Abstract
Although the effects of intense noise exposure on the peripheral and central auditory pathway have been well characterized, its effects on non-classical auditory structures in the brain, such as the hippocampus, are less well understood. Previously, we demonstrated that noise-induced hearing loss causes a significant long-term reduction in hippocampal neurogenesis and cell proliferation. Given the known suppressive effects of stress hormones on neurogenesis, the goal of the present study was to determine if activation of the stress response is an underlying mechanism for the long-term reduction in hippocampal neurogenesis observed following noise trauma. To accomplish this, we monitored basal and reactive blood plasma levels of the stress hormone corticosterone in rats for ten weeks following acoustic trauma, and quantified changes in hippocampal glucocorticoid and mineralocorticoid receptors. Our results indicate that long-term auditory deprivation does not cause a persistent increase in basal or reactive stress hormone levels in the weeks following noise exposure. Instead, we observed a greater decline in reactive corticosterone release in noise-exposed rats between the first and tenth week of sampling compared to control rats. We also observed a significant increase in hippocampal glucocorticoid receptor expression which may cause greater hippocampal sensitivity to circulating glucocorticoid levels and result in glucocorticoid-induced suppression of neurogenesis, as well as increased feedback inhibition on the HPA axis. No change in mineralocorticoid receptor expression was observed between control and noise exposed rats. These results highlight the adverse effect of intense noise exposure and auditory deprivation on the hippocampus.
Keywords: Noise-exposure, hearing loss, neurogenesis, corticosterone, glucocorticoid receptors, mineralocorticoid receptors
1. Introduction
High-intensity noise exposure has long been known to induce damage in the peripheral and central auditory system, often resulting in auditory pathologies such as hearing loss, tinnitus and hyperacusis (Syka, 2002, Roberts et al., 2010). Within the inner ear, structures including the cochlear hair cells, stria vascularis, and auditory nerve fiber terminals are all vulnerable to noise-induced damage (Henderson et al., 2006, Salvi et al., 2000a). Additionally, intense noise exposure impacts central auditory structures of the brain leading to changes in spontaneous firing rates, neural synchrony, tonotopic map reorganization, neural degeneration, and axonal sprouting (Salvi et al., 2000b, Norena and Eggermont 2003, Michler et al., 2002, Baizer et al., 2015, Salvi et al., 2000, Roberts et al., 2010, Illing et al., 2005).
Although the effects of intense noise exposure on the peripheral and central auditory pathway have been the focus of most research, its effects on non-classical auditory structures in the brain are less well understood. The ascending non-classical auditory pathway supplies information to a widely distributed network in the brain including structures of the limbic system such as the hippocampus (Moller, 2007, Moller and Rollins, 2002). The hippocampus is one of the few regions within the brain in which neurogenesis occurs during adulthood (Altman, 1962, Bayer et al., 1982, Eriksson et al., 1998, Gould et al., 1999), and mounting evidence suggests that the production and integration of new neurons into the circuitry of the adult hippocampus contributes to this structure’s role in spatial navigation, learning and memory (for review see: Deng et al., 2010).
Recently, a number of studies have identified the hippocampus as particularly vulnerable to intense noise exposure and hearing loss (Goble et al., 2009, Saljo et al., 2002, Kraus et al., 2010, Kovesdi et al., 2011, Kwon et al., 2011, Kamnaksh et al., 2012, Newman et al., 2015, Wang et al., 2018). For example, rats acutely exposed to a loud tone demonstrated dramatic alterations in hippocampal place cell activity (Goble et al., 2009). Furthermore, intense noise exposure and noise-induced hearing loss alters hippocampal gene expression, down-regulates cell proliferation and neurogenesis, induces cell death, and results in hippocampal-dependent cognitive deficits in animal models (Saljo et al., 2002, Kraus et al., 2010, Kovesdi et al., 2011, Kwon et al., 2011, Kamnaksh et al., 2012, Newman et al., 2015, Cheng et al., 2011, Cui et al., 2009, Sajja et al., 2012, Liu et al., 2016).
Previously, we demonstrated that a single high-intensity unilateral noise exposure that resulted in permanent hearing loss suppressed cell proliferation and neurogenesis in the hippocampus out to 10 weeks post-exposure (Kraus et al., 2010); however, the mechanisms responsible for these changes are poorly understood. One possibility is that the long-term reduction in hippocampal neurogenesis following intense noise exposure and hearing loss is related to a persistent level of stress. Chronic stress and elevated stress hormone levels have long been known to suppress hippocampal neurogenesis (Cameron and Gould, 1994, Alahmed and Herbert, 2008, Brummelte and Galea, 2010). Thus, the noise-induced reduction in hippocampal neurogenesis could be due to dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis that controls the release of stress-related hormones from the hypothalamus, pituitary, and adrenal glands. Importantly, the hippocampus contains one of the highest concentrations of stress hormone responsive receptors in the brain, the glucocorticoid (GR) and mineralocorticoid receptors (MR), which underlies the important role of the hippocampus in providing negative feedback regulation on HPA axis activity (Felt et al., 1984, Van Eekelen et al., 1988, Morimoto et al., 1996, Sapolsky et al., 1984). Given that intense noise can activate the HPA axis acutely (Samson et al, 2007, De Boer et al., 1988), it is conceivable that noise-induced hearing loss could also act as a chronic stressor (Garnefski and Kraaij, 2012, Kraus and Canlon, 2012, Gomaa et al., 2014) and alter basal and/or reactive corticosterone levels or the expression of GRs and MRs in the hippocampus. To test this hypothesis, we monitored basal and reactive blood plasma levels of the stress hormone corticosterone in rats for 10 weeks following intense noise exposure and assessed the expression of GR and MR in the hippocampus using immunohistochemical techniques.
2. Material and Methods
2.1. Subjects and Experimental Design
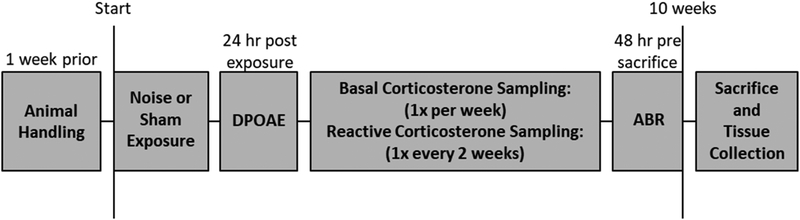
Adult male Sprague Dawley rats (Sasco, Charles River Laboratories International, Inc., Wilmington, MA, USA; 2–3 months old; n = 18) were used for this study; only males were used to avoid potential estrus-cycle confounds. Rats were divided evenly into noise-exposed (n = 9) and sham control (n = 9) groups. Four animals from each group were used for basal corticosterone blood sampling and five animals from each group were used for reactive corticosterone blood sampling. Animals were allowed to acclimate to the animal facility for at least two weeks prior to use. During the second week all animals were briefly handled each day. Rats were housed in pairs, maintained on a 12-hour light/dark cycle and allowed free access to food and water. The experimental design for this study is summarized in Figure 1. The use of animals in this project was approved by the Institutional Animal Care and Use Committee at the University of Buffalo and was carried out in accordance with National Institutes of Health guidelines.
Figure 1:
Experimental timeline. Animals were handled daily for one week prior to noise or sham exposure. Distortion product otoacoustic emissions (DPOAE) were assessed 24-hours post exposure to monitor hearing loss. Rats then underwent basal or reactive corticosterone sampling for a 10-week period. Hearing loss was again assessed 48 hours prior to sacrifice using auditory brainstem response (ABR) recordings. Following animal sacrifice, brain tissue was harvested and processed using immunohistochemical techniques.
2.2. Noise Trauma
Noise and sham exposures were carried out under isoflurane anesthesia (4% induction, 1–2% maintenance) in a sound attenuating booth. Prior to exposure, rats were examined with an otoscope to exclude animals with tympanic membrane perforation, middle ear infection and debris in the external auditory canal. During exposure, body temperature was maintained at 37 °C using a homeothermic heating pad. The left ear of each rat was exposed to a 126 dB SPL narrowband noise centered at 12 kHz (100 Hz bandwidth) for 2 hours. The noise was created using Tucker Davis Technologies hardware, amplified (Crown XLS-202), and presented via a free-field speaker positioned 2 cm from the entrance of the left ear canal. The stimulus was calibrated with a sound level meter (Larson Davis System 824) coupled to a half-inch condenser microphone (Larson Davis) before each exposure. During the exposure the opposite ear was plugged with a pediatric ear-probe filled with plumbers tack in order to restrict the noise exposure to the left ear alone. Control rats underwent the same treatment as noise-exposed rats; however, the speaker was turned off.
2.3. Distortion Product Otoacoustic Emissions (DPOAE)
Cochlear outer hair cell function was assessed using distortion product otoacoustic emissions (DPOAE) 24 hours post noise exposure as previously described (Chen et al., 2010). Rats were anesthetized with isoflurane (4% induction, 1–2% maintenance) and body temperature was maintained at 37 °C with a homeothermic heating pad and rectal probe during testing. The earpiece containing an ER10B+ microphone (Etymotic Research Inc.) and two sound delivery tubes were inserted into the ear canal. Two IHS-3738 high-frequency transducers (Intelligent Hearing System, Miami, FL) were used to deliver the primary tones, F1 and F2, to the ear canal via flexible tubes connected to the earpiece. The F2/F1 ratio was set at 1.2. The output of the microphone was fed to the input of the DPOAE system, digitized and evaluated using the system software. DPOAEs were recorded at 2F1–F2 using a Smart Distortion Product Otoacoustic Emission System (Intelligent Hearing System). DPOAE input/output functions were measured at F2 frequencies of 6, 11, and 24 kHz. The intensity of F1 was varied from 25 to 70 dB SPL in 5-dB steps and the intensity of F2 was 10 dB lower than that of F1. The output of the microphone was sampled at 40 kHz over a period of 204 ms; the spectrum of each sweep was computed and averaged over 32 non-rejected sweeps. Sweeps with an average noise level 10 dB above the initially measured noise floor were rejected. The noise floor was measured in a 24 Hz band surrounding 2F1–F2.
2.4. Auditory Brainstem Response (ABR)
Auditory brainstem responses (ABR) were performed under isoflurane anesthesia (4% induction, 1–2% maintenance, body temperature maintained at ~37 °C) as previously reported (Lobarinas et al., 2013). Briefly, the ABRs were obtained using a Tucker Davis Technologies System 3 Real Time Signal Processing System running BioSig32 and SigGen (Tucker Davis Technologies). Tone bursts (2 ms, 0.5 rise-fall time, 21/s) were presented at 6, 12, 16, 20, 24, and 32 kHz at intensities ranging from 0–100 dB SPL in 5 dB steps, and were delivered through a speaker (FT28D, Fostex) located approximately 10 cm in front of the ear being tested; the opposite ear was plugged with an earplug. The speaker was calibrated using a sound level meter (Larson Davis System 824) and a quarter-inch microphone (Larson Davis). Subdermal electrodes were placed at the ipsilateral pinna (inverting electrode) and the vertex (non-inverting electrode) with an electrode at the contralateral pinna serving as a ground. ABR evoked potentials were averaged over 1024 repetitions, amplified (RA16PA, Tucker Davis Technologies), filtered (100–3000 Hz bandpass) and digitized. Threshold at each frequency was defined as the lowest sound intensity to generate a visible waveform that was reproducible.
2.5. Blood Sampling and Corticosterone Quantification
Blood was collected from the saphenous vein of unanesthetized rats (Hem et al., 1998). Briefly, animals were held loosely in a towel with their back legs exposed. A single puncture of the vein with a sterile syringe needle was used to collect a few drops of blood into a sterile tube. The time from when the animal was removed from its cage to when sampling was complete was kept under 4 minutes to avoid the effects of handling and the sampling procedure on blood hormone levels. For rats that underwent basal corticosterone measurements, blood samples were collected once per week for ten consecutive weeks (at 9 am each day) in order to avoid circadian variation in hormone levels (Atkinson and Waddell, 1997, Tahera et al., 2007). For rats undergoing reactive corticosterone measurements from restraint stress, blood samples were collected once every two weeks for ten consecutive weeks, with each day of sampling consisting of (1) a pre-restraint sample (~9 am), (2) a sample taken immediately after removal from a 30-minute restraint session in a plastic rat restraint device (Brain Tree Scientific) (~9:30 am), and (3) a sample taken 60 minutes after removal from the restraint device (~10:30 am). Following collection of blood samples, plasma was separated by centrifugation at 6000 rpm and was immediately stored at −20 °C until further processing. Plasma corticosterone concentrations were determined using a commercially available EIA kit following the manufacturer’s instructions (Enzo Life Sciences).
2.6. Immunohistochemistry
At 10-weeks post-exposure, noise-exposed and sham control rats were deeply anesthetized with 86 mg/kg i.p. of Fatal-Plus (Vortech Pharmaceuticals Ltd), and perfused through the heart with 0.1 M phosphate buffered saline (PBS) followed by 4% paraformaldehyde (JT Baker Chemicals). Following perfusion, brains were removed and post-fixed in 4% paraformaldehyde overnight at 4 °C. The following day, tissue was cryoprotected in 15% followed by 30% sucrose in PBS until the tissue sank. Brain tissue was then cut into 40 μm thick coronal sections using a cryostat and stored in storage solution (30% ethylene glycol, 30% glycerol in 0.1M PBS) at −20 °C until further processing.
Immunolabeling was carried out on free-floating tissue sections. For every immunolabeling session, control and noise-exposed tissue sections were processed in parallel using the same solutions. Tissue sections were initially removed from cryoprotectant and rinsed in 0.1M phosphate buffered saline, pH 7.4 (PBS). Tissue sections were washed in 0.1 M PBS for 30 minutes at room temperature between each of the following incubation steps. For deactivation of endogenous peroxidase, sections were treated with 0.3% H2O2 (Fischer Scientific) in PBS for 10 minutes. Next, the sections were pre-treated with blocking solution containing 5% normal horse serum (Vector Laboratories) and 0.3% Triton X-100 (Fischer Scientific) in PBS for 1 hour. Sections were then incubated with primary antibody against the glucocorticoid receptor (GR) (1:250; Abcam, ab2768) or mineralocorticoid receptor (MR) (1:250; Santa Cruz, sc-6860) in blocking solution with 5% normal horse serum and 0.15% Triton X-100 overnight at 4 °C. Next, sections were incubated with biotinylated secondary antibody (1:100; anti-mouse or anti-goat IgG, Vector Laboratories) in blocking solution with 5% normal horse serum and 0.15% Triton X-100 for 2 hours at room temperature. Sections were processed using Vectastain ABC kits (Vector Laboratories) and labeling was visualized using the glucose oxidase modification of the diaminobenzidine (DAB) method (Van Der Gucht et al., 2006). Immunolabeled sections were mounted on Fisher Superfrost polarized slides and dried overnight. Slides were then dehydrated in increasing concentrations of alcohol, cleared in xylene and sealed using DPX (Fisher Scientific).
GR and MR expression was assessed in coronal sections throughout the rostral-caudal axis of the dorsal hippocampus using 6 sections per animal, and 4 animals from each of the two exposure groups. Images were acquired at 40X magnification under brightfield illumination (Axioskop, Carl Zeiss MicroImaging Inc.) using a digital camera (SPOT Insight; Diagnostic Instruments Inc.) with camera exposure and gain settings held constant for all images. Quantification of GR/MR expression was carried out using NIH ImageJ software as previously reported (Fournier et al., 2010, Fournier et al., 2012). Mean relative optical density was calculated for each region of interest containing the dentate gyrus in noise-exposed and control tissue sections. Background staining was controlled by calculating the average optical density levels from surrounding tissue and subtracting these values from the region of interest.
2.7. Statistical Analyses
Statistical analyses were conducted using either a two-way repeated measures analysis of variance (ANOVA) or t-test, depending on the comparison of interest (see Results section for the details of each specific comparison). All statistical comparisons used an alpha value of 0.05. When an ANOVA was used, post hoc testing was performed with Bonferroni post-tests to correct for multiple comparisons. Sigma Stat 3.5 was used for all statistical analyses. All results are presented as mean ± standard error of the mean (SEM).
3. Results
3.1. Distortion Product Otoacoustic Emission and Auditory Brainstem Response Recordings
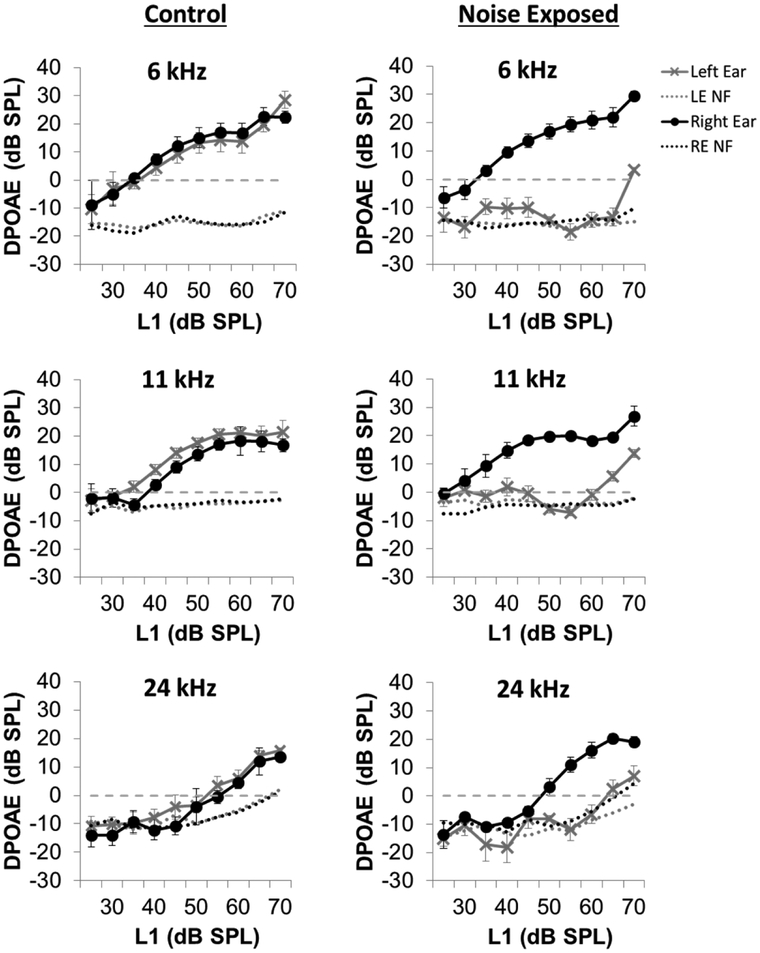
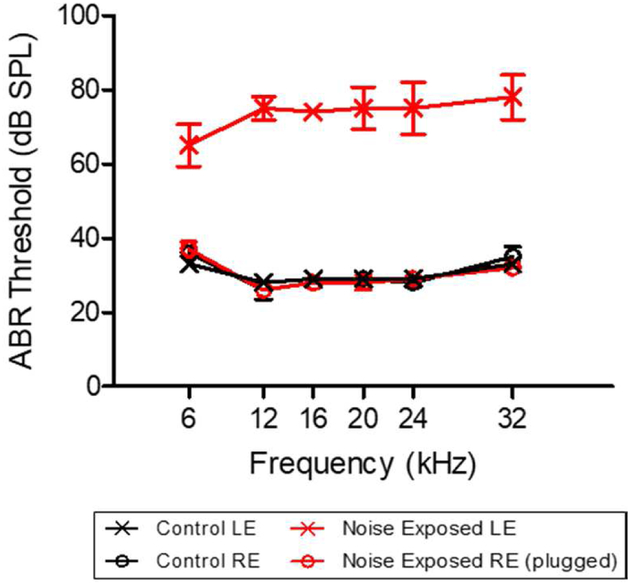
To test for noise-induced damage to outer hair cells (OHCs), the mean DPOAE input/output functions at F2 frequencies of 6, 11, and 24 kHz were measured 24 hours after the unilateral noise or sham exposure. As shown in Figure 2, noise exposure caused a dramatic reduction in DPOAE amplitude in the exposed left ear at each tested frequency, a physiological indicator of impaired OHC function. DPOAEs were nearly absent in the left-exposed ear while DPOAEs in the right unexposed ears were substantially larger and similar to the DPOAEs in the left and right ears of sham controls. ABR thresholds measured 10-weeks post-exposure confirmed the presence of permanent hearing loss in the left noise-exposed ears. ABR thresholds in the left noise-exposed ears were 40–45 dB higher than those in the right-unexposed ear and in the left and right sham control ears. There was a significant elevation of ABR thresholds observed in the noise-exposed ears at all tested frequencies (Figure 3; Two-way RM ANOVA, p < 0.001). ABR thresholds in the left and right ears of the sham control rats were virtually identical to those in the plugged, right ear of the noise-exposed rats. These findings indicate that our noise exposure protocol produced significant and permanent hearing loss in the left-exposed ears, but not in the right-unexposed ears, consistent with our prior studies using the same noise exposure (Kraus 2010).
Figure 2:
Distortion product otoacoustic emissions (DPOAEs), a measure of cochlear outer hair cell function, were assessed 24-hours post noise or sham exposure. DPOAEs were dramatically reduced in the left noise-exposed ears compared to unexposed ears at 6, 11 and 24 kHz, demonstrating cochlear damage from the noise exposure. (LE NF = left ear noise floor; RE NF = right ear noise floor).
Figure 3:
Auditory brainstem response (ABR) recordings were used to monitor permanent hearing loss following noise exposure at the end of the 10-week experimental timeline. The left noise-exposed ears demonstrated a significant threshold shift from 6–32 kHz compared to unexposed right ears, demonstrating the presence of a permanent hearing loss following the noise exposure protocol. (RE = right ear, LE = left ear). ABR thresholds in the left and right control ears were similar to the thresholds in the unexposed right ear.
3.2. Basal Corticosterone Levels
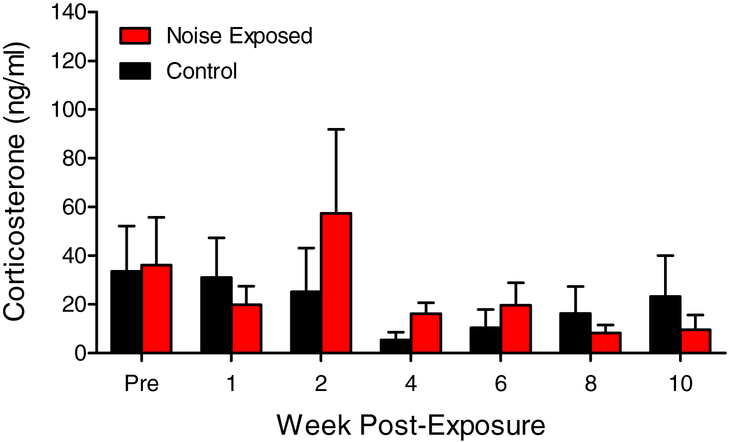
In order to determine if unilateral noise exposure and the resulting hearing loss serves as a long-term stressor, changes in basal corticosterone levels in noise-exposed and control animals were monitored over a 10-week period (Figure 4). The mean corticosterone levels were not significantly different between the noise group and control group, and did not change significantly over the 10 week period post noise/sham-exposure.
Figure 4:
Baseline corticosterone levels were monitored pre and post noise exposure and over the 10-week sampling period in noise-exposed and control animals. No significant differences in basal corticosterone levels were observed between noise-exposed and control animals (Two-way RM-ANOVA, p > 0.05, n = 4). Data represent mean ± SEM.
3.3. Reactive Corticosterone Levels
In addition to basal corticosterone levels, release of corticosterone in response to an acute stressor (reactive corticosterone level) was also quantified over the 10-week period post noise exposure. Blood samples were collected prior to (baseline), immediately after (post), and one hour after (1 hr post) a 30-minute session in a plastic restraint device. Compared to sham exposed animals, reactive corticosterone levels were significantly elevated in the noise-exposed group 1-week post exposure (Figure 5A; Two-way RM ANOVA, p < 0.05). For the remaining weeks of sampling, there was a trend for a reduction or blunting of the stress response in the noise-exposed group (Figure 5B–D); however, this difference was not statistically significant. Closer inspection of the corticosterone data immediately after restraint stress suggests that the magnitude of corticosterone decline between 1-week and 10-weeks post-exposure was greater in the noise-exposed group than the control group (Figure 5A–D). We measured the decrease in post-restraint corticosterone levels between week-1 and week-10 and found that corticosterone levels in the noise-exposed group declined 334 ± 73 ng/ml (643 ng/ml to 309 ng/ml) compared to a decline of 155 ± 31 ng/ml (542 ng/ml to 387 ng/ml) in the control group; the decrease in the noise-group was more than twice as large as the control group (one-tailed t-test, p < 0.05).
Figure 5:

Reactive corticosterone levels before (pre), immediately following (post), and 1-hour after (1 hr post) exposure to 30-minutes of restraint were monitored over the 10-week sampling period in noise-exposed and control animals. (A) Reactive corticosterone levels were significantly elevated in noise-exposed rats 1-week post exposure (Two-way RM-ANOVA, p < 0.05, n = 5). (B-D) In contrast, in weeks 4–10, there was a trend for a reduction (i.e., blunting) of the stress response in the noise exposed group of animals compared to the controls; however, this difference was not statistically significant. Data represent mean ± SEM.
3.4. Hippocampal Glucocorticoid and Mineralocorticoid Receptor Expression
Corticosterone binds to glucocorticoid (GR) and mineralocorticoid (MR) receptors with low and high affinity, respectively. Consequently, the response of the hippocampus to circulating corticosterone not only depends on corticosterone concentration, but also on the abundance of corticosterone receptors. To determine if noise exposure alters expression of these stress hormone receptors within the subgranular zone of the hippocampal dentate gyrus where neurogenesis occurs, we used immunolabeling to visualize the expression of GR and MR in the hippocampus of noise-exposed and control rats. Expression of GR and MR was then quantified by measuring the intensity of staining (relative optical density) on each processed tissue section.
Figure 6 shows representative photomicrographs of GR immunolabeling in the hippocampal dentate gyrus of a noise-exposed (A, C) and sham control (B, D) rat. Cells immunopositive for the glucocorticoid receptor can be seen throughout the length of the neurogenic zone of the dentate gyrus, while labeling outside of the neurogenic zone was more dispersed and less intense. Visual inspection clearly shows that GR labeling was much greater in the noise-exposed rat compared to the control rat (Figure 6A, B). To quantify these differences, we measured the relative optical density of immunolabeling in the dentate gyrus of all the sampled sections from the hippocampus of the noise-exposed and control groups. Consistent with the photomicrographs, we found a significant increase in GR expression in the noise-exposed group compared to the sham-exposed group; labeling intensity in the noise group was roughly twice as great as that in the control group (Figure 6E, two-tailed t-test, p < 0.05).
Figure 6:
Representative photomicrographs of hippocampal glucocorticoid receptor (GR) expression in noise-exposed (A, C) and control (B, D) rats. Cells immunopositive for GR can be seen spanning the length of the hippocampal dentate gyrus (DG). The arrows in panels A and B mark the location of the higher magnification images in panels C and D. Compared to control animals, unilaterally noise-exposed animals had a significant increase (~2 fold) in dentate gyrus GR expression (E; two-tailed t-test, p < 0.05). Data represent mean ± SEM.
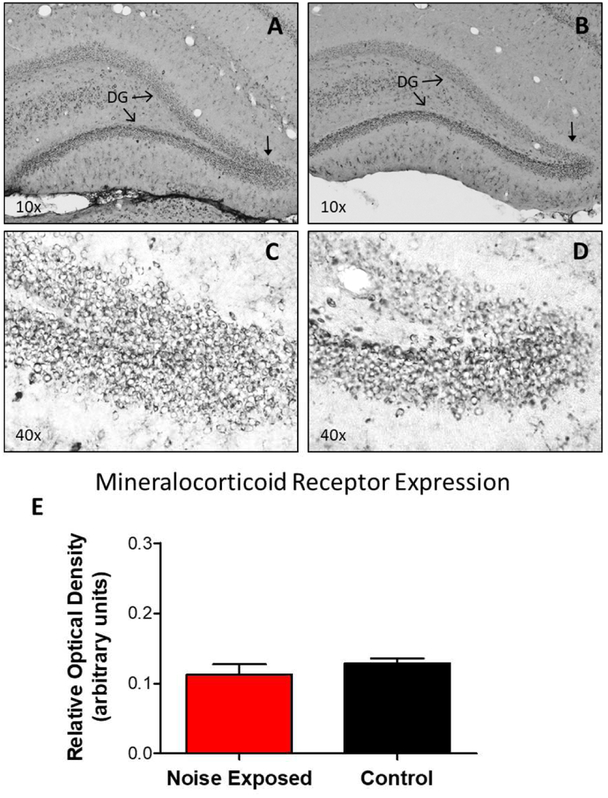
In Figure 7, representative photomicrographs of MR immunolabeling of the hippocampus from a noise-exposed (A-C) and a control (B-D) rat show a similar pattern of moderate labeling along the dentate gyrus, with less intense labeling in surrounding regions. To quantify these results, we measured the relative optical density of MR immunolabeling in the dentate gyrus in the noise-exposed group and the control group as described above. Consistent with the photomicrographs, MR labeling in the noise-exposed animals was not significantly different from the control group (Figure 7E).
Figure 7:
Representative photomicrographs of hippocampal mineralocorticoid receptor (MR) expression in noise-exposed (A, C) and control (B, D) rats. Cells immunopositive for MR can be seen spanning the length of the hippocampal dentate gyrus (DG). The arrows in panels A and B mark the location of the higher magnification images in panels C and D. No significant difference was observed in MR expression between noise-exposed and control animals (E; two-tailed t-test, p > 0.05). Data represent mean ± SEM.
4. Discussion
Accumulating evidence suggests that the hippocampus is sensitive to noise-induced hearing loss and other forms of sensory deprivation, which deprives the hippocampus of sensory information required for its normal operations such as spatial navigation and memory formation (Smith et al., 2005a, Smith et al., 2005b, Ciu et al., 2009, Cheng et al., 2011). Previous studies have shown that noise-induced hearing loss can suppress cell proliferation and neurogenesis in the hippocampus (Kraus et al., 2010, Newman et al., 2015). Because increased stress and elevated stress hormone levels can suppress neurogenesis, we investigated whether noise-induced hearing loss may serve as a stressor and alter basal corticosterone levels, reactive corticosterone levels and/or the expression levels of GRs and MRs in the dentate gyrus of the hippocampus.
4.1. Basal corticosterone and hippocampal MR expression
Within the brain, the high affinity mineralocorticoid receptor binds corticosterone at low concentrations and regulates the basal activity of the HPA axis important for maintaining bodily homeostasis under resting conditions (de Kloet et al., 1993). While exposure to intense noise is known to evoke an acute stress response (Samson et al, 2007, De Boer et al., 1988), little is known about the long-term effects of noise-induced hearing loss on resting state levels of stress hormones and the receptors that regulate it. In the present study, we found no significant change in basal corticosterone levels sampled over a 10-week period post-noise exposure (Figure 4). Similarly, noise-induced hearing loss also did not alter expression levels of the hippocampal MR responsible for regulating basal HPA axis activity (Figure 7). Thus, despite the ability of noise exposure to activate the stress response acutely, it does not result in altered levels of basal activity of the HPA axis.
4.2. Reactive corticosterone and hippocampal GR expression
Because dysregulation of HPA axis activity is often revealed under stressful rather than basal conditions (Sotnikov et al., 2014), we further explored the effects of noise-induced hearing loss on the stress response evoked by 30-minutes of restraint. Glucocorticoid receptors (GRs) are preferentially activated during such stressful conditions when corticosterone levels are high, and activation of GRs within the hippocampus results in negative feedback inhibition of the HPA axis in order to limit the magnitude and duration of the stress response (Sapolsky et al., 1984). In both noise-exposed and control animals, 30-minutes of restraint was a potent activator of the stress response, resulting in a roughly 10-fold increase of corticosterone levels immediately following restraint (Figure 5). One week post exposure, noise-exposed rats demonstrated a significantly greater corticosterone release in response to restraint compared to control rats. However, when comparing the level of corticosterone released following restraint between 1-week and 10-weeks post exposure, a greater decline was observed in the noise-exposed rats compared to the control group.
While exposure to intense noise has previously been shown to acutely activate the stress response (Samson et al, 2007, De Boer et al., 1988), our results provide insight on how HPA axis reactivity changes over time following acoustic trauma. The significant increase in reactive corticosterone release in noise-exposed rats one-week post exposure (Figure 5) demonstrates that even after one week, noise exposure can result in hyperactivity of the HPA axis. This suggests that the acute effects of noise exposure previously reported on HPA axis reactivity can last up to one-week following the exposure. Following this initial hyperactivity of the stress response at the one-week period, we observed a gradual hyporeactivity of the HPA axis in the noise-exposed animals, characterized by a larger decline in corticosterone release over the 10-week period. One explanation for the larger decline in stress-evoked corticosterone in the noise-exposed group is that the HPA axis became more responsive to the release of corticosterone resulting from restraint stress. One way in which this could occur is if there were a gradual increase in the number of GRs in the hippocampus between week-1 and week-10 post exposure which would cause greater negative feedback inhibition on HPA axis activity. Consistent with this interpretation, we found that GRs were expressed at a significantly higher level in the dentate gyrus of the noise-exposed group than the control group at 10-weeks post-exposure (Figure 6). While this interpretation of the corticosterone decline is intriguing, experimental data showing the GR level progressively increased between 1-week and 10-weeks of restraint stress are needed to support this view. However, a previous study supports this hypothesis, as repeated stress does affect GR expression in the hippocampus (Robertson, et al., 2005). In the Robertson et. al. 2005 study, rats repeatedly exposed to stress exhibited changes in glucocorticoid immunoreactivity in the dorsal hippocampus, but not in other brain regions (e.g. ventral hippocampus, frontal cortex, hypothalamus or parietal cortex). They also observed a bimodal temporal response in GR immunoreactivity in the dorsal hippocampus, whereby an initial decrease in GR expression was observed after 5 days of repeated stress, followed by an increase in GR immunoreactivity after 20 days. This suggests that repetitive stress-mediated changes in GR expression in the rat brain are both time-dependent as well as brain region specific. Furthermore, genetically modified mice with an overexpression of GRs within the brain also demonstrate enhanced feedback inhibition of the HPA axis and release significantly less corticosterone in response to stress with no change in basal corticosterone release (Ridder et al., 2005), highlighting the important relationship between the level of GR expression and activity of the HPA axis under stressful conditions.
4.3. Role of GR in neurogenesis
There is a large body of evidence supporting the hypothesis that activation of GRs has a suppressive effect on neurogenesis in the brain. Acute or chronic stress, as well as chronic glucocorticoid administration, all have inhibitory effects on newborn neurons and their survival in the brain (Cameron and Gould, 1994, Oomen et al., 2007, Yu et al., 2010). Prenatal stress is a known inhibitor of neurogenesis in the rat brain particularly in the dentate gyrus, with accompanying impairment in hippocampal-related spatial tasks (Lemaire et al., 2000). Additional studies point to a major role for glucocorticoid molecular signaling pathways in neurogenesis, as high concentrations of corticosterone, through activation of GR, reduce both cell proliferation and neuronal differentiation (Anacker et al., 2013). As further evidence, loss of GR function, through knockdown experiments, alter migration, integration and differentiation of newborn granule cells in the hippocampus (Fitzsimons, et al., 2013). Moreover, the GR antagonist mifepristone, when administered in a high stress environment, normalizes neurogenesis in rat brain (Oomen, et al., 2007). Taken together, activation of GRs has a suppressive effect on neurogenesis, an important factor to consider given the reduction in neurogenesis (Kraus et al., 2010) and increased expression of GRs (Figure 6) observed in the hippocampus of rats with noise-induced hearing loss. Our results provide a potential mechanism through which increased expression of hippocampal GRs following intense noise exposure would result in greater hippocampal sensitivity to corticosterone and impairments of hippocampal neurogenesis.
4.4. Auditory pathology and the stress response
Our findings of adverse effects of noise exposure on the hippocampus, including changes in GR expression and corticosterone release, are interesting in light of a number of recent human and animal studies linking altered HPA axis activity and hearing disorders. Interestingly, auditory pathologies such as hearing loss and tinnitus have been shown to occur in higher prevalence in individuals with long-lasting stress and emotional burnout (Hasson et al., 2011). Furthermore, hyporeactivity of the HPA axis has been documented in patients suffering from chronic tinnitus and hearing loss (Hebert and Lupien, 2007). In response to an acute psychosocial stress protocol, individuals with tinnitus and hearing loss secrete significantly less cortisol than control subjects without tinnitus. Our results provide a potential mechanism through which changes in hippocampal GR expression due to hearing loss or tinnitus may lead to a reduction in stress hormone release through enhanced negative feedback on the HPA axis. Although we did not test our noise-exposed animals for the presence of tinnitus in the present study, high-intensity noise exposure has long been used as a method of tinnitus induction in animal models (Heffner and Harrington, 2002, Roberts et al., 2010, Singer et al., 2013), making it possible that some of the noise-exposed rats in the present study were experiencing both hearing loss and tinnitus.
Recent animal studies of intense noise exposure have also implicated a role for the hippocampus and stress response in the development of tinnitus and cochlear damage (Singer et al., 2013, Singer et al., 2018). Elevated corticosterone at the time of noise exposure was found to influence both the level of auditory nerve fiber loss in the cochlea, as well as the adaptive response of the central auditory system to cochlear damage. Specifically, high levels of corticosterone were correlated with greater auditory nerve fiber loss and a failure to mobilize Arc mRNA (a putative marker of synaptic plasticity) in the hippocampus and auditory cortex of noise exposed rats, resulting in the development of tinnitus (Singer et al., 2013). Interestingly, administration of the GR antagonist mifepristone was found to attenuate the loss of auditory nerve fibers associated with high levels of corticosterone at the time of acoustic trauma (Singer et al., 2018). In future studies, it would be interesting to determine whether antagonism of GRs with mifepristone could also attenuate the impairment of neurogenesis within the hippocampus following noise exposure (Kraus et al., 2010), especially given the increase in hippocampal GR expression observed in the present study.
4.4. Conclusion
Clearly, the association between auditory deprivation, GR expression, and brain pathology is multifaceted, and future animal studies may help elucidate their underlying relationship. Our study was limited to investigating changes in GR expression in the hippocampus following noise-induced auditory deprivation; however, changes in GR expression in other areas of the brain including structures of the central auditory pathway have yet to be investigated. Our results add to a growing body of evidence demonstrating adverse effects of intense noise exposure and hearing loss on brain regions outside of the classical auditory pathway, as well as studies linking hearing problems with stress related disorders (Hasson et al., 2011, Hebert and Lupien, 2007, Hebert et al., 2012). Furthermore, future animal studies investigating the consequence of auditory deprivation-induced changes in hippocampal neurogenesis may help us better understand the relationship between cognitive decline and hearing loss observed in the human population (Lin et al., 2011).
Highlights.
We determined if stress causes noise-induced suppression of hippocampal neurogenesis
Noise exposed rats have greater reactive corticosterone 1-week post noise exposure
No long-term increase in basal/reactive corticosterone observed post noise exposure
Noise-induced hearing loss causes a long-term increase in hippocampal GR expression
Increased GR may cause hippocampal sensitivity to stress and reduce neurogenesis
Acknowledgements
This work was supported in part by National Institute of Health grants 5R01DC011808, R01DC014452, and R01DC014693. The authors would like to thank Dr. Kelly Radziwon and Dr. Gail Seigel for their helpful comments on earlier versions of the manuscript.
Abbreviations
- ABR
Auditory brainstem response
- DPOAE
Distortion product otoacoustic emissions
- GR
Glucocorticoid receptor
- HPA axis
Hypothalamic-pituitary-adrenal axis
- MR
Mineralocorticoid receptor
- OHC
Outer hair cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- Alahmed S, Herbert J 2008. Strain differences in proliferation of progenitor cells in the dentate gyrus of the adult rat and the response to fluoxetine are dependent on corticosterone. Neuroscience 157:677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J 1962. Are new neurons formed in the brains of adult mammals? Science 135:1127–1128. [DOI] [PubMed] [Google Scholar]
- Anacker C, Cattaneo A, Luoni A, Musaelyan K, Zunszain PA, Milanesi E, Rybka J, Berry A, Cirulli F, Thuret S, Price J, Riva MA, Gennarelli M, Pariante CM 2013. Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology 38:872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson HC, Waddell BJ 1997. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology 138:3842–3848. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Wong KM, Manohar S, Hayes SH, Ding D, Dingman R, Salvi RJ 2015. Effects of acoustic trauma on the auditory system of the rat: The role of microglia. Neuroscience 303:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Yackel JW, Puri PS 1982. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science 216:890–892. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LA 2010. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience 168:680–690. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E 1994. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 61:203–209. [DOI] [PubMed] [Google Scholar]
- Chen GD, Kermany MH, D’Elia A, Ralli M, Tanaka C, Bielefeld EC, Ding D, Henderson D, Salvi R 2010. Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res 265:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Wang SH, Chen QC, & Liao XM 2011. Moderate noise induced cognition impairment of mice and its underlying mechanisms. Physiol Behav, 104(5), 981–988. [DOI] [PubMed] [Google Scholar]
- Cui B, Wu M, & She X 2009. Effects of chronic noise exposure on spatial learning and memory of rats in relation to neurotransmitters and NMDAR2B alteration in the hippocampus. J Occup Health, 51(2), 152–158. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koopmans SJ, Slangen JL, Van der Gugten J 1989. Effects of fasting on plasma catecholamine, corticosterone and glucose concentrations under basal and stress conditions in individual rats. Physiol Behav, 45(5), 989–994. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Sutanto W, van den Berg DT, Carey MP, van Haarst AD, Hornsby CD, Meijer OC, Rots NY, Oitzl MS 1993. Brain mineralocorticoid receptor diversity: functional implications. J Steroid Biochem Mol Biol 47:183–190. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH 2010. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH 1998. Neurogenesis in the adult human hippocampus [see comments]. Nat Med 4:1313–1317. [DOI] [PubMed] [Google Scholar]
- Felt BT, Sapolsky RM, McEwen BS 1984. Regulation of hippocampal corticosterone receptors by a vasopressin analogue. Peptides 5:1225–1227. [DOI] [PubMed] [Google Scholar]
- Fitzsimons CP, van Hooijdonk LWA, Schouten M, Zalachoras I, Brinks V, Zheng T, … Vreugdenhil E 2013. Knockdown of the glucocorticoid receptor alters functional integration of newborn neurons in the adult hippocampus and impairs fear-motivated behavior. Mol Psychiatry, 18(9), 993–1005. [DOI] [PubMed] [Google Scholar]
- Fournier NM, Andersen DR, Botterill JJ, Sterner EY, Lussier AL, Caruncho HJ, Kalynchuk LE 2010. The effect of amygdala kindling on hippocampal neurogenesis coincides with decreased reelin and DISC1 expression in the adult dentate gyrus. Hippocampus 20:659–671. [DOI] [PubMed] [Google Scholar]
- Fournier NM, Lee B, Banasr M, Elsayed M, Duman RS 2012. Vascular endothelial growth factor regulates adult hippocampal cell proliferation through MEK/ERK- and PI3K/Akt-dependent signaling. Neuropharmacology 63:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnefski N, Kraaij V 2012. Cognitive coping and goal adjustment are associated with symptoms of depression and anxiety in people with acquired hearing loss. Int J Audiol 51:545–550. [DOI] [PubMed] [Google Scholar]
- Goble TJ, Moller AR, Thompson LT 2009. Acute high-intensity sound exposure alters responses of place cells in hippocampus. Hear Res 253:52–59. [DOI] [PubMed] [Google Scholar]
- Gomaa MA, Elmagd MH, Elbadry MM, Kader RM 2014. Depression, Anxiety and Stress Scale in patients with tinnitus and hearing loss. Eur Arch Otorhinolaryngol 271:2177–2184. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E 1999. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A 96:5263–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson D, Theorell T, Wallen MB, Leineweber C, Canlon B 2011. Stress and prevalence of hearing problems in the Swedish working population. BMC Public Health, 11, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert S, Canlon B, Hasson D 2012. Emotional Exhaustion as a Predictor of Tinnitus. Psychotherapy and Psychosomatics, 81(5), 324–326. [DOI] [PubMed] [Google Scholar]
- Hebert S, & Lupien SJ 2007. The sound of stress: Blunted cortisol reactivity to psychosocial stress in tinnitus sufferers. Neuroscience Letters, 411(2), 138–142. [DOI] [PubMed] [Google Scholar]
- Heffner HE, & Harrington IA (2002). Tinnitus in hamsters following exposure to intense sound. Hear Res, 170(1–2), 83–95. [DOI] [PubMed] [Google Scholar]
- Hem A, Smith AJ, Solberg P 1998. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guinea pig, ferret and mink. Lab Anim 32:364–368. [DOI] [PubMed] [Google Scholar]
- Henderson D, Bielefeld EC, Harris KC, Hu BH 2006. The role of oxidative stress in noise-induced hearing loss. Ear Hear 27:1–19. [DOI] [PubMed] [Google Scholar]
- Illing RB, Kraus KS, Meidinger MA 2005. Reconnecting neuronal networks in the auditory brainstem following unilateral deafening. Hear Res 206:185–199. [DOI] [PubMed] [Google Scholar]
- Kamnaksh A, Kwon SK, Kovesdi E, Ahmed F, Barry ES, Grunberg NE, Long J, Agoston D 2012. Neurobehavioral, cellular, and molecular consequences of single and multiple mild blast exposure. Electrophoresis 33:3680–3692. [DOI] [PubMed] [Google Scholar]
- Kovesdi E, Gyorgy AB, Kwon SK, Wingo DL, Kamnaksh A, Long JB, Kasper CE, Agoston DV 2011. The effect of enriched environment on the outcome of traumatic brain injury; a behavioral, proteomics, and histological study. Front Neurosci 5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus KS, Canlon B 2012. Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear Res 288:34–46. [DOI] [PubMed] [Google Scholar]
- Kraus KS, Mitra S, Jimenez Z, Hinduja S, Ding D, Jiang H, Gray L, Lobarinas E, Sun W, Salvi RJ 2010. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience 167:1216–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SK, Kovesdi E, Gyorgy AB, Wingo D, Kamnaksh A, Walker J, Long JB, Agoston DV 2011. Stress and traumatic brain injury: a behavioral, proteomics, and histological study. Front Neurol 2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN 2000. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA 97:11032–11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L 2011. Hearing loss and incident dementia. Archives of neurology 68:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LJ, Shen P, He TT, Chang Y, Shi LJ, Tao S … Wang J (2016). Noise induced hearing loss impairs spatial learning/memory and hippocampal neurogenesis in mice. Scientific Reports, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Hayes SH, Allman BL 2013. The gap-startle paradigm for tinnitus screening in animal models: limitations and optimization. Hear Res 295:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michler SA, & Illing RB 2002. Acoustic trauma induces reemergence of the growth- and plasticity-associated protein GAP-43 in the rat auditory brainstem. Journal of Comparative Neurology, 451(3), 250–266. [DOI] [PubMed] [Google Scholar]
- Moller AR, Rollins PR 2002. The non-classical auditory pathways are involved in hearing in children but not in adults. Neurosci Lett 319:41–44. [DOI] [PubMed] [Google Scholar]
- Moller AR (2007). The role of neural plasticity in tinnitus. Prog Brain Res, 166, 37–45. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M 1996. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res 26:235–269. [DOI] [PubMed] [Google Scholar]
- Newman AJ, Hayes SH, Rao AS, Allman BL, Manohar S, Ding D, Stolzberg D, Lobarinas E, Mollendorf JC, Salvi R 2015. Low-cost blast wave generator for studies of hearing loss and brain injury: blast wave effects in closed spaces. J Neurosci Methods 242:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norena AJ, Eggermont JJ 2003. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res 183:137–153. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Mayer JL, de Kloet ER, Joels M, Lucassen PJ 2007. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur J Neurosci 26:3395–3401. [DOI] [PubMed] [Google Scholar]
- Ridder S, Chourbaji S, Hellweg R, Urani A, Zacher C, Schmid W, … Gass P 2005. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. Journal of Neuroscience, 25(26), 6243–6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA 2010. Ringing ears: the neuroscience of tinnitus. J Neurosci 30:14972–14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DAF, Beattie JE, Reid IC, Balfour DJK 2005. Regulation of corticosteroid receptors in the rat brain: the role of serotonin and stress. European Journal of Neuroscience, 21(6), 1511–1520. [DOI] [PubMed] [Google Scholar]
- Sajja VS, Galloway MP, Ghoddoussi F, Thiruthalinathan D, Kepsel A, Hay K, Bir CA, VandeVord PJ 2012. Blast-induced neurotrauma leads to neurochemical changes and neuronal degeneration in the rat hippocampus. NMR Biomed 25:1331–1339. [DOI] [PubMed] [Google Scholar]
- Saljo A, Bao F, Jingshan S, Hamberger A, Hansson HA, Haglid KG 2002. Exposure to short-lasting impulse noise causes neuronal c-Jun expression and induction of apoptosis in the adult rat brain. J Neurotrauma 19:985–991. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Ding D, Wang J, Jiang H-Y. 2000a. Effects of selective inner hair cell loss on distortion product otoacoustic emissions, cochlear function and auditory evoked potentials. Noise & Health 6:9–25. [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D 2000b. Auditory plasticity and hyperactivity following cochlear damage. Hear Res 147:261–274. [DOI] [PubMed] [Google Scholar]
- Samson J, Sheeladevi R, Ravindran R, Senthilvelan M 2007. Stress response in rat brain after different durations of noise exposure. Neurosci Res 57:143–147. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, Krey LC, McEwen BS 1984. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc. Natl. Acad. Sci. USA 81:6174–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W, Zuccotti A, Jaumann M, Lee SC, Panford-Walsh R, Xiong H, Zimmermann U, Franz C, Geisler HS, Kopschall I, Rohbock K, Varakina K, Verpoorten S, Reinbothe T, Schimmang T, Ruttiger L, Knipper M (2013). Noise-Induced Inner Hair Cell Ribbon Loss Disturbs Central Arc Mobilization: A Novel Molecular Paradigm for Understanding Tinnitus. Mol Neurobiol, 47(1), 261–279. [DOI] [PubMed] [Google Scholar]
- Singer W, Kasini K, Manthey M, Eckert P, Armbruster P, Vogt MA, Jaumann M, Dotta M, Yamahara K, Harasztosi C, Zimmermann U, Knipper M, Ruttiger L (2018). The glucocorticoid antagonist mifepristone attenuates sound-induced long-term deficits in auditory nerve response and central auditory processing in female rats. Faseb Journal, 32(6), 3005–3019. [DOI] [PubMed] [Google Scholar]
- Smith PF, Horii A, Russell N, Bilkey DK, Zheng Y, Liu P, Kerr DS, Darlington CL 2005a. The effects of vestibular lesions on hippocampal function in rats. Prog Neurobiol 75:391–405. [DOI] [PubMed] [Google Scholar]
- Smith PF, Zheng Y, Horii A, Darlington CL 2005b. Does vestibular damage cause cognitive dysfunction in humans? J Vestib Res 15:1–9. [PubMed] [Google Scholar]
- Sotnikov S, Wittmann A, Bunck M, Bauer S, Deussing J, Schmidt M, Touma C, Landgraf R, Czibere L 2014. Blunted HPA axis reactivity reveals glucocorticoid system dysbalance in a mouse model of high anxiety-related behavior. Psychoneuroendocrinology 48:41–51. [DOI] [PubMed] [Google Scholar]
- Syka J 2002. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiological Reviews, 82(3), 601–636. [DOI] [PubMed] [Google Scholar]
- Van Eekelen JA, Jiang W, De Kloet ER, Bohn MC 1988. Distribution of the mineralocorticoid and the glucocorticoid receptor mRNAs in the rat hippocampus. J Neurosci Res 21:88–94. [DOI] [PubMed] [Google Scholar]
- Wang WH, Zinsmaier AK, Firestone E, Lin RZ, Yatskievych TA, Yang S, … Bao SW (2018). Blocking Tumor Necrosis Factor-Alpha Expression Prevents Blast-Induced Excitatory/Inhibitory Synaptic Imbalance and Parvalbumin-Positive Interneuron Loss in the Hippocampus. Journal of Neurotrauma, 35(19), 2306–2316. [DOI] [PubMed] [Google Scholar]
- Yu S, Patchev AV, Wu Y, Lu J, Holsboer F, Zhang JZ, Sousa N, Almeida OF 2010. Depletion of the neural precursor cell pool by glucocorticoids. Ann Neurol 67:21–30. [DOI] [PubMed] [Google Scholar]