Abstract
Background:
Sepsis is the leading cause of death in critically ill patients. Ulinastatin (UTI), a protease inhibitor, and rhubarb, used as a traditional Chinese medication, are proved to be effective in treating sepsis, but the effect of the combination therapy of these two drugs on sepsis remains unclear. This study aimed to investigate the effect of the combination treatment of UTI and rhubarb on sepsis patients.
Methods:
A total of 75 septic patients were randomly divided into control group, UTI group, Rhubarb group, and UTI plus Rhubarb group. Clinical data and score of Acute Physiology and Chronic Health Evaluation II (APACHE II) were collected; lymphocyte subtypes in the peripheral blood were analyzed before and after the 5-day treatment in the Intensive Care Unit.
Results:
All the therapeutic interventions (UTI alone, rhubarb alone, or UTI plus rhubarb) significantly reduced the levels of C-Reactive protein, white blood cell density, lactic acid, and APACH II scores, and elevated the levels of CD4/CD8, but only UTI plus rhubarb treatment obviously decreased the level of procalcitonin.
Conclusion:
This study suggested that the combination of UTI and rhubarb may be a promising therapeutic scheme to ameliorate sepsis.
Keywords: procalcitonin, rhubarb, sepsis, ulinastatin
1. Introduction
Sepsis, usually induced by infection, is a systemic inflammatory response with abnormalities all over the body.[1,2] Systemic inflammation that overwhelm the body's anti-inflammatory mechanisms, results in widespread vascular, endothelial, and organ dysfunction that is often fatal.[2] Despite improvements in antimicrobial therapy and supportive care, sepsis is still a major public health concern worldwide.[3] The incidence of severe sepsis is estimated to be more than 100 cases per 100,000 people, and the hospital mortality of severe sepsis is 48.7% in China.[4] Up to now, there is still no ideal method to prevent and treat this serious disease. Thus, identifying novel strategies for a better management of this disease is urgent.
Serine proteases are one of the main intermediaries in the systemic inflammation, which have a crucial role in regulation of inflammation by inter- or intracellular signaling pathways.[5,6] Protease inhibitors are produced by the body during inflammation to counter-regulate the effect of these proteases.[6] Ulinastatin (UTI) is one such important intrinsic broad-spectrum protease inhibitor, which is extracted and purified from human urine.[7] It inhibits polymorphonuclear granulocyte elastase, which is involved in the progression of several pathologic inflammatory processes.[8] Thus, UTI is believed to play an important anti-inflammatory role. On top of this, it is now also well established that UTI has a protective effect on many organs against various human diseases including sepsis.[9–13]
Traditional Chinese medicines have been used to treat critically ill patients for thousands of years. Rhubarb (Da Huang) is one of the most frequently used traditional Chinese herbs, and it is commonly used in Treatise on Febrile Diseases (Shan Han Lun) as a purgative and anti-bacterial agent to reduce fever, promote blood circulation, and cleanse the body.[14] During the last decades, modern pharmacological studies have revealed that rhubarb can be applied to infectious diseases due to its various pharmacological properties such as anti-inflammation, anti-infection, and immuno-enhancement. Findings from these studies imply that rhubarb has benefits in the control of sepsis.[15,16]
To the best of our knowledge, no previous studies have been conducted to evaluate the combined effect of UTI and rhubarb on human sepsis. Our previous study has revealed that combination of UTI and rhubarb significantly decreased the mortality than using UTI or rhubarb alone in a rat model of sepsis.[17] Therefore, we designed this prospective trial to evaluate and compare the efficacy of UTI, rhubarb, and combination of UTI and rhubarb in the treatment of human sepsis.
2. Material and methods
2.1. Patients
From September 2015 to March 2017, patients with sepsis admitted to the Intensive Care Unit (ICU) in the First Hospital of Hebei Medical University were enrolled, and a prospective, randomized, single-blind trial was performed. Written informed consent was obtained from all patients before the study. The study protocol was approved by the medical ethics committee.
2.2. Inclusion criteria
Guidelines for the Treatment of Severe Sepsis or Septic Shock in China (2014) were used as the diagnostic criteria in this study.[18] The inclusion criteria include clear or suspicious infection and some of the following clinical features:
-
1.
General clinical features: fever (body temperature > 38.3°C); hypothermia (body temperature < 36°C); heart rate > 90 beats/min, or greater than two standard deviations of normal value; shortness of breath; changes in mental status; obvious edema or positive fluid balance (24 h over 20 mL/kg); hyperglycemia [blood glucose > 7.7 mmol/L (140 mg/dL)] but with no history of diabetes.
-
2.
Inflammatory response indicators: leukocytosis (white blood cell [WBC] > 12,000/μL); leukopenia (WBC < 4000/μL); WBC count was normal but the total number of immature WBC exceeded 10%; serum C-reactive protein (CRP) > two standard deviations of normal value; serum procalcitonin (PCT) > two standard deviations of normal value.
-
3.
Hemodynamics: hypotension (systolic blood pressure < 90 mm Hg [1 mm Hg = 0.133 kPa], mean arterial pressure [MAP] < 70 mm Hg or adult systolic blood pressure dropped over 40 mm Hg or were below two standard deviations of normal value)].
-
4.
Organ dysfunction: hypoxemia (partial pressure of oxygen [PaO2]/fraction of inspired oxygen [FiO2] < 300 mm Hg); acute oliguria (even if the fluid recovery is sufficient, urine volume is still < 0.5 mL/kg·h and lasted for at least 2 h); serum creatinine > 44.2 μmol/L (0.5 mg/dL); coagulation abnormalities (international normalized ratio > 1.5 or APTT > 60 s); intestinal obstruction (intestinal squeak disappears); thrombocytopenia (platelet <100,000/μL); hyperbilirubinemia (plasma bilirubin > 70 μmol/L [4 mg/dL]).
-
5.
Tissue perfusion indicator: hyperlactosis (lactic acid > 1 mmol/L); decreased capillary reperfusion ability or ecchymosis.
2.3. Exclusion criteria
The exclusion criteria were:
-
1.
age is below 18 years old;
-
2.
severe malnutrition, claimed immune system diseases, and the use of immunosuppressant;
-
3.
malignant tumor patients, and patients in terminal state;
-
4.
pregnant or lactating women;
-
5.
allergic to rhubarb, UTI, or their components;
-
6.
unable or unsuitable for gastrointestinal administration;
-
7.
active liver disease or severe liver and kidney dysfunction.
2.4. Elimination criteria
The elimination criteria were:
-
1.
any patient dies during the trial;
-
2.
other conditions in which trial data could not be collected.
2.5. Study design
The enrolled patients were randomly divided into four groups, which were control group, UTI group, Rhubarb group, and UTI plus Rhubarb group. Patients in control group received basic treatment, including:
-
1.
regular care of the primary disease, antibacterial drugs according to the condition of the primary disease, and regular examination on blood routine, microbiology, and other evidence;
-
2.
appropriate respiratory support, maintaining circulation stability, and correcting the perfusion of tissues and organs;
-
3.
adjustment of the enteral nutrition support program according to the disease state and patient tolerance;
-
4.
timely correction of the electrolyte and acid-base balance disorders;
-
5.
treatment for existing diseases.
Patients in UTI group received the basic treatment and additional intravenous injection of 200,000 units of UTI (Tianpu Biochemical Pharmaceutical Co., Ltd., Guangdong, China) three times a day. Patients in Rhubarb group received the basic treatment and nasal or oral feeding of 12 g of rhubarb laxative granules (Chenpai Pharmaceutical Group Co., Ltd., Jiangsu, China) once a day. Patients in UTI plus Rhubarb group received the basic treatment, intravenous injection of UTI, and nasal or oral feeding of rhubarb laxative granules. The course of the treatment continued for 5 days. During the trial, observation data were recorded during the same period of time, and the standard method was used to collect blood samples for testing. The blood examinations included complete blood count, serum chemistries, CRP, PCT, and lactic acid. These laboratory values and organ specific parameters were also used to calculate the Acute Physiology and Chronic Health Evaluation (APACHE) II score, which is the most widely applied severity score for sepsis.[19] PCT is a precursor protein of the hormone calcitonin, which is induced in the blood of patients with severe infections or sepsis.[20] CRP is an acute phase protein produced by the liver, and its levels increase several fold after trauma, infection, inflammation and other stimuli within hours.[21]
2.6. Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were separated by density gradient centrifugation using Ficoll-Hypaque (Sigma). Cells were surface stained with anti-CD3-PerCP/CD4-FITC/CD8-PE (BD 18561) antibodies. Cells were analyzed using a FACSCalibur (BD) flow cytometry, and data were analyzed by Flowjo.
2.7. Statistical analysis
Data were expressed as mean ± SD. All statistical analyses were performed using the SPSS 19.0 software (Chicago, IL). The significance of difference between two groups was analyzed using a Student's t test. Multiple comparisons were performed by one-way ANOVA, and followed by Bonferroni post-test for comparison between two groups. The classification data was expressed as a percentage, and the comparison was performed using the χ2 test. A P value < .05 was considered significant.
3. Results
3.1. Clinical characteristics of patients
During the study period, 87 patients were diagnosed as sepsis. Among them, 75 patients who met the inclusion criteria were enrolled, and were randomly divided into control group (n = 16), UTI group (n = 19), Rhubarb group (n = 19), and UTI plus Rhubarb group (n = 21). Age, sex, and underlying diseases of sepsis were similar in the 4 groups (Table 1). The underlying diseases of sepsis were as follows: lung infection (33.3%), urinary tract infection (22.7%), abdominal infection (13.3%), skin soft tissue infection (8%), trauma (13.3%), and others (9.3%).
Table 1.
Baseline characteristics of the patients.
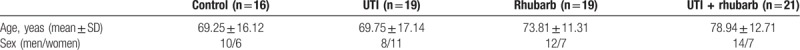
3.2. Comparison of indicators related to inflammation
Three inflammatory indicators, including CRP, PCT, and WBC, were comparable among groups before treatment (Table 2; Fig. 1). The CRP level in all groups showed significant reductions after treatment, and the CRP level in all experimental groups was markedly decreased compared with that in control group after treatment. The PCT level was distinctively reduced only in UTI plus Rhubarb group after treatment. The WBC level in all experimental groups showed significant reductions after treatment, and the WBC levels in all experimental groups also markedly reduced compared with that in control group after treatment.
Table 2.
Indicators related to inflammation.
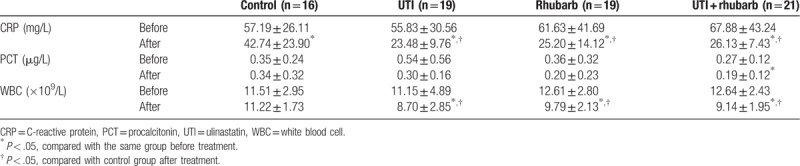
Figure 1.
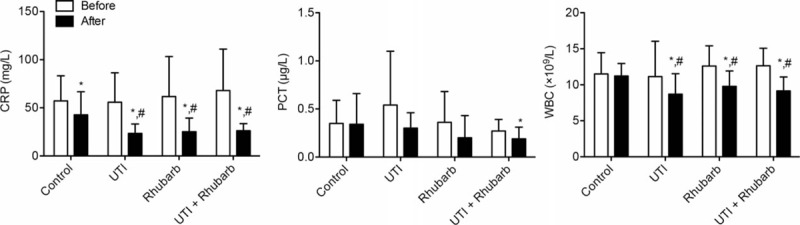
Indicators related to inflammation. ∗P < .05, compared with the same group before treatment; # P < .05, compared with control group after treatment.
3.3. Comparison of APACHE II score, tissue perfusion, and organ function
The level of APACHE II score, tissue perfusion, and organ function (liver/kidney) in different groups were similar before treatment (Table 3; Fig. 2). The APACHE II score in all groups were significantly reduced after treatment, and the APACHE II score in all experimental groups was markedly decreased compared with that in control group after treatment. Differences in APACHE II score were mirrored in the indicator of tissue perfusion (serum lactic acid). Liver and kidney function, measured by serum creatinine, AST, and ALT, were not obviously changed after treatment.
Table 3.
APACHE II score, tissue perfusion, and liver and kidney function.
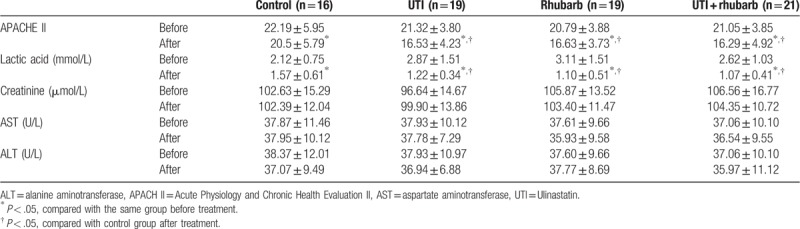
Figure 2.
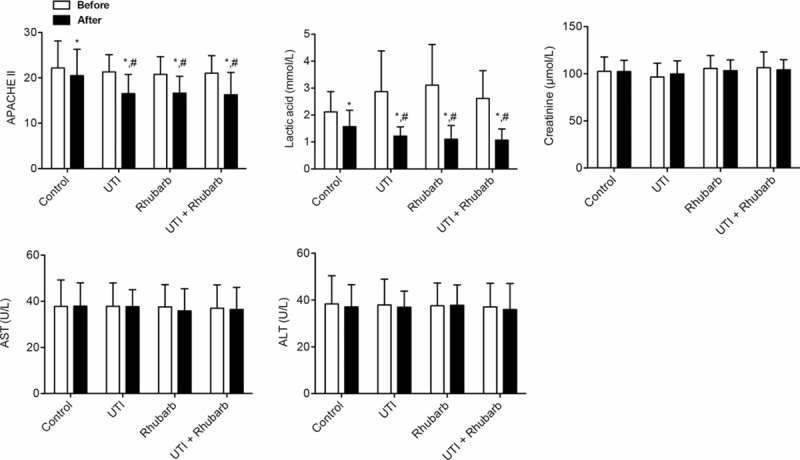
APACHE II score, tissue perfusion, and liver and kidney function. ∗P < .05, compared with the same group before treatment; # P < .05, compared with control group after treatment.
3.4. Comparison of lymphocyte subsets in peripheral blood
The percent of CD3, CD4 cells, and CD4/CD8 level were comparable among groups before treatment (Table 4; Fig. 3). The percent of CD3 cells was markedly elevated only in UTI group after treatment. There was an obvious elevation in the percent of CD4 cells in all experimental groups after treatment, and the percent of CD4 cells in UTI group was markedly increased than that in the control group after treatment. On the contrary, the percent of CD8 cells significantly declined in all experimental groups after treatment. The ratio of CD4 and CD8 cells distinctively elevated in all experimental groups after treatment.
Table 4.
Lymphocyte subsets in peripheral blood.
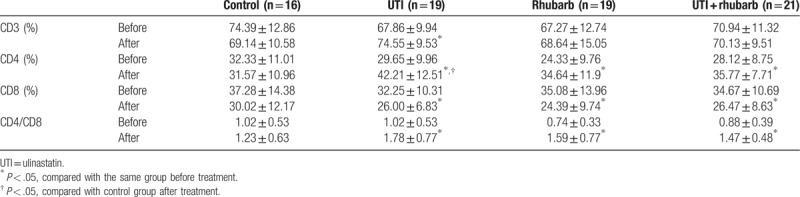
Figure 3.
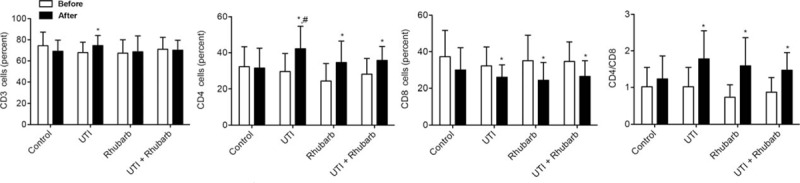
Lymphocyte subsets in peripheral blood. ∗P < .05, compared with the same group before treatment; # P < .05, compared with control group after treatment.
4. Discussion
Although the exact mechanism still remains elusive, sepsis is believed to be a systematic inflammation in the presence of infection.[22] Interaction between pathogen and host immune cells sets offs a series of pro-inflammatory responses, which is quickly followed by a concomitant anti-inflammatory response. Therefore, immunoregulatory therapy could be a promising strategy in controlling this highly fatal disease.[23] Unfortunately, using a single drug to inhibit a single inflammatory mediator, such as tumor necrosis factor α (TNF-α), has been shown to be ineffective.[24] Consequently, drugs that can suppress multiple aspects of systematic inflammation might be a preferable alternative, among which are UTI and rhubarb.
UTI attenuates inflammation by suppressing various serine proteases, and inhibiting the infiltration of neutrophils as well as the release of inflammatory mediators from neutrophils. It is also reported to inhibit the production of pro-inflammatory cytokines, such as TNF-α, IL-1, and IL-6. Previous studies have demonstrated that combining immunomodulatory therapy with UTI yields improved survival for patients and animals with sepsis through restoring the balance between pro-inflammatory and anti-inflammatory cytokines.[9–13] In this study, our data showed that combining conventional therapy with UTI significantly reduced the levels of CRP, WBC, lactic acid, and APACH II score, and elevated the levels of CD4/CD8. Thus, these studies imply that combining immunomodulatory therapy with UTI is a promising therapeutic strategy for the control of sepsis.
Traditional Chinese medicine, especially rhubarb, has been applied to treat critically ill patients for many years. It is reported that rhubarb can attenuate oxidative stress and inflammation, reduce microcirculatory disturbance of adhesion molecule expression, and inhibit leukocyte infiltration and cytokine release. Numerous studies have proved that rhubarb is effective in reducing organ dysfunction induced by sepsis in gastrointestinal tract, lung, and liver.[15,16] In this study, our data revealed that, similar to UTI, rhubarb also markedly decreased the levels of CRP, WBC, lactic acid, and APACH II score, and increased the levels of CD4/CD8. Therefore, these studies indicate that adjuvant treatment with rhubarb has additional benefits for septic patients.
Although numerous studies have shown that adjuvant treatment with UTI or rhubarb is effective in treating sepsis,[13,15,16] the clinical outcome when treating patients with both UTI and rhubarb remains to be reported. In this study, we found that, like using UTI or rhubarb alone, the combination of UTI and rhubarb also markedly reduced the levels of CRP, WBC, lactic acid, and APACH II score, and promoted the levels of CD4/CD8. Importantly, the PCT level only dropped in UTI plus rhubarb group after treatment. Serum PCT level has a positive correlation with the severity of infection, particularly in the infection caused by gram-negative Bacillus.[25] Our previous study also revealed that combination of UTI and rhubarb significantly decreased the mortality by recovering the immunosuppression than using UTI or rhubarb alone in a rat model of sepsis.[17] Thus, our study suggested that the combination of UTI and Rhubarb might improve the curative effect of sepsis. In addition, we noted that the before-treatment PCT measurement has a large S.D. (0.54 ± 0.56) in comparison to after-treatment data (0.30 ± 0.16). Thus, it is possible that the statistical insignificance could be due to the large variation before treatment.
5. Conclusions
In this study, a prospective trial was used to evaluate the efficacy of UTI, rhubarb, or combination of UTI and rhubarb for the treatment of sepsis. Our study highlighted that the combination of UTI and rhubarb might improve the curative effect on sepsis, but this finding needs to be validated in larger clinical trials.
Author contributions
Conceptualization: Fulei Meng, Xiufen Yang.
Data curation: Chongbo Du, Qingming Zhou, Liping Wu, Yanli Wang, Yuxiao Zhang.
Formal analysis: Liping Wu.
Investigation: Qingming Zhou.
Methodology: Fulei Meng, Chongbo Du.
Project administration: Fulei Meng, Yuxiao Zhang.
Resources: Liping Wu.
Software: Chongbo Du, Yanli Wang.
Supervision: Yanli Wang.
Visualization: Chongbo Du.
Writing – original draft: Fulei Meng, Shunyao Wang, Xiufen Yang.
Writing – review & editing: Fulei Meng, Xiufen Yang.
Footnotes
Abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II, CRP = C-Reactive protein, FiO2 = fraction of inspired oxygen, ICU = Intensive Care Unit, MAP = mean arterial pressure, PaO2 = partial pressure of oxygen, PBMCs = peripheral blood mononuclear cells, PCT = procalcitonin, TNF-α = tumor necrosis factor α, UTI = Ulinastatin, WBC = white blood cell.
How to cite this article: Meng F, Du C, Wang S, Zhou Q, Wu L, Wang Y, Zhang Y, Yang X. Protective effect of rhubarb combined with ulinastatin for patients with sepsis. Medicine. 2020;99:7(e18895).
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors have no conflicts of interest to disclose.
References
- [1].Fernando SM, Rochwerg B, Seely AJE. Clinical implications of the third international consensus definitions for sepsis and septic shock (Sepsis-3). CMAJ 2018;190:1058–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Moskowitz A, Omar Y, Chase M, et al. Reasons for death in patients with sepsis and septic shock. J Crit Care 2017;38:284–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Javed A, Guirgis FW, Sterling SA, et al. Clinical predictors of early death from sepsis. J Crit Care 2017;42:30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhou J, Qian C, Zhao M, et al. Epidemiology and outcome of severe sepsis and septic shock in intensive care units in mainland China. PLoS One 2014;9:e107181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sharony R, Yu PJ, Park J, et al. Protein targets of inflammatory serine proteases and cardiovascular disease. J Inflamm (Lond) 2010;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kessenbrock K, Dau T, Jenne DE. Tailor-made inflammation: how neutrophil serine proteases modulate the inflammatory response. J Mol Med (Berl) 2011;89:23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Atal SS, Atal S. Ulinastatin - a newer potential therapeutic option for multiple organ dysfunction syndrome. J Basic Clin Physiol Pharmacol 2016;27:91–9. [DOI] [PubMed] [Google Scholar]
- [8].Tsujino T, Komatsu Y, Isayama H, et al. Ulinastatin for pancreatitis after endoscopic retrograde cholangiopancreatography: a randomized, controlled trial. Clin Gastroenterol Hepatol 2005;3:376–83. [DOI] [PubMed] [Google Scholar]
- [9].Cao YZ, Tu YY, Chen X, et al. Protective effect of Ulinastatin against murine models of sepsis: inhibition of TNF-(and IL-6 and augmentation of IL-10 and IL-13. Exp Toxicol Pathol 2012;64:543–7. [DOI] [PubMed] [Google Scholar]
- [10].Li Yumin, Chen Hao, Li Xun, et al. A new immunomodulatory therapy for severe sepsis: Ulinastatin plus Thymosin alpha 1. J Intensive Care Med 2009;24:47–53. [DOI] [PubMed] [Google Scholar]
- [11].Huang N, Wang F, Wang Y, et al. Ulinastatin improves survival of septic mice by suppressing inflammatory response and lymphocyte apoptosis. J Surg Res 2013;182:296–302. [DOI] [PubMed] [Google Scholar]
- [12].Karnad DR, Bhadade R, Verma PK, et al. Intravenous administration of ulinastatin (human urinary trypsin inhibitor) in severe sepsis: a multicenter randomized controlled study. Intensive Care Med 2014;40:830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu D, Yu Z, Yin J, et al. Effect of ulinastatin combined with thymosin alpha1 on sepsis: a systematic review and meta-analysis of Chinese and Indian patients. J Crit Care 2017;39:259–66. [DOI] [PubMed] [Google Scholar]
- [14].Li P, Lu Q, Jiang W, et al. Pharmacokinetics and pharmacodynamics of rhubarb anthraquinones extract in normal and disease rats. Biomed Pharmacother 2017;91:425–35. [DOI] [PubMed] [Google Scholar]
- [15].Lai F, Zhang Y, Xie DP, et al. A systematic review of rhubarb (a Traditional Chinese Medicine) used for the treatment of experimental sepsis. Evid Based Complement Alternat Med 2015;2015:131283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang L, Chen J, Jiang D, et al. Adjuvant treatment with crude rhubarb for patients with systemic inflammation reaction syndrome/sepsis: a meta-analysis of randomized controlled trials. J Crit Care 2015;30:282–9. [DOI] [PubMed] [Google Scholar]
- [17].Tian H, Zhang M, Du C, et al. Effects of Rhubarb combined with ulinastatin on T-cell subsets in sepsis rats. Int J Clin Exp Med 2015;8:1234–40. [PMC free article] [PubMed] [Google Scholar]
- [18].Society of Critical Care Medicine, Chinese Medical Association. Guidelines for the treatment of severe sepsis/septic shock in China (2014). Chin Crit Care Med 2015;27:401–26. [Google Scholar]
- [19].Pathan N, Hemingway CA, Alizadeh AA, et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet 2004;363:203–9. [DOI] [PubMed] [Google Scholar]
- [20].Assicot M, Gendrel D, Carsin H, et al. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993;341:515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Castelli GP, Pognani C, Meisner M, et al. Procalcitonin and C-reactive protein during systemic inflammatory response syndrome, sepsis and organ dysfunction. Crit Care 2004;8:234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Artigas A, Wernerman J, Arroyo V, et al. Role of albumin in diseases associated with severe systemic inflammation: pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J Crit Care 2016;33:62–70. [DOI] [PubMed] [Google Scholar]
- [23].Minasyan H. Sepsis and septic shock: pathogenesis and treatment perspectives. J Crit Care 2017;40:229–42. [DOI] [PubMed] [Google Scholar]
- [24].Marshall JC. Such stuff as dreams are made on: mediator-directed therapy in sepsis. Nat Rev Drug Discov 2003;2:391–405. [DOI] [PubMed] [Google Scholar]
- [25].Rowland T, Hilliard H, Barlow G. Procalcitonin: potential role in diagnosis and management of sepsis. Adv Clin Chem 2015;68:71–86. [DOI] [PubMed] [Google Scholar]


