Abstract
Rationale:
In the diagnostics of neuroendocrine tumors (NETs), scintigraphy and Single Photon Emission Computed Tomography/Computed Tomography (SPECT/CT) with 111Indium-Octreotide occupy a prominent place.
The introduction in clinical practice of 68Gallium-labelled somatostatin analogues (DOTA-TOC, DOTA-TATE, DOTA-NOC) for Positron Emission Tomography/Computed Tomography (PET/CT), significantly improved NETs diagnostics due to greater sensitivity and improved lesion detection in addition to better patient convenience and decreased radiation dose.
Patient concerns:
We report a case of a patient who was diagnosed with a neuroendocrine tumor of the ileocecal valve.
Diagnoses:
Diagnosis was made by ultrasonography, CT, and colonoscopy. Hystology after surgery was G2 NET of ileo-cecal valve. Restaging was carried out by 111In-Octreotide SPECT/CT and, 1 month later, by 68Ga-DOTATOC PET/CT. 18F-FDG PET/C was also carried out.
Interventions:
68Ga-DOTATOC PET/CT showed larger disease that modified disease management from surgery to medical treatment.
Outcomes:
After an initial improvement in the patient clinical condition, the tumor caused a worsening with the appearance of ascites.
Lessons:
68Ga-DOTA-conjugate PET/CT is appropriate in low and intermediate NET (Ki67 index respectively ≤3% and 3%–20%) characterized by better survival and better response after Peptide Receptor Radionuclide Therapy.
18F-FDG is mostly useful in high grade (G3) of disease, so that 68Ga-DOTA-conjugate SUV and 18F-FDG SUV have an opposite trend in relation to the tumor grade. 68Ga-DOTATOC PET/CT changes, as in our case, therapeutic management in about 40% of cases.
Keywords: 111In-Octreotide, 18F-FDG, 68Ga-DOTATOC, ileocecal-NET, neuroendocrine tumors, PET/CT
1. Introduction
Neuroendocrine tumors (NETs) originate from the widespread neuroendocrine system and, therefore, can arise in any body district. In two thirds of cases they can be born in the gastroenteropancreatic tract. In terms of frequency, NETs are usually considered rare neoplasms when compared to the corresponding non-neuroendocrine neoplasms.
The frequency of NETs is low, even if increasing in the last years (1–5 new cases/100,000 inhabitants / year), but the prevalence is high (35 cases/100,000 inhabitants) because most of them are well differentiated and slowly growing tumors with an indolent clinical course.[1] In Italy, the overall incidence rate is 4.15%, with 2697 new cases in 2015, without significant differences between males and females, and reaches 11.27% in the age group over 65 years. The overall prevalence rate is 40.73%, with 23,937 estimated prevalent cases, with an average 5-year survival of approximately 75%. Small intestine and the bowel account for about a quarter of all cases (25.3%).[2] NETs express a variable amount of 5 different somatostatin receptors (SSTR1-5) which have been used as targets for diagnostic radionuclide imaging and therapy.
Radiolabelled somatostatin, which could be the best probe, cannot be used because of its in vivo very short half-life (1–3 minutes); therefore, the octreotide derivate as somatostatin analogues endowed with a sufficient long half-life are employed. Scintigraphic detection was performed first by radioiodinated analogues[3] followed by 111Indium (In)- and 99mTechnetium(Tc)-radiolabelled analogues.[4] Further technical improvements were achieved by the use of SPET/CT hybrid scanner[5] which improved detection via a better target-to-background ratio and better anatomical localization due to CT images.
The subsequent availability of positron emitters radionuclides like 68Gallium (Ga) for PET/CT scan led to synthesis of radiolabelled analogues in which, the interposition of specific chelator agents such as di-ethylene-triaminepentaacetate (DTPA) or 1,4,7,10-tetraazacyclo-dodecane- 1,4,7,10-tetraacetic acid (DOTA) between somatostatin analogue and radionuclide made the link more stable.
PET/CT hybrid imaging with 68Ga-DOTA-conjugated (DOTA-TOC, DOTA-TATE and DOTA-NOC) overcomes, moreover, technical limitations of SPECT studies improving resolution and efficiency with more accurate images.
The amount of uptake, which reflects the receptors expression, is measured by a semi-quantitative index, the Standardized Uptake Value (SUV). SUV is the most used index in clinical PET/CT imaging, because it overcomes the differences in patients weight and size and among the amount of the injected dose of radiopharmaceuticals.
It has also a specific role in assessing disease behavior and response to different treatments. In 68Ga-DOTA-conjugates PET/CT, SUV reflects the amount of somatostatin receptors in neuroendocrine tumor.
Radiolabelling by beta or beta-gamma emitters radionuclides 90Yttrium (Y) or 177Lutetium (Lu) paved the way to Peptide Receptor Radionuclide Therapy (PRRT).[6]
Despite the different affinity of the 3 68Ga-DOTA-conjugates towards somatostatin receptors,[7] they have a comparable diagnostic value in the detection of NETs lesions, and nevertheless the approximately 10-fold higher affinity in binding SSTR2 of 68Ga-DOTA-TATE, which did not prove to be clinically relevant, SUVmax measurements with 68Ga-DOTA-TOC tended to be higher than their 68Ga-DOTA-TATE counterparts.[8]
68Ga-DOTA-conjugates proved to be superior to 111In-Octreotide SPECT/CT in NETs diagnostics, with a significant improvement in investigation time (90 minutes vs 24 hours), decreased radiation dose (3 mSv vs 9 mSv) and therefore, better patient convenience. In bowel NETs, moreover, the rate of positive lesions detected by 68Ga-DOTATATE PET/CT and 111In-Pentetreotide SPECT/CT was respectively 49/51 (96.1%) and 7/51 (13.7%) that led to change the management of 32.8% patients.[9]
18Fluoro-deoxy-glucose (F-FDG) PET/CT is also used in NETs diagnostics, but it is reserved to less differentiated and more aggressive tumors.[10] In 18F-FDG PET/CT, SUV reflects increasing of glucose metabolism by cancer cells and consequently the tumor proliferative activity.
18F-FDG and 68Ga-DOTA-conjugates have therefore different indications and their SUV values have an opposite trend in relation to the tumor grade.[11] In this report, we present a case of a patient with neuroendocrine tumor arisen on ileo-cecal valve who underwent a complete radionuclide diagnostic imaging beyond standard radiological imaging.
2. Case report
A 68-year-old man, due to anemia, performed ultrasound and abdominal CT which showed a voluminous 10-cm nodule in the right hepatic lobe and retropancreatic bulky disease imprinting the inferior vena cava at the renal confluence. Clear thickening of the walls of the last ileal loop was associated with involvement of the caecal fundus and of the root of the mesentery.
Colonoscopy showed an easily bleeding polylobed vegetative mass, which involved the ileocecal valve and almost completely obstructed the visceral lumen.
Laboratory tests showed chromogranin A levels > 50 nmol/L.
Biopsy and hystology results showed an ulcerated neuroendocrine tumor of the ileocecal valve with Ki67 index approximately 12% (G2 sec. WHO 2010) and hepatic metastasis.
Immunohistochemistry showed positivity for Cromogranin A, synaptophysin, NSE and CD56.
The tumor was therefore staged (TNM/AJCC) as pT4N1M1.
Patient underwent surgery with right hemicolectomy and right hepatectomy and subsequent treatment by “cold” somatostatin analogues.
One month after surgery, the patient underwent 111In-Octreotide SPECT-CT scan (118 MBq i.v.) which showed retropancreatic increased expression of somatostatin receptors without any other uptake areas (Fig. 1a).
Figure 1.
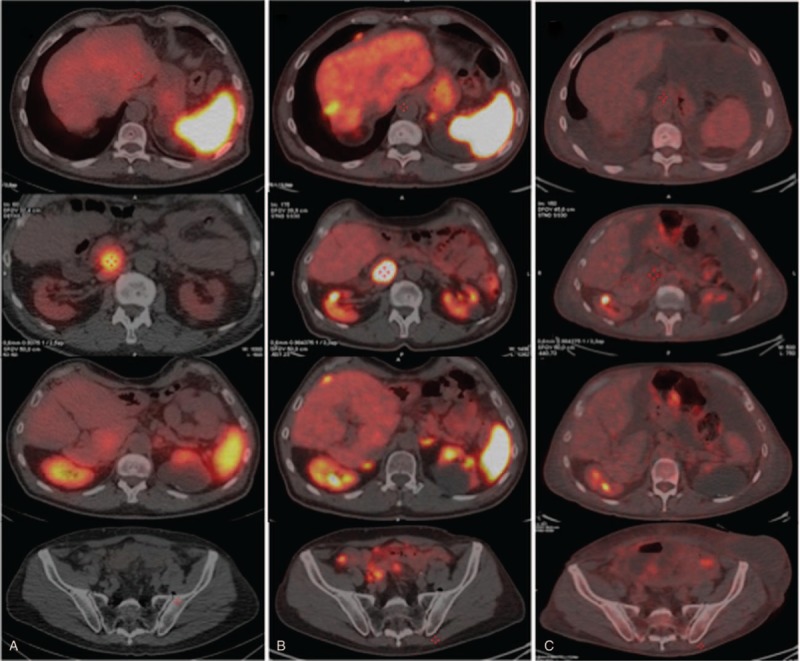
Restaging with 111In-Octreotide SPECT/CT scan (column a), 68Gallium-DOTATOC (column b), and 18F-FDG PET/CT scan (column c) of the patient, performed after colectomy and partial right hepatectomy and subsequent treatment by “cold” somatostatin. Since NET is a G2 tumor (Ki67 index 12%), positivity can observed in columns a and b. 111In-Octreotide SPECT-CT scan (column a) showed persistence of the retropancreatic uptake without other significant uptake areas. 68Ga-DOTATOC PET/CT scan (column b) confirmed uptake behind the pancreas head (SUV max 56.8) and showed multiple nodules of peritoneal implants in the periglissonian space, omentum, mesenteric region and pelvis (SUV max 13.8-15.4). 18F-FDG PET/CT scan (column c): metabolic activity in retropancreatic lesion was absent (SUV max 1.9) and mild pathological nodal uptake was observed in mesenteric region, with a conspicuous ascitic and pleural effusions.
The SPECT images were acquired on a dual-head gamma-camera (Discovery NM/CT 670, GE Healthcare, Little Chalfont, UK). Iterative method using ordered-subset expectation maximization (OSEM), with 2 iterations and 10 subsets, and Butterworth post-filter (0.48 critical frequency, power 10) were used for reconstruction procedure.
About a month later, the patient also underwent to 68Ga- DOTATOC PET/CT examination, while he was prepared for the surgical removal of the known retropancreatic mass. PET/CT scan was carried out by Discovery 710PET/CT (GE Healthcare, Little Chalfont, UK).
This radiopharmaceutical was synthesized in accordance with the European Pharmacopoeia (Gallium[68Ga] Edotreotide injection 01/2013: 2482 corrected 8.6) and 158 MBq were intravenously administrated to the patient.
Scan showed the known gross lymphnode mass in the right para-caval site, behind the head of the pancreas (SUV max 56.8) but also showed multiple nodular areas, as from peritoneal implants, in the peri-glissonian, omental, mesenteric and pelvic area (Fig. 1b). Moreover, inside the medial lip of the left adrenal gland, in the context of physiologic radiopharmaceutical uptake (SUV max 14.9), a nodular area compatible with adenoma was shown (SUV max 27.5) (Fig. 2).
Figure 2.
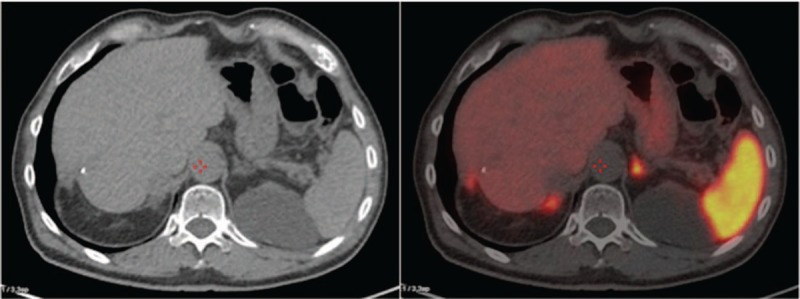
68Ga-DOTATOC PET/CT scan: as additional finding, in the medial lip of the left adrenal gland, surrounded by physiological uptake (SUV max 14.9), was found a nodular area suggesting adenoma (SUV max 27.5), confirmed by surgery.
These results modified the therapeutic course from surgical (curative secondary resection) to pharmacological treatment.
For completeness, 1 month later, we decided to carry out a 18F-FDG PET/CT scan (256 MBq i.v) (Fig. 1c) which showed a small but intense uptake (SUV max 7.8) in the left paracardiac area at the anterior segment of the lower lobe of the left lung, and uptake in some mesenteric nodes (Fig. 3).
Figure 3.
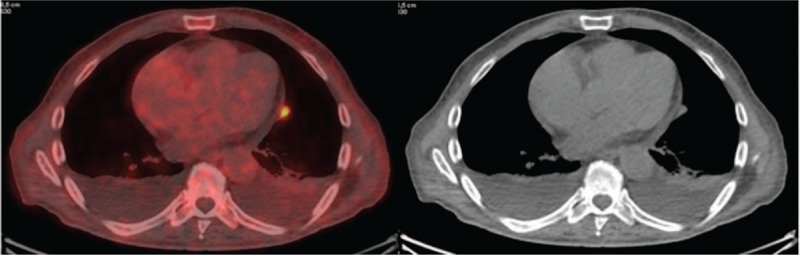
18F-FDG-PET/CT scan: small uptake in the left paracardiac area at the anterior segment of the lower lobe of the left lung not shown by 68Ga-DOTATOC PET/CT scan.
The Maximum Intensity Projection (MIP) images (Fig. 4), also known as volumetric images, summarize the physiological and pathological body distribution of the 2 PET radiopharmaceuticals (68Ga-DOTA-TOC on the left, 18F-FDG on the right).
Figure 4.
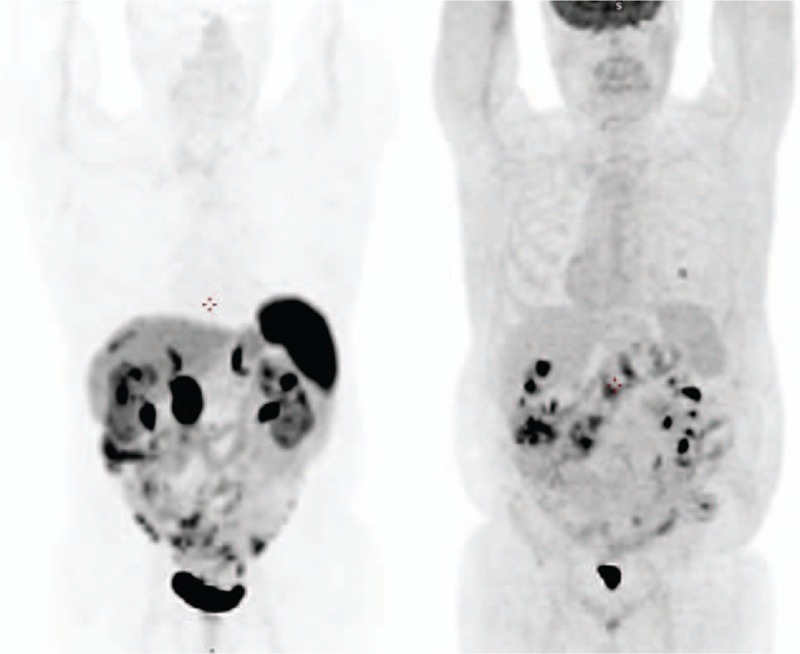
Maximum Intensity Projection (MIP) of 68Ga-DOTATOC PET/CT (left) and 18F-FDG PET/CT (right) showing the body physiological and pathological distribution of the 2 radiopharmaceuticals: the 2 images can be considered as complementary showing low and intermediate grade lesions (left image) and high grade lesions (right image) that can derive from tumor cell dedifferentiation or, as in the case of the pulmonary nodule, primary.
3. Discussion
Neuroendocrine neoplasms (NEYs) are a heterogeneous group of tumors that originate from neuroendocrine cells and grow in a lot of different organs, mainly in the gastrointestinal tract and the lungs.
Most of NETs express somatostatin receptors (SSTR), which can be used as targets for radionuclide imaging and therapy.
68Ga-DOTA-conjugates PET/CT showed higher sensitivity than 111In-Octreotide scintigraphy (96.1% vs 13.7% in bowel NETs)[9] due to its better intrinsic and physical resolution.
Another issue is the correct indication of use of these radiopharmaceuticals. For this purpose, it is very important to know the proliferative activity of the tumor, which is measured by the Ki67 index.
Radiolabelled somatostatin analogues are appropriate in NETs with low or intermediate proliferative activity grade. (Ki67 index respectively ≤3% and 3%–20%). Ki67 index well correlates with 69Ga-DOTA-conjugates uptake expressed by SUVmax, whereas 18F-FDG uptake is higher in tumors with high proliferative activity (Ki67 index >20%).[11]
68Ga-DOTA-conjugates SUVmax is, therefore, an important prognostic factor in G1 and G2 NETs because it well correlates with progression-free survival.[12,13]
Primary indications of radiolabelled somatostatin analogues are in diagnosis and staging, re-staging, monitoring response to treatments and more generally to determine the receptor status in order to adopt correct management decisions as well as patients selection for PRRT (with 177Lu or 90Y–DOTA-conjugates).
Secondary indication is to assess non-NETs with low or varying SSTR expression.[7,14]
PET/CT with 68Ga-DOTA-conjugates has a significant impact on general management which, in most of cases, about 40% in midgut NETs, was nonsurgical and more conservative.[15]
However, functional imaging is a high sensitive method and false-positive findings are always possible. These outcome triggers further tests with increased costs. Therefore, higher care must be taken to correlate the findings with the patient clinical conditions.[16]
The presented case as an excellent example of the diagnostic advantages of 68Ga-DOTA-TOC PET/CT in comparison with 111In-Octreotide SPECT/CT, and 18F-FDG PET/CT helps to highlight the various degrees of tumor differentiation during its spread. In the same patient we found also an adrenal adenoma. Even if adrenal medulla has a physiologic uptake of 68Ga-DOTA-conjugates, it is not rare to find, in course of PET/CT with these radiopharmaceuticals, other pathologic neuroendocrine tissue characterized by a higher SUVmax value[17,18] such as adenoma suggesting possible undiagnosed familiar NET syndromes.
Finally, 68Ga-DOTA-conjugates uptake, besides being a favorable prognostic indicator, is also a good prognostic index of response to PRRT.[19]
3.1. Statement
Patient has provided written informed consent for publication of the case. The authors would like to thank the patient for authorizing the use the medical information contained in the present article.
Author contributions
Conceptualization: Anna Tolomeo, Vincenzo Dimiccoli and Antonio Scilimati.
Data curation: Sergio Modoni.
Formal analysis: Sergio Modoni.
Investigation: Sergio Modoni.
Methodology: Sergio Modoni.
Supervision: Anna Tolomeo, Gaetano Lopolopo, Vincenzo Dimiccoli, Luana Perioli and Antonio Scilimati.
Validation: Gaetano Lopolopo, Vincenzo Dimiccoli, Luana Perioli and Antonio Scilimati.
Writing – original draft: Anna Tolomeo, Sergio Modoni.
Writing – review & editing: Anna Tolomeo, Gaetano Lopolopo, Vincenzo Dimiccoli, Luana Perioli and Antonio Scilimati.
Footnotes
Abbreviations: DOTA = 1,4,7,10-tetraazacyclododecane- 1,4,7,10-tetraacetic acid, DTPA = diethylenetriaminepentaacetate, MIP = Maximum Intensity Projection, NETs = neuroendocrine tumors, NOC = 1-Nal3-octreotide, PET/CT = Positron Emission Tomography(Computed Tomography, PRRT = Peptide Receptor Radionuclide Therapy, SPECT/CT = Single Photon Emission Computed Tomography/Computed Tomography, SUV = standardized uptake value, TATE = Tyr3-octreotate, TOC = Tyr3-octreotide.
How to cite this article: Tolomeo A, Lopopolo G, Dimiccoli V, Perioli L, Modoni S, Scilimati A. Impact of 68Ga-DOTATOC PET/CT in comparison to 111In-Octreotide SPECT/CT in Management of Neuro-Endocrine Tumors: a case report. Medicine. 2020;99:7(e19162).
The authors report no conflicts of interest.
References
- [1].Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes inpatients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brusco S, BUnozzi C, Mallone S, et al. Italian cancer figures – Report 2015: The burder of rare cancer in Italy. Epidemiologia e Prevenzione 2016;40:90–3. [DOI] [PubMed] [Google Scholar]
- [3].Krenning EP, Bakker WH, Breeman WA, et al. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet 1989;1:242–4. [DOI] [PubMed] [Google Scholar]
- [4].Bakker WH, Albert R, Bruns C, et al. [111In-DTPA-D-Phe1]-octreotide, a potential radiopharmaceutical for imaging of somatostatin receptor-positive tumors: synthesis, radiolabeling and in vitro validation. Life Sci 1991;49:1583–91. [DOI] [PubMed] [Google Scholar]
- [5].Perri M, Erba P, Volterrani D, et al. Octreo-SPECT/CT imaging for accurate detection and localization of suspected neuroendocrine tumors. Q J Nucl Med Mol Imaging 2008;52:323–33. [PubMed] [Google Scholar]
- [6].Krenning EP, Kwekkeboom DJ, Valkema R, et al. Peptide receptor radionuclide therapy. Ann NY Acad Sci 2004;1014:234–45. [DOI] [PubMed] [Google Scholar]
- [7].Bozkurt MF, Virgolini I, Balogova S, et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur J Nucl Med Mol Imaging 2017;44:1588–601. [DOI] [PubMed] [Google Scholar]
- [8].Poeppel TD, Binse I, Petersenn S, et al. 6868Ga-DOTATOC Versus 68Ga-DOTATATE PET/CT in Functional Imaging of Neuroendocrine Tumors. J Nucl Med 2011;52:1864–70. [DOI] [PubMed] [Google Scholar]
- [9].Sadowski SM, Neychev V, Millo C, et al. Prospective Study of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites. J Clin Oncol 2016;34:588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ezziddin S, Adler L, Sabet A, et al. Prognostic stratification of metastatic gastroenteropancreatic neuroendocrine neoplasms by 18F-FDG PET: feasibility of a metabolic grading system. J Nucl Med 2014;55:1260–6. [DOI] [PubMed] [Google Scholar]
- [11].Kayani I, Bomanji JB, Groves A, et al. Functional imaging of neuroendocrine tumors with combined PET/CT Using 68Ga-DOTATATE (Dota-DPhe1,Tyr3-octreotate) and 18F-FDG. Cancer 2008;112:2447–55. [DOI] [PubMed] [Google Scholar]
- [12].Campana D, Ambrosini V, Pezzilli R, et al. Standardized uptake values of (68)Ga-DOTANOC PET: a promising prognostic tool in neuroendocrine tumors. J Nucl Med 2010;51:353–9. [DOI] [PubMed] [Google Scholar]
- [13].Ambrosini V, Campana D, Polverari G, et al. Prognostic value of 68Ga-DOTANOC PET/CT SUVmax in patients with neuroendocrine tumors of the pancreas. J Nucl Med 2015;56:1843–8. [DOI] [PubMed] [Google Scholar]
- [14].Sundin A, Arnold R, Baudin E, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: radiological, nuclear medicine & hybrid imaging. Neuroendocrinology 2017;105:212–44. [DOI] [PubMed] [Google Scholar]
- [15].Evangelia Skoura E, Michopoulou S, Mohmaduvesh M, et al. The impact of 68ga-dotatate pet/ct imaging on management of patients with neuroendocrine tumors: experience from a national referral center in the United Kingdom. J Nucl Med 2016;57:34–40. [DOI] [PubMed] [Google Scholar]
- [16].Yu R, Wachsman A. Imaging of neuroendocrine tumors: indications, interpretations, limits, and pitfalls. Endocrinol Metab Clin North Am 2017;46:795–814. [DOI] [PubMed] [Google Scholar]
- [17].Hofman MS, Lau E, Hicks RJ. Somatostatin receptor imaging with 68ga dotatate pet/ct: clinical utility, normal patterns, pearls, and pitfalls in interpretation. RadioGraphics 2015;35:500–16. [DOI] [PubMed] [Google Scholar]
- [18].Chang CA, Pattison DA, Tothill RW, et al. 68Ga-DOTATATE and 18F-FDG PET/CT in paraganglioma and pheochromocytoma: utility, patterns and heterogeneity. Cancer Imaging 2016;16:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kratochwil C, Stefanova M, Mavriopoulou E, et al. SUV of [68Ga]DOTATOC-PET/CT predicts response probability of PRRT in neuroendocrine tumors. Mol Imaging Biol 2015;17:313–8. [DOI] [PubMed] [Google Scholar]


