Abstract
Aim:
To determine the risk factors related to hemorrhagic transformation in Chinese patients with acute ischemic stroke treated with intravenous thrombolysis.
Methods:
Studies published in different languages were retrieved by systematically searching PubMed, EMBASE, Vip, CNKI, and WanFang Data from the establishment of the library to December 31, 2018, as well as manually examining the references of the original articles. The outcome measures of efficacy covered risk factors. Safety evaluation was measured by relative ratio of complications.
Results:
A total of 36 studies involving 5597 participants were covered in this meta-analysis. The results indicated that age [WMD = 2.44, 95% CI (1.39,3.48)], male [OR = 1.21, 95% CI (1.02, 1.44)], diabetes [OR = 2.05, 95%CI (1.72,2.44)], atrial fibrillation [OR = 2.85, 95%CI (2.40, 3.39)], previous stroke [OR = 1.8, 95%CI (1.33, 2.44)], onset to treatment time (OTT) [WMD = 3.74, 95%CI (2.91, 4.58)], National Institute of Health stroke scale scores (NIHSS) [WMD = 4.17, 95% CI (3.37, 4.97)], infarct size [WMD = 4.11, 95% CI (3.15, 5.37)], ischemic signs of computed tomography (CT) [OR = 3.49, 95%CI (2.47, 4.93)] were associated with increased risk of hemorrhagic transformation after intravenous thrombolysis.
Conclusion:
The systematic review showed that male, age, diabetes, NIHSS, OTT, atrial fibrillation, post stroke, infarct size, and ischemic signs of CT were significantly correlated with hemorrhagic transformation (HT).
PROSPERO Registration number:
CRD42019127499.
Keywords: acute ischemic stroke, hemorrhagic transformation, intravenous thrombolysis, meta-analysis, risk factor
1. Introduction
Stroke is prevalent worldwide, which has been listed as the third most deadly disease.[1] According to the report of World Health Organization (WHO), there are 15 million people suffering from stroke every year.[2] Acute ischemic stroke is the most common type,[3] with the rate mortality >50%.[4] Thrombolysis is an effective treatment of acute ischemic stroke. The safety and efficacy of intravenous thrombolysis for acute ischemic stroke have been studied and recognized by multinational guidelines.[5] However, the data from the Chinese National Stroke Registry indicates that only 1.6% of patients receive rt-PA treatment in China.[6] One of the main reasons for therapy is that intravenous thrombolysis increases the risk of hemorrhagic transformation (HT). It can affect the efficacy and safety of thrombolysis, cause rapid deterioration and death of patients, and hinder the promotion of thrombolytic therapy. Some meta-analyses identified older age, higher neurological impairment, higher plasma glucose, antiplatelets, statins, computed tomography changes of acute ischemic stroke, leukoaraiosis, and the presence of atrial fibrillation, diabetes, previous ischemic heart or cerebral vascular diseases, and congestive cardiac failure as potential risk factor for HT,[7]which were based on Western populations. However, the influential factors may vary with race, geography, and others. Hence, a systematic review of studies relevant to present practice based on a Chinese population study was conducted to clearly identify risk factors that may cause HT. If these risk factors can be decrease, it may reduce the occurrence of cerebral hemorrhage transformation in Chinese patients after thrombolysis.
2. Materials and methods
2.1. Search strategy
A systematic and comprehensive search of data was performed on PubMed, EMB-A-SE, Vip, CNKI, and Wanfang Database in terms of the literature published till December 31, 2018, without language restriction. Five search themes were combined using the Boolean operator “and” and “or.” Search terms included “stroke or ischemic stroke or cerebrovascular disorders or brain infarction or cerebral infarction or intracranial embolism or thrombosis” and “intravenous thrombolysis or thrombolysis or tissue plasminogen activator or alteplase” and “brain hemorrhage or bleeding or HT” and “risk factor or correlative factor or relevant factor” and “Chinese or China.” This study is a system review, without intervention and control measures, and has no effect on patients. Therefore, ethical requirements are unnecessary.
2.2. Eligibility criteria
Inclusion criteria:
-
1.
Study type: case–control study or cohort study; retrospective or prospective design;
-
2.
Subject: Intravenous thrombolysis;
-
3.
Exposure factors: having similar definitions regarding each exposure factor, including demographic characteristics: age, gender, past health Condition: Hypertension, diabetes, heart disease (atrial fibrillation, coronary atherosclerotic heart disease, congestive heart failure or myocardial infarction), history of cerebrovascular disease (transient ischemic attack, hemorrhage or ischemic stroke);
-
4.
Outcome indicators: factors related to cerebral hemorrhage conversion.
Exclusion criteria:
-
1.
Cross-sectional study, no control, and only with clues of risk factors;
-
2.
Outcome indicators include extracranial hemorrhage events;
-
3.
Surgical intervention;
-
4.
Secondary study: such as a review of the original study or a literature review;
-
5.
Repeated reports; comments; abstracts, or no data that can be extracted from the study or with data error.
2.3. Studies
The research design was based on community, population or registered longitudinal cohort studies that reported relative impact estimates, such as Odds Ratio (ORs).
2.4. Study selection
Two authors independently evaluated potentially eligible studies that were identified by our search. Articles were screened for eligibility based on a review of title and abstract only, and disagreements were resolved by consensus. Regarding the remaining papers, their full text was accessed and read independently by the two reviewers mentioned above. The differences of opinion between reviewers were resolved by discussion with a third member of the research team, and the consensus was thereby reached.
2.5. Data collection
A standardized data collection sheet was used to extract all data. One author (Wen) extracted data from the included studies and another (Zhang) used statistical software to check the accuracy of inclusion. Any disagreement was resolved through discussions with the other authors. The data were extracted from each eligible study as follows: first author, year of publication, the number of cases, and risk factors. HT was defined according to the Neurological Disorders and Stroke (NINDS) criteria.[8]
2.6. Statistical analysis
For studies with sufficient quality data and similar simulation learning and result measurements, we used Newcastle Ottawa scale (NOS) for quality assessment of case–control or cohort studies in the current meta-analysis and combined the data in a meta-analysis to provide a summary effect estimate. All data were entered into RevMan 5.3, and the normalized deviation and 95% CI were calculated. Pooled ORs for categorical data, weighted mean differences (WMDs) for continuous data and 95% CI were estimated.[9]
For meta-analyses, I2 statistics were used for heterogeneity testing. The degree of inconsistency between the measurements was roughly interpreted as the proportion of total variation between studies attributable to heterogeneity rather than chance. When I2 < 50%, a fixed effect model was applied. A random effect model was performed when the existing statistical heterogeneity was measured by I2 > 50%.
3. Result
The document retrieval and screening process are shown in the flowchart (Fig. 1). The electronic search of PubMed, EMBASE, Vip, CNKI, and Wanfang Database provided a total of 768 citations, and 13 citations were found manually. After removing duplicate manuscripts, 559 studies remained, among which 423 were excluded according to the review of title and abstract. Then 136 studies were left for full-text reviews regarding eligibility, 55 of which were excluded because of unqualified study design and result measurements. A total of 81 studies met all the criteria and were selected for initial inclusion; after review, 45 studies were excluded for duplicates, reviews, and literature reports. Eventually, 36 articles[10–45] were included in the final analysis (Table 1).
Figure 1.
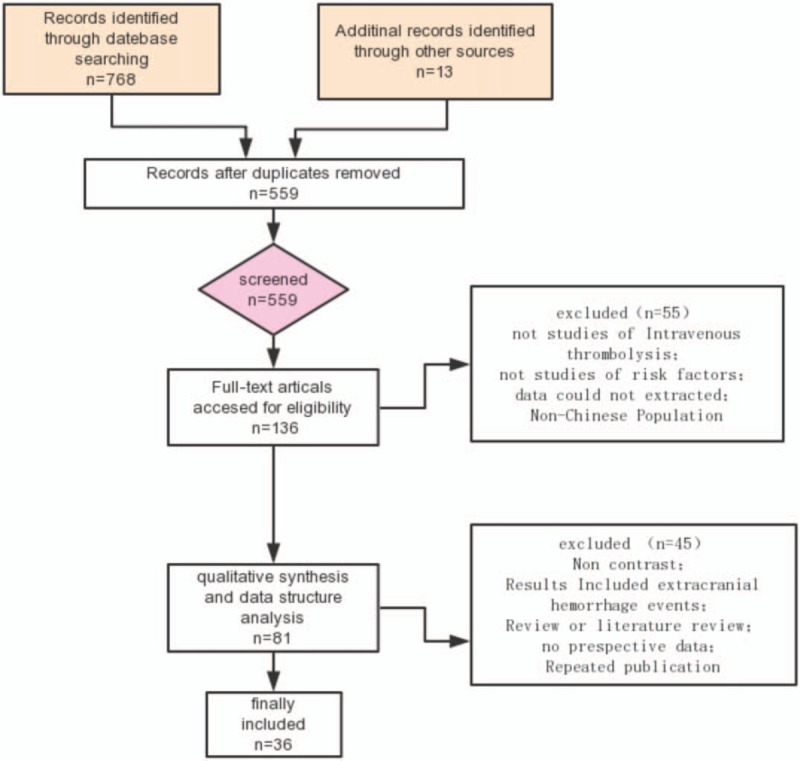
Flow diagram of retrieved, screened, and included studies.
Table 1.
Basic characteristics of the included studies.
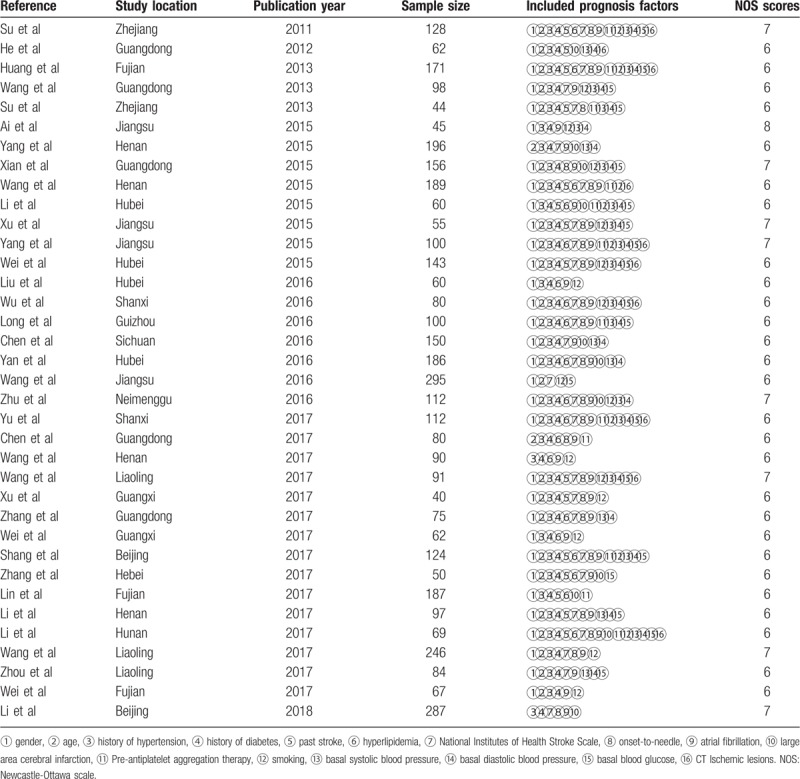
3.1. Study characteristics
For the risk factors mentioned in the 36 studies, the selected studies included 4575 cases, HT total of 1023 cases. A total of 22 risk factors related to cerebral hemorrhage after ischemic stroke were investigated. Among them, 16 factors such as age were mentioned in more than 10 studies, which hence were further analyzed and discussed; other risk factors were only mentioned in a few studies, and hence excluded in order to avoid the occurrence of bias. Table 1 depicts the basic characteristics and risk factors for HT of the included studies.
3.2. Meta-analysis results
3.2.1. Age
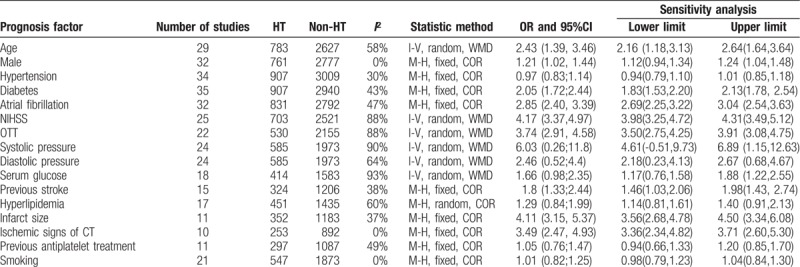
Total of 29 studies reported age. The heterogeneity among the studies was high (X2 = 66.98, I2 = 58%), a random effect model was applied. The result found that older age (WMD = 2.43, 95% CI [1.39, 3.46]) was significantly associated with HT.
3.2.2. Sex
Total of 32 studies reported sex (male). There was no heterogeneity among the studies (X2 = 30.45, I2 = 0%), the fixed effect model was used. The results found that male (OR = 1.21, 95% CI [1.02, 1.44]) was associated with risk of HT.
3.2.3. Hypertension and diabetes
Total of 35 articles studied hypertension and diabetes. There was less heterogeneous among the trials (X2 = 48.44, I2 = 30%) and (X2 = 57.72, I2 = 43%), a fixed effect model were used. The results of meta-analysis demonstrated that hypertension (OR = 0.97, 95%CI [0.83, 1.14]) was not associated with HT, diabetes (OR = 2.05, 95%CI [1.72, 2.44]) was associated with HT.
3.2.4. Atrial fibrillation
Total of 32 studies considered atrial fibrillation. There was moderate heterogeneity (X2 = 59.04, I2 = 47%), a fixed effect model was used for analysis. The results found that atrial fibrillation (OR = 2.85, 95% CI [2.40, 3.39]) was related to HT.
3.2.5. Stroke severity
Total of 25 studies reported the National Institute of Health stroke scale (NIHSS). The heterogeneity among the studies was high (X2 = 202.56, I2 = 88%), a random effect model was used. The results show that NIHSS (WMD = 4.17, 95% CI [3.37, 4.97]) was related to HT.
3.2.6. Onset to treatment time
Total of 22 studies evaluated onset to treatment time (OTT). The heterogeneity among the studies was high (X2 = 178.02, I2 = 88%), a random effect model was applied. The results showed that OTT (WMD = 3.74, 95%CI [2.91, 4.58]) was associated with HT obviously.
3.2.7. Infarct size
Total of 11 studies evaluated large-area infarction. The heterogeneity among the studies was high (X2 = 15.89, I2 = 37%), a fixed effect model was used. The results found that the infarct size (WMD = 4.11, 95% CI [3.15, 5.37]) was associated with an increased risk of HT.
3.2.8. Ischemic signs of CT
Total of 10 studies reported ischemic signs of CT. There was no heterogeneity among the studies (X2 = 3.55, I2 = 0%), a fixed effect model was used. The results found that early ischemic signs of CT (OR = 3.49, 95% CI [2.47, 4.93]) were significantly associated with HT.
3.2.9. Previous stroke
A total of 15 studies analyzed previous stroke. There was low heterogeneity among the studies (X2 = 22.5; I2 = 38%), a fixed effect model was used. The results indicated that previous stroke (OR = 1.8, 95%CI [1.33, 2.44]) was associated with HT.
3.2.10. Blood pressure and serum glucose level on admission
Systolic blood pressure, diastolic blood pressure, and blood glucose levels were studied in a partial inclusion study. The heterogeneities (X2 = 237.19, I2 = 90%), (X2 = 63.64, I2 = 64%) and (X2 = 260.63, I2 = 93%) were high among the studies, the random effect model were adopted. Meta-analysis showed that systolic blood pressure (WMD = 6.03, 95%CI [0.26, 1.8]), diastolic blood pressure (WMD = 2.46, 95%CI [0.52, 4.4]), and basal blood glucose (WMD = 1.66, 95%CI [0.98, 2.35]) were not associated with HT.
3.2.11. Previous antiplatelet treatment
The results indicated that previous antiplatelet treatment (OR = 1.05, 95%CI [0.76, 1.47]) was not significantly associated with HT.
3.2.12. Hyperlipidemia
The results indicated that hyperlipidemia (OR = 1.29, 95%CI [0.84, 1.99]) was not associated with HT.
3.2.13. Smoking
The results found that smoking (OR = 1.01, 95%CI [0.82, 1.25]) was not significantly associated with HT.
3.3. Sensitivity analysis and meta-regression
We conducted a sensitivity analysis by excluding every single study to explore the stability of the combined results. The range of the combined ORs or WWMDs for potential risk factors is shown in Table 2.
Table 2.
Heterogeneity and sensitivity analysis of prognosis factors among included studies.
4. Discussion
HT is considered to be the most serious complication of intravenous thrombolysis in ischemic stroke. The incidence rate of symptomatic intracranial hemorrhage is 2.2% to 8% across the world and 4.87% to 7.3% in China,[46] increasing disability and mortality. Therefore, it is important to find out the risk factors of HT. Some studies on HT risk factors have drawn different conclusions. For example, Su[10] and others proposed that the history of atrial fibrillation and hyperlipidemia were important factors of HT, but Chen[26] and others failed to find such a correlation. These disputes and other similar divergences indicate the need for a meta-analysis of this important topic. The meta-analysis was performed among 4575 patients undergoing thrombolytic therapy. It showed that age, male, diabetes, NIHSS, OTT, atrial fibrillation, stroke, infarct size, and ischemic signs of CT were significantly correlated with HT; in contrast, targeting Western populations, meta-analyses showed that older age, higher neurological impairment, higher plasma glucose, antiplatelets, statins, computed tomography changes of acute ischemic stroke, leukoaraiosis, and the presence of atrial fibrillation, diabetes, previous ischemic heart or cerebral vascular diseases, and congestive cardiac failure were significantly correlated with HT.[7]
The high incidence of HT after thrombolysis in patients with high NIHSS before thrombolysis was confirmed by many large studies at home and abroad.[47,48] NINDS studies have shown that the baseline stroke severity as indicated by NIHSS was an independent risk factor for HT, and an NIHSS score >25 was considered a contraindication to thrombolysis.[49]
The European Collaborative Acute Stroke Study (ECASS) I phase study also suggested that NIHSS score before thrombolysis was significantly correlated with HT, and that the disadvantage of rt-PA treatment for an ischemic area >1/3 of the middle cerebral artery distribution was more obvious than the advantage.[48,50] Therefore, according to the results of these studies, ischemic stroke with high NIHSS score (NIHSS score > 18) should be carefully weighed for intravenous thrombolysis.
The studies by Xuemei Mu et al[51] calculated the incidence of HT in patients with massive cerebral infarction to be 29.4%, much higher than that in patients with lacunar infarction (0.94%). The reason may be that that the larger the area of cerebral infarction, the secondary cerebral edema to oppress the surrounding blood vessels, and the corresponding autophagy and elevated plasma TAT levels may lead to the heavier secondary injury in patients with cerebral hemorrhage.[52] It hence is more likely to increase the permeability of the vascular wall and cause post-reperfusion hemorrhage when the vessels are recanalized.
This study found that the incidence of HT after thrombolysis was higher in patients with early ischemic changes on CT before thrombolysis. Tanne et al[50] found that the incidence of HT in patients with early ischemic changes on CT before thrombolysis was 5.63 times higher than that in patients without ischemic changes. Many studies at home and abroad confirmed that ischemic changes on CT before thrombolysis were an independent risk factor for HT after thrombolysis.[47,53,54] Therefore, this study suggested that thrombolysis should be carefully selected for patients with early ischemic changes on CT before thrombolysis, so as to reduce the occurrence of HT and improve the thrombolytic effect.
Leukoplakia was also considered as a risk factor for risk of hemorrhagic stroke in Reference 55.[55] However, there is less research literature, so no analysis.
If some of these risk factors can be avoided or prevented, it will help to better select thrombolytic indications and improve the safety of thrombolytic therapy for Chinese patients, thus reducing bleeding and enhancing efficacy.
5. Limitations
There are some differences in the experimental design among the included studies. In some studies, specific values for infarct size were not mentioned, and some of the inter-study analysis indicators were not uniform, which may affect the quality of meta-analysis. In order to demonstrate its significant advantages, subgroup analysis is needed to identify these findings.
6. Conclusion
The systematic review showed that male, age, diabetes, NIHSS, OTT, atrial fibrillation, post stroke, infarct size, and ischemic signs of CT were significantly associated with a higher risk of HT. Given the risk of bias, these results should not justify withholding intravenous thrombolysis.
Acknowledgments
We thank the authors and participants of the included studies for their important contributions.
Author contributions
Conceptualization: Xiaoyun Zhang.
Data curation: Song Zhang.
Formal analysis: Song Zhang, Kunzhen Wan.
Funding acquisition: Xiaoyun Zhang, Hong Zhang.
Software: Kunzhen Wan.
Validation: Xiaoyun Zhang, Hong Zhang.
Visualization: Xiaoyun Zhang, Kunzhen Wan.
Writing – original draft: Kunzhen Wan.
Footnotes
Abbreviations: CI = confidence interval, CT = computed tomography, ECASS = European Collaborative Acute Stroke Study, HT = hemorrhagic transformation, NIHSS = National Institute of Health stroke scale, NINDS = Neurological Disorders and Stroke, NOS = Newcastle Ottawa scale, OR = odds ratio, OTT = Onset to treatment time, WHO = World Health Organization, WMD = weighted mean differences.
How to cite this article: Wen L, Zhang S, Wan K, Zhang H, Zhang X. Risk factors of haemorrhagic transformation for acute ischaemic stroke in Chinese patients receiving Intravenous thrombolysis: A meta-analysis. Medicine. 2020;99:7(e18995).
Key R&D Projects in Sichuan Province (Clinical Program Optimization and Evaluation of Major Chronic or Refractory Diseases in Prevention and Treatment of Traditional Chinese Medicine [TCM]): Evaluation and Optimization of TCM Comprehensive Treatment Program in Acute Cerebral Hemorrhage2019YFS0082.
The authors have no conflicts of interest to disclose.
References
- [1].Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016;133:e38–60. [DOI] [PubMed] [Google Scholar]
- [2].European Stroke Initiative Executive EUSI Writing Committee, Olsen TS, Langhorne P, et al. European stroke initiative recommendations for stroke management-update 2003. Cerebrovasc Dis 2003;16:311–37. [DOI] [PubMed] [Google Scholar]
- [3].Feigin VL, Lawes CM, Bennett DA, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009;8:355–69. [DOI] [PubMed] [Google Scholar]
- [4].Feigin V, Lawes C, Bennett D, et al. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol 2003;2:43–53. [DOI] [PubMed] [Google Scholar]
- [5].Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004;363:768–74. [DOI] [PubMed] [Google Scholar]
- [6].Liu L, Wang D, Wong KS, et al. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke 2011;42:3651–4. [DOI] [PubMed] [Google Scholar]
- [7].William N. Whiteley, Karsten Bruins Slot, Peter Fernandes, et al. Risk factors for intracranial hemorrhage after rtPA. Stroke 2012;43:2904–9. [DOI] [PubMed] [Google Scholar]
- [8].Kwiatkowski TG, Libman RB, Frankel M, et al. Effects of tissue plasminogen activator for acute ischemic stroke at one year. N Engl J Med Overseas Ed 1999;340:1781–7. [DOI] [PubMed] [Google Scholar]
- [9]. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. Available at: http://training.cochrane.org/handbook [Accessed November 14, 2011]. doi: 10.1002/14651858.CD008754. [Google Scholar]
- [10].Su M, Yang W, Wang WH, et al. Risk factors of hemorrhagic transformation and risk induced by intravenous thrombolysis with recombinant tissue plasminogen activator in acute ischemic stroke. Chin J Neurol 2011;44:754–8. [Google Scholar]
- [11].He JJ, Shang WJ, Wu Q, et al. Clinical analysis of hemorrhagic transformation after intravenous thrombolytic therapy for cerebral infarction. Chin J Neuroimmunol Neurol 2012;19:170–8. [Google Scholar]
- [12].Huang YH, Li MM, Chen ZJ, et al. Risk factors of hemorrhage transformation after intravenous thrombolysis in acute ischemic stroke [M]. Chin J Neurol Psychiatric Dis 2013;39:581–6. [Google Scholar]
- [13].Wang SF, Xiao WM, Wu ZQ. Analysis of related factors of early cerebral hemorrhage transformation after intravenous thrombolysis in acute cerebral infarction. J Gannan Med Univ 2013;33:199–202. [Google Scholar]
- [14].Su LJ, Xu J, Xu HY, et al. Clinical analysis of intravenous thrombolysis for hemorrhagic transformation after cerebral infarction. Prevent Treatment Cardio Cerebral Vasc Dis 2013;13:390–1. [Google Scholar]
- [15].Ai HJ. Intracerebral hemorrhage post-thrombolytic therapy in acute ischemic stroke. Jiangsu Southeast Univ 2015;13:390–1. [Google Scholar]
- [16].Yang LJ. Risk factors of intravenous thrombolytic hemorrhage after acute stroke. Chin J Pract Neurol Dis 2015;18:94–5. [Google Scholar]
- [17].Xian WC, Zhu YC, Zhang HT, et al. Hemorrhagic transformation after intravenous thrombolysis in patients with ischemic stroke. Chin J Gerontol 2015;35:110–1. [Google Scholar]
- [18].Wang Q. Analysis of related factors affecting hemorrhagic transformation after intravenous thrombolysis in patients with acute ischemic stroke. Med Forum Mag 2015;36:115–6. [Google Scholar]
- [19].Li M, Li HH, Luo WJ, et al. Risk factors and risk of hemorrhage transformation after intravenous thrombolysis in cerebral infarction [M]. Neural Injury Funct Reconstr 2015;10:284–7. [Google Scholar]
- [20].Xu YP, Sun YM, Liu CF. Arteplase intravenous thrombolysis for acute cerebral infarction analysis of related factors of transformation of cerebral hemorrhage. Jiangsu Med J 2015;41:2297–300. [Google Scholar]
- [21].Yang RH, Fan WZ, Zhang LH, et al. Influencing factors of hemorrhagic transformation in patients with acute cerebral infarction after intravenous thrombolysis. Pract J Cardiac Cerebral Pneumal Vasc Dis 2015;23:16–9. [Google Scholar]
- [22].Wei H, Yu YF, Zhou R, et al. Clinical study on predicting hemorrhagic transformation after thrombolysis in acute ischemic stroke by HAT, SEDAN score and related risk factors of cerebrovascular disease. Chin J Mod Neurol Dis 2015;15:126–32. [Google Scholar]
- [23].Liu X, Li XR, Wen HB, et al. risk factors of hemorrhagic transformation after intravenous thrombolysis in patients with acute cerebral infarction. Med Recapitulate 2016;22:1228–30. [Google Scholar]
- [24].Wu J, Fu T. Analysis of related factors of intracerebral hemorrhagic transformation in patients with acute cerebral infarction after intravenous thrombolysis with urokinase. Mod J Integr Tradit Chin Western Med 2016;25:2758–60. [Google Scholar]
- [25].Long HC, Peng GG, Chen Y, et al. Influencing factors of hemorrhage transformation after thrombolysis in patients with cerebral infarction. China J Mod Med 2016;26:107–10. [Google Scholar]
- [26].Chen J. Hemorrhagic transformation after intravenous thrombolysis in patients with ischemic stroke. Med Frontier 2016;6:172–3. [Google Scholar]
- [27].Yan XQ, Tan Q, Yu DF, et al. risk factors of hemorrhagic transformation after intravenous thrombolysis in patients with acute cerebral infarction. Neural Injury Funct Reconstr 2016;11:432–3. [Google Scholar]
- [28].Wang C, Chen GF, Liu WW, et al. Risk factors of hemorrhagic transformation after thrombolysis in patients with cerebral infarction. Pract J Cardiac Cerebral Pneumal Vasc Dis 2016;24:37–9. [Google Scholar]
- [29].Zhu RX, Yuan J, Li P, et al. Analysis of related factors of intracerebral hemorrhage transformation after intravenous thrombolysis in acute cerebral infarction. Beijing Med 2016;38:429–32. [Google Scholar]
- [30].Yu F, Wang XP. Independent risk factors for hemorrhagic transformation in patients with acute cerebral infarction after intravenous thrombolysis. J Clin Med Pract 2017;21:87–8. [Google Scholar]
- [31].Chen Y, Dou Z, Xu WW, et al. Risk factors and risk of symptomatic hemorrhage transformation in patients with severe acute ischemic stroke after intravenous thrombolysis. Chin J Geriatr Heart Brain Vessel Dis 2017;19:914–7. [Google Scholar]
- [32].Wang H, Wang LC. Analysis of risk factors related to cerebral hemorrhage transformation after intravenous thrombolysis in acute cerebral infarction. China Foreign Med Treatment 2017;5:47–9. [Google Scholar]
- [33]. Wang CL. Risk factors and predictive model of hemorrhage transformation after thrombolysis in cerebral infarction. Liaoning, China: China Medical University; 2017. [Google Scholar]
- [34].Xu HY, Zhong SW, Li JF. Influencing factors of hemorrhage transformation after thrombolysis in patients with acute cerebral infarction. Prevent Treatment Cardio Cerebral Vasc Dis 2017;17:395–7. [Google Scholar]
- [35].Zhang JY, Guan QS, Zhou HH. Relevant factors of hemorrhage after thrombolysis in patients with acute cerebral infarction. J Mathemat Med 2017;30:320–2. [Google Scholar]
- [36].Wei XY, Wei SR. Analysis of related factors of hemorrhage transformation after intravenous thrombolysis in patients with cerebral infarction. Contemp J Clin Med 2017;30:2896–7. [Google Scholar]
- [37].Shang JY, Li XF, Zhao H, et al. Analysis of related factors of hemorrhagic transformation after intravenous thrombolysis of ateplase in patients with acute cerebral infarction. J Guangxi Med Univ 2017;34:1009–12. [Google Scholar]
- [38].Zhang J, Fu HX, Zhao XL, et al. risk factors of hemorrhage transformation after intravenous thrombolysis in cerebral infarction. Hebei Med J 2017;39:3737–40. [Google Scholar]
- [39].Lin SL. Risk factors of hemorrhagic transformation after intravenous thrombolysis in acute ischemic stroke. Chin J School Doctor 2017;31:371–2. [Google Scholar]
- [40].Li F. Analysis of the effect of intravenous thrombolysis with ateplase and the influencing factors of hemorrhagic transformation in 97 patients with acute cerebral infarction. Shanghai Medical Journal 2017;38:25–7. [Google Scholar]
- [41].Li YP. Relevant factors of intravenous thrombolysis for transformation of cerebral hemorrhage after acute cerebral infarction. Chin J Clin Ration Drug Use 2017;10:24–6. [Google Scholar]
- [42].Wang MX, Fan TP, Wang XD, et al. risk factors of hemorrhage transformation after intravenous thrombolysis in patients with acute cerebral infarction. Chin J Postgrad Med 2017;40:731–4. [Google Scholar]
- [43].Zhou BB, Wang XH, Yang J. Risk factors of hemorrhagic transformation after intravenous thrombolysis with recombinant tissue plasminogen activator in patients with acute cerebral infarction. J China Med Univ 2017;46:1101–4. [Google Scholar]
- [44].Wei XF, Che CH, Liu CY, et al. Risk factors of hemorrhagic transformation after intravenous thrombolysis in patients with acute ischemic stroke. Chin J Clin Ration Drug Use 2017;10:15–6. [Google Scholar]
- [45].Li JY, Zhang CP, Wang SA, et al. Analysis of related factors of early cerebral hemorrhage transformation after intravenous thrombolysis in acute cerebral infarction. J Clin Emerg (China) 2018;19:456–9. [Google Scholar]
- [46].Liu M, Pan Y, Zhou L, et al. Predictors of post-thrombolysis symptomatic intracranial hemorrhage in Chinese patients with acute ischemic stroke. PLoS One 2017;12:e0184646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Aviv RI, d’Esterre CD, Murphy BD, et al. Hemorrhagic transformation of ischemic stroke: prediction with CT perfusion. Radiology 2009;250:867–77. [DOI] [PubMed] [Google Scholar]
- [48].Singer OC, Berkefeld J, Lorenz MW, et al. Risk of symptomatic in tracerebral hemorrhage in patients treated with intra-arterial thrombolysis. Cerebrovasc Dis 2009;27:368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA Therapy for lschenfic Stroke. Stroke 1997;28:2109–18. [DOI] [PubMed] [Google Scholar]
- [50].Cocho D, Borrell M, Martí-Fàbregas J, et al. Pretreatment hemostatic markers of symptomatic intracerebral hemorrhage in patients treated with tissue plasminogen activator. Stroke 2006;37:996–9. [DOI] [PubMed] [Google Scholar]
- [51].Wu C, Yan X, Liao Y, et al. Increased perihematomal neuron autophagy and plasma thrombin–antithrombin levels in patients with intracerebral hemorrhage: an observational study. Medicine 2019;98:e17130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mu XM. Analysis of related factors of hemorrhagic transformation of cerebral infarction i. Med Theory Pract 2006;19:1292. [Google Scholar]
- [53].Tanne D, Kasner SE, Demchuk AM, et al. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice the ulticenter rt-PA Stroke Survey. Circulation 2002;105: j 1679-1685. [DOI] [PubMed] [Google Scholar]
- [54].Larrue V, von Kummer RR, Müller A, et al. risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (CASS II). Stroke 2001;32:438–41. [DOI] [PubMed] [Google Scholar]
- [55].Liang Y, Zhang J. Stroke and white matter disease. Neurol Injury Funct Reconstr 2014;9:512–4. [Google Scholar]


