Supplemental Digital Content is available in the text
Keywords: adjuvant, influenza vaccine, meta-analysis, MF59
Abstract
Background:
Influenza is a severe disease burden among all age groups. This study aimed to review the efficacy of inactivated influenza vaccines with MF59 adjuvant and non-adjuvanted inactivated influenza vaccines among all age groups against specific influenza vaccine strains.
Methods:
Literature search of PubMed, Embase, Medline, OVID, and Cochrane Library Trials (CENTRAL) was implemented up to March 1, 2019. Homogeneity qualified studies were included for
-
(1)
inoculation of licensed MF59 influenza vaccines,
-
(2)
subject age over 6 months old, and
-
(3)
assessment of immunogenicity outcome for at least 1 type or subtype.
Data were extracted such as study country location, demographic characteristics, and measure outcomes, and were analyzed by a random effect model and sensitivity analyses to identify heterogeneity. Risk of bias was evaluated using the Cochrane Risk of Bias Tool.
Results:
We retrieved 1,021 publications and selected 31 studies for full review, including 17 trials for meta-analysis and 6 trials for qualitative synthesis. MF59-adjuvanted influenza vaccines demonstrated better immunogenicity against specific vaccine virus strains compared to non-adjuvanted influenza vaccine both in healthy adult group (RR = 2.10; 95% CI: 1.28–3.44) and the healthy aged (RR = 1.26; 95% CI: 1.10–1.44).
Conclusion:
The quality of evidence is moderate to high for seroconversion and seroprotection rates of influenza vaccine. MF59-adjuvanted influenza vaccines are superior to non-adjuvanted influenza vaccines to enhance immune responses of vaccination in healthy adults and older adults, and could be considered for routine use especially the monovalent prepandemic influenza vaccines.
1. Introduction
Influenza vaccines were first developed to prevent the attack of influenza virus in 1938, and then recommended to be inoculated annually based on the results from randomized clinical trials (RCTs) and laboratory detection showing effectiveness and safety.[1] A previous meta-analysis of RCTs showed no evidence for decreasing protection with annually repeated influenza vaccination.[2] Inoculation of influenza vaccine should result in effective and safe protection. Present efforts mainly focus on the prevention of seasonal and pandemic influenza. However, it is urgent to enhance the efficacy of influenza vaccines to decrease flu-related morbidity and mortality in all age groups.[3] The antibody titer levels would decrease to levels without protection no more than a year after the inoculation. Moreover, age-associated weakening of innate and adaptive immune responses would lead to impairment of influenza vaccine responses.[4–7] Lower T-cell responses are companied with a decrease in the antibody titers postvaccination with aging, and the level of cytolytic effector CD8+ T-cells is a vital factor for the clinical protection against influenza.[8] The degradation of immunity related with aging limits vaccine effects (VE) in healthy older people. These obstacles emphasize the importance to develop adjuvants to distinctly improve the efficacy of conventional influenza vaccines.
Addition of the adjuvants is a powerful approach to enhance the immunogenicity of influenza vaccines and decrease the content of hemagglutinin protein. In addition, the adjuvants would balance humoral immunity and cellular immunity, which may trigger the quicker and durable immune response.[9] Microfluidized emulsion 59 (MF59) is a squalene-based adjuvant, which is an oil-in-water emulsion. MF59 recruits the antigen present cells (APCs) at the injected site, subsequently transmits immune activation signals to lymphocyte T cells and B cells. Ansaldi et al concluded that seasonal trivalent influenza vaccines adjuvanted with MF59 provided a greater advantage compared to nonadjuvanted vaccines in protecting against a broader range of virus strains during the influenza season.[10]
The effectiveness and safety of influenza vaccines adjuvanted with MF59 had been evaluated in several systematic analysis.[11–14] One study showed that influenza vaccines adjuvanted with MF59 were no better than conventional vaccines in some people.[15] In addition, RCTs comparing the effectiveness of MF59-adjuvanted influenza vaccines with conventional non-adjuvanted vaccines reported inconsistent consequences.[16] Therefore, the priority of MF59 adjuvant did not reach a consensus for extensive application.
Up to now, no meta-analysis and systematic review has systematically inspected the relation between the addition of MF59 adjuvant and immunogenicity outcomes in all age groups inoculated with adjuvanted vaccines. Therefore, we performed a systematic review and meta-analysis to evaluate the effect of MF59 adjuvant on humoral immune responses among healthy people over 6 months old inoculated with influenza vaccines.
2. Methods
Our systematic review and meta-analysis was performed following the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) guidelines (Table S1).
We retrieved the following databases up to March 2019: PubMed, Embase, Medline, OVID, and Cochrane Library Trials (CENTRAL) using medical subject heading (MeSH) terms and free words of the keywords “influenza vaccine”, “MF59” and “adjuvant”. The search strategy was shown in Table S2. Only articles with English language were retrieved, and the reference lists from all retrieved studies and the most recent review articles were reviewed to collect additional undetected published studies.
The protocol of this study was registered: PROSPERO (Prospective register of systematic reviews) registration No. CRD42019129579. No ethics approval was necessary because this was a meta-analysis.
2.1. Study selection and methodological quality assessment
The retrieved articles would be identified based on the inclusion criteria:
-
1.
Healthy people over 6 months old,
and the exclusion criteria:
-
1.
animal studies,
-
2.
people allergic to eggs and other vaccines,
-
3.
people vaccinated with adjuvant influenza vaccine 6 months before enrollment,
-
4.
people with immunodeficiency,
-
5.
people infected with influenza virus within 12 months,
-
6.
participated in any other clinical studies,
-
7.
pregnant and lactating women, and
-
8.
people with acute, chronic or serious diseases.
The quality assessment of the included studies was conducted independently by 2 researchers employing the Cochrane Review Risk of Bias Assessment Tool. The methodological heterogeneity was evaluated by the strategies of randomization, allocation concealment, and blinding. Subsequently, the data integrity was assessed by dropout rate, complete outcome data, selective data reporting. Risk of bias of every portion was classified into low, high, or unclear.
2.2. Data extraction and analysis
The data were extracted from the included studies by 2 investigators (Jing Yang and Jiayou Zhang), respectively, including first author's name, time of publication, country of experimental locations, number of subjects, age of subjects, sex ratio of subjects, measures of intervention, information of vaccine, type of comparison vaccine, local and systemic adverse events, clinical and laboratorial outcome measures. The immunogenicity of influenza vaccine with MF59 adjuvant was evaluated for Geometric Mean Titer (GMT), and then the seroconversion and seroprotection rates were extracted from the studies. HI antibody titer was the maximum dilution capable of inhibiting the agglutination of red blood cells with the influenza viruses under standardized conditions. Seroconversion rate was defined as the percentage of subjects per group achieving at least a 4-fold increase in HI titer from a seropositive prevaccination titer (>10) or a rise from <10 to 40 in those who were originally seronegative. Seroprotection rate was defined as the proportion of subjects achieving an influenza antibody titer greater than or equal to 40 in HI laboratory detection.
Licensure criteria of influenza vaccines for adults were slightly different between Center for Biologics Evaluation and Research (CBER) and Committee for Medicinal Products for Human Use (CHMP) guidelines (Table 1). Immunogenicity of the influenza vaccine in our studies should at least satisfy the requirements of the CBER guidelines, which set valid levels of vaccine immunogenicity for a population over the age of 65 years as at least a 30% seroconversion rate, at least a 60% subjects achieving antibody titer ≥40. As for the population less than 65 years, the rate of seroconversion should be no less than 40%, or at least a 70% subjects achieving antibody titer ≥40. Each vaccine antigen should meet at least 1 of the following criteria in the CBER guidelines.
Table 1.
CBER (US) and CHMP (European) licensure criteria for hemagglutination inhibition (HI) immunogenicity analyses in adult subjects.

Meta-analysis was implemented as the trials had acceptable clinical homogeneity and statistical heterogeneity. A random-effect model was employed on account of the significant heterogeneity expected among the studies. Dichotomous data were analyzed using a Risk Ratio (RR) with 95% confidence intervals (CI) for identifying the differences in effectiveness and immunogenicity between the intervention and the control groups. Heterogeneity was quantified using the Cochrane Q TEST and I2 statistics. Potential publication bias was assessed by observing the symmetry of funnel plots and by using Egger test. Meta-analysis was performed using Review Manager (RevMan) program Version 5.3.
3. Results
3.1. Description of studies and quality assessment
Search process and outcomes were shown in Figure 1. Of the 1021 publications, 17 RCTs without duplication satisfied the inclusion criteria.[1,4,15–29] The 17 publications included 21 trials (some studies contained 2 trails to testify appropriate antigen doses of the adjuvanted influenza vaccines in different age group), and involved 8932 healthy subjects. The majority of studies conducted a 2-dose schedule vaccination (3–4 weeks interval),[4,15–23,26,29] only a few used 1-dose schedule.[1,18,27–29] The subjects of the 17 studies ranged from young children (6 to 72 months old) to older adults (over 64 years old). The subjects of 3 studies were healthy young children,[16,20,29] while more attention was paid to healthy adults or healthy older adults in other studies.
Figure 1.
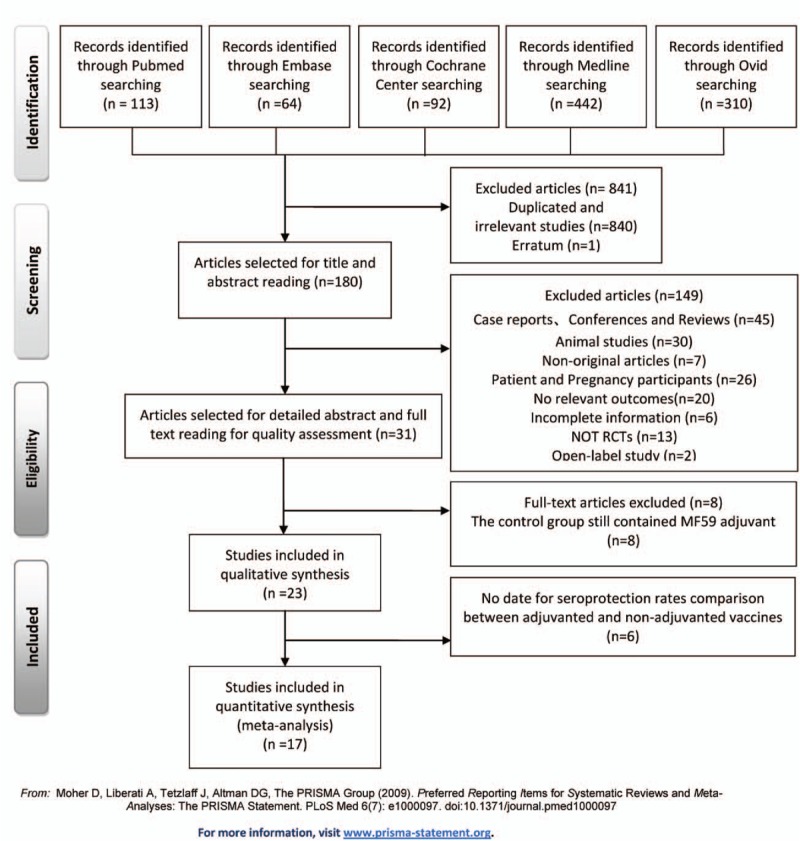
Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) flow diagram.
Eleven RCTs focused on monovalent prepandemic influenza vaccine,[4,15,17–22,24–26] while 7 RCTs focused on trivalent seasonal influenza vaccine.[1,16,23,25,27–29] Only 1 study focused on the efficacy and safety of combined vaccination of monovalent and trivalent vaccines.[25] Although 1 study had slightly different definition of outcome indicators for the seroconversion and seroprotection, we still collected the valuable data in our review.[17]
The Cochrane Risk of Bias Tool was used to evaluate the quality of included studies, and most of the included studies had no significant bias. The assessment of each included study was shown in Table S3. There was no distinct publication bias within the studies (Supplemental Fig. S1-S7).
3.2. Demographics
The main characteristics of the included studies are presented in Table 2. The RCTs were implemented worldwide, with 7 in the USA,[4,15,17–20,23] 3 in Germany[1,24,25] and Korea,[21,27,28] 2 in the UK[22,26] and Mexico,[20,29] 1 in Sweden,[1] Belgium,[24] Switzerland,[24] and Guatemala.[16] Three RCTs enrolled healthy young children,[16,20,29] eleven RCTs enrolled healthy adults,[4,15,17–19,21–26] and 6 RCTs enrolled healthy older adults.[1,4,21,24,27,28]
Table 2.
Characteristics of RCTs using MF59-adjuvanted influenza vaccines.
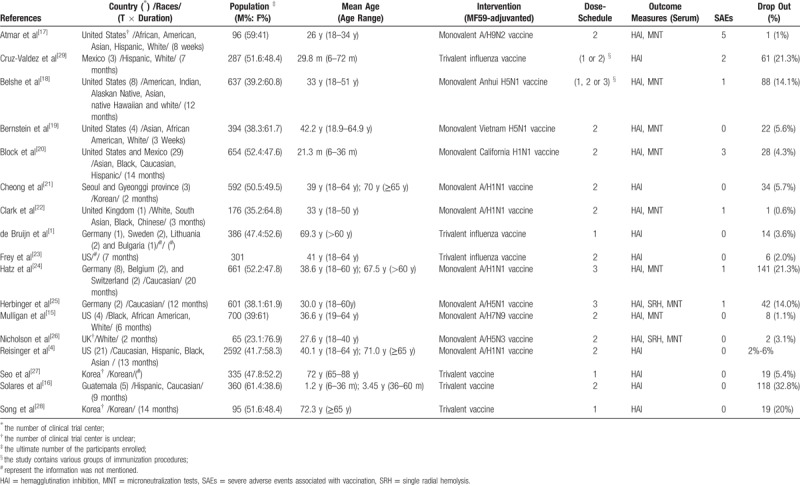
Among 8932 participants the male to female ratio was 0.82, which showed favorable gender balance.
3.3. Intervention
Fourteen RCTs used monovalent prepandemic influenza vaccine,[4,15,17–22,25,26] and 6 RCTs selected trivalent seasonal influenza vaccine[1,16,23,25,27–29] to identify the immunogenicity and safety of MF59 adjuvant. One study compared 3 different combined vaccination strategies.[25] Another study explored an appropriate dosage of MF59 adjuvant through various dose range trials.[4]
The standard MF59 adjuvant was supplied in the same formulation according to the included studies, which contained 9.75 mg squalene, 1.175 mg polysorbate 80, 1.175 mg sorbitan trioleate, 0.66 mg sodium citrate, 0.04 mg citric acid.
Vaccination strategies ranged from 1 dose immunization to 1 or 2 booster immunization for better immune responses. Among the 8932 participants included, 14 individuals had severe adverse effects related to vaccination.
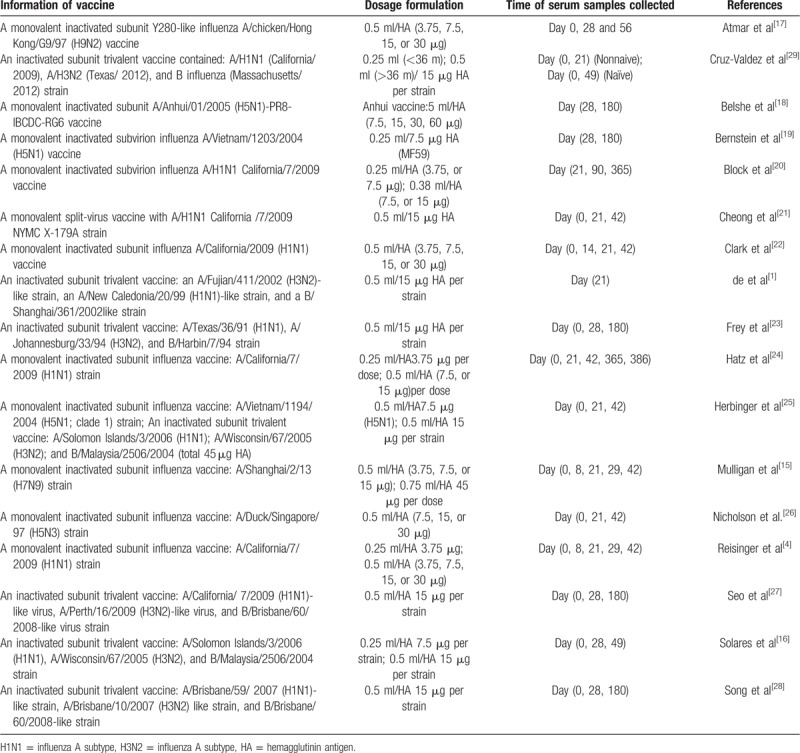
Most of RCTs chose inoculation with a monovalent inactivated subunit influenza vaccine, while 2 trials chose a monovalent inactivated sub-virion influenza vaccine.[19,20] Six RCTs used trivalent seasonal influenza vaccine to testify the benefit of MF59 adjuvant.[1,16,23,27–29] The main vaccine information of included RCTs was shown in Table 3.
Table 3.
Vaccine information of included studies.
3.4. Outcome measurement
One RCT was excluded from our meta-analysis because the objective of this study was to identify the immunogenicity and safety of combined inoculation of prepandemic and seasonal influenza vaccine with MF59 adjuvant.[25]
3.5. Comparison of immunogenicity between MF59-adjuvanted influenza vaccines and non-adjuvanted influenza vaccines against specific vaccine strains
The data from 10 RCTs[1,4,16,20–23,27–29] including 2341 participants were pooled for the analysis of seroconversion rate against H1N1 strain, and 8 RCTs[1,16,20–22,24,27,28] including 1515 subjects were enrolled for our meta-analysis to identify seroprotection rate against HIN1 strain. Six studies[1,16,23,27–29] including 993 subjects provided enough data for the analysis of seroconversion rate against H3N2 strain, and 4 studies[1,16,27,28] including 594 subjects were enrolled for our meta-analysis to estimate seroprotection rate against H3N2 strain. For B strain, the data from 6 RCTs[1,16,23,27–29] including 993 participants were pooled for the analysis of seroconversion rate, and a total of 613 participants from 4 RCTs[1,16,27,28] were enrolled to evaluate seroprotection rate. The seroconversion rates in those inoculated with MF59-ajuvanted H1N1, H3N2, and influenza B vaccines were 78.9%, 69.9%, and 61.0%, respectively. The overall seroprotection rates for MF59-adjuvanted H1N1, H3N2, and influenza B vaccines were 88.6%, 92.3%, and 61.9%, respectively.
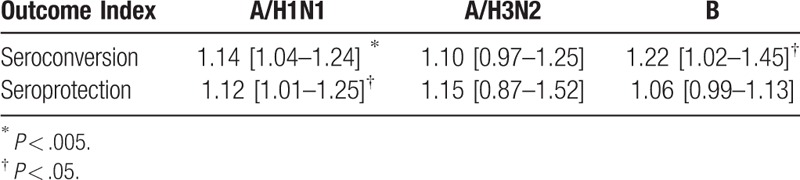
The differences in Immunogenicity were shown between those inoculated with MF59-adjuvanted influenza vaccines and those incubated with non-adjuvanted influenza vaccines. For influenza vaccine against H1N1 strain, the RR for seroconversion was 1.14, with a 95% CI of 1.04 to 1.24, I2 = 70%, P = .003, the RR for seroprotection was 1.12, with a 95% CI of 1.01 to 1.25, I2 = 86%, P = .03 (Fig. 2). For the vaccine against H3N2 strain, we found no significant differences in the seroconversion rate (RR = 1.10, 95% CI = 0.97–1.25, I2 = 57%, P = .12) and the seroprotection rate (RR = 1.15, 95% CI = 0.87–1.25, I2 = 97%, P = .34) (Fig. 3). In addition, for the vaccine against influenza B strain, we found a significant difference in the seroconversion rate (RR = 1.22, 95% CI = 1.02–1.45, I2 = 63%, P = .03), but not in the seroprotection rate (RR = 1.06, 95% CI = 0.99–1.13, I2 = 0%, P = .11) (Fig. 4). Furthermore, from the 2 outcome measures (seroconversion and seroprotection rates) utilized to assess the effectiveness of MF59 adjuvant on 3 influenza vaccine strains, subjects had the best response to B strain by seroconversion rate, followed by A/H1N1 strain, and had no statistical difference in H3N2 seroconversion rate (Table 4).
Figure 2.
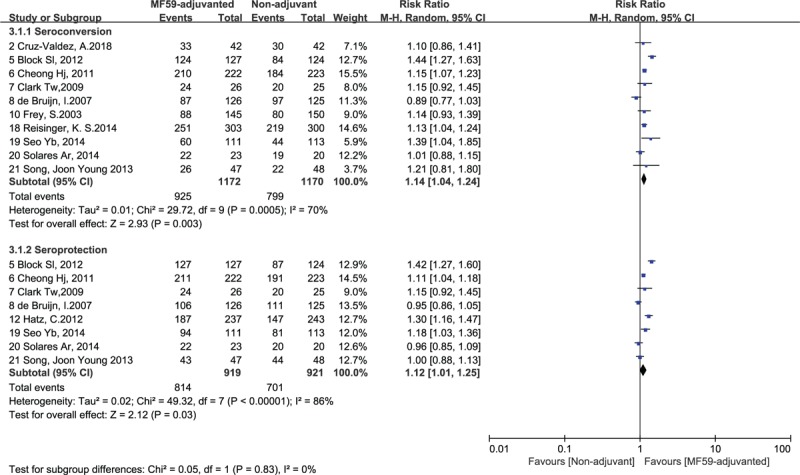
Forest plots of seroconversion and seroprotection rate of H1N1 strain. The bold data represent total participants of all included studies and the Risk Ratio (RR) between the MF59-adjuvanted group and the non-adjuvant group. The diamond stands for the pooled RR. Weights are from random-effects model. CI: confidence interval.
Figure 3.
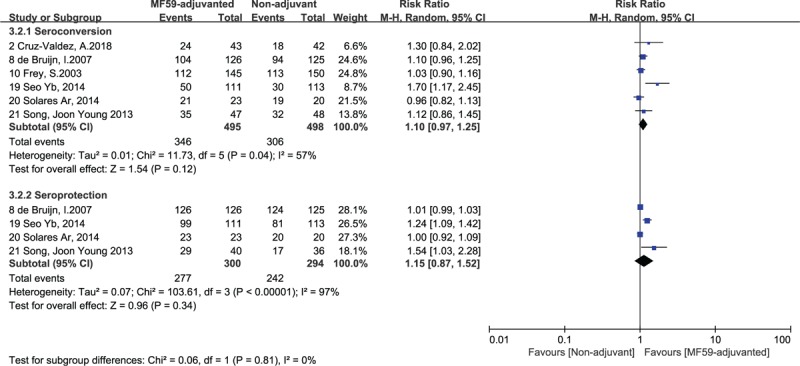
Forest plots of seroconversion and seroprotection rate of H3N2 strain. The bold data represent total participants of all included studies and the Risk Ratio (RR) between the MF59-adjuvanted group and the nonadjuvant group. The diamond stands for the pooled RR. Weights are from random-effects model. CI: confidence interval.
Figure 4.
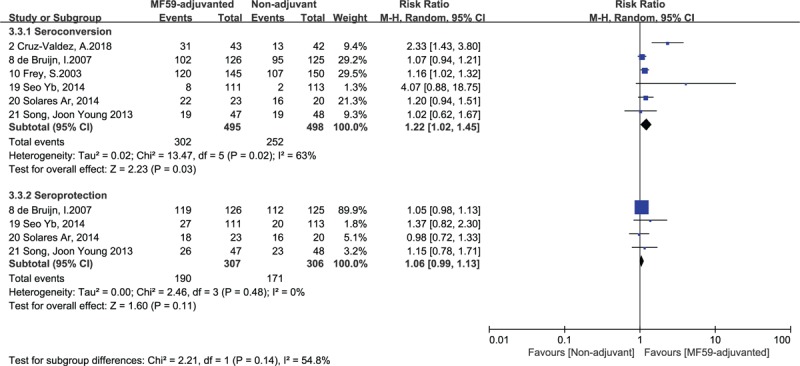
Forest plots of seroconversion and seroprotection rate of B strain. The bold data represent total participants of all included studies and the Risk Ratio (RR) between the MF59-adjuvanted group and the non-adjuvant group. The diamond stands for the pooled RR. Weights are from random-effects model. CI: confidence interval.
Table 4.
Subgroup analysis of Risk Ratio of seroprotection and seroconversion rates for different influenza vaccine strains.
3.6. Subgroup analysis of immunogenicity of influenza vaccines with MF59 adjuvant in different age groups
A subgroup meta-analysis was conducted based on the different age groups of the healthy subjects. The participants included were divided into the following 3 age groups: healthy young children (6 to 72 months old), healthy adults (7 to 64 years old), and healthy older adults (over 64 years old). The heterogeneity of the subgroups seroconversion rate was low in 2 extreme age groups (Fig. 5). We used the outcome measure of seroconversion rate to assess the effects of MF59 adjuvant on influenza vaccine strains, participants from the second age group with the RR for seroconversion rate was 2.10, with a 95% CI of 1.28 to 3.44, I2 = 94%, P = .003, which benefited the most from inoculation of MF59-adjuvanted influenza vaccine followed by the healthy older adults, but the young children (36–72 months old) group had no significant differences in immune responses between MF59-adjuvant and Non-adjuvant influenza vaccine.
Figure 5.
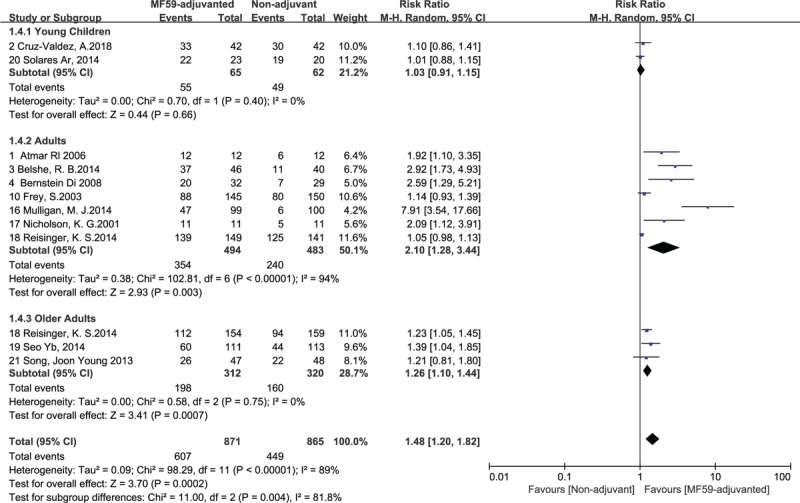
Forest plots of seroconversion rate in different age groups. The bold data represent total participants of all included studies and the Risk Ratio (RR) between the MF59-adjuvanted group and the non-adjuvant group. The diamond stands for the pooled RR. Weights are from random-effects model. CI: confidence interval.
3.7. Subgroup analysis of immunogenicity differences of influenza vaccines with MF59 adjuvant between the monovalent prepandemic vaccine and trivalent vaccine
Because of high heterogeneity of seroconversion rates in adult group, another subgroup analysis was conducted in accordance with the type of influenza vaccine. In the H1N1 seroconversion rate of trivalent seasonal influenza vaccine, the results showed low heterogeneity (RR = 1.18, 95% CI = 1.05–1.13, I2 = 45%, P = .12) after grouping adult subjects into 2 categories: monovalent MF59-adjuvanted prepandemic influenza vaccines and trivalent MF59-adjuvanted seasonal influenza vaccine (Fig. 6). Based on the seroconversion rate, we found more favorable effect in the MF59-ajuvanted monovalent influenza vaccine group (RR = 1.59, 95% CI = 1.40–1.80, I2 = 44%, P = .15) (Fig. 6).
Figure 6.
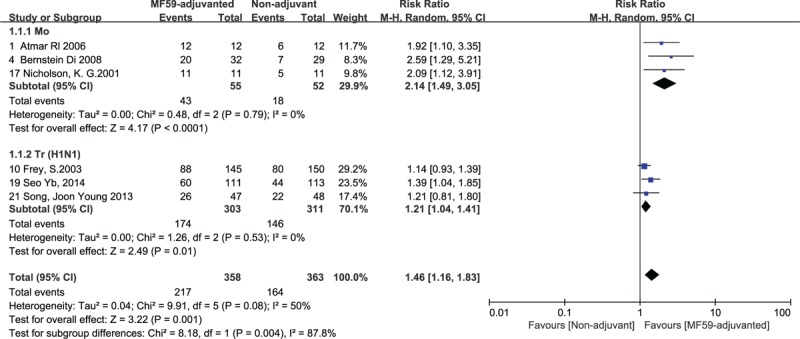
Forest plots of seroconversion rate of the monovalent and trivalent influenza vaccine (against H1N1 strain) in adult group. The bold data represent total participants of all included studies and the Risk Ratio (RR) between the MF59-adjuvanted group and the nonadjuvant group. The diamond stands for the pooled RR. Weights are from random-effects model. CI: confidence interval.
3.8. Subgroup analysis of immunogenicity of influenza vaccines with MF59 adjuvant in different content of HA antigen
Furthermore, to investigate appropriate content of HA antigen with standard dose MF59 adjuvant and the necessity of 2-dose schedule vaccination, we performed a subgroup analysis of the content of HA antigen, and compared the seroconversion rate between second dose vaccination and first dose vaccination with same vaccine formulation that contained 7.5 μg HA antigen with standard dose MF59 adjuvant.
Both 15 μg HA antigen group and 7.5 μg HA antigen group showed high homogeneity after dividing participants into MF59-adjuvanted influenza vaccines containing 15 μg HA antigen and MF59-adjuvanted influenza vaccines containing 7.5 μg HA antigen groups. Based on the seroconversion rate of MF59-adjuvanted influenza vaccine, we found more favorable effect in the 7.5 μg HA antigen group (RR = 1.30, 95% CI = 1.15–1.47, I2 = 54%, P < .0001) (Fig. 7a).
Figure 7.
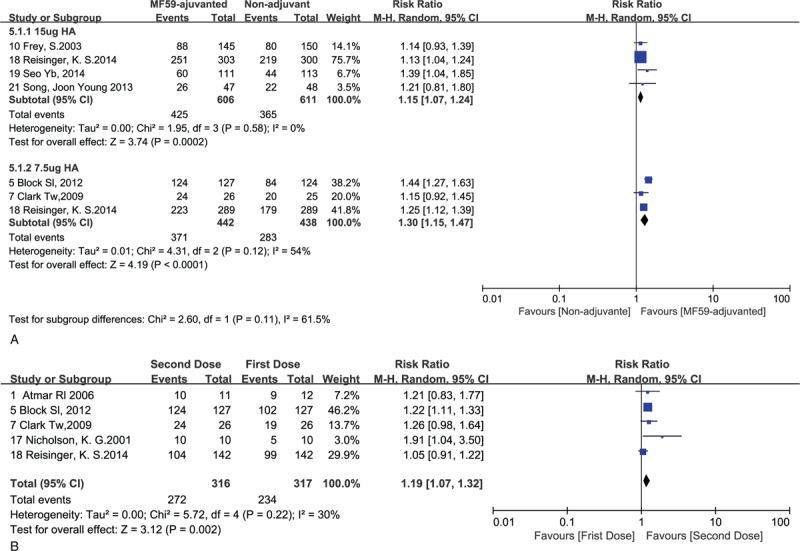
a. Forest plots of seroconversion rate in different content of HA antigen in adult group. b. Forest plots of seroconversion rate for the second dose versus the first dose. The bold data represent total participants of all included studies and the Risk Ratio (RR) between the MF59-adjuvanted group and the non-adjuvant group. The diamond stands for the pooled RR. Weights are from random-effects model. CI: confidence interval.
In addition, we found a significant difference in seroconversion rate between the second inoculation and the first inoculation (RR = 1.19, 95% CI = 1.15–1.47, I2 = 30%, P = .002) (Fig. 7b).
4. Discussion
To our knowledge, this is the first systematic review and meta-analyzed on the efficacy of MF59 adjuvant in different types of influenza vaccines among various age groups. We found that MF59 adjuvant enhanced the immunogenicity against specific influenza viral strains H1N1, H3N2, B strains, H9N2, and H5N1, but not the H7N9 strain.[15] The seroconversion rates from included RCTs showed better immune protective effect in the participants inoculated with MF59 adjuvanted influenza vaccine against vaccine homologous H1N1 and B strain, and the seroprotection rate showed significantly enhanced efficacy of MF59-adjuvanted influenza H1N1 vaccine.
In this study, the subjects inoculated with MF59-adjuvanted influenza vaccine met at least 1 of the CBER license criteria for the influenza vaccine strains (seroprotection rates and seroconversion rates against the specific influenza viral strains), and had superior seroconversion rates against the B strain, H1N1, H9N2 and H5N1 strains compared to non-adjuvanted groups. In this meta-analysis, only1 study[17] might have slightly overestimated the seroconversion rate against H9N2, as the HI titer ≥ 32, and a >4 fold increase was used as the standard, which would not affect the final results by the sensitivity analysis.
The efficacy of MF59 adjuvant for enhancing the effectiveness of influenza vaccines did not reach a consistent conclusion in previous studies. Our results show that the majority of the 17 enrolled RCTs reached a consistent conclusion, and only 1 trial[1] showed opposite results. The inconsistency might be attributed to study design, dose-schedule, immune procedure, as well as the demographic characteristics of the subjects enrolled such as health status and age. We attempted to analyze the potential mixed effect of these variables on MF59-adjuvanted influenza vaccine. The age and influenza vaccine type had significant effects on the participants’ immune responses to MF59-adjuvanted influenza vaccines. Subjects from adult group were more sensitive to the stimulation of MF59-adjuvanted influenza vaccines. Nevertheless, young children group did not show positive effects from MF59 adjuvanted influenza vaccines as this age group is in the development of immune system and sensitive to any kind of antigens. This systematic review and meta-analysis showed that both healthy adults and healthy older adults benefited from MF59 adjuvanted influenza vaccines, compared with young children group. Our results provide solid evidence for clinical practices to make appropriate recommendations for different age groups to strengthen influenza vaccine efficacy.
Although compliance of the participants might be a confounding factor in RCTs. In this study, the compliance was recorded in all RCTs and most of the studies showed good compliance, and 7 studies further confirmed the immune persistence of MF59-adjuvanted influenza vaccines half or 1 year after inoculation. Only 1 study declared no good compliance, and the dropout rate was over 30%.[16] Furthermore, a subgroup meta-analysis was performed on different types of influenza vaccine in adult group, we found that MF59-adjuvanted monovalent inactivated influenza vaccines had distinguish positive effects on immunogenicity changes against all kinds of influenza vaccine strains except H7N9 strain. On the contrary, MF59 adjuvant showed no significant difference in the efficacy in young children group. Further investigation is needed to identify the influences of lower MF59 adjuvant content in young children.
The potential mechanisms of MF59 adjuvant to enhance immune efficacy of influenza vaccine strains remain to be elucidated. In cellular immunity, MF59 adjuvant can recruit neutrophil, phagocyte, and NK cells[30] and promote IgA secretion to the injection site to enhance the vaccine effects.[31] In addition, Th1 cells produce interferon γ, which has antiviral effects.
No previous meta-analysis has compared the efficacy of MF59 adjuvant in various age groups in enhancing the immunogenicity of various types of influenza vaccines. In this meta-analysis, subgroup analysis revealed that monovalent MF59 adjuvanted influenza vaccine in adult group achieved more immunogenicity changes. Consequently, the addition of MF59 adjuvant might be a significant approach to increase the immune responses to influenza vaccines.
Low heterogeneity of the pooled analysis is the advantage of this study. Furthermore, the RCTs included in this analysis were retrieved from various databases and included publications in English from different perspectives and cultural viewpoints. However, our meta-analysis has several limitations. First, we only used the rates of seroprotection and seroconversion as outcome measures, and did not use the changes in antibody geometric mean titer (GMT) because none of the included studies recorded GMT with a standard deviation before and after vaccination. Second, only 1 trial was included for subgroup analysis of different MF59 adjuvant content, therefore, the comparison of different MF59 adjuvant content was limited. Third, due to the limited number of included RCTs and the lack of sufficient data on basic immune status and original antibody titers against influenza, it was impossible to conduct more subgroup analysis to identify the effects of these variables. Finally, the medications used by the subjects might be confound factors. However, no medication records had been reported in the included studies.
5. Conclusions
Our meta-analysis showed that MF59 adjuvant can improve the immunogenicity of influenza vaccines in healthy adults and the aged. We propose that MF59-adjuvanted influenza vaccines can be widely inoculated both in healthy adults and the aged, especially monovalent pre-pandemic influenza vaccines. Further large RCTs focusing on the optimal dose, inoculation interval, and the antagonism effect of a combination of MF59 adjuvant and trivalent seasonal influenza vaccine are required to testify these findings.
Author contributions
Conceptualization: Xiaoming Yang, Jing Yang, Jiayou Zhang.
Data curation: Xiaoming Yang, Jing Yang, Jiayou Zhang.
Formal analysis: Xiaoming Yang, Jing Yang, Jiayou Zhang.
Investigation: Jing Yang, Jiayou Zhang, Tian Han, Xinghang Li, Baifeng Yang.
Methodology: Jing Yang, Jiayou Zhang, Chen Liu, Luyao Yan.
Project administration: Xiaoming Yang.
Supervision: Xiaoming Yang.
Writing – original draft: Jing Yang, Jiayou Zhang.
Writing – review & editing: Jing Yang, Jiayou Zhang, Xiaoming Yang.
Xiaoming Yang orcid: 0000-0002-2481-555X.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: MF59 = microfluidized emulsion 59, RCTs = randomized clinical trials, VE = vaccine effects.
How to cite this article: Yang J, Zhang J, Han T, Liu C, Li X, Yan L, Yang B, Yang X. Effectiveness, immunogenicity and safety of influenza vaccines with MF59 adjuvant in healthy people of different age groups: A systematic review and meta-analysis. Medicine. 2020;99:7(e19095).
Authors have no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].de Bruijn I, Meyer I, Gerez L, et al. Antibody induction by virosomal, MF59-adjuvanted, or conventional influenza vaccines in the elderly. Vaccine 2007;26:119–27. [DOI] [PubMed] [Google Scholar]
- [2].Beyer WE, de Bruijn IA, Palache AM, et al. Protection against influenza after annually repeated vaccination: a meta-analysis of serologic and field studies. Arch Intern Med 1999;159:182–8. [DOI] [PubMed] [Google Scholar]
- [3].Karch C, Burkhard P. Vaccine technologies: from whole organisms to rationally designed protein assemblies. Biochem Pharmacol 2016;120:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Reisinger KS, Holmes SJ, Pedotti P, et al. A dose-ranging study of MF59(®)-adjuvanted and non-adjuvanted A/H1N1 pandemic influenza vaccine in young to middle-aged and older adult populations to assess safety, immunogenicity, and antibody persistence one year after vaccination. Hum Vaccin Immunother 2014;10:2395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schaffner W, van Buynder P, McNeil S, et al. Seasonal influenza immunisation: strategies for older adults. Int J Clin Pract 2018;72:e13249. [DOI] [PubMed] [Google Scholar]
- [6].Tsai TF. Fluad®-MF59®-Adjuvanted Influenza Vaccine in older adults. Infect Chemother 2013;45:159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kang I, Hong MS, Nolasco H, et al. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol 2004;173:673–81. [DOI] [PubMed] [Google Scholar]
- [8].Kumar A, McElhaney JE, Walrond L, et al. Cellular immune responses of older adults to four influenza vaccines: results of a randomized, controlled comparison. Hum Vaccin Immunother 2017;13:2048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Baudner BC, Ronconi V, Casini D, et al. MF59 emulsion is an effective delivery system for a synthetic TLR4 agonist (E6020). Pharm Res 2009;26:1477–85. [DOI] [PubMed] [Google Scholar]
- [10].Ansaldi F, Zancolli M, Durando P, et al. Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine 2010;28:4123–9. [DOI] [PubMed] [Google Scholar]
- [11].Domnich A, Arata L, Amicizia D, et al. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: a systematic review and meta-analysis. Vaccine 2017;35:513–20. [DOI] [PubMed] [Google Scholar]
- [12].Beyer WE, Nauta JJ, Palache AM, et al. Immunogenicity and safety of inactivated influenza vaccines in primed populations: a systematic literature review and meta-analysis. Vaccine 2011;29:5785–92. [DOI] [PubMed] [Google Scholar]
- [13].Nicolay U, Heijnen E, Nacci P, et al. Immunogenicity of aIIV3, MF59-adjuvanted seasonal trivalent influenza vaccine, in older adults >/=65 years of age: meta-analysis of cumulative clinical experience. Int J Infect Dis 2019;85S:S1–9. [DOI] [PubMed] [Google Scholar]
- [14].Banzhoff A, Nacci P, Podda A. A new MF59-adjuvanted influenza vaccine enhances the immune response in the elderly with chronic diseases: results from an immunogenicity meta-analysis. Gerontology 2003;49:177–84. [DOI] [PubMed] [Google Scholar]
- [15].Mulligan MJ, Bernstein DI, Winokur P, et al. Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. JAMA 2014;312:1409–19. [DOI] [PubMed] [Google Scholar]
- [16].Solares Ar ACGPRUP-CDS-CVPMG.N.. Safety and immunogenicity profiles of an adjuvanted seasonal influenza vaccine in Guatemalan children. J Infect Dev Ctries 2014;8:1160. [DOI] [PubMed] [Google Scholar]
- [17].Atmar Rl KWAPSMKJMSDESHPJCTRCR.B.. Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. Clin Infect Dis 2006;43:1135. [DOI] [PubMed] [Google Scholar]
- [18].Belshe RB, Frey SE, Graham IL, et al. Immunogenicity of avian influenza A/Anhui/01/2005(H5N1) vaccine with MF59 adjuvant: a randomized clinical trial. JAMA V 312 2014;1420–8. [DOI] [PubMed] [Google Scholar]
- [19].Bernstein DI, Edwards KM, Dekker CL, et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis 2008;197:667. [DOI] [PubMed] [Google Scholar]
- [20].Block SL, Ruiz-Palacios GM, Guerrero ML, et al. Dose-range study of MF59-adjuvanted versus nonadjuvanted monovalent A/H1N1 pandemic influenza vaccine in six- to less than thirty-six-month-old children. Pediatr Infect Dis J 2012;31:e92. [DOI] [PubMed] [Google Scholar]
- [21].Cheong HJ, Song JY, Heo JY. Immunogenicity and safety of the influenza A/H1N1 2009 inactivated split-virus vaccine in young and older adults: mF59-adjuvanted vaccine versus nonadjuvanted vaccine. Clin Vaccine Immunol 2011;18:1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Clark TW, Pareek M, Hoschler K. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med 2009;361:2424. [DOI] [PubMed] [Google Scholar]
- [23].Frey S, Poland G, Percell S, et al. Comparison of the safety, tolerability, and immunogenicity of a MF59-adjuvanted influenza vaccine and a non-adjuvanted influenza vaccine in non-elderly adults. Vaccine 2003;21:4234–7. [DOI] [PubMed] [Google Scholar]
- [24].Hatz C, von Sonnenburg F, Casula D, et al. A randomized clinical trial to identify the optimal antigen and MF59(®) adjuvant dose of a monovalent A/H1N1 pandemic influenza vaccine in healthy adult and elderly subjects. Vaccine 2012;30:3470–7. [DOI] [PubMed] [Google Scholar]
- [25].Herbinger KH, von Sonnenburg F, Nothdurft HD, et al. A phase II study of an investigational tetravalent influenza vaccine formulation combining MF59®: adjuvanted, pre-pandemic, A/H5N1 vaccine and trivalent seasonal influenza vaccine in healthy adults. Hum Vaccin Immunother 2014;10:92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nicholson KG, Colegate AE, Podda A, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 2001;357:1937–43. [DOI] [PubMed] [Google Scholar]
- [27].Seo YB, Choi WS, Lee J. Comparison of the immunogenicity and safety of the conventional subunit, MF59-adjuvanted, and intradermal influenza vaccines in the elderly. Clin Vaccine Immunol 2014;21:989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Song JY, Cheong HJ, Noh JY, et al. Long-term and cross-reactive immunogenicity of inactivated trivalent influenza vaccine in the elderly: MF59-adjuvanted vaccine versus unadjuvanted vaccine. J Med Virol 2013;85:1591–7. [DOI] [PubMed] [Google Scholar]
- [29].Cruz-Valdez A, Valdez-Zapata G, Patel SS, et al. MF59-adjuvanted influenza vaccine (FLUAD®) elicits higher immune responses than a non-adjuvanted influenza vaccine (Fluzone®): a randomized, multicenter, Phase III pediatric trial in Mexico. Hum Vaccin Immunother 2018;14:386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ko E-J, Lee Y-T, Kim K-H, et al. Effects of MF59 adjuvant on induction of isotype-switched IgG antibodies and protection after immunization with T-dependent influenza virus vaccine in the absence of CD4+ T cells. J Virol 2016;90:6976–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Khurana S, Verma N, Yewdell JW. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med 2011;3:85ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


