Abstract
Objective
Multidrug resistance-associated protein 2 (MRP2), encoded by ABCC2 gene, is involved in the efflux of certain anticancer drugs. Here we observed whether the ABCC2 (G1249A) polymorphism impacts the transport abilities of MRP2-dependent paclitaxel, docetaxel, and doxorubicin in recombinant LLC-PK1 cell lines.
Methods
LLC-PK1 cell lines transfected with ABCC21249G wild-type and ABCC21249A variant alleles were used to evaluate the sensitivity, intracellular accumulation, and transmembrane transport of paclitaxel, docetaxel, and doxorubicin.
Results
The recombinant ABCC21249A variant cell line showed higher IC50 values for paclitaxel and doxorubicin than ABCC21249G wild-type cell system (p<0.01). Intracellular accumulations of paclitaxel and doxorubicin in cells transfected with ABCC21249A variant allele were significantly decreased compared to cells transfected with ABCC21249G wild-type allele (p<0.01). The efflux ratios of paclitaxel and doxorubicin across ABCC21249A cell line were significantly increased compared with ABCC21249G cell system (p<0.01). However, ABCC2 (G1249A) polymorphism had no effect on the transport activity of MRP2-mediated docetaxel.
Conclusion
Our results indicate that ABCC2 (G1249A) polymorphism affects the transport activities of MRP2-dependent paclitaxel and doxorubicin, resulting in greater efflux of these anticancer drugs.
Keywords: ABCC2, polymorphism, sensitivity, accumulation, transport
Introduction
Paclitaxel, docetaxel, and doxorubicin are important anticancer drugs for the treatment of breast cancer, advanced renal cell carcinoma and other cancers.1–4 However, chemotherapy often leads to the occurrence of multidrug resistance (MDR) in various tumor cells.5 Impacts of anticancer drugs on MDR cancers might be greatly decreased due to the high expression of multidrug resistance-associated protein 2 (MRP2) transporting agents from tumor cells.6 The efflux of anticancer drugs from cancer cells may decrease their anticancer efficacy even more significant for protecting the tumor cells directly. Paclitaxel, docetaxel, and doxorubicin are transported from cancer cells by MRP2, which belongs to the ATP-binding cassette transporter, subfamily C; and MRP2 is encoded by the ABCC2 gene.7,8 The overexpression of MRP2 in tumor cells has promoted the ability to extrude a large number of anticancer drugs.9
Single nucleotide polymorphisms (SNPs) in genes which encode metabolic anticancer drug enzymes and transporters that pump compounds out of tumor cells have been found to result in the inter-individual differences.10 Genetic mutations in transporter proteins might change the pharmacokinetics and further affect the absorption and distribution.11,12 A variety of SNPs within ABCC2 gene have been reported, such as C-24T, G1249A, C2366T, and G3542T and so on, with different allele frequencies across ethnic and racial individuals.13 Early researches concluded somewhat controversial results concerning the extent to which ABCC2 SNPs change the function and activity of MRP2.
G1249A variant is one of the most common ABCC2 SNPs, which was reported to be associated with the inter-individual differences in chemotherapy response and severe toxicity.14 A clinical study has indicated that subjects expressing the ABCC2 (G1249A) variant could increase MRP2 transport activity in enterocytes.12 This was considered as a mechanism to explain the decreased absorption of the β1-selective blocker and MRP2 substrate, talinolol, in individuals carrying ABCC2 (G1249A) mutation. In fact, results from in vitro studies have stated inconsistent findings. Recent research indicates that ABCC2 (G1249A) SNP has higher efflux activity of MRP2-mediated sorafenib.15 In other study, ABCC2 (G1249A) was found to reduce the apparent affinity for glutathione and glucuronide-conjugated substrates.16 However, some researchers demonstrated that ABCC2 (G1249A) polymorphism had no influence on the efflux of MRP2-dependent 17β-estradiol-D-glucuronide and leukotriene C4 and 2,4-dinitrophenyl-S- glutathione in the recombinant LLC-PK1 cell line.17 These different data from previous studies indicated that the effects of ABCC2 SNPs on the transport ability of MRP2-mediated substrates show a specific-drug manner. Therefore, in the current research, we aimed to verify the effect of ABCC2 (G1249A) polymorphism on the transport activity of MRP2-mediated anticancer drugs, including paclitaxel, docetaxel, and doxorubicin.
Materials and Methods
Materials
Paclitaxel, docetaxel, and doxorubicin were purchased from Sigma-Aldrich (St Louis, MO, USA). MK-571 and puromycin were obtained from Selleck (Shanghai, China). All drugs were dissolved in DMSO. Cell Counting Kit-8 (CCK8) was purchased from Pepro Tech (Wuhan, China). Pig kidney cells (LLC-PK1) were purchased from Wuhan Bafeier Biological Co. Ltd (Wuhan, China). LLC-PK1 stable transfected with empty vector (pcDNA3.1), ABCC21249G wild-type allele and ABCC21249A variant allele were obtained from Biofavor Biotech (Wuhan, China). Dulbecco’s modified eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from PAN-Biotech (Aidenbach, Germany). MRP2 and β-actin monoclonal antibody were purchased from Abcam (Cambridge, UK). HRP-conjugated IgG was also obtained from Abcam (Cambridge, UK). BCA protein assay kit was obtained from Innova Biosciences (Cambridge, UK).
Cell Culture
The four types of LLC-PK1 cell lines were cultured in DMEM medium supplemented with 10% FBS, 1% antibiotic-antimycotic solution and 2 μmol/L puromycin. Puromycin was used to screen the stable recombinant cells. Both cell lines were grown at 37°C in a moderately humid atmosphere of 5% CO2.
Characterization of MRP2 Expression by Western Blotting
After cells were cultured for 48 h, the medium was removed and sterile PBS was used to wash cells. Then, cells were decomposed on ice for 30 min by RIPA lysis buffer (Beyotime, Shanghai, China). The cell lysate was centrifuged at 12,000 g for 12 min at 4°C, and then the concentration of total protein in the supernatant was detected by BCA kit. The loading buffer was added and mixed with supernatant, then boiled for 5 min at 100°C. 50 μg of total protein in each sample was loaded on 8% gels at 70 V for 3 h; then, protein was transferred onto a 0.45-μm NC membrane at 275 mA for 90 min. 5% of non-fat milk was used to block the non-specific binding for 1 h. Blots were incubated with primary MRP2 antibody (dilution 1:1000) and β-actin antibody (dilution 1:1000) overnight. Next, goat anti-rabbit IgG secondary antibody (dilution 1:3000) was used to incubate with blots at room temperature for 1.5 h. Protein expressions were measured by chemiluminescence detection system (Thermo Scientific).
Cytotoxicity of Anticancer Drugs
The CCK8 assay was carried out to detect the half maximal inhibitory concentration (IC50) of several anticancer drugs in the four types of LLC-PK1 cell lines. Briefly, the four types of LLC-PK1 cell lines (1×104 cells/well) were grown in 96-well plates and cultured for 24 h. The medium was then replaced by fresh medium containing different contents of paclitaxel (10–400 nM), docetaxel (1–60 nM), and doxorubicin (10–300 nM). After treatment with 24 h, 100 μL medium containing 10 μL CCK8 reagent was added into each well, and continue to incubate for 1 h at 37°C in a moderately humid atmosphere of 5% CO2. Absorbance of cells in each well was detected using a Victor 31420 Multilabel Counter (PerkinElmer, MA, USA). The IC50 values of each drug were assessed based on dose–inhibition curve.
Intracellular Accumulation of Anticancer Drugs
The four types of LLC-PK1 cell lines (2×105 cells/well) were grown in 6-well plates and cultured for 24 h. After cells reached at least 90% confluence, the old medium was replaced with 1 mL of fresh medium containing paclitaxel (50 nM), docetaxel (10 nM), and doxorubicin (30 nM). Then, the cells were incubated for 4 h to allow intracellular accumulation of these drugs. After medium was discarded, cells were washed twice with sterile PBS and collected into microcentrifuge tubes. Cells were decomposed on ice for 30 min by RIPA lysis buffer and centrifuged at 12,000 g for 12 min at 4°C, and then the concentration of total protein in the supernatant was detected by BCA kit to normalize the levels of drugs. Anticancer drugs in supernatant were acquired using liquid-liquid extraction technology (tertiary butyl methyl ether). The contents of drugs were determined by UPLC-MS/MS analyses (Agilent, Japan).
Transepithelial Efflux Assay
To determine paclitaxel, docetaxel, and doxorubicin efflux across LLC-PK1 monolayers, the four types of LLC-PK1 cell lines (2×105 cells/well) were grown in transwell insert-plate unit in 500 μL of complete medium for 24 h. The transepithelial electrical resistances (TEER) of monolayer LLC-PK1 cells were measured using a Millicell-ERS (Millipore, Billerica, MA). Efflux assay contained the transmembrane efflux of apical-to-basal (A→B) and (B→A), and paclitaxel (50 nM), docetaxel (10 nM), and doxorubicin (30 nM) were added into the A chamber and B chamber, respectively. MRP2 inhibitor (MK-571, 2 μM) was used to assess the suppressed test of transmembrane efflux. Briefly, cells were incubated with MK-571 for 120 min, and then anticancer drugs were added to intervene cells for 4 h. Apparent permeability values (Papp) were calculated to evaluate the efflux ability of drugs.18
Statistical Analysis
Data were shown as mean ± SD and analyzed by SPSS19.0 software (La Jolla, CA, USA). Student’s independent t-test was performed when the difference between means was significant. P <0.05 were considered as statistical significant.
Results
Levels of MRP2 Overexpression in ABCC21249G and ABCC21249A Recombinant Cell Lines
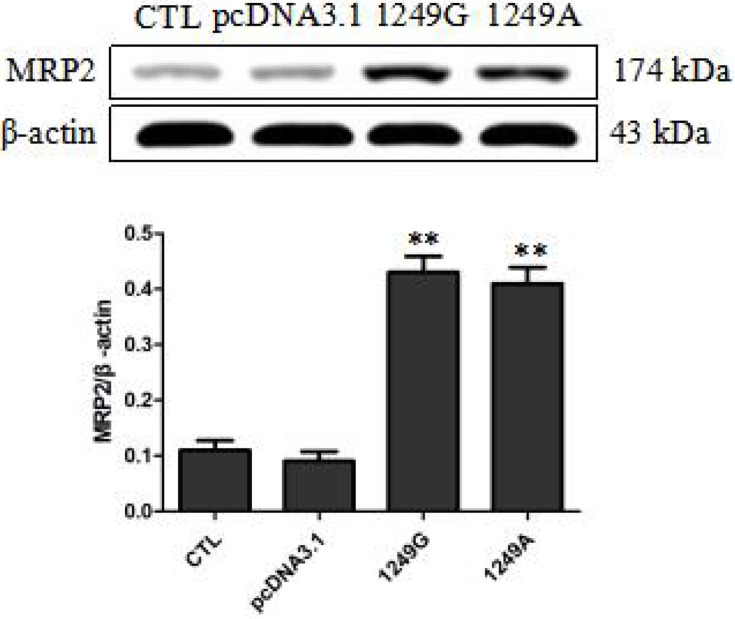
Similar expression levels of MRP2 in ABCC21249G wild-type and ABCC21249A variant recombinant cell lines were observed by Western blot (Figure 1). MRP2 expression levels in cells transfected with empty vector were significantly lower than recombinant cells transfected with 1249G wild-type allele and 1249A variant allele (p<0.01). These data confirmed the overexpression of ABCC21249G wild-type and ABCC21249A variant genes in LLC-PK1 cells, indicating that ABCC2 (G1249A) SNP does not impact the expression of this protein.
Figure 1.
Overexpression levels of wild-type and variant MRP2 in stable recombinant LLC-PK1 cell lines. Compared with pcDNA3.1 group, **p<0.01.
Abbreviations: CTL, untransfected LLC-PK1 cells; pcDNA3.1, transfected with empty vector; 1249G, transfected with 1249G wild-type allele; 1249A, cells transfected with 1249A variant allele.
Recombinant ABCC21249G and ABCC21249A Cell Lines Show Altered Resistance to Anticancer Drugs
The sensitivity of the four types of LLC-PK1 cells to paclitaxel, docetaxel, and doxorubicin was assesses based on IC50 values. The two types of recombinant cells transfected with ABCC21249G and ABCC21249A alleles displayed higher IC50 values for these anticancer drugs than cells transfected with empty vector (p<0.01, Table 1). IC50 values for paclitaxel was 2.09-fold higher in ABCC21249A cells than in ABCC21249G cells (p<0.01). IC50 values for doxorubicin was 2.78-fold higher in ABCC21249A cells than in ABCC21249G cells (p<0.01). However, the IC50 values for docetaxel in ABCC21249A cells was similar to ABCC21249G cells. These data suggested that ABCC2 (G1249A) SNP affects the sensitivity of cells to anticancer drugs with a drug-specific manner.
Table 1.
ABCC2 (G1249A) Polymorphism Alters the Sensitivity of Recombinant Cell Systems to Anticancer Drugs
| LLC-PK1 Cells | IC50 (nM)±SD | |||
|---|---|---|---|---|
| CLT | pDNA3.1 | 1249G | 1249A | |
| Paclitaxel | 45.6±6.7 | 39.8±5.9 | 136.4±7.3** | 285.4±6.7**,## |
| Docetaxel | 9.3±1.4 | 11.6±1.2 | 39.4±3.7** | 34.5±5.2** |
| Doxorubicin | 30.4±3.8 | 28.4±3.1 | 84.6±3.5** | 236.4±5.2**,## |
Notes: Compared with pcDNA3.1 group, **p<0.01; compared with 1249G group, ##p<0.01.
Abbreviations: CTL, cells untransfected LLC-PK1 cells; pcDNA3.1, transfected with empty vector; 1249G, transfected with 1249G wild-type allele; 1249A, cells transfected with 1249A variant allele.
ABCC21249A Variant Allele Impacts the Intracellular Accumulation of Anticancer Drugs
Intracellular accumulation of these anticancer drugs was assessed in the four types of LLC-PK1 cells. The two types of recombinant cells transfected with ABCC21249G and ABCC21249A alleles displayed lower intracellular accumulation for these anticancer drugs than cells transfected with empty vector (p<0.01, Table 2). Intracellular accumulation of paclitaxel was 3.11-fold lower in ABCC21249A cells than in ABCC21249G cells (p<0.01). Intracellular accumulation of doxorubicin was 2.68-fold lower in ABCC21249A cells than in ABCC21249G cells (p<0.01). However, the Intracellular accumulation of docetaxel in ABCC21249A cells was similar to ABCC21249G cells. These data suggested that ABCC2 (G1249A) SNP affects the transport abilities of anticancer drugs with a drug-specific manner.
Table 2.
Effects of ABCC2 (G1249A) Polymorphism on the Intracellular Accumulation of Paclitaxel, Docetaxel, Sorafenib, and Doxorubicin
| LLC-PK1 Cells | C (ng/mg Protein)±SD | |||
|---|---|---|---|---|
| CLT | pDNA3.1 | 1249G | 1249A | |
| Paclitaxel | 253.1±8.4 | 238.1±9.5 | 95.4±6.5** | 30.7±5.7**,## |
| Docetaxel | 76.3±6.4 | 79.4±5.9 | 34.1±4.7** | 37.9±5.4** |
| Doxorubicin | 198.6±8.4 | 208.8±7.2 | 82.3±6.4** | 30.7±5.4**,## |
Notes: Compared with pcDNA3.1 group, **p<0.01; compared with 1249G group, ##p<0.01.
Abbreviations: CTL, cells untransfected LLC-PK1 cells; pcDNA3.1, transfected with empty vector; 1249G, transfected with 1249G wild-type allele; 1249A, cells transfected with 1249A variant allele.
ABCC21249A Variant Allele Affects the Transport Ability of Anticancer Drugs
The efflux ratios (Papp (B→A)/Papp (A→B)) of paclitaxel, docetaxel, and doxorubicin across the four types of LLC-PK1 monolayers were also assessed. The two types of recombinant cells transfected with ABCC21249G and ABCC21249A alleles displayed higher efflux ratios for these anticancer drugs than cells transfected with empty vector (p<0.01, Table 3). The efflux ratio of paclitaxel was 3.32-fold higher in ABCC21249A cells than in ABCC21249G cells (p<0.01). The efflux ratio of doxorubicin was 3.17-fold higher in ABCC21249A cells than in ABCC21249G cells (p<0.01). However, the efflux ratio of docetaxel in ABCC21249A cells was similar to ABCC21249G cells. These data suggested that ABCC2 (G1249A) SNP affects the efflux activities of MRP2-mediated anticancer drugs with a drug-specific manner.
Table 3.
Effects of ABCC2 (G1249A) Polymorphism on the Efflux Activity of Paclitaxel, Docetaxel, Sorafenib, and Doxorubicin in Recombinant LLC-PK1 Monolayers
| LLC-PK1 Cells | Papp (B→A)/Papp (A→B) | |||
|---|---|---|---|---|
| CLT | pDNA3.1 | 1249G | 1249A | |
| Paclitaxel | 0.87±0.07 | 0.81±0.06 | 3.64±0.57** | 12.08±0.69**,## |
| Paclitaxel+MK-571 | 0.96±0.06 | 0.89±0.05 | 1.54±0.07*,† | 1.79±0.10*,† |
| Docetaxel | 1.34±0.16 | 1.47±0.18 | 5.06±0.34** | 4.69±0.41** |
| Docetaxel+MK-571 | 1.46±0.19 | 1.54±0.21 | 2.12±0.24*,† | 1.96±0.26†† |
| Doxorubicin | 0.54±0.06 | 0.67±0.05 | 4.19±0.14** | 13.27±0.24**,## |
| Doxorubicin+MK-571 | 0.65±0.05 | 0.58±0.03 | 3.64±0.24*,† | 3.88±0.29*,† |
Notes: Compared with pcDNA3.1 group, *p<0.05, **p<0.01; compared with 1249G group, ##p<0.01; compared with without inhibitor group, †p<0.05, ††p<0.01.
Abbreviations: CTL, cells untransfected LLC-PK1 cells; pcDNA3.1, transfected with empty vector; 1249G, transfected with 1249G wild-type allele; 1249A, cells transfected with 1249A variant allele.
Discussion
Acquired paclitaxel, docetaxel, and doxorubicin resistance of breast cancer cells is involved in the overexpression of MRP2 transporter.19–21 Genetic polymorphism of ABCC2 gene response to anticancer drug chemotherapy in breast tumor patients.22 ABCC2 (G1249A) polymorphism was demonstrated to be associated with the risk of resistance to multiple drugs, such as antiepileptic agents.23 Here we present direct proof indicating that the common G1249A SNP in ABCC2 gene substantially induces the transport ability of MRP2-mediated paclitaxel and doxorubicin, decreases the content of these drugs that accumulates within the four types of cell lines. Since MRP2 could lead to multidrug resistance, the present findings might be helpful for improving such resistance in case of paclitaxel and doxorubicin as well as other chemotherapeutic drugs. Our data also provide testable assumption concerning why patients with cancers can differ so widely in the response to paclitaxel and doxorubicin. The cell line used in our research, LLC-PK1 derived fro, a pig kidney, has been shown to be useful to observe transport proteins functionality.24 Therefore, we selected LLC-PK1 cell line to study the effects of ABCC2 polymorphism on the transport of paclitaxel, docetaxel and doxorubicin. We first used different concentrations of anticancer drugs to intervene LLC-PK1 cells to assess the cytotoxicity of agents. The IC50 values of paclitaxel, docetaxel, and doxorubicin in LLC-PK1 control cells were 45.6, 9.3, and 30.4 nM, respectively. Then, we used paclitaxel (50 nM), docetaxel (10 nM), and doxorubicin (30 nM) to intervene recombinant cell systems to Conduct follow-up experiments, and these drugs did not affect the cell morphology.
We conducted control tests to make sure that the investigated alterations in the transport of paclitaxel and doxorubicin in the recombinant ABCC21249G and ABCC21249A cell lines were not due to difference in the expression levels of MRP2. Our results from Western blot assay displayed that MRP2 was overexpressed at similar levels in ABCC21249G and ABCC21249A cell systems. Our results are consistent with previous studies in vivo,22,25 but disagree with other researches.26,27 As one of the usual in vitro methods to help transporter observation to data, recombinant cell systems over-expressing MRP2 have some limitations, for instance, the different expression levels of MRP2 in different laboratories may result in altered results, and endogenous efflux activity in cells might also complicate the interpretation of results. Nevertheless, the present on vitro systems make the observation of characteristics of a single protein feasible and lower the complexity. The differences might reflect the complexity of the in vivo environment, as well as changes among the in vivo and in vitro contexts. Therefore, it makes us to further verify our results in other recombinant cell system, including cancer cells, as well as studies in vivo.
These data indicated that the ABCC2 (G1249A) SNP, which leads to a Val-Ile replacement in the MRP2 protein. This mutation may change the interaction among the specific substrate-recognizing domain of MRP2 and drugs,28 and it can explain that 1249A SNP increases the transport activities of MRP2-mediated paclitaxel and doxorubicin, but not docetaxel. Our results showed that intracellular accumulations of paclitaxel and doxorubicin were 3.11-fold and 2.68-fold lower in cells transfected with 1249A allele than cells transfecting with 1249G, respectively. Therefore, this was sufficient to result in a dramatic decrease in sensitivity to paclitaxel and doxorubicin. This may reflect the resistance of cancer cells to paclitaxel and doxorubicin.
Since the MRP2 might pump paclitaxel, docetaxel and doxorubicin out of cells,29 we further check that the effects of ABCC2 (G1249A) SNP on transmembrane transport of these anticancer cells. Moreover, we observed the efflux ratios of paclitaxel, docetaxel and doxorubicin in the presence and absence of the MRP2 antagonist, MK-571. Our data suggested that nearly the efflux of these drugs was eliminated in the presence of MRP2 antagonist, indicating that MRP2 was responsible for most of the transport investigated in this test. However, our results displayed that ABCC2 (G1249A) SNP has no effect on the efflux of MRP2-mediated docetaxel. Since MRP2 displays altered affinities for different substrates,30 it may be necessary to explain our findings. Indeed, an early study has demonstrated that ABCC2 (G1249A) polymorphism may impact MRP2 activity in a substrate-specific manner.16 In addition, we observed the efflux ratios of paclitaxel, docetaxel and doxorubicin in the presence and absence of the MRP2 antagonist and discovered that efflux of these drugs was eliminated in the presence of MRP2 antagonist. But there are still significant statistical differences between the efflux ratios of two types of recombinant cells and cells transfected with empty vector. It would be explained that the discrepancy was resulted from insufficient inhibition of MRP2 by antagonist or other transportation mechanisms. Furthermore, our study turned out conflicting results of paclitaxel and docetaxel which are the same group of anticancer drugs. This phenomenon may be explained by the fact that paclitaxel and docetaxel can interact with different domains of MRP2 protein, and further effect protein conformation.
Conclusions
Collectively, more detailed researches of structure-activity associations in MRP2 protein are needed to verify the functional roles of ABCC2 (G1249A) polymorphism and other SNPs. Our in vitro recombinant cell lines may provide a useful tool for investigating the cause of poor chemotherapy response and drug resistance.
Acknowledgments
The authors would like to thank the Department of Pharmacy, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science. Guo Lian and Jia Yuan are co-first authors.
Disclosure
All authors declare that there is no conflict of interest.
References
- 1.Tiainen L, Tanner M, Lahdenperä O, et al. Bevacizumab combined with docetaxel or paclitaxel as first-line treatment of HER2-negative metastatic breast cancer. Anticancer Res. 2016;36(12):6431–6438. doi: 10.21873/anticanres.11241 [DOI] [PubMed] [Google Scholar]
- 2.Carbognin L, Sperduti I, Nortilli R, et al. Balancing activity and tolerability of neoadjuvant paclitaxel- and docetaxel-based chemotherapy for HER2-positive early stage breast cancer: sensitivity analysis of randomized trials. Cancer Treat Rev. 2015;41(3):262–270. doi: 10.1016/j.ctrv.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 3.Bronte G, Andreis D, Bravaccini S, et al. Sorafenib for the treatment of breast cancer. Expert Opin Pharmacother. 2017;18(6):621–630. doi: 10.1080/14656566.2017.1309024 [DOI] [PubMed] [Google Scholar]
- 4.Shafei A, El-Bakly W, Sobhy A, et al. A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer. Biomed Pharmacother. 2017;95:1209–1218. doi: 10.1016/j.biopha.2017.09.059 [DOI] [PubMed] [Google Scholar]
- 5.Hida K, Kikuchi H, Maishi N, Hida Y. ATP-binding cassette transporters in tumor endothelial cells and resistance to metronomic chemotherapy. Cancer Lett. 2017;400:305–310. doi: 10.1016/j.canlet.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Sun X, Feng Y, et al. Dihydromyricetin reverses MRP2-mediated MDR and enhances anticancer activity induced by oxaliplatin in colorectal cancer cells. Anticancer Drugs. 2017;28(3):281–288. doi: 10.1097/CAD.0000000000000459 [DOI] [PubMed] [Google Scholar]
- 7.Vos K, Sciuto CL, Piedade R, et al. MRP2/ABCC2 C1515Y polymorphism modulates exposure to lumefantrine during artemether-lumefantrine antimalarial therapy. Pharmacogenomics. 2017;18(10):981–985. doi: 10.2217/pgs-2017-0032 [DOI] [PubMed] [Google Scholar]
- 8.Andersen V, Svenningsen K, Knudsen LA, et al. Novel understanding of ABC transporters ABCB1/MDR/P-glycoprotein, ABCC2/MRP2, and ABCG2/BCRP in colorectal pathophysiology. World J Gastroenterol. 2015;21(41):11862–11876. doi: 10.3748/wjg.v21.i41.11862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliveira C, Joshee L, Bridges CC. MRP2 and the transport kinetics of cysteine conjugates of inorganic mercury. Biol Trace Elem Res. 2018;184(1):279–286. doi: 10.1007/s12011-017-1163-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platt A, Xia Z, Liu Y, Chen G, Lazarus P. Impact of nonsynonymous single nucleotide polymorphisms on in-vitro metabolism of exemestane by hepatic cytosolic reductases. Pharmacogenet Genom. 2016;26(8):370–380. doi: 10.1097/FPC.0000000000000226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abla N, Chinn LW, Nakamura T, et al. The human multidrug resistance protein 4 (MRP4, ABCC4): functional analysis of a highly polymorphic gene. J Pharmacol Exp Ther. 2008;325(3):859–868. doi: 10.1124/jpet.108.136523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haenisch S, May K, Wegner D, Caliebe A, Cascorbi I, Siegmund W. Influence of genetic polymorphisms on intestinal expression and rifampicin-type induction of ABCC2 and on bioavailability of talinolol. Pharmacogenet Genom. 2008;18(4):357–365. doi: 10.1097/FPC.0b013e3282f974b7 [DOI] [PubMed] [Google Scholar]
- 13.Wen X, Joy MS, Aleksunes LM. In vitro transport activity and trafficking of MRP2/ABCC2 polymorphic variants. Pharm Res. 2017;34(8):1637–1647. doi: 10.1007/s11095-017-2160-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han B, Gao G, Wu W, et al. Association of ABCC2 polymorphisms with platinum-based chemotherapy response and severe toxicity in non-small cell lung cancer patients. Lung Cancer. 2011;72(2):238–243. doi: 10.1016/j.lungcan.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 15.Wei D, Zhang H, Peng R, Huang C, Bai R. ABCC2 (1249G > A) polymorphism implicates altered transport activity for sorafenib. Xenobiotica. 2016;47:1–7. [DOI] [PubMed] [Google Scholar]
- 16.Megaraj V, Zhao T, Paumi CM, Gerk PM, Kim RB, Vore M. Functional analysis of nonsynonymous single nucleotide polymorphisms of multidrug resistance-associated protein 2 (ABCC2). Pharmacogenet Genomics. 2011;21(8):506–515. doi: 10.1097/FPC.0b013e328348c786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirouchi M, Suzuki H, Itoda M, et al. Characterization of the cellular localization, expression level, and function of SNP variants of MRP2/ABCC2. Pharm Res. 2004;21(5):742–748. doi: 10.1023/B:PHAM.0000026422.06207.33 [DOI] [PubMed] [Google Scholar]
- 18.Dessilly G, Elens L, Panin N, Benjamin RS. ABCB1 1199G>A polymorphism (rs2229109) affects the transport of imatinib, nilotinib and dasatinib. Pharmacogenomics. 2016;17(8):883–890. doi: 10.2217/pgs-2016-0012 [DOI] [PubMed] [Google Scholar]
- 19.Němcová-Fürstová V, Kopperová D, Balušíková K, et al. Characterization of acquired paclitaxel resistance of breast cancer cells and involvement of ABC transporters. Toxicol Appl Pharmacol. 2016;310:215–228. doi: 10.1016/j.taap.2016.09.020 [DOI] [PubMed] [Google Scholar]
- 20.Ehrlichova M, Vaclavikova R, Ojima I, et al. Transport and cytotoxicity of paclitaxel, docetaxel, and novel taxanes in human breast cancer cells. Naunyn Schmiedebergs Arch Pharmacol. 2005;372(1):95–105. doi: 10.1007/s00210-005-1080-4 [DOI] [PubMed] [Google Scholar]
- 21.Chaisit T, Siripong P, Jianmongkol S. Rhinacanthin-C enhances doxorubicin cytotoxicity via inhibiting the functions of P-glycoprotein and MRP2 in breast cancer cells. Eur J Pharmacol. 2017;795:50–57. doi: 10.1016/j.ejphar.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 22.Tecza K, Pamula-Pilat J, Lanuszewska J, Grzybowska E. Genetic polymorphisms and response to 5-fluorouracil, doxorubicin and cyclophosphamide chemotherapy in breast cancer patients. Oncotarget. 2016;7(41):66790–66808. doi: 10.18632/oncotarget.11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen P, Yan Q, Xu H, Lu A, Zhao P. The effects of ABCC2 G1249A polymorphism on the risk of resistance to antiepileptic drugs: a meta-analysis of the literature. Genet Test Mol Biomarkers. 2014;18(2):106–111. doi: 10.1089/gtmb.2013.0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77(4):491–502. doi: 10.1016/0092-8674(94)90212-7 [DOI] [PubMed] [Google Scholar]
- 25.Jabir RS, Naidu R, Annuar MA, Ho GF, Munisamy M, Stanslas J. Pharmacogenetics of taxanes: impact of gene polymorphisms of drug transporters on pharmacokinetics and toxicity. Pharmacogenomics. 2012;13(16):1979–1988. doi: 10.2217/pgs.12.165 [DOI] [PubMed] [Google Scholar]
- 26.Kiyotani K, Mushiroda T, Kubo M, Zembutsu H, Sugiyama Y, Nakamura Y. Association of genetic polymorphisms in SLCO1B3 and ABCC2 with docetaxel-induced leukopenia. Cancer Sci. 2008;99(5):967–972. doi: 10.1111/j.1349-7006.2008.00765.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun N, Sun X, Chen B, et al. MRP2 and GSTP1 polymorphisms and chemotherapy response in advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2010;65(3):437–446. doi: 10.1007/s00280-009-1046-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subramanian N, Condic-Jurkic K, O’Mara ML. Structural and dynamic perspectives on the promiscuous transport activity of P-glycoprotein. Neurochem Int. 2016;98:146–152. doi: 10.1016/j.neuint.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 29.van Hasselt JG, van Calsteren K, Heyns L, et al. Optimizing anticancer drug treatment in pregnant cancer patients: pharmacokinetic analysis of gestation-induced changes for doxorubicin, epirubicin, docetaxel and paclitaxel. Ann Oncol. 2014;25(10):2059–2065. doi: 10.1093/annonc/mdu140 [DOI] [PubMed] [Google Scholar]
- 30.Ellis LC, Hawksworth GM, Weaver RJ. ATP-dependent transport of statins by human and rat MRP2/Mrp2. Toxicol Appl Pharmacol. 2013;269(2):187–194. doi: 10.1016/j.taap.2013.03.019 [DOI] [PubMed] [Google Scholar]