Abstract
Elderly individuals with non-dipper hypertension are at high risk of cardiovascular disease because of increased stiffness of peripheral arteries. Since, vitamin D deficiency is prevalent in elderly Chinese. We examined whether reduced plasma levels of 25-hydroxyvitamin D [25(OH)D] may help promote this stiffness.
Hypertensive patients at least 60 years old without history of peripheral arterial disease at our hospital were retrospectively divided into dipper and non-dipper groups according to the results of 24-hour ambulatory blood pressure monitoring. Plasma levels of 25(OH)D were measured by enzyme immunoassay. Peripheral arterial stiffness was measured based on the cardio-ankle vascular index (CAVI).
Of the 155 patients enrolled, 95 (61.3%) were diagnosed with non-dipper hypertension and these patients had significantly lower plasma levels of 25(OH)D than the 60 patients with dipper hypertension (19.58 ± 5.97 vs 24.36 ± 6.95 nmol/L, P < .01) as well as significantly higher CAVI (8.46 ± 1.65 vs 7.56 ± 1.08 m/s, P < .01). Vitamin D deficiency was significantly more common among non-dipper patients (57.9% vs 31.7%, P < .01). Multivariate regression showed that age and 25(OH)D were independently related to CAVI, with each 1-ng/ml decrease in 25(OH)D associated with a CAVI increase of +0.04 m/s.
Non-dipper hypertension is associated with vitamin D deficiency and reduced plasma levels of 25(OH)D. The latter may contribute to stiffening of peripheral arteries, increasing the risk of cardiovascular disease.
Keywords: 25-Hydroxyvitamin D, Elderly individuals, Non-dipper hypertension, Peripheral arterial stiffness
1. Introduction
Hypertension in elderly individuals is a major risk factor for adverse cardiovascular events, including stroke, acute myocardial infarction and heart failure.[1] This is particularly true in individuals with a “non-dipper” blood pressure pattern in which pressure falls by less than 10% at night. The non-dipper pattern has been associated with accelerated stiffening of peripheral arteries.[2–4] Studies suggest that abnormal activation of the renin-angiotensin-aldosterone system (RAAS) may cause the “non-dipper” phenomenon and that this may accelerate vascular endothelial damage[5,6]
Another potential contributor to the “non-dipper” phenomenon is deficiency in vitamin D, commonly assayed as its metabolite 25-hydroxyvitamin D [25(OH)D]. Patients with non-dipper hypertension show lower plasma levels of vitamin D than patients with dipper hypertension.[7,8] Vitamin D deficiency has been associated with increased arterial blood pressure and vascular endothelial damage.[9,10] This suggests that vitamin D deficiency may contribute to the arterial stiffening observed in patients with non-dipper hypertension.
To examine this possibility, we compared Chinese elderly with dipper or non-dipper hypertension in terms of plasma levels of 25(OH)D and peripheral arterial stiffness. Since, vitamin D deficiency is prevalent in China: a study of more than 10,000 individuals aged 40 to 75 years indicated a prevalence of 75%,[11] compared to 33% among people aged 18 years and older in the UK.[12]
2. Methods
2.1. Study population
This retrospective study involved individuals at least 60 years old who were diagnosed between January 2017 and June 2017 with essential hypertension at the Affiliated Hospital of North Sichuan Medical College (Nanchong, China). Hypertension was defined as resting systolic or diastolic blood pressure (DBP) ≥140/90 mmHg on two clinical visits or prescription of antihypertensive medication. Patients were excluded if they had arteritis and peripheral vascular stent implantation, cancer, severe hepatorenal or kidney insufficiency, or a disease affecting bone metabolism (hyper- or hypoparathyroidism, hyperthyroidism). Patients were also excluded if they had taken glucocorticoid or calcitonin in the previous 3 months, or if they were taking drugs that affect calcium or vitamin D status. The study was approved by the Ethics Committee of the Affiliated Hospital of North Sichuan Medical College and performed according to the Declaration of Helsinki. All participants gave written informed consent.
2.2. Clinical and laboratory examinations
For all patients, clinical and demographic data were collected on the same day that blood was sampled and arterial stiffness was assessed. These data included age, height, weight, and duration of hypertension. Body mass index (BMI) was calculated as body weight/height2 (kg/m2). Serum levels of triglycerides, cholesterol, high-density lipoprotein, low-density lipoprotein, fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), and creatinine were measured after patients had fasted for at least 8 hours. Plasma levels of 25(OH)D, considered the best index of vitamin D status,[13] were measured using an enzyme immunoassay kit (Immunodiagnostic Systems, Fountain Hills, AZ). The results were used to classify patients as having vitamin D deficiency (<12 ng/mL), insufficient vitamin D (12–20 ng/mL), or sufficient vitamin D (>20 ng/mL).[14]
2.3. 24-hour ambulatory blood pressure
This measurement was made in all patients automatically every 30 min during the day (06:00–18:00) and every 60 min during the night (18:00–06:00) using a cuff (Welch Allyn, USA) attached to the patient's right upper arm. The effective 24-hour ambulatory blood pressure monitoring defined as more than 80% of the measurements obtained with this cuff were judged reliable based on the cuff manufacturer's internal quality control criteria. The average systolic blood pressure (SBP) and average DBP were calculated from the 24-hour ambulatory blood pressure measurements. The mean arterial pressures (MAPs) for day- and night-time were calculated as DBP + (SBP − DBP)/3. The percentage reduction in MAP at night-time was calculated as 100 × [1 − (night-time MAP/daytime MAP)]. If the night-time MAP declined by ≥10%, patients were classified as ‘dippers’; otherwise, they were classified as ‘non-dippers’.[1]
2.4. Arterial stiffness measurement
Peripheral arteriosclerosis was measured in terms of the cardio-ankle vascular index (CAVI)[2] using a blood pressure pulse measuring device (VS-1000 system, Beijing Futian Electronic Medical Equipment, China). The brachial and ankle arterial pulse waveforms were recorded and calculated as electro- and phonocardiograms. To achieve this, patients lay in a supine position on a pillow in a quiet environment for 5 to 10 minutes, after which blood pressure was measured using a cuff on the upper arm and another on the ankle; the cuffs were tightened so that only one finger could still fit under it. The heart monitor was then placed on the sternum at the level of the second ribs, and the electrocardiography electrodes were installed on both hands. The R-CAVI and L-CAVI were calculated automatically according to the formula:
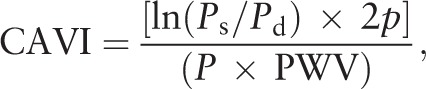 |
where Ps represents systolic blood pressure; Pd, diastolic blood pressure; p, blood density; P, pulse pressure; and PWV, pulse wave velocity for the aortic valve to the ankle artery. Measurements were performed automatically twice at 10 min apart, then averaged to give the final result.
2.5. Statistical analysis
Data are expressed as the mean ± standard deviation for continuous variables, or as frequency (percentage) for categorical variables. Inter-group differences in continuous variables were assessed for significance using Student t test, while differences in categorical variables were assessed using the chi-squared test. Risk factors of poor CAVI were identified using multivariable regression involving variables associated with P < .05 in univariate regression. Statistical analyses were performed using SPSS for Windows 22.0 (IBM, Chicago, IL). All P values are two-sided, and differences associated with P < .05 were considered statistically significant.
3. Results
Of the 200 patients at least 60 years old who were diagnosed with essential hypertension at our hospital during the study period, 45 were excluded for the following reasons:
-
(1)
they had arteritis and peripheral vascular stent implantation (n = 7);
-
(2)
they had cancer or severe hepatorenal or kidney insufficiency (n = 5);
-
(3)
they had hyperparathyroidism, hypoparathyroidism, hyperthyroidism, or other diseases affecting bone metabolism (n = 12);
-
(4)
they had taken glucocorticoids or calcitonin in the previous three months; or
-
(5)
they were taking drugs that affect calcium or vitamin D status (n = 10).
Eleven patients refused to participate in the study. In the end, 155 patients (63 women, 40.6%) were included in the study, with an average age of 66.8 ± 4.47 years (Table 1).
Table 1.
Clinico-demographic characteristics and plasma levels of 25(OH)D in elderly Chinese with dipper or non-dipper hypertension.
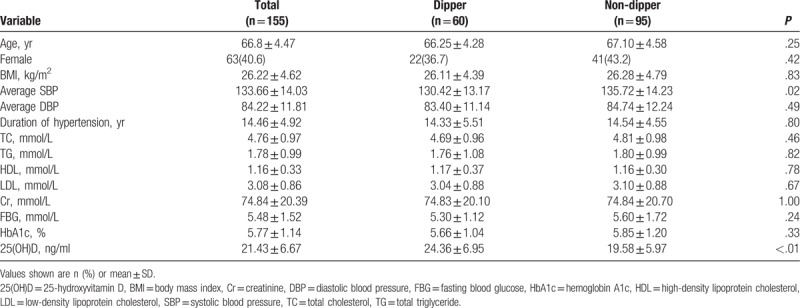
Based on 24-hour ambulatory blood pressure, 60 patients (38.7%), of whom 22 (36.7%) were women, were diagnosed with dipper hypertension. Average SBP was significantly higher among those with non-dipper hypertension (135.72 ± 14.23 vs 130.42 ± 13.17 mmHg, P = .02). The two groups were similar in age, BMI, duration of hypertension, triglycerides, cholesterol, high-density lipoprotein, low-density lipoprotein, creatinine, FBG, and HbA1c.
Plasma levels of 25(OH)D were significantly lower among those with non-dipper hypertension than among those with dipper hypertension (19.58 ± 5.97 vs 24.36 ± 6.95 ng/mL, P < .01; Table 1). Conversely, incidence of vitamin D deficiency was significantly higher among those with the non-dipper pattern (57.9% vs 31.7%, P < .01). The percentage of patients with a 25(OH)D level below 12 ng/mL was 10.5% in the non-dipper group but 0 in the dipper group (Fig. 1).
Figure 1.
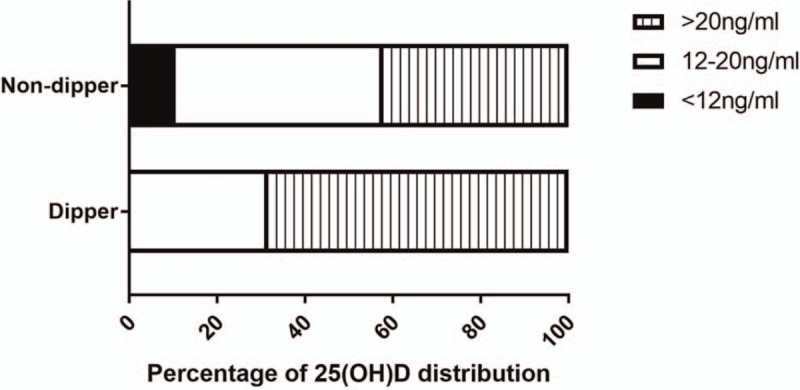
Distribution of plasma levels of 25(OH)D in elderly Chinese with dipper or non-dipper hypertension. 25(OH)D = 25-hydroxyvitamin D.
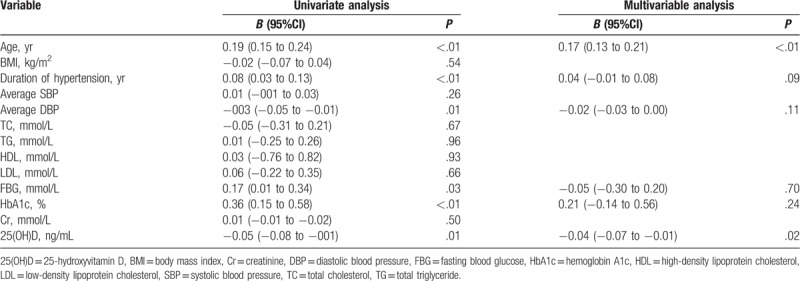
Patients with non-dipper hypertension showed significantly higher CAVI (8.46 ± 1.65 vs 7.56 ± 1.08 m/s, P < 0.01; Fig. 2). We first performed univariate analysis to identify factors potentially associated with higher CAVI: in this analysis, the dependent variable was CAVI, while age, average DBP, duration of hypertension, FBG, HbA1c, 25(OH)D, BMI, average SBP, triglycerides, cholesterol, high-density lipoprotein, low-density lipoprotein, and creatinine were independent variables (Table 2). The first six factors emerged as significant in this analysis, and entering them in multivariable regression identified only age and 25(OH)D as related to CAVI. Each 1-ng/mL decrease in 25(OH)D level was associated with a CAVI increase of 0.04 m/s.
Figure 2.

Comparison of the CAVI between elderly Chinese with dipper or non-dipper hypertension. CAVI = cardio-ankle vascular index.
Table 2.
Screening of potential risk factors of elevated cardio-ankle vascular index.
4. Discussion
The present study suggests that among elderly Chinese, vitamin D deficiency is more common in individuals with non-dipper hypertension than in those with dipper hypertension. We further show evidence that non-dipper hypertension is associated with lower plasma levels of 25(OH)D and greater peripheral arterial stiffness. This is consistent with the idea that 25(OH)D deficiency promotes arterial stiffening in non-dipper hypertension.
The large, long-term US-based National Health and Nutrition Examination Survey provides strong evidence that plasma levels of 25(OH)D were negatively associated with blood pressure.[15,16] The present study further found that vitamin D deficiency is more common in elderly Chinese with non-dipper hypertension compared with those with dipper hypertension. This could be partly explained by the cross talk between vitamin D and RAAS. Vitamin D deficiency causes the non-dipper hypertensive pattern by boosting secretion of parathyroid hormone, especially at middle night, which consequently stimulates adrenal glands to secrete aldosterone and renders the target tissues more sensitive to the hormone, resulting in an increase in blood pressure.[17,18] Lower levels of 25(OH)D have been linked to substantial up-regulation of renin and angiotensin II, leading to stimulation of the RAAS,[19] angiotensin II in turns reduces renal klotho expression resulting in modulations of 1-α hydroxylase activity, which directly influenced plasma levels of 25(OH)D.[20] Future work should explore the cross talk in detail, especially as it relates to potential therapies.
Previous studies have demonstrated that non-dipper hypertension was associated with accelerated peripheral arterial stiffness, such that affected individuals show thicker carotid intima-media and faster brachial-ankle pulse wave velocity.[21,22] The present study also confirmed this point and further found that 25(OH)D deficiency promotes arterial stiffening in non-dipper hypertension. The underline mechanisms could be explained as follows: lower plasma levels of 25(OH)D resulted hyperactive RAAS, that can damage vasculature and stiffen arteries.[23] Vitamin D deficiency may also lead to vessel stiffening through other mechanisms, since vitamin D suppresses proliferation of vascular smooth muscle cells[24] and activation of macrophages.[25] Vitamin D also suppresses T-cell proliferation and inflammatory cytokine production, mitigating inflammation.[26] Any or all of these pathways may help explain why non-dipper hypertension with vitamin D deficiency is associated with faster progression of peripheral arteriosclerosis. As a result, these individuals are at higher risk of cardiovascular disease than those with dipper hypertension.
4.1. Clinical implications
The present study of elderly Chinese provides evidence that vitamin D deficiency is more prevalent among those with a non-dipper hypertensive pattern than those with a dipper pattern, and that the non-dipper pattern is associated with greater stiffness of peripheral arteries. Consistent with these findings, vitamin D supplementation can inhibit atherosclerosis in animal studies[27,28] and can slow arterial stiffening in patients who are overweight or diabetic or have chronic kidney disease.[29–31] However, further study is needed to optimize the dose, frequency, and duration of vitamin D supplementation,[32] and the present study highlights the need for such work on patients with non-dipper hypertension.
4.2. Limitations
There are limitations in our study. Although we did not compare the two groups in propensity score matching style, we actually minimized potential confounding through multivariable analysis, which may reduce the impact of data comparison. Furthermore, this was a cross-sectional analysis, and thus, the relationship between changes in vitamin D status and progression of arterial stiffness in elderly individuals with non-dipper hypertension cannot be established in the present study. Future work is also needed to further examine whether vitamin D status is linked to arterial stiffness in normotensive individuals.
5. Conclusions
Vitamin D deficiency is more prevalent among elderly Chinese with non-dipper hypertension than among those with dipper hypertension. The non-dipper pattern is associated with lower plasma levels of 25(OH)D and greater peripheral arterial stiffness.
Author contributions
Conceptualization: Jian-Wei Gu, Ju-Hua Liu, Yun-Feng Yang, Li Liu, Cheng-Shi He, Bi-Hua Wu.
Data curation: Jian-Wei Gu, Ju-Hua Liu, Hui-Neng Xiao, Yun-Feng Yang, Wen-Ju Dong, Quan-Bo Zhang, Li Liu, Cheng-Shi He, Bi-Hua Wu.
Formal analysis: Jian-Wei Gu, Ju-Hua Liu, Hui-Neng Xiao, Yun-Feng Yang, Wen-Ju Dong, Li Liu, Cheng-Shi He, Bi-Hua Wu.
Funding acquisition: Jian-Wei Gu, Ju-Hua Liu, Wen-Ju Dong, Quan-Bo Zhang, Bi-Hua Wu.
Investigation: Jian-Wei Gu, Ju-Hua Liu, Bi-Hua Wu.
Methodology: Jian-Wei Gu, Ju-Hua Liu, Hui-Neng Xiao, Yun-Feng Yang, Quan-Bo Zhang, Cheng-Shi He, Bi-Hua Wu.
Project administration: Jian-Wei Gu, Ju-Hua Liu, Hui-Neng Xiao, Bi-Hua Wu.
Resources: Bi-Hua Wu.
Software: Yun-Feng Yang, Bi-Hua Wu.
Supervision: Jian-Wei Gu, Ju-Hua Liu, Yun-Feng Yang, Wen-Ju Dong, Bi-Hua Wu.
Validation: Jian-Wei Gu, Ju-Hua Liu, Hui-Neng Xiao, Wen-Ju Dong, Quan-Bo Zhang, Li Liu, Bi-Hua Wu.
Visualization: Jian-Wei Gu, Ju-Hua Liu, Hui-Neng Xiao, Yun-Feng Yang, Wen-Ju Dong, Quan-Bo Zhang, Li Liu, Cheng-Shi He, Bi-Hua Wu.
Writing – original draft: Jian-Wei Gu, Ju-Hua Liu, Yun-Feng Yang, Wen-Ju Dong, Quan-Bo Zhang, Li Liu, Cheng-Shi He, Bi-Hua Wu.
Writing – review & editing: Jian-Wei Gu, Ju-Hua Liu, Cheng-Shi He, Bi-Hua Wu.
Footnotes
Abbreviations: 25(OH)D = 25-hydroxyvitamin D, BMI = body mass index, CAVI = cardio-ankle vascular index, DBP = diastolic blood pressure, FBG = fasting blood glucose, HbA1c = glycosylated hemoglobin, MAPs = mean arterial pressures, RAAS = renin-angiotensin-aldosterone system, SBP = systolic blood pressure.
How to cite this article: Gu JW, Liu JH, Xiao HN, Yang YF, Dong WJ, Zhang QB, Liu L, He CS, Wu BH. Relationship between plasma levels of 25-hydroxyvitamin D and arterial stiffness in elderly Chinese with non-dipper hypertension: an observational study. Medicine. 2020;99:7(e19200).
J-WG and J-HL contributed equally and shall be considered as co-first authors.
The study was approved by the Ethics Committee of the Affiliated Hospital of North Sichuan Medical College and conducted according to the Declaration of Helsinki. All participants gave written informed consent.
This study was supported by the Research Grant Council of the Sichuan Medical Association, China (2017SHD019) and the Cadre Research Health Project of Sichuan Province (2017-1601).
The authors have no conflicts of interest to disclose.
References
- [1].Viera AJ. Hypertension update: current guidelines. FP Essent 2018;469:11–5. [PubMed] [Google Scholar]
- [2].Chen Y, Liu JH, Zhen Z, et al. Assessment of left ventricular function and peripheral vascular arterial stiffness in patients with dipper and non-dipper hypertension. J Investig Med 2018;66:319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lee HT, Lim YH, Kim BK, et al. The relationship between ambulatory arterial stiffness index and blood pressure variability in hypertensive patients. Korean Circ J 2011;41:235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Amah G, Ouardani R, Pasteur-Rousseau A, et al. Extreme-dipper profile, increased aortic stiffness, and impaired subendocardial viability in hypertension. Am J Hypertens 2017;30:417–26. [DOI] [PubMed] [Google Scholar]
- [5].Yang K, Wang Y, Ding Y, et al. Valsartan chronotherapy reverts the non-dipper pattern and improves blood pressure control through mediation of circadian rhythms of the renin-angiotensin system in spontaneous hypertension rats. Chronobiol Int 2019;1–4. [DOI] [PubMed] [Google Scholar]
- [6].Kim S, Kim NH, Kim YK, et al. The number of endothelial progenitor cells is decreased in patients with non-dipper hypertension. Korean Circ J 2012;42:329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Karadag MK, Secen O. Relationship of vitamin D and parathyroid hormone with the nocturnal blood pressure decline in hypertension. Blood Press Monit 2017;22:322–7. [DOI] [PubMed] [Google Scholar]
- [8].Sabio JM, Vargas-Hitos JA, Martínez-Bordonado J, et al. Association between non-dipper hypertension and vitamin D deficiency in women with systemic lupus erythematosus. Clin Exp Rheumatol 2019;37:286–92. [PubMed] [Google Scholar]
- [9].Bozic M, Alvarez A, de Pablo C, et al. Impaired vitamin D signaling in endothelial cell leads to an enhanced leukocyte-endothelium interplay: implications for atherosclerosis development. PLoS One 2015;10:e0136863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vishnu A, Ahuja V. Vitamin D and blood pressure among U.S. adults: a cross-sectional examination by race/ethnicity and gender. Am J Prev Med 2017;53:670–9. [DOI] [PubMed] [Google Scholar]
- [11].Zhen D, Liu L, Guan C, et al. High prevalence of vitamin D deficiency among middle-aged and elderly individuals in northwestern China: its relationship to osteoporosis and lifestyle factors. Bone 2015;71:1–6. [DOI] [PubMed] [Google Scholar]
- [12].Crowe FL, Jolly K, MacArthur C, et al. Trends in the incidence of testing for vitamin D deficiency in primary care in the UK: a retrospective analysis of The Health Improvement Network (THIN), 2005–2015. BMJ Open 2019;9:e028355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Farrell CJ, Herrmann M. Determination of vitamin D and its metabolites. Best Pract Res Clin Endocrinol Metab 2013;27:675–88. [DOI] [PubMed] [Google Scholar]
- [14].Sahota O. Understanding vitamin D deficiency. Age Ageing 2014;43:589–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2007;167:1159–65. [DOI] [PubMed] [Google Scholar]
- [16].Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens 2007;20:713–9. [DOI] [PubMed] [Google Scholar]
- [17].Jubiz W, Canterbury JM, Reiss E, et al. Circadian rhythm in serum parathyroid hormone concentration in human subjects: correlation with serum calcium, phosphate, albumin, and growth hormone levels. J Clin Invest 1972;51:2040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tomaschitz A, Ritz E, Pieske B, et al. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease. Metabolism 2014;63:20–31. [DOI] [PubMed] [Google Scholar]
- [19].Li YC, Kong J, Wei M, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002;110:229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].de Borst MH, Vervloet MG, ter Wee PM, et al. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol 2011;22:1603–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ozdemir E, Yildirimturk O, Cengiz B, et al. Evaluation of carotid intima-media thickness and aortic elasticity in patients with nondipper hypertension. Echocardiography 2014;31:663–8. [DOI] [PubMed] [Google Scholar]
- [22].Kim HL, Seo JB, Chung WY, et al. The association between ambulatory blood pressure profile and brachial-ankle pulse wave velocity in untreated hypertensive subjects. Blood Press 2015;24:139–46. [DOI] [PubMed] [Google Scholar]
- [23].Gonçalves I, Edsfeldt A, Colhoun HM, et al. Association between renin and atherosclerotic burden in subjects with and without type 2 diabetes. BMC Cardiovasc Disord 2016;16:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Valcheva P, Cardus A, Panizo S, et al. Lack of vitamin D receptor causes stress-induced premature senescence in vascular smooth muscle cells through enhanced local angiotensin-II signals. Atherosclerosis 2014;235:247–55. [DOI] [PubMed] [Google Scholar]
- [25].Zhang X, Zhao Y, Zhu X, et al. Active vitamin D regulates macrophage M1/M2 phenotypes via the STAT-1-TREM-1 pathway in diabetic nephropathy. J Cell Physiol 2019;234:6917–26. [DOI] [PubMed] [Google Scholar]
- [26].Sheikh V, Kasapoglu P, Zamani A, et al. Vitamin D3 inhibits the proliferation of T helper cells, downregulate CD4(+) T cell cytokines and upregulate inhibitory markers. Hum Immunol 2018;79:439–45. [DOI] [PubMed] [Google Scholar]
- [27].Xiang W, Hu ZL, He XJ, et al. Intravenous transfusion of endothelial progenitor cells that overexpress vitamin D receptor inhibits atherosclerosis in apoE-deficient mice. Biomed Pharmacother 2016;84:1233–42. [DOI] [PubMed] [Google Scholar]
- [28].Szeto FL, Reardon CA, Yoon D, et al. Vitamin D receptor signaling inhibits atherosclerosis in mice. Mol Endocrinol 2012;26:1091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Raed A, Bhagatwala J, Zhu H, et al. Dose responses of vitamin D3 supplementation on arterial stiffness in overweight African Americans with vitamin D deficiency: a placebo controlled randomized trial. PLoS One 2017;12:e0188424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Anandabaskar N, Selvarajan S, Dkhar SA, et al. Effect of vitamin D supplementation on vascular functions and oxidative stress in type 2 diabetic patients with vitamin D deficiency. Indian J Endocrinol Metab 2017;21:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Levin A, Tang M, Perry T, et al. Randomized controlled trial for the effect of vitamin D supplementation on vascular stiffness in CKD. Clin J Am Soc Nephrol 2017;12:1447–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Papadimitriou DT. The big vitamin D mistake. J Prev Med Public Health 2017;50:278–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


