Your first patient of the day is a teenager with high-risk asthma recently discharged from the pediatric intensive care unit, his second admission this year. He grudgingly glances up from his smartphone, insisting he feels great, takes his controllers, but still needs his albuterol several times a day. The pulmonary function tests show a low FEV1% predicted that does not fully normalize after albuterol. He is a poor perceiver of symptoms and at risk of permanently diminished lung function as an adult. Ten minutes later, he is walking out the door after you unload rapid-fire education, update his asthma action plan, and refill his medications. You implore him to call the office next week to check in and schedule a visit in 3 months. Meanwhile, you know he is at risk for rehospitalization or even death. You delivered standard of care, but is this enough? Do we really understand his asthma control, risk, or impairment? What could more personalized data tell us that we did not have time to collect or incorporate?
During a 3-month lag between 30-minute clinic visits, a patient with asthma has approximately 2190 hours of experiencing and treating (or not treating) their asthma symptoms. Within a 15-minute visit, providers are using 0.0005% of the patient experience to understand and treat a complex disease. Only a fraction of data is captured in the electronic health record (EHR). We know that environmental factors, medication adherence, a patient’s home location, emotional and economic stress, and many other social determinants of health are critical causes for uncontrolled asthma and are not adequately captured in the EHR. Big Data (ie, large data sets from many sources), if used effectively at the point of care, could be transformational in concert with traditional EHR data. Furthermore, as health care shifts into the e-health and telehealth realm, with care provided outside the traditional office visit, demand for devices that can facilitate remote “examination” and diagnosis will continue to increase. Data produced by thoughtful digital health innovation can personalize management of asthma, potentially improving timeliness of care and capture both symptoms and objective measures of treatment response.
Our patients and the pace of data growth are compelling us to incorporate insights from Big Data to inform care. Predictive analytics, using machine learning (ML) and artificial intelligence (AI), has revolutionized many industries, working in the background in ways we cannot even recognize. Predictive analytics uses logistic regression, ML, or other methods to automate knowledge discovery from large, complex data sets. ML algorithms can incorporate diverse data to yield increased prediction accuracy over conventional statistical methods. These tools collect, interpret, and present disparate data in powerful ways, finding patterns and trends. Outcomes must be mathematically and clinically relevant, which demands thoughtful, longitudinal, and well-funded collaboration between clinicians, clinical researchers, data scientists, and computer scientists.
Generations of clinical, scientific, and social research have confirmed for us that asthma is a heterogeneous and complex disease that affects patients from cradle to grave with high prevalence. The potential of ML to improve asthma management is significant, including predicting exacerbations, assistance in decreasing medication doses during stable periods, tracking and minimizing cumulative steroid doses, triggering treatment path- ways on the basis of weather conditions, and more. ML has also been used to better understand the pathology of asthma, and correlate mechanistic insights with clinical phenotypes.1
Prediction of asthma exacerbations and patterns of health care service utilization for individuals and across populations is within technical and clinical reach.2In particular, ML algorithms can find these patterns by mining and aggregating repositories of data (including EHR, environmental, continuous physiologic data captured via remote telemonitoring, even social media feeds).3 Ram et al4 used multiple external data sources to help predict emergency department utilization in asthma in near real time with 70% precision. Finkelstein and Jeong3 pioneered the use of home-based telemonitoring data to predict asthma exacerbations within a 7-day window, building on similar ML studies in asthma exacerbation prediction that have yielded potentially actionable sensitivity.2,5 As depicted in Fig 1, the current and near-future application of ML and AI to currently inaccessible Big Data (including patient, population, and environmental sources) can drastically improve our diagnosis, management, and mechanistic understanding of asthma.
FIG 1.
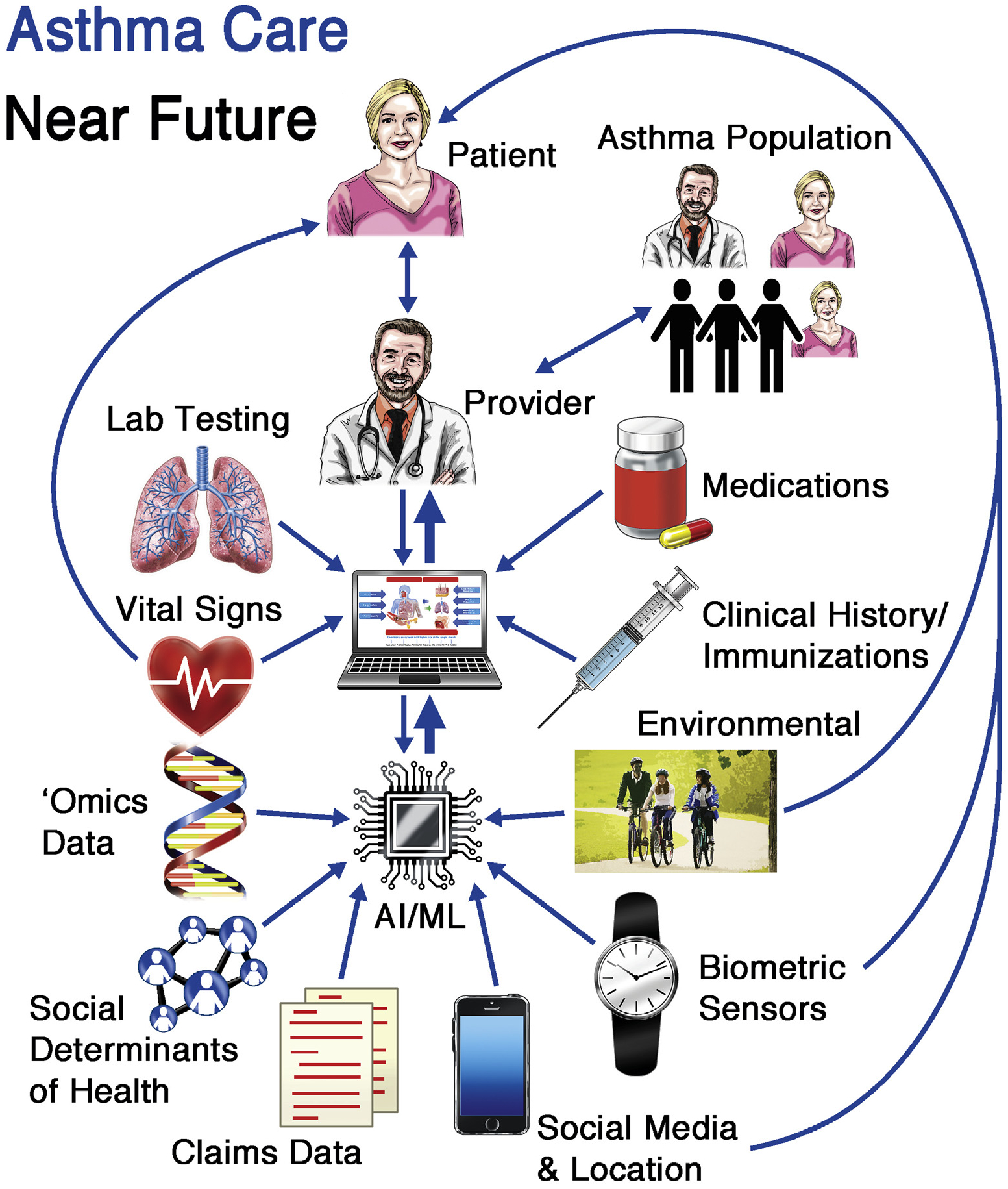
This diagram illustrates the potential of ML and AI to improve asthma care, with emphasis on bidirectional information exchange and data application for patients, their providers, and the health system.
Prediction tools must identify not only who is likely to experience an asthma attack but also when that attack might occur and can allow optimization of personalized management of health care resources.3 Clinical prediction tools for asthma exacerbation or asthma hospitalization, even those that use ML, have a limited window of prediction and insufficient sensitivity and specificity.2 To address these limitations, the inclusion of more variables, increased population size, novel physiologic measures (eg, heart rate variability and actigraphy), and environmental or epidemiologic data (eg, air quality, season, and viral infection rates) may help.
Asthma research has accelerated in recent years, due in large part to longitudinal birth cohort studies and multicenter collaborations such as the Severe Asthma Research Program and have contributed understanding about asthma pathophysiology, natural history, phenotypes, seasonality, genetics and epigenetics, and biomarkers.6 Importantly, significant research focus is now centering on the longitudinal effects of childhood disease (including lung function and rate of decline of function) and how the environmental, genetic, social, and clinical milieu sets the stage for severe adult asthma and declining lung functions.7
Work to associate symptoms to key pathogenic mechanisms has aided advances in treatment using anti-inflammatory biologic therapies. ML and other data-driven methods have successfully been applied to endotype discovery, using ML applied to large longitudinal data sets to cluster patients into mechanistic and causal categories that incorporate genetic, molecular, and immune phenotypes.1 Important work in genomics and pharmacogenomics related to asthma, combined with analysis of the airway microbiome and genetic mapping of environmental influences (epigenetics), is on the cusp of translation into higher fidelity prediction of exacerbations and true precision medicine for both adults and children. This work, if translated into clinical practice, can potentially link genetic traits to phenotypes that can, for example, predict response, or nonresponse, to medications such as albuterol and steroids or identify an individual’s risk for cortisol suppression.8
As Haider and Custovic9 suggest in their excellent review, much of our clinical and scientific insights about asthma have been produced in silos, limiting the hypotheses that can be formed or the outcomes that can be tracked. They instead urge that a truly integrative paradigm, when coupled with “rigorous scientific scrutiny, can lead to a more nuanced understanding of asthma.”9 Data being generated and interpreted across different domains for patients with asthma are increasing, but challenges include lack of data standards and disease definitions, difficulty with data interoperability/sharing, and concerns about data quality and fidelity, all of which have frustrated progress. Furthermore, the exchange and collaboration between data scientists and computer engineers and clinical researchers and providers is hindered by the lack of common language. However, recent developments in the AI field of deep learning have embraced this data complexity within health care, producing compelling predictions about inpatient mortality, length of stay, readmission risk, and even diagnoses using real, messy, uncurated EHR data.9,10
Another important challenge with use of ML and AI we carefully must consider in health care applications is the issues of fairness, bias, privacy, and medical bioethics. Even in Big Data sets, an automated prediction result can be biased; for instance, if a subpopulation does not have enough patients, or data itself are collected in a biased way. The most accurate ML models are often a black box to both computer scientists and clinicians; however, solutions for this are also emerging, including automated explanation algorithms. Issues of legal accountability and medical responsibility within adoption of algorithms into practice must also be carefully considered. With increased automated clinical decision making, safety, privacy, and use of evidence- based practice must be rigorously maintained to protect both medical providers and patients. Distribution of the potential benefits of digital health innovation in the broader health care system is also likely to be unequal given the relative expense of these tools, magnifying uncomfortable but familiar inequalities relating to social justice and care access.
Furthermore, we must as clinicians and researchers constructively transform the concern and lack of understanding many clinicians have about digital health, ML, and AI into educated and critical engagement. We need our most experienced clinical experts at the table, to innovate alongside data and computer scientists to create, validate, and implement these powerful tools, so that the patient, their outcomes, and well-being remain primary. We know the single human brain is limited in its ability to recognize longitudinal patterns, aggregate, and distill large amounts of disparate data into a cogent, evidence-based treatment plan. In this world of data overload, with terabytes of input daily and demands for increasingly complex data outputs, clinicians need help. Our job now is to use ML and AI tools to understand and predict how asthma affects patients and help us make decisions, at the patient and population levels, to treat it better. The future is bright, there is much work to be done, to really see and understand our patients with asthma beyond the clinical encounter.
Footnotes
Disclosure of potential conflict of interest: A. I. Messinger was funded by the National Institutes of Health (NIH) and the National Heart, Lung, and Blood Institute (NHLBI) (Abman T-32 training grant no. 2T32HL007670-26). G. Luo was partially supported by the NHLBI of the NIH (award no. R01HL142503). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. R. R. Deterding is cofounder and board member of Triple Endoscopy, Inc (Patent Nasal Endoscopy Scope); is on the Pediatric Interstitial Lung Disease Advisory Board for Boehringer Ingelheim; is cofounder and board member of Now Vitals, Inc; has the patents (1) Computing Systems for Determining Vital Information and(2) Personalized Health Care Wearable Sensor System; and receives support from Colorado Advanced Industries Accelerator (AIA).
REFERENCES
- 1.Saglani S, Custovic A. Childhood asthma: advances using machine learning and mechanistic studies. Am J Respir Crit Care Med 2019;199:414–22. [DOI] [PubMed] [Google Scholar]
- 2.Luo G, He S, Stone BL, Nkoy FL, Johnson MD. Developing a Model to Predict Hospital Encounters for asthma in asthmatic patients: secondary analysis. JMIR Med Inform. 2020. [In press]. doi: 10.2196/16080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkelstein J, Jeong IC. Machine learning approaches to personalize early prediction of asthma exacerbations. Ann N Y Acad Sci 2017;1387:153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ram S, Zhang W, Williams M, Pengetnze Y. Predicting asthma-related emergency department visits using big data. IEEE J Biomed Health Inform 2015;19:1216–23. [DOI] [PubMed] [Google Scholar]
- 5.Mohktar MS, Redmond SJ, Antoniades NC, Rochford PD, Pretto JJ, Basilakis J, et al. Predicting the risk of exacerbation in patients with chronic obstructive pulmonary disease using home telehealth measurement data. Artif Intell Med 2015;63:51–9. [DOI] [PubMed] [Google Scholar]
- 6.Buhl R, Korn S, Menzies-Gow A, Aubier M, Chapman KR, Canonica GW, et al. Assessing biomarkers in a real-world severe asthma study (ARIETTA). Respir Med 2016;115:7–12. [DOI] [PubMed] [Google Scholar]
- 7.Szefler SJ. Asthma across the lifespan: time for a paradigm shift. J Allergy Clin Immunol 2018;142:773–80. [DOI] [PubMed] [Google Scholar]
- 8.Slob EM, Maitland-Van der Zee AH, Koppelman GH, Pijnenburg MW. Precision medicine in childhood asthma. Curr Opin Allergy Clin Immunol 2019;19:141–7. [DOI] [PubMed] [Google Scholar]
- 9.Haider S, Custovic A. Breaking down silos in asthma research: the case for an integrated approach. EMJ Innov 2019;3:82–92. [Google Scholar]
- 10.Rajkomar A, Oren E, Chen K, Dai AM, Hajaj N, Hardt M, et al. Scalable and accurate deep learning with electronic health records. NPJ Digital Med 2018;1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]


