Abstract
Primary tumor resection (PTR) for unresectable metastatic colorectal cancer (mCRC) patients has been documented to be associated with postoperative hyper-neovascularization and enhanced growth of metastases, which may be prevented by bevacizumab. This study aimed to investigate the survival outcome of PTR in patients who received palliative bevacizumab-containing chemotherapy (BCT).
From January 2006 to December 2018, medical records of 240 mCRC patients who received palliative BCT at a single tertiary colorectal cancer center were retrospectively reviewed. Patients were classified into three groups: PTR-a (PTR before BCT, n = 60), PTR-b (PTR during BCT, n = 17), and BCT-only group (n = 163). Resectable mCRCs or recurrent diseases were excluded, and the end-point was overall survival (OS) rate.
Three groups had similar age, cell differentiation, location of the primary tumor, and the number of metastatic organs. More than two-thirds of patients who received PTR experienced disease-progressions (PD) during their postoperative chemotherapy-free time (PTR-a vs PTR-b; 66.7% vs 76.5%, P = .170), but OS was not inferior to the BCT-only group (PTR-a vs BCT-only; HR 0.477 [95% CI 0.302–0.754], P = .002/PTR-b vs BCT-only; HR 0.77 [95% CI 0.406–1.462], P = .425). The postoperative chemotherapy-free time was similar between PTR-a and PTR-b (median 32.0 [14–98] days vs 41.0 [18–71] days, P = .142), but non-obstructive indications (perforation, bleeding, pain) were the more frequent in the PTR-b than PTR-a. Young age, the number of BCT, and PTR-a were the independent factors for OS.
The efficacy of the PTR for unresectable mCRC has been controversial, but this study demonstrated that PTR should be considered for the unresectable mCRC patients regardless before and during BCT.
Keywords: bevacizumab, colorectal cancer, primary tumor resection
1. Introduction
Colorectal cancer is the second leading cause of cancer death in the USA in 2017.[1] And approximately 20% to 25% of patients with colorectal cancer presented with synchronous metastases, which are unresectable in 75% to 90% of these patients, at the time of diagnosis.[2,3] For these unresectable metastatic colorectal cancer (mCRC) patients, systemic chemotherapy is recommended to increase the survival time.[4] The current approach for treating mCRC includes doublet combinations, as well as the triplet combination combined with a biological agent targeting either the vascular endothelial growth factor in an unselected population or the epidermal growth factor receptor in patients with RAS wild-type tumors.[4]
Bevacizumab (BV) is a recombinant humanized monoclonal antibody against VEGF, and it was approved for treating mCRC by the US Food and Drug Administration in February 2004.[5] BV inhibits tumor growth by suppressing the growth of new blood vessels, reducing interstitial fluid pressure, and enhancing the efficacy of chemotherapeutic agents.[6] The substantial clinical efficacy of BV has been demonstrated in several studies; it prolongs overall and progression-free survival when used in combination with other chemotherapy regimens in the first- or second-line treatment of mCRC.[7–10]
Primary tumor resection (PTR) for unresectable mCRC has been controversial because of the possibility of delayed initiation of systemic chemotherapy. As a result, most of the asymptomatic patients who had unresectable mCRC have been indicated for palliative systemic chemotherapy. By the way, several reports documented postoperative flare-up of neovascularization and enhanced growth of metastatic foci after PTR,[11–13] which might be prevented by BV. Indeed, most of the articles published after approvement of BV, showed a positive association between PTR and survival outcomes.[14–17] As far as we know, few studies have investigated the effects of PTR alone in patients who received bevacizumab-containing chemotherapy (BCT). So we aimed to analyze the survival outcome of the PTR in the patients who received BCT.
2. Materials and methods
2.1. Study population
Our study cohort comprised 344 consecutive patients with initially unresectable mCRC who underwent palliative 1st-line or 2nd-line BCT at the Chonnam National University Hwasun Hospital from January 2006 to December 2018. Patients who received curative resection followed by conversion chemotherapy, who diagnosed to a recurrent colorectal cancer, who had a resectable mCRC, and who received only the best supportive care were excluded (Table 1).
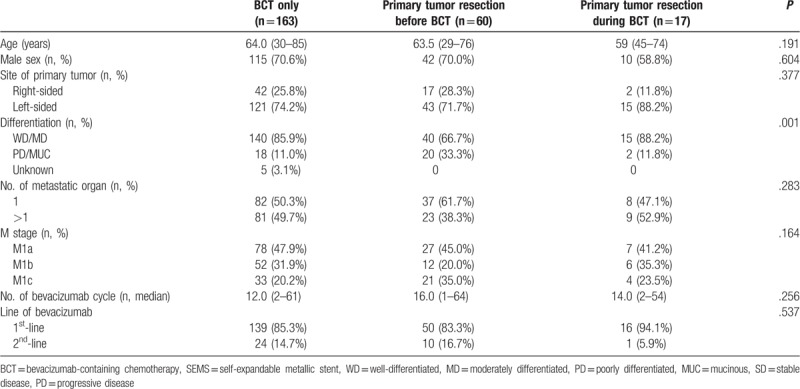
Table 1.
Patient characteristics.
2.2. Data collection
The following parameters were retrospectively collected using medical records: age, sex, tumor differentiation, the location of the primary lesion, the number of BV dose, a line of BV administration, the indication of PTR, postoperative disease-progression status, and mortality. For primary tumor sidedness, right-colon was defined as tumors of the cecum, ascending colon, and transverse colon and left-colon was defined as tumors of the splenic flexure, descending colon, sigmoid colon, rectosigmoid colon, and rectum. All of the 1st-line chemotherapy regimens were BV plus FOLFOX or FOLFIRI, and distant metastases were recategorized to M stage according to National Comprehensive Cancer Network (NCCN) guidelines, 2018.[4]
2.3. Study endpoints and the follow-up
The follow-up period started from the date of surgery or chemotherapy, and it ended when the subjects were expired or decided to receive best supportive care only instead of palliative treatments or lived beyond December 31, 2018. The primary endpoint was the time to death, and the death was confirmed by referencing the Korea National Death Registry. Postoperative chemotherapy-free time and mortality and postoperative disease-progression status were investigated as a second endpoint. The chemotherapy-free time was defined as an interval, from the day of PTR to the postoperative chemotherapy or last follow-up day in patients who did not receive postoperative chemotherapy. The disease-progression status was evaluated by computed tomography scan and analyzed using revised Response evaluation criteria in solid tumors guideline, version 1.1.[18]
2.4. Statistical analysis
Fisher exact test and Pearson χ2 test were used for the analysis of categorical variables. Continuous variables were compared using the Mann–Whitney U test and independent samples t test, and the Kruskal–Wallis test was performed for non-parametric ANOVA. Overall survival (OS) was defined as the interval between the date of mCRC diagnosis and the date of death from all causes; survivors at the date of data cut-off (December 31, 2018) were censored. The Kaplan–Meier method was used to estimate OS; differences in survival were assessed using the generalized Wilcoxon test, and multivariate Cox proportional hazards regression models were used to evaluate the prognostic impact of PTR on OS by adjusting for several clinical factors. Data are presented as numbers of patients, percentages (%), or hazard ratios (HR) and 95% confidence intervals (CI), as indicated. A P-value < .05 was considered statistically significant. All statistical analyses were performed using the using IBM SPSS statistics version 22.0 (SPSS, Inc., Chicago, IL).
3. Results
3.1. Patient characteristics
Among 240 unresectable mCRC patients who received palliative BCT, 163 included to CT only group, and 60, 17 patients included to PTR-a and PTR -b groups (Fig. 1). Median follow-up duration was BCT-only: 13.0 months, PTR-a: 17.0 months, 19.0 months (P = .081, Table 1). Three groups had similar age, sex, the location of the primary tumor, but poorly differentiated (PD) and mucinous adenocarcinomas (MUC) were more included in PTR-a group than the others (P = .001, Table 1). Three groups had a similar number of metastases and M stage, and BV was commonly administrated as the 1st-line treatment in three groups (Table 1). Liver, lung, peritoneum, and remote lymph node (LN) were the frequent lesions of metastasis, and about half of the metastases existed in a single organ (Tables 1 and 2).
Figure 1.
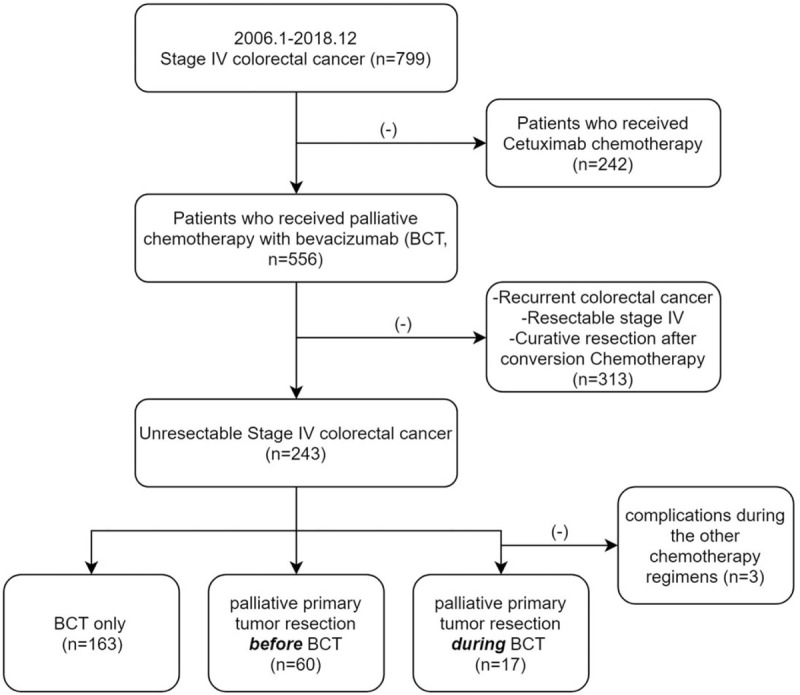
Flow chart of patient selection.
Table 2.
Site of the metastases.

3.2. Indication and the outcome of palliative surgery (Table 3)
Table 3.
Indications and the outcome of palliative surgery.
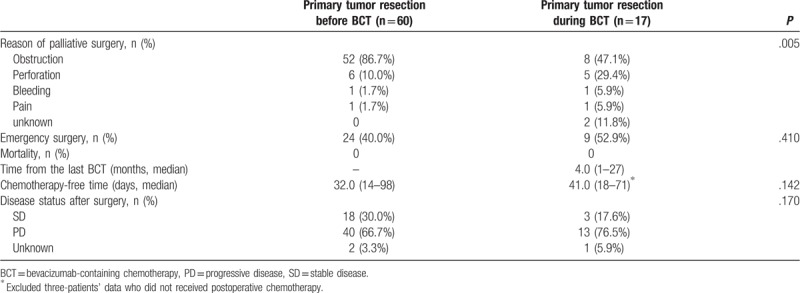
The obstruction was the most common indication in both of PTR-a and PTR-b. However, non-obstructive causes were significantly frequent in PTR-b than in PTR-a (P = .005). Among 180 patients who received BCT initially (163 BCT-only + 17 PTR-b), the incidence rate of PTR-b was 9.4% (17 PTR-b/ [163 BCT-only + 17 PTR-b]), and the PTR-b was performed median 4.0 months (1–27) later from the day of first BV administration. Emergency surgery was performed in about half of patients commonly in both group (P = .410). Postoperative chemotherapy-free periods (time interval between the date of surgery to the systemic chemotherapy) were similar between two groups (PTR-a; 32 days, PTR-b; 41 days, P = .142), and there were no postoperative mortalities in both groups. In postoperative computed tomography for re-staging, more than two-third of patients in both surgery group, commonly experienced disease-progressions (PTR-a vs PTR-b: 66.7% vs 76.5%, P = .170).
3.3. Survival outcome
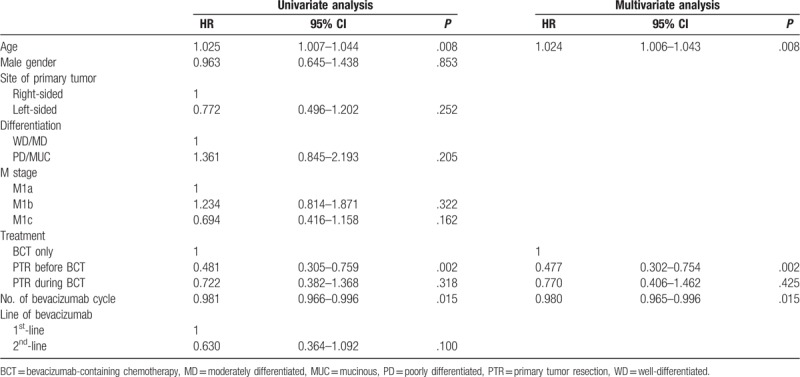
Median survival time was BCT-only: 23.0 months, PTR-a: 40.0 months, PTR-b: 31.0 months (P = .005, Fig. 2). Compared with BCT-only group, PTR-a and PTR-b associated with longer survival (PTR-a: adjusted HR 0.477, 95% CI 0.302–0.754, P = .002/PTR-b: adjusted HR 0.770, 95% CI 0.406–1.462, P = .425) (Fig. 2). In univariate analysis, left-sided tumor, well/moderately differentiated tumor (WD/MD), M1c, 2nd-line BV were associated with longer survival without statistical significances (Left-sided tumor: HR 0.772, PD/MUC: HR 1.361, M1c: HR 0.694, 2nd-line BV: HR 0.630). In multivariate analysis, young age, PTR-a, and the number of BV use were the independently associated factors for the longer survival (Age: HR 1.024, PTR-a: HR 0.477, No. of BV use: HR 0.980) (Table 4).
Figure 2.
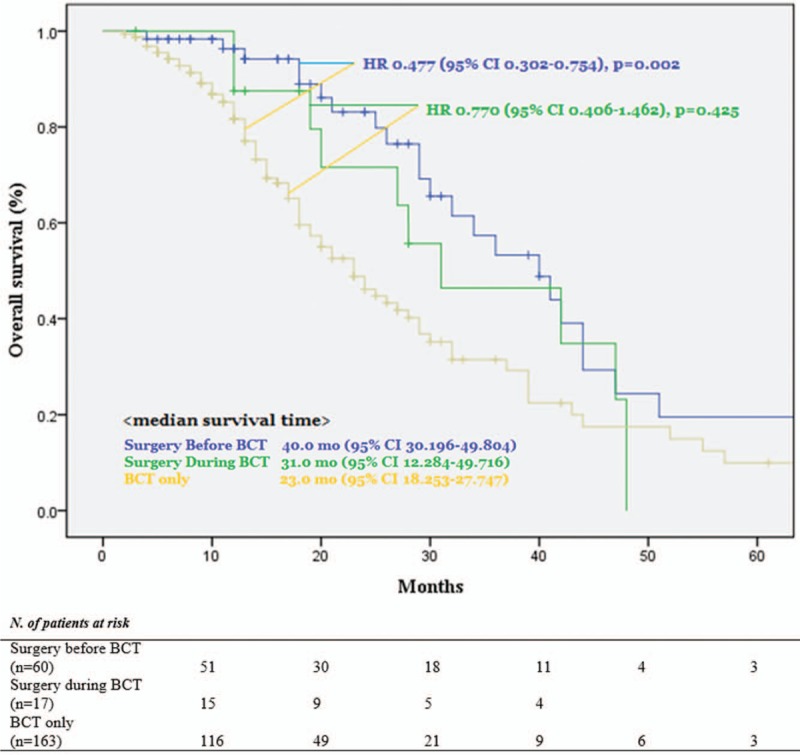
Overall survival.
Table 4.
Univariate and multivariate analysis for associated factors to overall survival.
4. Discussion
This study demonstrated the conflicting results of the PTR in patients who received BCT: regardless of the order between PTR and chemotherapy, PTR needed more than one month of postoperative chemotherapy-free time, and more than two-thirds of patients experienced disease-progressions during their postoperative chemotherapy-free time T. However, interestingly, the OS was not inferior to that of the BCT-only patients. Rather, the PTR-a showed statistically significantly longer survival month, and the PTR-b had also similar survival month to the BCT-only group. This retrospective study also demonstrated that about 10% of BCT-only patients had a risk of PTR during the BCT. Previous articles reported the increased survival outcome of the PTR in the unresectable mCRC patients.[14–17,19–24] However, we had three patients (17.6%) who did not continue systemic chemotherapy. The reasons were due to the persistent BV complication (53 yr/M) and the loss of willing for further chemotherapy (74 yr/F, 66 yr/M). Taking into account the probability of treatment discontinuance after PTR, it looks like that PTR-a can be regarded as a more proper option than the PTR-b. But PTR-a also has a risk of complications.
PTR-a has been studied to avoid discontinuation of palliative systemic chemotherapy due to a tumor-related complication during the chemotherapy, but the PTR-a has also been controversial because it also causes delayed or discontinuation of chemotherapy due to complications[25–27] or results in a disturbance of homeostasis, which may lead to immunosuppression and faster growth of metastases.[28] An important concern of the opposites to the PTR was that PTR stimulates peritumoral neovascularization and, thus, accelerated growth of the metastatic lesion. Indeed, it was reported that suppressed production of endogenous anti-angiogenesis inhibitors (angiostatin and endostatin), and a flare-up in vessel neoformation.[29] Our study also demonstrated that the majority of the patients who underwent PTR experienced disease-progressions (PTR-a: 66.7%, PTR-b: 76.5%), but they survived longer than the BCT-only patients. PTR may be associated with the accelerated disease-progressions, but decreased tumor burden and concentrated chemotherapy to metastatic lesions by PTR looks increased survival time than non-PTR patients.
Another possible factor for the increased survival months of the PTR may be the BV administration. BV is a monoclonal antibody that targets vascular endothelial growth factor which suppresses neovascularization nearby cancer, and the effectiveness for 1st-line, 2nd-line, and maintenance therapy in combination with or without other chemotherapy regimens have been well-established by previous studies.[7–10,30,31] BV may have a synergistic effect when it used for patients who underwent PTR, to control accelerated neovascularization of metastases. Indeed, a lot of previous studies which were published before approvement of the BV concluded that PTR before chemotherapy had no survival benefit than patients who did not undergo PTR,[21–23] but recent papers and meta-analyses are recommending PTR before palliative chemotherapy.[14–17] Recently, a population-based cohort study in 2017, reported that PTR was not associated with improved survival compared with systemic chemotherapy, but they used old data of the National Cancer Data Base of the USA between 2003 and 2005.[20]
A recent meta-analysis of seventy-seven studies demonstrated a low rate of mortality (4.5%) and the major adverse events (10.2%) after the PTR-a,[17] and a high-incidence of anastomotic leakage of 26.6% was reported postoperatively from a large-single center's retrospective study.[32] However, the studies reported that the complications from BV seem not associated with survival outcome. In actually, while BV has been largely hypothesized to increase perioperative complications in resected colorectal cancer patients. But this association has not achieved statistical significance in presently available trials and complication-associated survival differences also. This retrospective study also showed statistically significant longer survival months of the PTR-a than the BCT-only, and no one experienced surgical-site complications as like anastomotic dehiscence during BCT following PTR in this study.
Interestingly, PTR during BCT (PTR-b) was not related to the prolonged postoperative chemotherapy-free time compared to PTR-a. And PTR-b also did not compromise the survival outcome than that of the BCT-only patients. By the way, the survival curve of the PTR-b was closer to the survival curve of PTR-a, than of BCT-only, although the P-value was not significant between PTR-b and BCT-only. Many series have reported BV-related complications like perforation, bleeding, and fistula, which could be associated with tumor shrinkage,[33–37] and several studies have reported that good responders to the chemotherapy had a high-survival rate.[38,39] In the present study, patients with those complications also significantly more included in the PTR-b, and this can be a bias to interpret the favorable survival outcome of the PTR-b. Thus, the favorable outcome of PTR-b in this study seems not to be used as a basis for supporting BCT-only. Further researches will be necessary which are including evaluation of the relationship between the complications during postoperative BCT, and the effect to survival outcome after PTR in the patients who received BCT. PTR-a seems to be considered for the unresectable mCRC patients until the reliable negative pieces of evidence are accumulated.
This study has several limitations. First, this study is a small retrospective study, suggesting a possible bias in decision-making for individual patients, which may have influenced oncologic outcomes although the factors that may have affected the selection of patients for surgery to remain unclear. Second, there is a possibility that PTR during BCT, was indicated for ‘more favorable’ patients and consequently showed better prognosis. On the other hand, patients with poor physical status could be included in BCT-only group. And the outcome of the patient group who received PTR before BV administration could be exaggerated, because patients’ data who did not receive BV administration after PTR were excluded, who might have severe morbidity or poor performance status. Although, severe complication rate after PTR-a is expected to be rare. Third, the molecular features of tumors as like MSI, RAS/BRAF mutation status, and several clinicopathological factors were not included in the analyses of this retrospective study. However, the effects of the variables are also not clear, and our variables as like young age, male, left-sidedness of the primary tumor, well differentiation, M1c, 2nd-line BV administration showed similar associations and hazard-ratios to OS compared to recent large-scale trials.[14,17,40–42]
The efficacy of PTR for unresectable mCRC is controversial. However, PTR in the patients who received BCT was associated with longer survival than that of the BCT-only patients, and PTR during BCT was also not associated with a significant delay of postoperative chemotherapy. PTR seems to be a good palliative treatment option for the unresectable mCRC patients to increase survival outcome, regardless of before and during the BCT. In our knowledge, this is the first study, which focused on the outcome of PTR in the patients who received BCT, and further studies should be performed to determine the optimal treatment approach for the unresectable mCRC patients.
Author contributions
Conceptualization: Seung-Seop Yeom, Young Jin Kim, Hyeong Rok Kim.
Data curation: Seung-Seop Yeom, Soo Young Lee, Young Jin Kim, Hyeong Rok Kim.
Formal analysis: Seung-Seop Yeom, Chang Hyun Kim, Hyeong Rok Kim.
Funding acquisition: Seung-Seop Yeom, Hyeong Rok Kim.
Investigation: Seung-Seop Yeom, Hyeong Rok Kim.
Methodology: Seung-Seop Yeom, Young Jin Kim, Hyeong Rok Kim.
Project administration: Seung-Seop Yeom, Hyeong Rok Kim.
Resources: Seung-Seop Yeom.
Software: Seung-Seop Yeom.
Supervision: Seung-Seop Yeom, Young Jin Kim, Hyeong Rok Kim.
Validation: Seung-Seop Yeom, Han Deok Kwak.
Visualization: Seung-Seop Yeom.
Writing – original draft: Seung-Seop Yeom.
Writing – review & editing: Seung-Seop Yeom.
Seung-Seop Yeom orcid: 0000-0003-3651-222X.
Footnotes
Abbreviations: BCT = bevacizumab-containing chemotherapy, BV = bevacizumab, mCRC = metastatic colorectal cancer, OS = overall survival, PTR = primary tumor resection.
How to cite this article: Yeom SS, Lee SY, Kwak HD, Kim CH, Kim YJ, Kim HR. The outcome of primary tumor resection in the unresectable stage IV colorectal cancer patients who received the bevacizumab-containing chemotherapy. Medicine. 2020;99:7(e19258).
All procedures were performed following the ethical standards of the institutional research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Institutional Review Board at the Chonnam National University Hwasun Hospital, Hwasun, South Korea approved the study (reference number CNUHH-2019-071) and was eligible for exemption of informed consent. No animal experiments were performed for this study.
This study was supported by a grant (HCRI 190005) Chonnam National University Hwasun Hospital Institute for Biomedical Science.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].de Mestier L, Neuzillet C, Pozet A, et al. Is primary tumor resection associated with a longer survival in colon cancer and unresectable synchronous metastases? A 4-year multicentre experience. Eur J Surg Oncol 2014;40:685–91. [DOI] [PubMed] [Google Scholar]
- [3].Kim CW, Baek JH, Choi GS, et al. The role of primary tumor resection in colorectal cancer patients with asymptomatic, synchronous unresectable metastasis: study protocol for a randomized controlled trial. Trials 2016;17:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw 2018;16:359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol 2009;10:559–68. [DOI] [PubMed] [Google Scholar]
- [6].Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res 2007;67:2729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 2013;14:29–37. [DOI] [PubMed] [Google Scholar]
- [8].Hegewisch-Becker S, Graeven U, Lerchenmuller CA, et al. Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol 2015;16:1355–69. [DOI] [PubMed] [Google Scholar]
- [9].Petracci E, Scarpi E, Passardi A, et al. Efficacy of bevacizumab in second-line versus first-line treatment of metastatic colorectal cancer: results from a new methodological approach based on the ITACa strategy trial. J Clin Oncol 2017;35:3546–13546. [Google Scholar]
- [10].Simkens LH, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet 2015;385:1843–52. [DOI] [PubMed] [Google Scholar]
- [11].Peeters CF, de Waal RM, Wobbes T, et al. Outgrowth of human liver metastases after resection of the primary colorectal tumor: a shift in the balance between apoptosis and proliferation. Int J Cancer 2006;119:1249–53. [DOI] [PubMed] [Google Scholar]
- [12].O’Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994;79:315–28. [DOI] [PubMed] [Google Scholar]
- [13].O’Reilly MS, Holmgren L, Chen C, et al. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med 1996;2:689–92. [DOI] [PubMed] [Google Scholar]
- [14].Ha GW, Kim JH, Lee MR. Meta-analysis of oncologic effect of primary tumor resection in patients with unresectable stage IV colorectal cancer in the era of modern systemic chemotherapy. Ann Surg Treat Res 2018;95:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee KC, Ou YC, Hu WH, et al. Meta-analysis of outcomes of patients with stage IV colorectal cancer managed with chemotherapy/radiochemotherapy with and without primary tumor resection. Onco Targets Ther 2016;9:7059–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Petrelli F, Coinu A, Ghilardi M, et al. Efficacy of oxaliplatin-based chemotherapy + bevacizumab as first-line treatment for advanced colorectal cancer: a systematic review and pooled analysis of published trials. Am J Clin Oncol 2015;38:227–33. [DOI] [PubMed] [Google Scholar]
- [17].Simillis C, Kalakouti E, Afxentiou T, et al. Primary tumor resection in patients with incurable localized or metastatic colorectal cancer: a systematic review and meta-analysis. World J Surg 2019;43:1829–40. [DOI] [PubMed] [Google Scholar]
- [18].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [19].Chan TW, Brown C, Ho CC, et al. Primary tumor resection in patients presenting with metastatic colorectal cancer: analysis of a provincial population-based cohort. Am J Clin Oncol 2010;33:52–5. [DOI] [PubMed] [Google Scholar]
- [20].Alawadi Z, Phatak UR, Hu CY, et al. Comparative effectiveness of primary tumor resection in patients with stage IV colon cancer. Cancer 2017;123:1124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Michel P, Roque I, Di Fiore F, et al. Colorectal cancer with non-resectable synchronous metastases: should the primary tumor be resected? Gastroenterol Clin Biol 2004;28:434–7. [DOI] [PubMed] [Google Scholar]
- [22].Tebbutt NC, Norman AR, Cunningham D, et al. Intestinal complications after chemotherapy for patients with unresected primary colorectal cancer and synchronous metastases. Gut 2003;52:568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Watanabe A, Yamazaki K, Kinugasa Y, et al. Influence of primary tumor resection on survival in asymptomatic patients with incurable stage IV colorectal cancer. Int J Clin Oncol 2014;19:1037–42. [DOI] [PubMed] [Google Scholar]
- [24].Raouf S, Bertelli G, Ograbek A, et al. Real-world use of bevacizumab in metastatic colorectal, metastatic breast, advanced ovarian and cervical cancer: a systematic literature review. Future Oncol 2019;15:543–61. [DOI] [PubMed] [Google Scholar]
- [25].Kleespies A, Fuessl KE, Seeliger H, et al. Determinants of morbidity and survival after elective non-curative resection of stage IV colon and rectal cancer. Int J Colorectal Dis 2009;24:1097–109. [DOI] [PubMed] [Google Scholar]
- [26].Konyalian VR, Rosing DK, Haukoos JS, et al. The role of primary tumour resection in patients with stage IV colorectal cancer. Colorectal Dis 2007;9:430–7. [DOI] [PubMed] [Google Scholar]
- [27].Stelzner S, Hellmich G, Koch R, et al. Factors predicting survival in stage IV colorectal carcinoma patients after palliative treatment: a multivariate analysis. J Surg Oncol 2005;89:211–7. [DOI] [PubMed] [Google Scholar]
- [28].Watt DG, Horgan PG, McMillan DC. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery 2015;157:362–80. [DOI] [PubMed] [Google Scholar]
- [29].Peeters CF, de Geus LF, Westphal JR, et al. Decrease in circulating anti-angiogenic factors (angiostatin and endostatin) after surgical removal of primary colorectal carcinoma coincides with increased metabolic activity of liver metastases. Surgery 2005;137:246–9. [DOI] [PubMed] [Google Scholar]
- [30].Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42. [DOI] [PubMed] [Google Scholar]
- [31].Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013–9. [DOI] [PubMed] [Google Scholar]
- [32].Bong JW, Lee JL, Kim CW, et al. Risk factors and adequate management for complications of bevacizumab treatment requiring surgical intervention in patients with metastatic colorectal cancer. Clin Colorectal Cancer 2018;17:e639–45. [DOI] [PubMed] [Google Scholar]
- [33].Bege T, Lelong B, Viret F, et al. Bevacizumab-related surgical site complication despite primary tumor resection in colorectal cancer patients. Ann Surg Oncol 2009;16:856–60. [DOI] [PubMed] [Google Scholar]
- [34].Black JM, Hodari KT, Rogers N, et al. Exudative, nonhealing scalp: a complication of systemic chemotherapy with capecitabine and bevacizumab. Arch Dermatol 2011;147:134–5. [DOI] [PubMed] [Google Scholar]
- [35].Eveno C, Passot G, Goere D, et al. Bevacizumab doubles the early postoperative complication rate after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2014;21:1792–800. [DOI] [PubMed] [Google Scholar]
- [36].Ley EJ, Vukasin P, Kaiser AM, et al. Delayed rectovaginal fistula: a potential complication of bevacizumab (avastin). Dis Colon Rectum 2007;50:930. [DOI] [PubMed] [Google Scholar]
- [37].Seet RC, Rabinstein AA, Lindell PE, et al. Cerebrovascular events after bevacizumab treatment: an early and severe complication. Neurocrit Care 2011;15:421–7. [DOI] [PubMed] [Google Scholar]
- [38].Colloca GA, Venturino A, Guarneri D. Early tumor shrinkage after first-line medical treatment of metastatic colorectal cancer: a meta-analysis. Int J Clin Oncol 2019;24:231–40. [DOI] [PubMed] [Google Scholar]
- [39].Ito M, Kusaba H, Mukaide S, et al. Early tumor shrinkage indicates a favorable response to bevacizumab-based first-line chemotherapy for metastatic colorectal cancer. Anticancer Drugs 2017;28:1166–73. [DOI] [PubMed] [Google Scholar]
- [40].John SK, Robinson SM, Rehman S, et al. Prognostic factors and survival after resection of colorectal liver metastasis in the era of preoperative chemotherapy: an 11-year single-centre study. Dig Surg 2013;30:293–301. [DOI] [PubMed] [Google Scholar]
- [41].Kim MS, Park EJ, Kang J, et al. Prognostic factors predicting survival in incurable stage IV colorectal cancer patients who underwent palliative primary tumor resection. Retrospective cohort study. Int J Surg 2018;49:10–5. [DOI] [PubMed] [Google Scholar]
- [42].Stillwell AP, Ho YH, Veitch C. Systematic review of prognostic factors related to overall survival in patients with stage IV colorectal cancer and unresectable metastases. World J Surg 2011;35:684–92. [DOI] [PubMed] [Google Scholar]


