Abstract
Background:
Patients with advanced gastric or gastro-oesophageal junction cancer (GC/GEJC) that fail to respond to prior chemotherapy have poor clinical prognosis. Lately, many trials have paid much attention on the oncological outcomes of immune checkpoint inhibitors (ICI). A new therapy based on programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) inhibitors has recognized as promising prospects for advanced GC/GEJC. We assessed efficacy and safety of PD-L1 antibody versus chemotherapy alone in previously treated non-small cell lung cancer.
Methods:
Computerized literature search was done on the published trials in: Pubmed, Embase, Cochrane library updated on June 2019. Randomized controlled trials were selected investigating chemotherapy plus PD-1/PD-L1 versus chemotherapy alone.
Results:
Three randomized controlled trails were included. The pooled analysis of overall survival (OS) was longer with anti-PD1/PD-L1 than with chemotherapy alone in the OS (OR = 0.66, 95%CI = 0.47–0.92, P = .02) and sub-group OS of GEJC (OR = 0.73, 95%CI = 0.58–0.93, P = .01). Whereas, there is no significant difference in progression-free survival (OR = 0.93, 95%CI = 0.62–1.39, P = .72). The pooling adverse events (AE) data did not achieve advantage in the PD-1/PD-L1 targeted agents (OR = 0.53, 95%CI = 0.13–2.10, P = .36), the same as the treatment-related AE of grade 3 to 5 (OR = 0.53, 95%CI = 0.16–1.74, P = .30).
Conclusions:
Treatment of patients with advanced GC/GEJC with PD-1/PD-L1 targeted did result in an improvement in some but not all survival endpoints. Moreover, it had a comparable toxicity profile as compared with chemotherapy alone. More well designed studies are needed to develop a database of all anti-PD1/PD-L1 sub-groups and their individual impact on the differing anti-PD1/PD-L1 treatments.
Keywords: advanced GC/GEJC, meta-analysis, PD-1, PD-L1
1. Introduction
Gastric/gastro-oesophageal junction cancer (GC/GEJC) is known to be the fifth leading cause of cancer-related death globally.[1] The chemotherapy with a platinum and fluoro-pyrimidine is emerged as the standard regimen for these patients. However, initial chemotherapy is frequently unsuccessful, most GC/GEJC cases develop disease relapse and a tendency to systemic metastasis, necessitating secondary treatment.[2–4] For patients with advanced or metastatic GC/GEJC, therapy options include docetaxel, paclitaxel, or irinotecan mono-therapy,[5,6] trastuzumab for patients with HER-2 positive disease and the anti-vascular endothelial growth factor receptor 2 antibody ramucirumab as either monotherapy or in combination with paclitaxel.[7,8]
Although chemotherapy regimens for these patients have recently developed, the prognosis of advanced GC/GEJC is still disappointing. Lately, immune checkpoint inhibitors (ICI) have been recommended as therapies for GC/GEJC cancer because these cancers have the high mutational burden and overexpression of immune checkpoint proteins, and programmed death-ligand 1 (PD-L1) pathway is a promising therapeutic option in treatment of patients with advanced GC/GEJC.[9–11]
The programmed death 1 (PD-1) receptor is an immune-checkpoint protein expressed on tumor cells and tumor-infiltrating immune cells that down-regulate T-cell activation and evade immune response against tumor cells.[12] Many studies have demonstrated the effect of anti-PD1/PD-L1 for advanced GC/GEJC on oncological outcomes, while the significance has been controversial. Some studies have found that not all but some patients were able to achieve survival benefit, while other reports have shown the effect on survival of anti-PD1/PD-L1 for advanced gastric or gastro-oesophageal junction cancer is doubtful.[13] It is, thus, a key controversy whether or not an anti-PD1/PD-L1 have oncological outcomes benefits for advanced GC/GEJC.
The purpose of this study is to analyze the significance of anti-PD1/PD-L1 for advanced GC/GEJC.
2. Materials and methods
2.1. Ethics statement
As the present meta-analysis was performed based on previously published studies, no ethical approval and patient consent are required.
2.2. Search strategy
Two investigators conducted a systematic search of the Pubmed, Embase, Cochrane library up to June 2019 independently, using the MeSH terms and free key words “Anti PD-1” OR “Anti PD-L1”AND “gastric cancer”, AND “gastro-oesophageal junction cancer”. We also identified the reference materials for further evaluation.
2.3. Inclusion criteria
Studies were included in the meta-analysis relating to: (1) the studies are designed as random control trials (RCTs); (2) patients were underwent using chemotherapy plus PD-1/PD-L1 versus chemotherapy alone; (3) patients were clinical diagnosis of advanced G/GEJ progresses on chemotherapy after failure of prior therapy; (4) the interested outcomes were efficacy and toxicity; (5) the full-text papers were only included.
2.4. Risk-of-bias assessments
The risk of bias was evaluated by two investigators, separately. Study quality was justified using the Cochrane Collaboration's “Risk of bias” tool.
2.5. Data selection
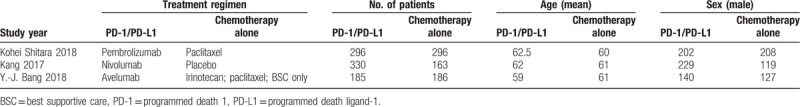
Two researchers independently extracted the extract contents from each trial. In case of disagreement, a third investigator helps resolve the disagreement or through discussion. We extracted the main categories based on the following: lead author, publication year, treatment regimen, patient number, age, sex number, and outcome measures.
2.6. Statistical analysis
The Review Manager version 5.3 software (Revman; The Cochrane collaboration Oxford, United Kingdom) used for statistical analysis. The chi-square was used to assess the significance of heterogeneity, and the degree of heterogeneity across studies was then examined using the I2 statistic.[14]I2 value larger than 50% suggested heterogeneity was significant, and the random-effects model was used. Otherwise, the fixed-effect model was used.[15] A P-value <.05 was identified as statistically significant difference.
3. Results
3.1. Overview of literature search and study characteristics
A total of 212 studies were retrieved. Eight irrelevant citations were evaluated based on the review of titles and abstracts, but some did not provide enough detail of results of two approaches. Therefore, a final total of three RCTs[16–18] were further eliminated. The search process is described in Fig. 1. Table 1 presents a brief description of these eligible studies.
Figure 1.
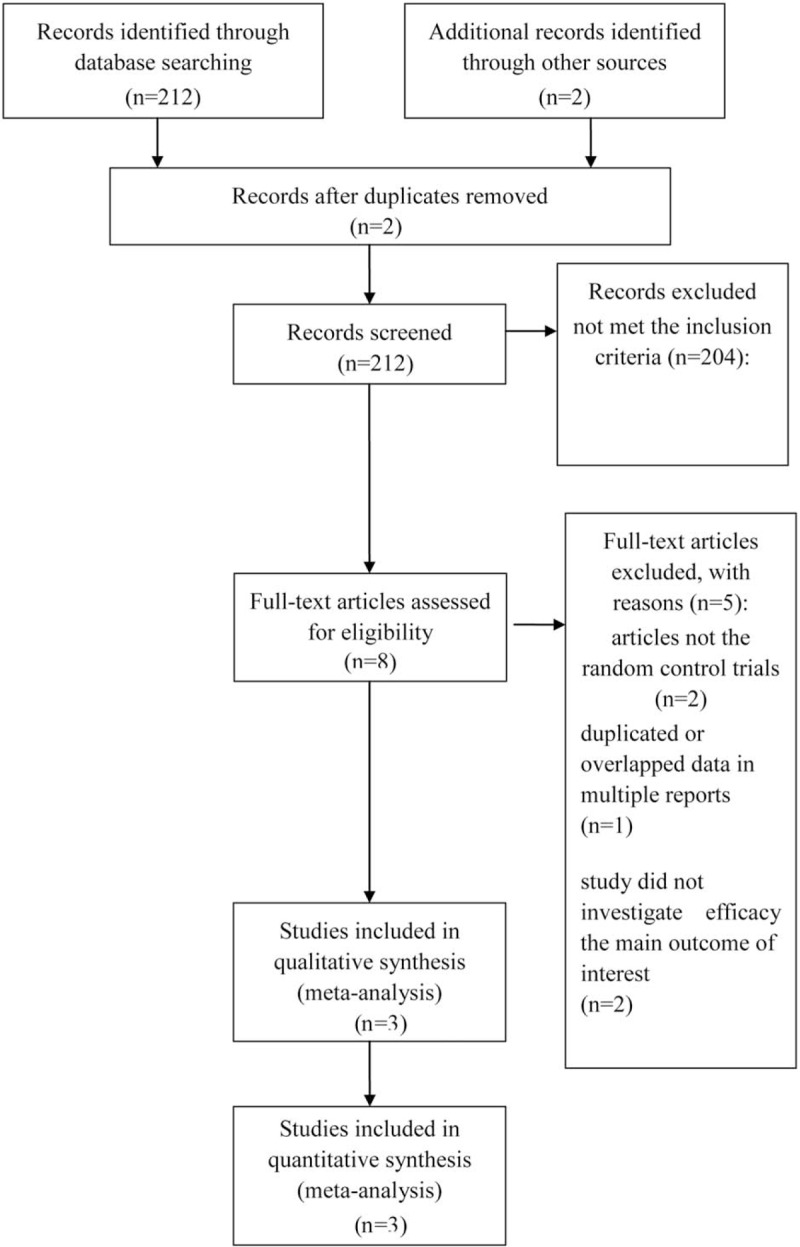
PRISMA flow chart of selection process to identify studies eligible for pooling.
Table 1.
Brief description of included eligible studies.
3.2. Outcomes and synthesis of results
-
1.
Pooled analysis of overall survival (OS) comparing chemotherapy plus PD-1/PD-L1 with chemotherapy alone
Pooling the OS demonstrated that PD-1/PD-L1 targeted agents did lead to an OS advantage (OR = 0.66, 95%CI = 0.47–0.92, P = .02). Also, subgroup analysis revealed GEJC (OR = 0.73, 95%CI = 0.58–0.93, P = .01) was associated with better OS, but the GC group (OR = 0.88, 95%CI = 0.64–1.20, P = .41). Results were shown in Fig. 2.
-
2.
Pooled analysis of progression-free survival (PFS) comparing chemotherapy plus PD-1/PD-L1 with chemotherapy alone
Pooled estimates of effect sizes showed that the difference of PFS between two groups was no statistically significant (OR = 0.93, 95%CI = 0.62–1.39, P = .72). Results were shown in Fig. 3.
-
3.
Pooled analysis of AE comparing chemotherapy plus PD-1/PD-L1 with chemotherapy alone
Figure 2.
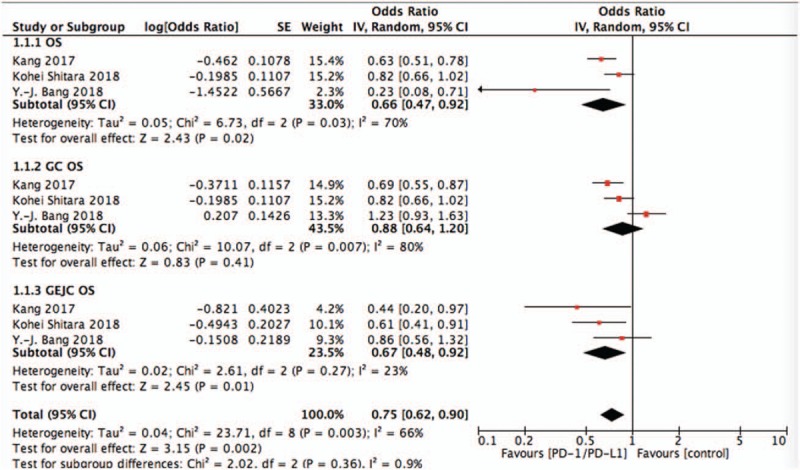
Pooled analysis of OS comparing chemotherapy plus PD-1/PD-L1 with chemotherapy alone. OS = overall survival, PD-1 = programmed death 1, PD-L1 = programmed death ligand 1.
Figure 3.
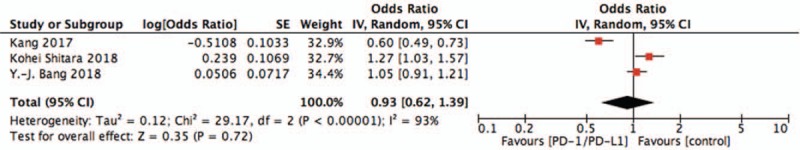
Pooled analysis of PFS comparing chemotherapy plus PD-1/PD-L1 with chemotherapy alone. PD-1 = programmed death 1, PD-L1 = programmed death ligand 1.
The pooling AE data did not achieve advantage in the PD-1/PD-L1 targeted agents (OR = 0.53, 95%CI = 0.13–2.10, P = .36) (Fig. 4). And results showed that the difference of grade 3 to 5 serious adverse events between two groups was no statistically significant (OR = 0.53, 95%CI = 0.16–1.74, P = .30) (Fig. 5).
Figure 4.
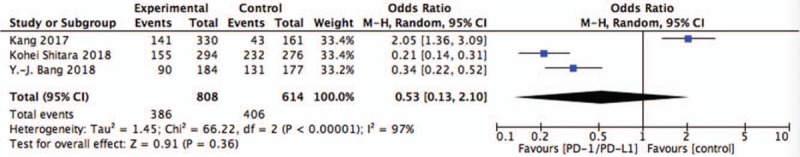
Pooled analysis of AE comparing chemotherapy plus PD-1/PD-L1 with chemotherapy alone. AE = adverse events, PD-1 = programmed death 1, PD-L1 = programmed death ligand 1.
Figure 5.
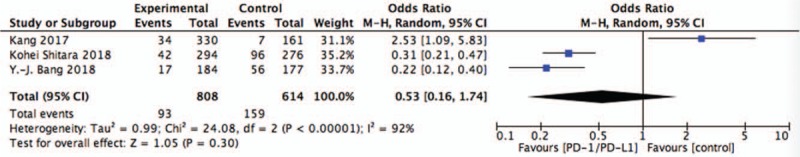
Pooled analysis of SAE comparing chemotherapy plus PD-1/PD-L1 with chemotherapy alone. PD-1 = programmed death 1, PD-L1 = programmed death ligand 1, SAE = serious adverse events.
4. Discussion
Patients diagnosed with advanced GC/GEJC cancer have a poor performance status despite the use of standard treatments, such as chemotherapy and biologic agents. Previous studies are currently focused on evaluating combinations of ICI, standard chemotherapy, and biologic agents as well as novel biomarkers to prolong survival and improve quality of life. While, most chemotherapy therapeutic approaches fail to provide substantial efficacy benefits.[3,4] Our meta-analysis will provide the evidence of anti-PD1/PD-L1 for advanced GC/GEJC cancer treatment in clinical practice.
In case of the efficacy, the Bangs[16] study did not achieve benefit of the OS or PFS, indicated that potentially identified patient subgroups most likely to benefit from checkpoint inhibitors. Kang[18] reported that the survival benefit with nivolumab was independent in pretreated patient population, regardless of primary tumor site, Lauren classification, or PD-L1 positivity. While, consistent with findings of KEYNOTE-059[7] and report results for pembrolizumab in other tumor types,[19–21] a relationship between greater PD-L1 expression and a greater treatment effect for pembrolizumab was found in KEYNOTE-061. PD-L1 expression in Kohei Shitara's study[17] was assessed as a better predictor of outcomes for selecting patients for therapy with pembrolizumab mono-therapy. Therefore, the pattern and prognostic implication of PD-1/PD-L1 expression need further investigation.
According to The Cancer Genome Atlas research network, there are four specific molecular subtypes of gastric cancer: Epstein-Barr virus-positive (EBV+), high microsatellite instability (MSI-H), genomically stable and chromosomally instable.[22] MSI-H is associated with high PD-L1 tumor expression and induces innate anti-tumor immune responses and make tumors more sensitive to immunotherapy.[23,24]EBV+ types are associated with high-response rates to immunotherapies in other solid tumors.[22,25] Tumor mutation burden has also been recognized as a predictor of response to PD-1 blockade, with patients with higher mutational loads had better effect of response.[26]
Furthermore, GC/GEJC is biologically heterogeneous, which increases the difficulty of treatment. Our study indicated that GEJC was better associated with OS, than the GC group. Potential differences in patient cohorts, immunohistochemistry methods, and end points may account for these results. The biologic characteristics of molecular subtypes of advanced GC/GEJC make these subtypes excellent candidates for anti-PD-1-directed ICI treatment.[27]
Moreover, regarding the safety profile, we concluded that the safety profile of anti-PD1/PD-L1 in patients with advanced GC/GEJC was manageable. Therefore, it can be considered that anti-PD1/PD-L1 treatment was safe. In addition, we found that the adverse effect like hypothyroidism, hyperthyroidism, pneumonitis, colitis, hepatitis, hypopituitarism, which were more likely to appear grade ≥3 TRAEs, although the incidences were not high. The occurrence of these TRAEs was associated with ICI immunotherapy. They were called “TRAEs of special interest” or “immune-mediated adverse events”. Thus, particular attention should be given to these adverse reactions.
Moderate evidence has been gathered in our study, but there is still limitation in our study. Considering the lack of patient-level data, clinical heterogeneity among studies, the optimal option for incorporating checkpoint inhibitors into the continuum of care for patients with advanced GC/GEJC is still under debate. More high-quality researches with further data on the different subtypes and larger RCTs are strongly in-needed to confirm the effectiveness and safety of alternative anti-PD-1/PD-L1 therapy in an effort to treat patients with advanced GC/GEJC.
5. Conclusion
Our study confirms that patients treated with anti-PD-1/PD-L1 therapy had a better superior survival benefit with some but not all survival endpoints, and with a comparable adverse event for advanced GC/GEJC. From an efficacy standpoint, further trials into immune checkpoint therapy that will benefit patients by specific molecular subtype and genomic alterations, which can be instructive in driving therapy decisions, while conferring with manageable safety profile. To further validate this treatment, the effect and safety of PD-1/PD-L1 agents should systematically sub-group analyzed in the near future.
Author contributions
Conceptualization: Chang-Peng Zou.
Data curation: Ying Guo.
Writing – original draft: Zheng Zheng, Chang-Peng Zou.
Writing – review & editing: Zheng Zheng, Chang-Peng Zou.
Footnotes
Abbreviations: EBV+ = Epstein-Barr virus-positive, GC/GEJC = gastric or gastro-oesophageal junction cancer, ICI = immune checkpoint inhibitors, MSI-H = high microsatellite instability, PD-1 = programmed death 1, PD-L1 = programmed death ligand 1.
How to cite this article: Zheng Z, Guo Y, Zou CP. Oncological outcomes of addition of anti-PD1/PD-L1 to chemotherapy in the therapy of patients with advanced gastric or gastro-oesophageal junction cancer: a meta-analysis. Medicine. 2020;99:7(e18332).
ZZ and YG contributed equally to this paper.
The authors have no funding and conflicts of interest to disclose.
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2]. Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. [Google Scholar]
- [3].John Wiley & Sons, Ltd., Wagner AD, Grothe W, Behl S, et al. Chemotherapy for advanced gastric cancer. The Cochrane Library. 2002. [Google Scholar]
- [4].Digklia A, Wagner AD. Advanced gastric cancer: current treatment landscape and future perspectives. World J Gastroenterol 2016;22:2403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ford HER, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78–86. [DOI] [PubMed] [Google Scholar]
- [6].Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer – a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011;47:2306–14. [DOI] [PubMed] [Google Scholar]
- [7].Fuchs CS, Tomasek J, Schwartz JD, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31–9. [DOI] [PubMed] [Google Scholar]
- [8].Wilke H, Muro K, Van CE, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35. [DOI] [PubMed] [Google Scholar]
- [9].Amatatsu M, Arigami T, Uenosono Y, et al. Programmed death-ligand 1 is a promising blood marker for predicting tumor progression and prognosis in patients with gastric cancer. Cancer Sci 2018;109:814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kawazoe A, Kuwata T, Kuboki Y, et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer 2017;407–151. [DOI] [PubMed] [Google Scholar]
- [11].Yuan J, Zhang J, Zhu Y, et al. Programmed death-ligand-1 expression in advanced gastric cancer detected with RNA in situ hybridization and its clinical significance. Oncotarget 2016;7:39671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sharma P, Allison J. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015;161:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].De Mello RA, Lordick F, Muro K. Current and future aspects of immunotherapy for esophageal and gastric malignancies. Gastrointest (Noncolorectal) Cancer 2019;39:237–47. [DOI] [PubMed] [Google Scholar]
- [14].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [15].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol 2018;29:2052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392:123–33. [DOI] [PubMed] [Google Scholar]
- [18].Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461–71. [DOI] [PubMed] [Google Scholar]
- [19].Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- [20].Daud AI, Wolchok JD, Robert C, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol 2016;34:4102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Balar AV, Castellano D, O"Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:1483–92. [DOI] [PubMed] [Google Scholar]
- [22].Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dudley JC, Lin MT, Le DT, et al. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res 2016;22:813–20. [DOI] [PubMed] [Google Scholar]
- [24].Ma C, Patel K, Singhi AD, et al. Programmed death-ligand 1 expression is common in gastric cancer associated with Epstein-Barr Virus or microsatellite instability. Am J Surg Pathol 2016;40:1496–506. [DOI] [PubMed] [Google Scholar]
- [25].Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Johnson DB, Frampton GM, Rioth MJ, et al. Targeted next generation sequencing identifies markers of response to PD-1 blockade. Cancer Immunol Res 2016;4:2326–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 2018;24:1449–58. [DOI] [PubMed] [Google Scholar]


