (MDs) using the method of the inverse variance. All the estimates were calculated as having 95% confidence intervals(CIs). I-squared statistics (I2) were used to assess heterogeneity. A fixed-effect model was adopted if no significant heterogeneity existed (I2 < 50%); a random-effect model was adopted if significant heterogeneity existed. Publication bias was assessed through funnel plots. Subgroup analysis were performed if the primary outcome demonstrated statistically significant differences between the 2 groups.
3. Results
Our primary retrieval found 3269 references, and 1904 references were repeated and were excluded. After reading titles and abstracts, the other 656 references were excluded due to repeated literature, experimental studies, retrospective studies, reviews, case reports. This left 182 full texts to be reviewed, and 149 of them were excluded because: they were not RCTs (n = 52), had a short treatment duration or small sample size (n = 23), participants did not meet the inclusion criteria (n = 36), or the intervention included other TCM therapy (n = 38). Finally, 33 RCTs[20–52] were included (Fig. 1).
Figure 1.
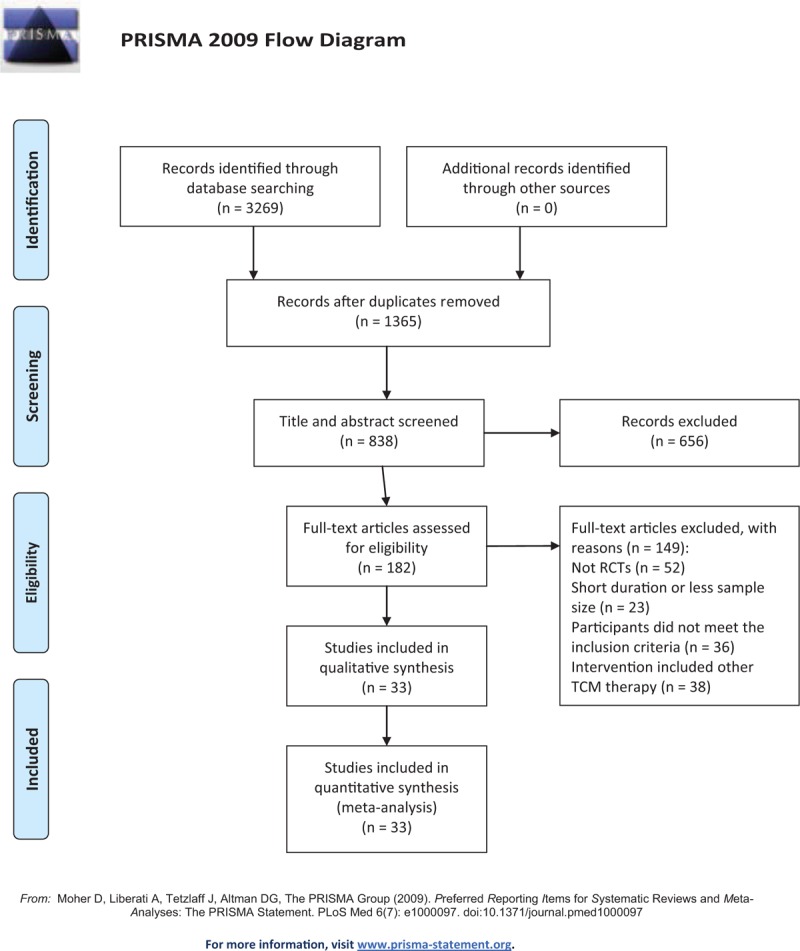
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of literature.
3.1. Description of the included trials
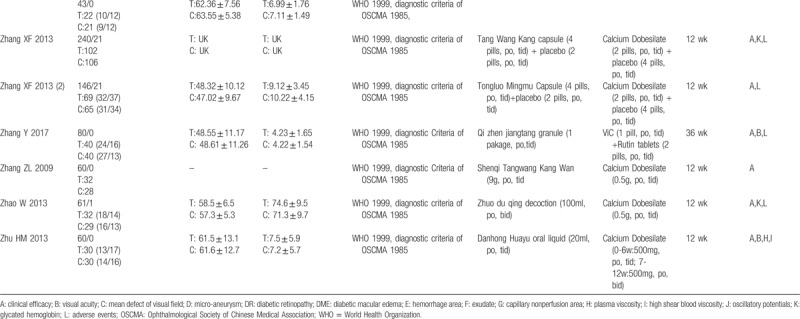
A total of 3430 participants were included (1846 of the intervention group and 1584 of the control group). The sample size ranged from 40 to 360 participants. All the enrolled participants suffered from DM, and most were diagnosed with DR according to diagnostic criteria established in 1985 by the Ophthalmological Society of Chinese Medical Association (OSCMA) or the International Disease Severity Scale for DR, proposed by the Global Diabetic Retinopathy Project Group in 2002. Of the trials, 19 mentioned the syndrome of DR patients according to traditional Chinese medical theory. All were RCTs with 2 parallel arms. In total, 26 trials compared the TCM formula with CM (mainly Calcium Dobesilate), 3 trials compared the TCM formula with a placebo treatment, and 4 trials compared the TCM formula with a blank treatment. Basic treatment (BT) was concomitantly given in both groups to control glycemia. treatment durations varied from 12 to 36 weeks (Table 1 ).
Table 1.
Characteristics of trials included in this review.
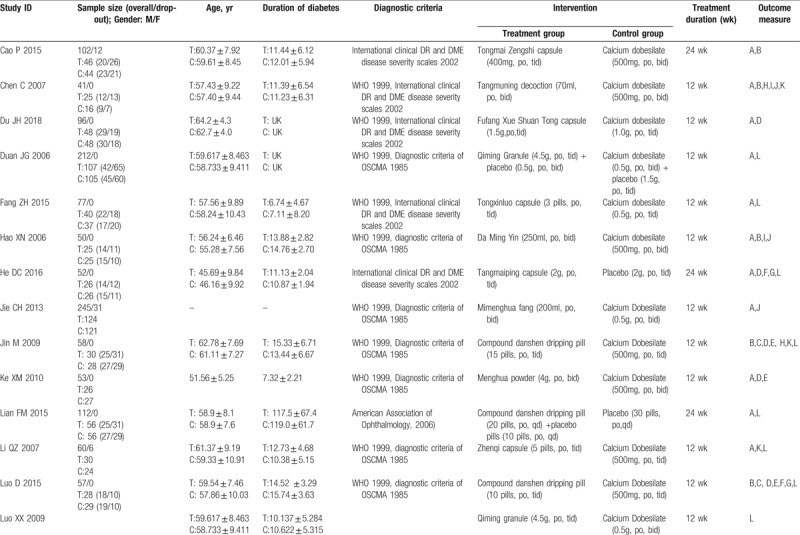
Table 1 (Continued).
Characteristics of trials included in this review.
3.2. Methodological quality
Fifteen trials described methods of randomization using a random number table or stratified blocked randomization. The remaining trials only indicated “randomly allocating,” with no specific methods of randomization were mentioned. Two trials[28,41] stated how allocation concealment was performed. Eight trials[20,26,29,38,41,43,47–48] used a placebo to conduct the blinding. All trials described the similarities between the intervention and control group. Nine trials[22,23,33,36,37,39,41,43,47] reported dropouts or withdrawals, 3 of whom[22,37,39] reported no drop-out or withdrawal. Selective reporting was difficult to assess, because trial protocols were unavailable (Fig. 2).
Figure 2.
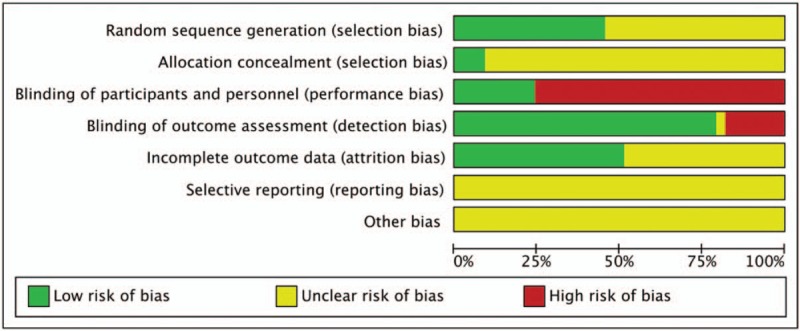
Risk of bias graph.
3.3. Progression of retinopathy
None of the 33 studies reported progression of retinopathy.
3.4. Visual acuity
Thirteen trials reported visual acuity data. A pooled analysis of 11 trials showed a statistically significant increase in visual acuity with TCM, compared to the CM group (n = 899, MD −0.10, 95% CI −0.16 to −0.05, P = .0001; I2 = 72%), while visual acuity differed insignificantly between the TCM and blank groups (n = 164, MD 0.03, 95% CI −0.42 to 0.48, P = .90; I2 = 98%) (Fig. 3).
Figure 3.
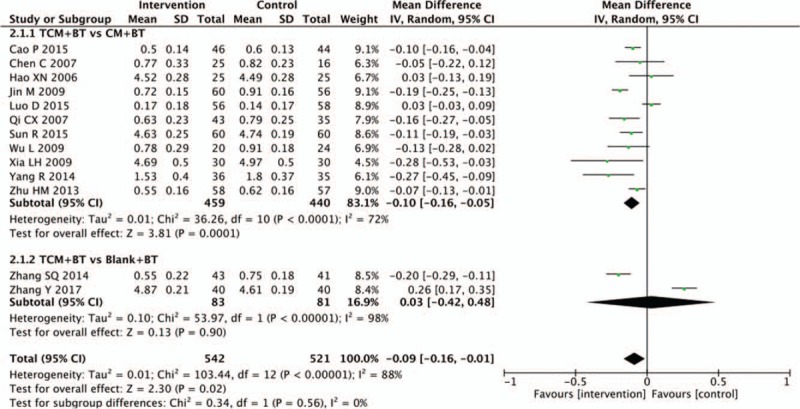
Effects of TCM versus controls on visual acuity. TCM = traditional Chinese medicine.
3.5. Mean defect of visual field
Four trials provided the improvement of the mean defect of the visual field. Three trials between the TCM and CM groups showed significant differences (n = 308, MD −1.43, 95%CI −2.17 to −0.68, P = .0002; I2 = 94%). In the TCM versus the blank subgroup, only 1 trial reported the mean defect of visual field. There was a significant difference between the 2 groups (n = 68, MD −0.87, 95% CI −0.95 to −0.79, P < .00001) (Fig. 4).
Figure 4.
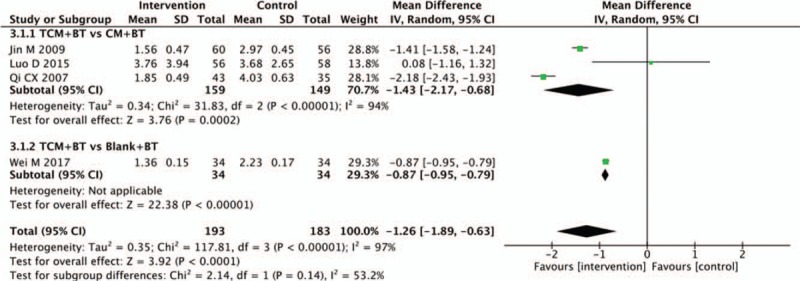
Effects of TCM versus controls on mean defect of visual field. TCM = traditional Chinese medicine.
3.6. Micro-aneurysm
Eight trials provided the data concerning the number of micro-aneurysms. The number of micro-aneurysms significantly decreased in the TCM group, compared with those in the CM group (n = 470, MD −4.51, 95% CI −6.23 to −2.79, P < .00001; I2 = 81%). There was also a significant difference between the subgroups of the TCM and placebo groups (n = 52, MD −4.35, 95% CI −6.25 to −2.45, P <−.00001) and the TCM and blank groups (n = 152, MD −3.35, 95% CI −4.73 to −1.97, P <−.00001; I2 = 0%) (Fig. 5).
Figure 5.
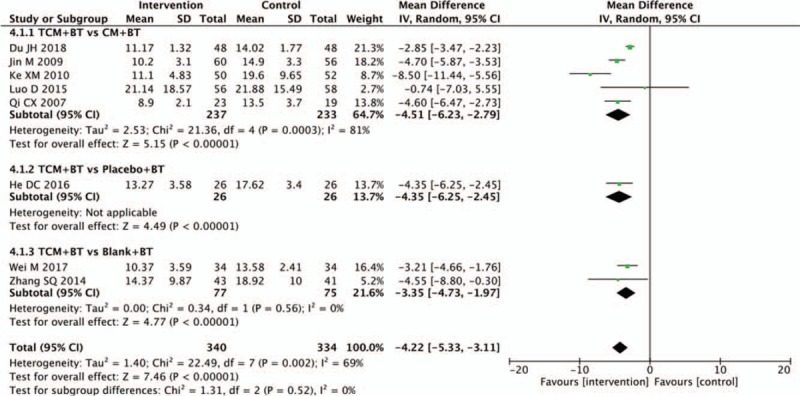
Effects of TCM versus controls on micro-aneurysm. TCM = traditional Chinese medicine.
3.7. Hemorrhage area
Six trials found the hemorrhage area to be the outcome. The pooled analysis of 4 trials in the subgroup of the TCM versus the CM groups showed a statistically significant reduction in the hemorrhage area (n = 316, MD −0.62, 95% CI −1.06 to −0.19, P = .005; I2 =94%). In 2 trials of TCM, in comparison with blank treatment, results indicated that there was no statistical difference (n = 111, MD −0.49, 95% CI −1.09 to 0.11, P = .11; I2 = 93%) (Fig. 6).
Figure 6.
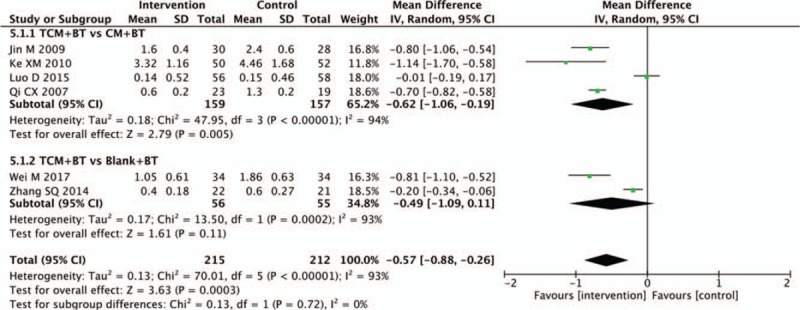
Effects of TCM versus controls on hemorrhage area. TCM = traditional Chinese medicine.
3.8. Exudates
In 5 trials, data for the area of retinal exudation were provided. The TCM group was not statistically different than the CM group in decreasing the retinal exudation (n = 174, MD −0.03, 95% CI −0.13 to 0.06, P = .49; I2 = 61%), and no significant difference existed between the TCM and blank groups (1 trial; n = 43, MD −0.09, 95% CI −0.20 to 0.02, P = .11). Results showed that there was statistical difference between the TCM and placebo groups in decreasing the exudation area (n = 98, MD −0.17, 95% CI −0.31 to −0.03, P = .02; I2 = 51%) (Fig. 7).
Figure 7.
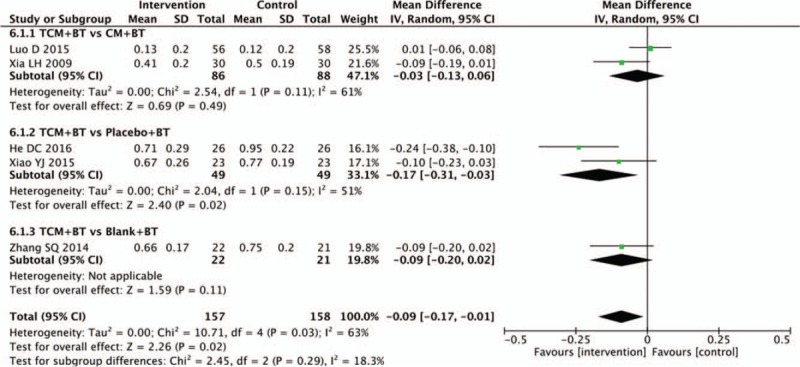
Effects of TCM versus controls on exudates. TCM = traditional Chinese medicine.
3.9. Capillary nonperfusion area
In 4 trials, data for the capillary nonperfusion area were measured. Pooled analysis of 2 trials showed that the TCM group was not statistically different than the control groups in decreasing the capillary nonperfusion area (n = 174, MD −0.03, 95% CI −0.13 to 0.07, P = 0.59; I2 = 55%). However, a significant difference was found between the TCM and placebo groups (n = 98, MD −0.18, 95% CI −0.31 to −0.04, P = .010; I2 = 59%) (Fig. 8).
Figure 8.
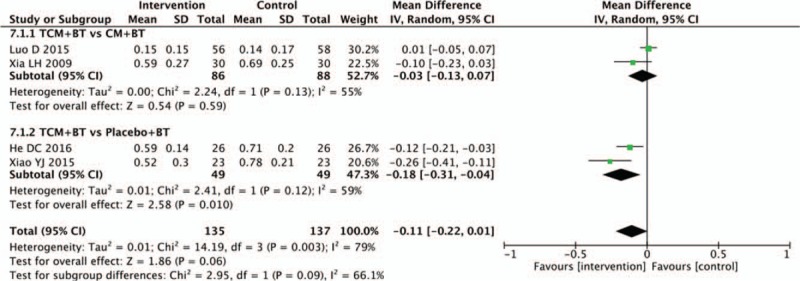
Effects of TCM versus controls on capillary nonperfusion area. TCM = traditional Chinese medicine.
3.10. Hemorheological indicators
Nine trials recorded changes in hemorheology indicators. In this review, we mainly assessed plasma viscosity and high shear blood viscosity. There was no statistical difference between the TCM and CM groups in decreasing plasma viscosity (n = 467, MD −0.10, 95% CI −0.20 to 0.00, P = .05; I2 = 90%). Pooled analysis showed that the TCM group was not statistically different than the CM group in decreasing the high shear blood viscosity (n = 522, MD −0.08, 95% CI −0.41 to 0.24, P = .62; I2 = 91%) (Figs. 9 and 10).
Figure 9.
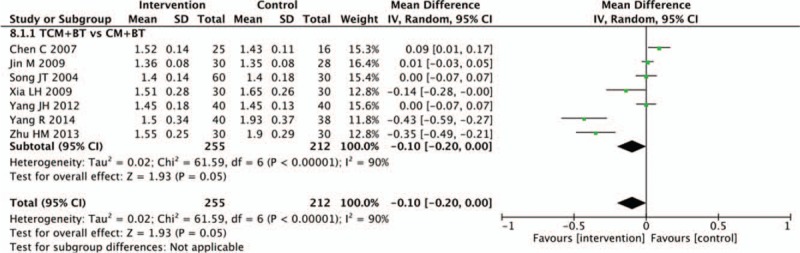
Effects of TCM versus controls on plasma viscosity. TCM = traditional Chinese medicine.
Figure 10.
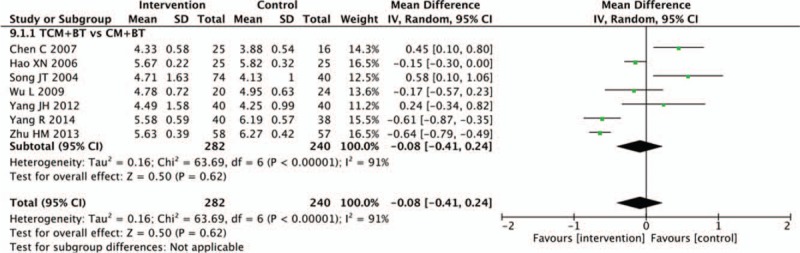
Effects of TCM versus controls on high shear blood viscosity. TCM = traditional Chinese medicine.
3.11. Oscillatory potentials
Five trials compared the effects on oscillatory potentials. Pooled analysis indicated that oscillatory potentials in the TCM group had improved more significantly than in the CM group (n = 539, MD −4.68, 95% CI −8.51 to −0.85, P = .02; I2 = 0%) (Fig. 11).
Figure 11.
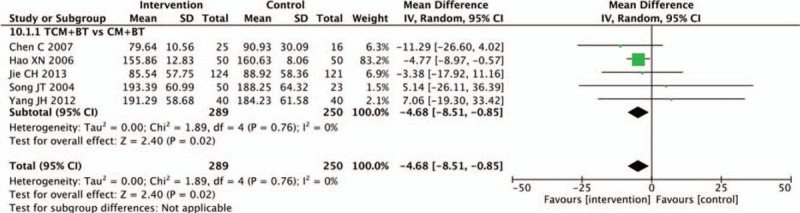
Effects of TCM versus controls on oscillatory potentials. TCM = traditional Chinese medicine.
3.12. HbA1C
Nine trials recorded HbA1c data. Seven trials compared the HbA1c of the TCM to the CM groups, and a meta-analysis demonstrated that participants treated with TCM decreased more significantly than participants receiving CM (n = 519, MD −0.22, 95% CI −0.42 to −0.03, P = .02; I2 = 0%). Results of 1 trial showed that there was a statistical difference between the TCM and placebo groups in decreasing the HbA1c level (n = 47, MD −0.88, 95% CI −1.44 to −0.32, P = .002). Results of another trial indicated that there was a statistical difference between the TCM and blank groups in decreasing the HbA1c level (n = 43, MD −0.46, 95% CI −0.80 to −0.12, P = .009) (Fig. 12).
Figure 12.
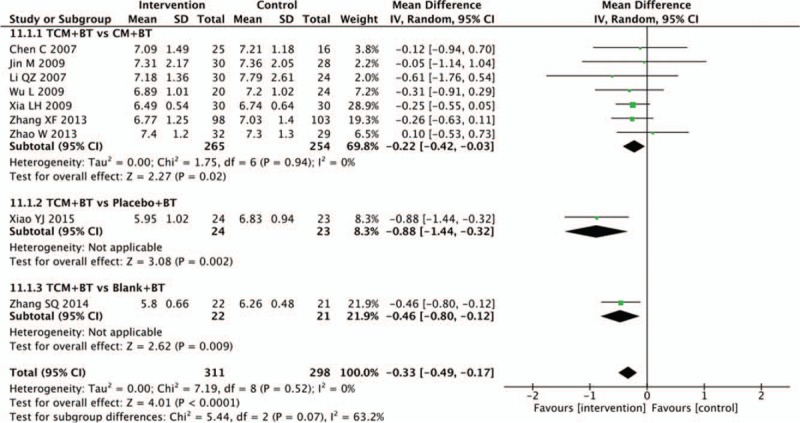
Effects of TCM versus controls on glycated hemoglobin. TCM = traditional Chinese medicine.
3.13. Adverse events
Adverse events (AEs) were reported in 20 trials. Eleven trials[21,27,31,33,37,39,40,41,42,46,51] reported that the TCM groups experienced no AEs, while nine trials recorded the condition of AEs, which were shown in (Fig. 13). Pooled analysis of 5 trials showed that there was a significant difference in the frequency of AEs comparing the TCM with CM group (n = 1228, RR 0.15, 95% CI 0.06 to 0.38, P <.0001; I2 = 0%). Three trials indicated that there was a significant difference in the frequency of AEs (n = 210, RR 3.67, 95% CI 1.05 to 12.86, P = 0.04; I2 = 46%) between the TCM and placebo groups. One trial indicated that there was no significant difference in AEs between the TCM and blank group (n = 80, RR 0.67, 95% CI 0.26 to 1.70, P = .40).
Figure 13.
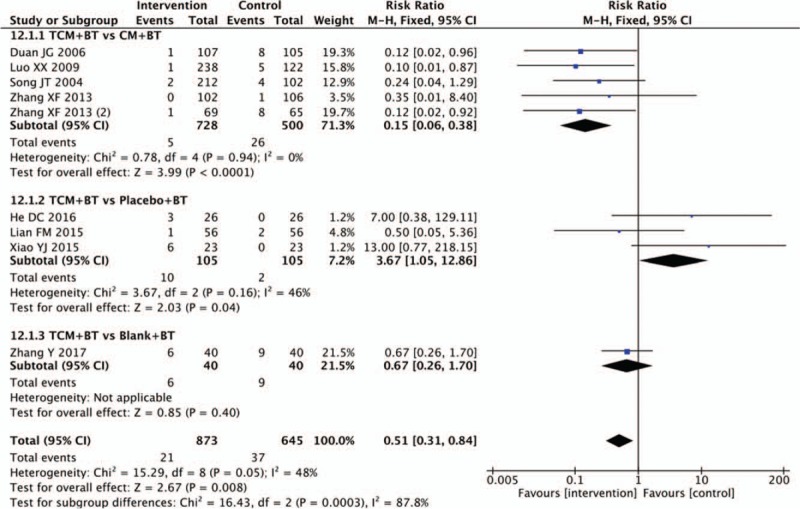
Effects of TCM versus controls on adverse events. TCM = traditional Chinese medicine.
Regarding individual AEs, 14 types of AEs were reported in 5 trials that compared TCM with CM. Nausea and vomiting, gastrointestinal fullness, and appetite loss were the 3 most frequently AEs in patients receiving CM. In the subgroup of TCM versus placebo, mild diarrhea was a more frequent AEs in patients receiving TCM. Special information on AEs in Lian FM[20] was unknown. In the subgroup of TCM versus blank, 6 types of AEs were reported, and there was no significant difference between the groups (n = 40, RR 0.73, 95% CI 0.30 to 1.79, P = .49) (Table 2).
Table 1 (Continued).
Characteristics of trials included in this review.
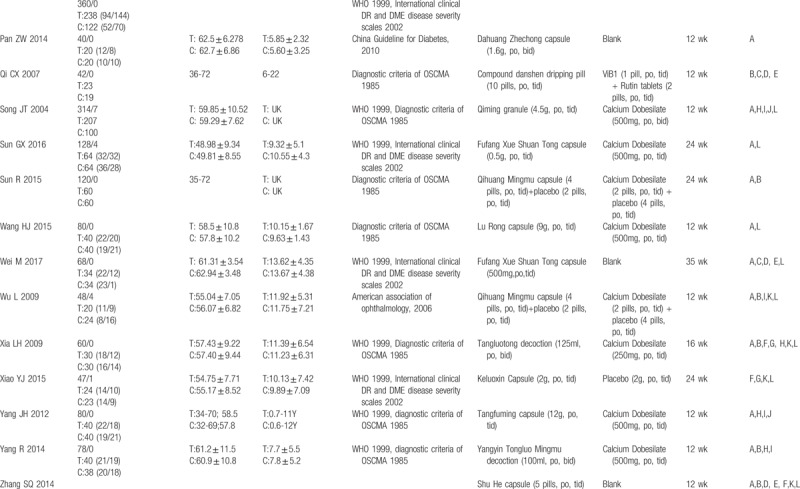
Table 2.
Incidence of adverse events.
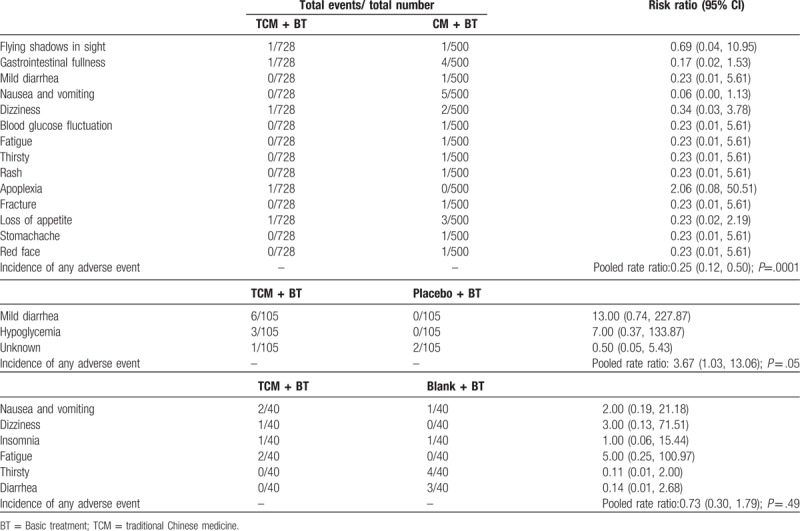
3.14. Publication bias
We performed a funnel plot for clinical efficacy. The funnel shape of the plot was not completely symmetrical, indicating a potential publication bias (Fig. 14).
Figure 14.
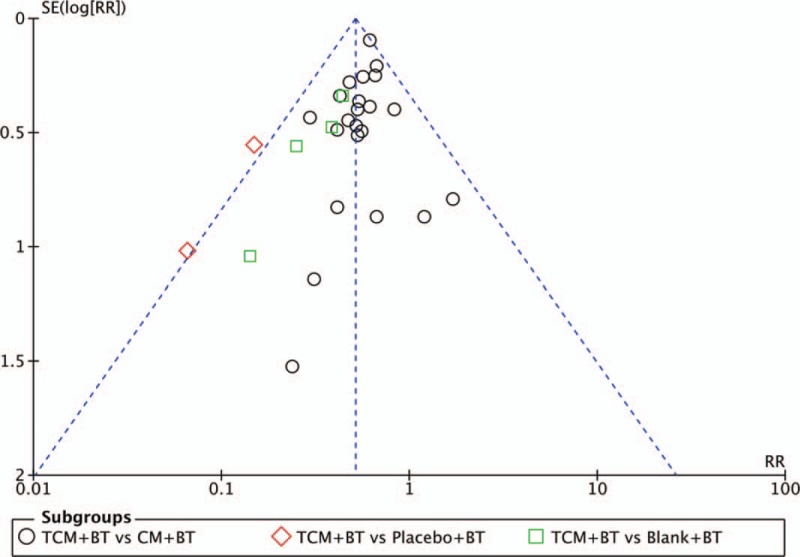
Funnel plot for assessing publication bias.
4. Discussion
4.1. Summary of evidence
This systematic review enrolled 33 trials involving 3373 participants. The main findings were that, no study reported any data on the progression of DR. Compared with CM, TCM was significantly better at improving visual acuity and oscillatory potentials (OPs), and at reducing the mean defect of visual field, micro-aneurysms, hemorrhage area, plasma viscosity, and HbA1c. Compared with the placebo groups, the interventions of TCM were also associated with a decline in the numbers of micro-aneurysms, exudates, capillary nonperfusion area, and HbA1c. Compared with the blank groups, TCM was superior at decreasing the mean defect of visual field and the numbers of micro-aneurysms. The incidence of AEs in the CM group was higher than that in the TCM group, and nausea and vomiting, gastrointestinal fullness and appetite loss were the 3 most common AEs. Compared with the placebo group, there was a significant difference in the frequency of AEs in the TCM group, and mild diarrhea was the most frequently reported AEs in patients of the TCM group. No statistical significance existed between the TCM and blank groups.
The results suggested participants who took TCM were associated with the increased likelihood of improving visual acuity compared with conventional medication, which was similar to a previous finding.[7] TCM may have been more likely to decrease the number of micro-aneurysms compared to participants who did not take these herbs but used conventional intervention, placebo, or non-treatment. The mechanism may be related to the fact that activating blood herbal medicine possessed the effects of improvement of microcirculation, production of the retinal vascular endothelium, anti-inflammation, anti-oxidation, and so on. In addition, Chinese herbal medicine possessed definite antihyperglycemic effects in decreasing the blood glucose level,[8,53–54] so the meta-analysis results of the HbA1c indicated that TCM was superior to conventional medication, placebo, and non-treatment. From the results of the article, conventional medication had more gastrointestinal side effects, which maybe 1 of the reasons why Chinese patients preferred to choose TCM.
All the included participants were clearly diagnosed with NPDR, but the diagnostic criteria differed, such as the diagnostic criteria of OSCMA established in 1985 and the International Disease Severity Scale for DR proposed by the Global Diabetic Retinopathy Project Group in 2002, and so on. Additionally, 25 types of compound Chinese herbal medicine were included. Although they varied in their herbal components, the formulated prescriptions were based on the principle of “activating the blood circulation and removing stasis”, and formed part of a “group” of herbal medicines with effects of antihyperglycemia, improvement of microcirculation, antiinflammation, and antioxidation designed to decrease blood glucose levels, improve blood rheology, and protect retinal vascular endothelial function.[8–10] The formulations of included TCM contained capsules (16 trials), decoction (6 trials), granules (4 trials), pills (5 trials), powder (1 trials) and oral liquid (1 trial), which were various and highly heterogenous. Regarding the primary outcome, there was a lack of reports on the progression of DR and blinding events. In future research, the primary outcomes should include the occurrence of endpoint events and the progression of DR. The endpoint event was considered as a blinding event. The progression of DR strictly refers to the current international or domestic criteria for the classification or staging of DR, judgement of the progress of DR grading or staging by fundus examination results, and detailed reports on changes in DR grading or staging after treatment. Secondary outcomes mainly focused on laboratory examination and AEs, but the results on assessment of quality of life and disease expenses were rare. The asymmetrical funnel plot demonstrates the potential publication bias. Funnel plots are a visual aid to identify publication bias or systematic heterogeneity. All of the 33 trials were included in these funnel plots, recognizing the heterogeneity of the treatment, trial size, and design. None of the trials found a negative effect, indicating publication bias. Although we undertook extensive searches for unpublished literature, we found no negative trials. However, trials with large positive results are often much easier to publish than trials with negative results. Therefore, it is likely that publication bias is present, affecting the reliability of the meta-analysis.
All the RCTs included in this review were of low quality in terms of design, reporting, and methodology. This provided limited descriptions of study design, randomization and allocation concealment, although all trials stated the randomization procedure they used, only 15 trials provided sufficient information to judge whether randomization was conducted properly and 3 trials stated how allocation was concealed. 8 trials conducted the blinding of participants and personnel, although most of the included trials conducted the blinding of outcome assessment. Half of included trials reported withdrawals or dropouts, and none of the trials mentioned intention-to-treat analysis or had a pre-trial estimation of sample size. Based on the above reasons, the evidences must be interpreted with caution.
4.2. Limitation
Several limitations are noteworthy. First, some heterogeneity was found. Although we only included NPDR participants, there was also some heterogeneity in different ingredients, formulations, and dosages of compound Chinese herbal medicine, or different treatment durations across studies, making fully reliable comparisons difficult. Second, regarding the choice of outcomes, the standard of objective assessment is necessary and lacking. Blinding events, the progression of DR, the assessment of quality of life, and disease expenses should be focused on more. Third, the long-term efficacy and safety of TCM on DR are not known. Hence, the pooled results should be treated with caution.
5. Conclusion
Preliminary evidence indicated that activating blood compound Chinese herbal medicine may improve the clinical efficacy and may also be associated with the increased likelihood of improving visual acuity and visual function (OPs and mean defect of visual field), compared with conventional medication, and decreased the numbers of micro-aneurysms and HbA1c. However, the methodological quality of trials included in this review were of poor quality. Despite the apparently positive findings, it is premature to conclude the effectiveness of activating blood compound Chinese herbal medicine for the treatment of DR due to the heterogeneity of the included trials and the generally low methodological quality of the included trials. Multi-center, double-blinded, and placebo-controlled RCTs are required to provide stronger evidence.
Author contributions
Conceptualization: Bing Pang, Xiao-lin Tong, Qing Ni.
Data curation: Ya-li Qin, Shuo Feng.
Formal analysis: Guang-tong Dong, Jia Wang.
Methodology: Qing-wei Li.
Resources: Xiao-lin Tong, Qing Ni.
Software: Shuo Feng, Jia Wang.
Writing – original draft: Bing Pang.
Writing – review and editing: Bing Pang, Qing-wei Li, Qing Ni.
Footnotes
Abbreviations: CI = confidence interval, CM = conventional medicine, DM = diabetes mellitus, DR = diabetic retinopathy, HbA1c = glycated hemoglobin, MD = mean difference, NPDR = non- proliferative diabetic retinopathy, Ops = oscillatory potentials, OSCMA = ophthalmological society of Chinese medical association, RCT = randomized controlled trial, RRs = risk ratios, TCM = traditional Chinese medicine.
How to cite this article: Pang B, Li QW, Qin YL, Dong GT, Feng S, Wang J, Tong XL, Ni Q. Traditional chinese medicine for diabetic retinopathy: A systematic review and meta-analysis. Medicine. 2020;99:7(e19102).
SYSTEMATIC REVIEW REGISTRATION: PROSPERO CRD42016039367.
BP and QL contributed equally to this study as first authors.
This is a systematic review, the original data of clinical trials was not included, so ethics approval is not applicable.
The results will be disseminated through peer-reviewed journal articles and presented abstracts and posters at scientific conferences in the field of diabetes and traditional Chinese medicine, as well as the general public through internet and newspaper.
All data included in this study are available upon request by contact with the corresponding author.
This study was supported by grants from the National Natural Science Foundation of China (81774296); from Special program for excellent scientific personnel training of Chinese Academy of traditional Chinese Medicine (ZZ13-YQ-032); from Institutional Research Foundation of Guang’ anmen Hospital, China Academy of Chinese Medical Science (59957).
The authors have no conflicts of interest to disclose.
References
- [1].Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet 2010;376:124–36. [DOI] [PubMed] [Google Scholar]
- [2].Wang FH, Liang YB, Zhang F, et al. Prevalence of diabetic retinopathy in rural China: the Handan Eye Study. Ophthalmology 2009;116:461–7. [DOI] [PubMed] [Google Scholar]
- [3].Cui J, Ren JP, Chen DN, et al. Prevalence and associated factors of diabetic retinopathy in Beijing, China: a cross-sectional study. BMJ Open 2017;7:e015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sun L, Wang GX. Interpretation of cardiovascular events in the latest Sino-American diabetes diagnosis and treatment guidelines. Zhong Guo Yi Xue Qian Yan Za Zhi 2015;7:10–5. [Google Scholar]
- [5].Valencia WM, Florez H. How to prevent the microvascular complications of type 2 diabetes beyond glucose control. BMJ 2017;356:i6505. [DOI] [PubMed] [Google Scholar]
- [6].Xiao E, Luo L. Alternative therapies for diabetes: a comparison of western and traditional Chinese medicine (TCM) approaches. Curr Diabetes Rev 2018;14:487–96. [DOI] [PubMed] [Google Scholar]
- [7].Zhang HW, Zhang H, Grant SJ, et al. Single herbal medicine for diabetic retinopathy. Cochrane Database Syst Rev 2018;12:CD007939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pang B, Zhou Q, Zhao TY, et al. Innovative thoughts on treating diabetes from the perspective of traditional Chinese medicine. Evid Based Complement Med 2015;2015:905432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Behl T, Kotwani A. Chinese herbal drugs for the treatment of diabetic retinopathy. J Pharm Pharmacol 2017;69:223–35. [DOI] [PubMed] [Google Scholar]
- [10].He L, Wang H, Gu G, et al. Administration of traditional Chinese blood circulation activating drugs for microvascular complications in patients with type 2 diabetes mellitus. J Diabetes Res 2016;2016:1081657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dong W, Xie RP, Liu Q. Research progress on traditional Chinese medicine treatment of diabetic retinopathy. Zhongguo Zhongyi Yanke Za Zhi 2017;27:131–3. [Google Scholar]
- [12].Zhao YQ, Li QS, Xiang MH, et al. Distribution of traditional Chinese medicine syndromes of diabetic retinopathy and correlation between symptoms. Zhongguo Zhongyao Za Zhi 2017;42:2796–801. [DOI] [PubMed] [Google Scholar]
- [13]. Zhuang Feng. Clinical research on pathogenesis identification and related factors of blood stasis in type 2 diabetic retinopathy. Nanjing University of Traditional Chinese Medicine, 2016:12–18. (Dissertation). [Google Scholar]
- [14].Ling YJ, Qin YH, Sun ZQ. Study on the mechanism of blood stasis in diabetic retinopathy. Zhongguo Zhongyi Jichu Yixue Za Zhi 2001;7:50–1. [Google Scholar]
- [15].Wang F, Song HL, Ma XY, et al. Systematic review and meta-analysis on effect of compound traditional Chinese medicine on non-proliferative diabetic retinopathy. Zhongguo Shi Yan Fang Ji Xue Za Zhi 2017;23:203–10. [Google Scholar]
- [16].Yan XY, Jia LY, Zhao NQ, et al. Systematic review of safety on randomized controlled trials of Chinese medicine for diabetic retinopathy. Yao Wu Liu Xing Bing Xue Za Zhi 2017;26:153–60. [Google Scholar]
- [17].Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated March 2013). The Cochrane Collaboration, 2013. Available from handbook.cochrane.org. [Google Scholar]
- [19].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lian FM, Wu L, Tian JX, et al. The effectiveness and safety of a danshen-containing Chinese herbal medicine for diabetic retinopathy: a randomized, double-blind, placebo-controlled multicenter clinical trial. J Ethnopharmacol 2015;164:71–7. [DOI] [PubMed] [Google Scholar]
- [21].Luo D, Qin Y, Yuan W, et al. Compound danshen dripping pill for treating early diabetic retinopathy: a randomized, double-dummy, double-blind study. Evid Based Complement Med 2015;2015:539185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Luo XX, Duan JG, Liao PZ, et al. Effect of Qiming granule on retinal blood circulation of diabetic retinopathy: a multicenter clinical trial. Chin J Integr Med 2009;15:384–8. [DOI] [PubMed] [Google Scholar]
- [23].Cao P, Tong JA, Wang XM, et al. Clinical observation of Tongmai Zengshi Capsule in the treatment of non-proliferative diabetic retinopathy. Zhong Cheng Yao 2015;37:1143–5. [Google Scholar]
- [24].Eye Hospital China Academy of Chinese Medical Sciences, Chen C. Clinical and experimental study of Tangmuning in the treatment of early diabetic retinopathy. 2007. [Google Scholar]
- [25].Du JH, Zhang Z, Li JQ, et al. Effect of compound Xueshuantong on blood lipids and fundus micro-aneurysm in patients with diabetic retinopathy. Xian Dai Zhong Xi Yi Jie He Za Zhi 2018;27:663–5. [Google Scholar]
- [26].Duan JG, Liao PZ, Wu L, et al. A double-blind, double-dummy, randomized controlled multi-center clinical study of Qiming Granule in the treatment of diabetic retinopathy. Cheng Du Zhong Yi Yao Da Xue Xue Bao 2006;29:1–5. [Google Scholar]
- [27].Fang ZH, Zhao JD, Ni YQ, et al. Clinical efficacy and safety of Tongxinluo capsule in treatment of non-proliferative diabetic retinopathy with deficiency of Qi and Yin and blood stasis syndrome. The conference paper collection of the 11th International Conference on Collateral Diseases 2015;05:230–3. [Google Scholar]
- [28].Hei Long Jiang University of Traditional Chinese Medicine, Hao XN. Clinical study on the treatment of Da Ming Ying to diabetic retinopathy of non-proliferative phase. 2006. [Google Scholar]
- [29].He DC, Zhang Y, Xiao JJ. Clinical observation of Tangmaiping on fundus lesions in diabetic patients. Hua Nan Guo Fang Yi Xue Za Zhi 2016;30:677–8. [Google Scholar]
- [30].Jie CH, Gao JS, Yan J, et al. Effect of Mimenghua prescription on visual function in non-proliferative diabetic retinopathy. Zhong Guo Zhong Yi Yan Ke Za Zhi 2013;23:157–60. [Google Scholar]
- [31].Jin M, Deng H, Yuan W, et al. Clinical observation of compound Danshen dropping pills in the treatment of early diabetic retinopathy. Zhong Guo She Qu Yi Shi 2009;25:32–3. [Google Scholar]
- [32].Ke XM, Zhang YT, Wang SZ, et al. Therapeutic effect of Menghuasan on I and II phase of diabetic retinopathy. Shan Dong Zhong Yi Yao Da Xue Xue Bao 2010;34:144–5. [Google Scholar]
- [33].Li QZ, Li R, Li KL, et al. Clinical study of Zhenqi Capsules on non-proliferative diabetic retinopathy of syndrome with deficiency of both qi and yin. Zhong Guo Zhong Yi Yan Ke Za Zhi 2017;17:319–22. [Google Scholar]
- [34].Pan ZW, Zhao SF, Li CY. Therapeutic effect of Dahuang Zhechong capsule on non-proliferative diabetic retinopathy. Bei Fang Yao Xue 2014;11:40–1. [Google Scholar]
- [35].Qi CX, Tan XH, Li QG, et al. Clinical study of diabetic retinopathy treated by compound Danshen dripping pills. Zhong Yao Cai 2007;30:375–7. [PubMed] [Google Scholar]
- [36].Chengdu University of Traditional Chinese Medicine, Song JT. A randomized, positive drug-controlled, multicenter clinical study of Qiming granule in the treatment of diabetic retinopathy. 2004. [Google Scholar]
- [37]. Sun GX. Clinical observation of compound Xueshuantong capsule in the treatment of non-proliferative diabetic retinopathy with qi-yin dual deficiency and stasis syndrome. Zhejiang University of Traditional Chinese Medicine, 2016:9–10. (Dissertation). [Google Scholar]
- [38].Sun R, Hui SY. Effect of Qihuang Mingmu capsule on visual evoked potential P100 in patients with early diabetic retinopathy. Shi Jie Zhong Yi Yao 2015;10:1704–7. [Google Scholar]
- [39].Wang HJ, Xu HE, Zhang J. Randomized parallel controlled study of Lurong recipe in the treatment of non-proliferative diabetic retinopathy. Shi Yong Zhong Yi Nei Ke Za Zhi 2015;29:30–2. [Google Scholar]
- [40].Wei M, Chen CZ, Liao TT, et al. Clinical effect of compound Xueshuantong on early diabetic retinopathy and serum levels of VEGF, IGF-1 and NSE. Xian Dai Sheng Wu Yi Xue Jin Zhan 2017;17:3901–4. [Google Scholar]
- [41].Wu L, Yan F, Su H, et al. Clinical study on Qihuang Mingmu granule for the treatment of diabetic retinopathy (DR). Zhong Guo Zhong Yi Yan Ke Za Zhi 2009;19:74–8. [Google Scholar]
- [42]. Xia LH. Clinical study of Tangluotong decoction in the treatment of nonproliferative diabetic retinopathy. Shandong University of Traditional Chinese Medicine, 2004:9–14. (Dissertation). [Google Scholar]
- [43]. Xiao YJ. Clinical research of Keluoxin on non-proliferative diabetic retinopathy. Hubei University of Traditional Chinese Medicine, 2015:17–26. (Dissertation). [Google Scholar]
- [44]. Zhang SQ. Clinical research on the treatment of Shu He capsule on patients with non-proliferative diabetic retinopathy. Guangzhou University of Traditional Chinese Medicine, 2014:13–19. (Dissertation). [Google Scholar]
- [45].Yang R, Wei ZQ, Liu YX, et al. Clinical observation on forty cases of non-proliferative diabetic retinopathy treated by Yangyin Tongluo Mingmu prescription. Xin Zhong Yi 2014;46:123–5. [Google Scholar]
- [46].Guangzhou University of Traditional Chinese Medicine, Zhang SQ. Clinical research on the treatment of Shu He capsule on patients with non-proliferative diabetic retinopathy. 2014;13–19. (Dissertation). [Google Scholar]
- [47].Zhang XF, Zhao BL, Yang LB, et al. A phase II clinical trial of TangWangKang capsules for the treatment of diabetic retinopathy. Shi Yong Yao Wu Yu Lin Chuang 2013;16:818–20. [Google Scholar]
- [48].Zhang XF, Zhao BL, Yang LB, et al. A randomized, double-blind and placebo-controlled study of Tong Luo Ming Mu capsule in treating diabetic retinopathy. Xi Bu Zhong Yi Yao 2013;26:88–90. [Google Scholar]
- [49].Zhang Y. Clinical study on Qi Zhen Jiangtang granule in treating non-proliferative liver and kidney yin deficiency syndrome of diabetic retinopathy. Zhong Yi Xue Bao 2017;32:2354–8. [Google Scholar]
- [50].Zhang ZL, Meng LC. Clinical observation of Shenqi Tangwankang pill in the treatment of diabetic retinopathy. Shan Dong Zhong Yi Za Zhi 2009;28:15–6. [Google Scholar]
- [51].Zhao W, Li SL, Tang AH, et al. Clinical study of Zhuoduqing in the treatment of non-proliferative diabetic retinopathy. Shizhen Guoyi Guoyao 2013;24:2691–2. [Google Scholar]
- [52].Zhu HM, Jiang Y, Li L, et al. Efficacy of Danhong Huayu in the treatment of diabetic retinopathy. Zhong Guo Shi Yan Fang Ji Xue Za Zhi 2013;19:320–3. [Google Scholar]
- [53].Pang B, Guo J, Zhao LH, et al. Retrospective study of traditional Chinese medicine treatment on diabetes mellitus. J Tradit Chin Med 2016;36:307–13. [DOI] [PubMed] [Google Scholar]
- [54].Li WL, Zheng HC, Bukuru J, et al. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol 2004;92:1–21. [DOI] [PubMed] [Google Scholar]


