Abstract
The gastrointestinal (GI) tract has multifold tasks of ingesting, processing, and assimilating nutrients and disposing of wastes at appropriate times. These tasks are facilitated by several stereotypical motor patterns that build upon the intrinsic rhythmicity of the smooth muscles that generate phasic contractions in many regions of the gut. Phasic contractions result from a cyclical depolarization/repolarization cycle, known as electrical slow waves, which result from intrinsic pacemaker activity. Interstitial cells of Cajal (ICC) are electrically coupled to smooth muscle cells (SMCs) and generate and propagate pacemaker activity and slow waves. The mechanism of slow waves is dependent upon specialized conductances expressed by pacemaker ICC. The primary conductances responsible for slow waves in mice are Ano1, Ca2+-activated Cl− channels (CaCCs), and CaV3.2, T-type, voltage-dependent Ca2+ channels. Release of Ca2+ from intracellular stores in ICC appears to be the initiator of pacemaker depolarizations, activation of T-type current provides voltage-dependent Ca2+ entry into ICC, as slow waves propagate through ICC networks, and Ca2+-induced Ca2+ release and activation of Ano1 in ICC amplifies slow wave depolarizations. Slow waves conduct to coupled SMCs, and depolarization elicited by these events enhances the open-probability of L-type voltage-dependent Ca2+ channels, promotes Ca2+ entry, and initiates contraction. Phasic contractions timed by the occurrence of slow waves provide the basis for motility patterns such as gastric peristalsis and segmentation. This chapter discusses the properties of ICC and proposed mechanism of electrical rhythmicity in GI muscles.
Keywords: Interstitial cells of Cajal, Pacemaker, Ca2+ transient, Slow wave, SIP syncytium, ANO1 channels, T-type Ca2+ channels, Electrophysiology, Gastrointestinal motility
1.1. Introduction
GI smooth muscles are complex tissues composed of multiple cell types. Smooth muscle cells (SMCs) provide the motor responsible for force development and movement of nutrients and waste products, but at least two additional types of cells, known as interstitial cells, are electrically coupled to SMCs and provide moment-to-moment modulation of SMC excitability. Together with SMCs, interstitial cells of Cajal (ICC) and platelet-derived growth factor receptor α positive (PDGFRα+) cells form a multicellular syncytium known as the SMC, ICC, and PDGFRα+ cell (SIP) syncytium [1]. SIP cells each express intrinsic electrophysiological mechanisms and a variety of receptors for neurotransmitters, hormones, paracrine substances, and inflammatory mediators. SIP cells are innervated by enteric motor neurons and receive and transduce neurotransmitter signals. The integrated output of the SIP syncytium sets the moment-to-moment excitability of SMCs [2]. The contractile behavior in most smooth muscle regions of the GI tract is phasic in nature, consisting of a rhythmic contraction-relaxation cycle. Phasic contractions are the basis for the major motility patterns of the GI tract, such as segmentation and peristalsis, and the phasic nature of contractions is intrin sic to the muscle tissues. Phasic contractions of smooth muscle cells (SMCs) are driven by electrical slow waves, which are generated by ICC [3–5]. There are several types of ICC present in the GI tracts of mammals and humans. Common nomenclature used to identify the different classes of ICC and categorize their functions is provided in Table 1.1.
Table 1.1.
Nomenclature for ICC in the GI tract
| Anatomical location | Common name | Organ distribution | Functions |
|---|---|---|---|
| Plane of the myenteric plexus between circular and longitudinal muscle layers | ICC-MYa | STM, SI, CLN | Pacemaker activity, innervated by motor neurons in CLN |
| Intramuscular localization, within muscle bundles and in close contact with varicose processes of enteric motor neurons | ICC-IMb | ESG (smooth muscle portion), STM, SI, CLN |
Express receptors for and provide transduction for neurotransmitters released by enteric motor neurons; mediators of responses to stretch |
| Intramuscular-type ICC within plane of the deep muscular plexus in small intestine | ICC- DMPc |
SI | Express receptors for and provide transduction for neurotransmitters released by enteric motor neurons |
| Submucosal border of circular muscle layer | ICC-SM | CLN, STM | Pacemaker activity in CLN; limited number of cells in STM and function of STM cells unknown |
| Serosal surface of longitudinal muscle layer | ICC-SS | CLN | Unknown function at present time |
| Septal spaces between muscle bundles in larger animals | ICC-SEP | STM, SI, CLN | Appear to be extensions of ICC-MY or ICC-SM networks and actively propagate slow waves in thicker GI muscles of large mammals and humans |
Organ abbreviations: Esophagus (ESG); stomach (STM); small intestine (SI); colon (CLN)
Also referred to as ICC-MP by some authors, but this is misleading because these cells do not penetrate and are not part of the myenteric plexus. They are distributed around the ganglia and tertiary plexus
Some authors have broken this term down to specify in which muscle layer the cells are found (e.g., ICC-CM for cells in the circular muscle layer and ICC-LM for cells in the longitudinal muscle layer). Since no functional differences have been reported for the cells in these different locations, the term ICC-IM is used throughout this chapter
ICC-DMP are most likely the ICC-IM of the small intestine. They show a distinctive localization in laboratory animals and have received considerable experimental attention, so they are designated separately. Larger animals tend to have ICC-IM distributed through the circular muscle layer, as observed in the stomach and colon of laboratory animals.
ICC are organized into networks in the pacemaker regions of the GI tract. Once a slow wave is generated, it regenerates cell to cell, propagating actively through the ICC network. Slow waves conduct passively into SMCs, because SMCs do not express the unique conductances that contribute to slow waves and therefore have no means for their regeneration (i.e., depolarization of a SMC does not produce a slow wave-like event). Depolarization of SMCs by slow waves enhances the open-probability of L-type voltage-dependent Ca2+ channels that are ubiquitously expressed in GI SMCs. In some SMCs of the small bowel and colon activation of L-type Ca2+ channels results in generation of Ca2+ action potentials, which are superimposed upon the peaks of slow waves. In the stomach slow waves depolarize SMCs but action potentials are not typically generated. In either case Ca2+ entry into SMCs initiates contraction (excitation-contraction coupling), and in both cases the slow wave depolarization/repolarization cycle determines the period of enhanced open probability of L-type Ca2+ channels in SMCs (contraction) and the period of time in which Ca2+ channel open probability is low (relaxation). This chapter discusses the characteristics of slow waves in GI muscles, the apparatus required for slow waves, the mechanism of slow wave generation and propagation, and how nerves and other factors influence cells of the SIP syncytium to increase or decrease the gain for excitation-contraction coupling. It should also be noted that in some regions of the GI tract, such as the internal anal sphincter [6], slow waves occur at sufficient frequencies to cause summation of cytoplasmic Ca2+ and tonic contraction (similar to a partial tetanus), but this topic is covered in another chapter.
1.2. Nature of Electrical Rhythmicity in GI Smooth Muscles
Contractile rhythmicity in GI organs was likely recognized as soon as the abdomens of freshly killed animals were sliced open. Gut motility persists for various periods of time after the death of an animal because it is not driven by circulating factors in blood or by neural input from the central or enteric nervous systems. The basic motility patterns are intrinsic to the cells and tissues of the tunica muscularis, and cells of the SIP syncytium appear to be rather resistant to the hypoxia that rapidly kills the heart and brain. Placing metal electrodes on organs of the gut allowed electrical recording of gut activity nearly 100 years ago [7, 8], but it is likely that these recordings were heavily contaminated by movements At the time of the initial recordings the electrophysiological basis for muscle contraction was unknown, and it was not possible to block movements independently of upstream mechanisms. Therefore, we have no way of knowing whether the first electrical recordings of GI muscle activity contained electrophysiological information (i.e., events based on changes in transmembrane potentials in cells within the muscles or organs) or were “biopotentials” resulting from muscle movements [9]. Better techniques developed with time, such as sucrose gap, which was a means of obtaining pseudo-transmembrane potential recording, provided valuable information about the waveforms of electrical slow waves in the gut [10, 11]. Voltage-clamping of GI muscle strips was attempted with sucrose gaps, and several ideas about the mechanism of slow waves were developed based on these experiments [12, 13]. However, true voltage-control (and space clamp) of the many electrically coupled cells in the SIP syncytium may have been difficult to accomplish with this approach.
Eventually cell impalement techniques were adopted to directly measure transmembrane potential in a dynamic manner [14–17]. Small SMCs are difficult to impale, and impalements of cells among moving muscle cells are difficult to maintain, but this technique provided, and still provides, the most accurate means of measuring resting membrane potentials, slow waves, and action potentials in intact muscles (Fig. 1.1). Microelectrode studies allowed investigators to better understand the ionic mechanisms that cause slow waves, the responses to neurotransmitters, and the effects of bioactive compounds. Information obtained from these recordings is dependent upon many voltage-and non-voltage-dependent and receptor-operated ion channels expressed in cells of the SIP syncytium [18]. It remains technically difficult to voltage-clamp intact GI muscles, except perhaps with small bundles of cells, as used by David Hirst and collaborators in many studies of smooth muscle tissues [19–21]. It should be reemphasized that GI muscles are syncytial in nature, and the complete syncytium (SIP syncytium) contains SMCs, ICC, and PDGFRα+ cells [1]. Thus, intracellular recording from a single cell within the SIP syncytium is complex and contains membrane potential information not only from the impaled cell but also from electrically coupled cells. The complexity of the SIP syncytium was unknown to investigators during the early use of both sucrose gap and intracellular microelectrode recording, and several behaviors believed to be intrinsic to SMCs are now known to originate in cells other than SMCs (e.g., slow waves, fast (purinergic) inhibitory junction potentials, cholinergic excitatory junction potentials (EJPs); see [4, 5, 22, 23]).
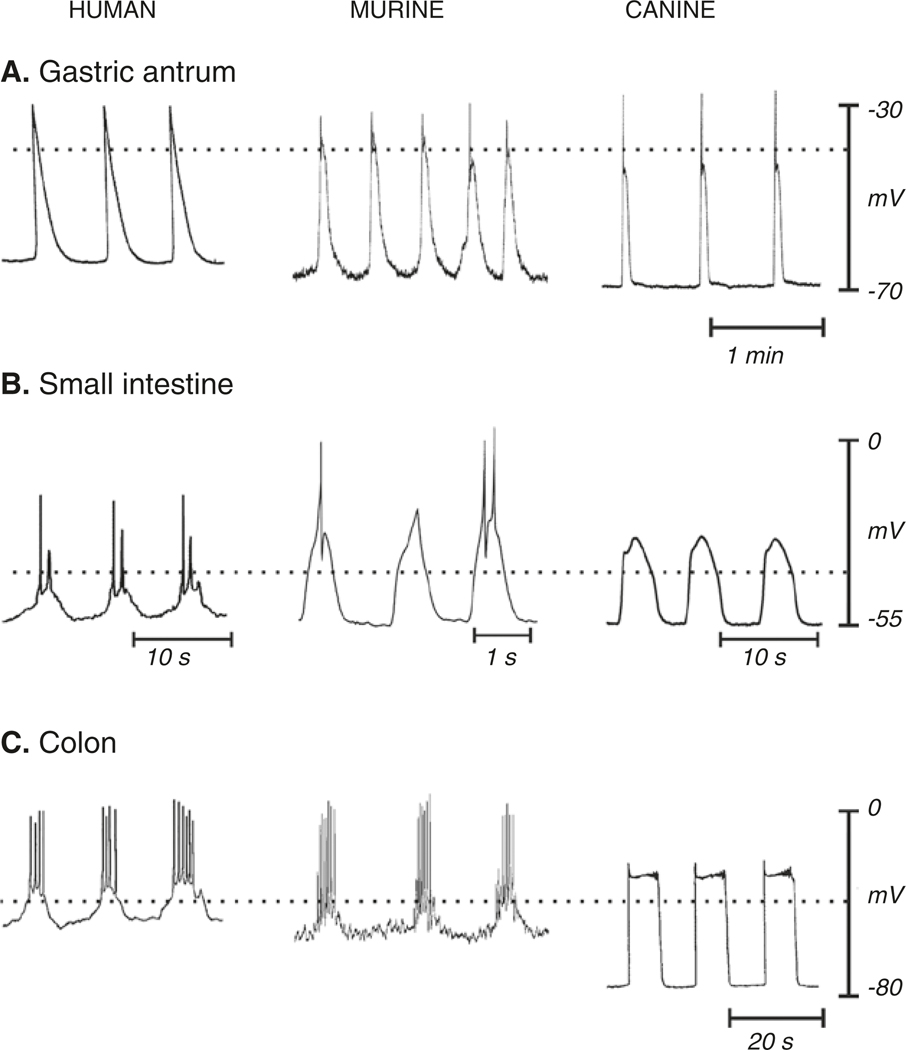
Fig. 1.1.
Electrical activity recorded from stomach, small bowel, and colon of three species. Recordings were made with intracellular microelectrodes from the circular muscle layers of isolated strips of muscle from the antrum, ileum or jejunum and proximal colon. The major features of electrical activity that vary in waveform in different regions of the GI tract and in different species are displayed. From a relatively stable membrane potential between slow waves (resting membrane potential), a sharp upstroke depolarization occurs when a propagating slow wave reaches the point of recording. The upstroke typically repolarizes quickly to a pseudo-stable plateau potential that can last for several seconds before repolarization to the resting potential. Resting potentials vary, making it necessary for slow waves in different regions to depend upon different voltage-dependent Ca2+ channels to carry the main current during the upstroke (see text for details). The plateau potential depends upon sustained activation of Ano1 channels that are activated by Ca2+ release events in the ER of ICC. In some regions slow waves initiate Ca2+ action potentials in SMCs. These are initiated in the small bowel and colon when the depolarization reaches about −40 mV (dotted lines in each panel). Ca2+ action potentials are superimposed upon the slow wave plateau phase. Slow waves with or without superimposed action potentials generate phasic contractions. Copied with permission from [2]
1.2.1. Terminology Used for Rhythmic Electrical Potentials in GI Muscles
Electrical rhythmicity in GI muscles has been given many terms throughout the history of electrical recording, including pacesetter potentials [24], pacemaker potentials [25], electrical control activity [15, 26], basic electrical rhythm (BER) [27], slow waves [28], and action potentials [16, 29]. Recognizing the syncytial connectivity between SMCs and ICC, David Hirst and coworkers developed even more specific terminology, with different terms applied to slow waves recorded from different cell types [30–32]. For example, events recorded from SMCs of the circular muscle of the guinea pig antrum were called slow waves, events recorded from ICC in proximity to myenteric plexus (ICC-MY) were referred to as driving potentials, and those recorded from the longitudinal muscle were called follower potentials. Use of specific terms to distinguish events in different cells may be more precise, because depolarization of SMCs activates local voltage-dependent conductances that sculpt waveforms into voltage transients with unique profiles. However, since all of these events originate from a common source in a given tissue [2, 3, 5, 33–35], we prefer to use the term slow waves because this is the common term used in the modern literature.
1.2.2. Waveform Features of Electrical Slow Waves
Slow waves have two basic components that have been given various descriptive terms by different authors, and the two components have been attributed to a variety of mechanisms. This review will focus mainly on recent information and not address the many mechanisms proposed in older studies or in older reviews [12, 36, 37]. In Tadao Tomita’s concept, the first component of the slow wave was the driving potential that propagates through the tissue. The second component was called the regenerative potential and was thought to be initiated locally by the depolarization caused by the driving potential. The second component was later thought to result from activation of conductances in intramuscular interstitial ICC (ICC-IM) [33, 38]. In Joseph Szurszewski’s concept, the first and second components were termed the upstroke depolarization and plateau potential, respectively. These events have now been attributed to specific conductances expressed by ICC and will be described in greater detail later in this review.
Slow waves occur without inputs from nerves, hormones, or paracrine substances in GI muscles, and therefore these muscles are referred to as autonomous and slow waves as myogenic. Slow waves occur for many hours in vitro and persist in isolated muscles for many days in organotypic cultures [39]. The slow wave cycle typically contains a period of relatively stable resting potential (aka diastolic period or period of most negative membrane potential), although a gradual, inter-slow wave depolarization is observed in some intracellular electrical recordings. In most cases recordings from cells in intact muscle strips represent propagating slow waves, so there is only a brief exponential foot before development of the upstroke potential [16]. When recordings are made from impalements of SMCs the upstroke potential occurs at a maximum of about 1 V/s, but in many regions of muscle the upstroke velocity of slow waves in SMCs is only about 100 mV/s. The upstroke depolarization is transient, and after reaching a peak, partial repolarization occurs before a pseudo-stable state known as the plateau phase is reached [16]. The plateau phase can last from a second to many seconds depending upon the region of the GI tract and species [36]. Membrane potential eventually escapes from the plateau phase, and repolarization causes restoration of the resting potential, thus completing the cycle. Slow wave frequency varies from up to 80 cycles per minute, in phasic muscles that utilize summation of excitable events to generate tone [40], to just a few events per minute in muscles with well-defined phasic contractions. Slower frequencies allow complete relaxation between contractions and/or time for propulsive events to propagate for many cm. Frequency is an important parameter of slow wave activity because regional sites of pacemaker dominance resides in cells that generate the highest frequency of pacemaker activity. The factors that set slow wave frequency and why and how frequencies change in disease states are poorly understood at the present time. However, this is an important area of investigation for future studies because abnormal frequencies, generation of ectopic pacemakers (emergence of atypical dominant pacemaker sites), breakdown in natural frequency gradients, and lack of ability of the normal dominant pacemaker region to drive slow waves downstream appear to be fundamental to GI motility disorders, such as gastroparesis [41–43].
The vast majority of intracellular electrical recordings reported in the literature have been made by impalement of SMCs, but a few skilled investigators have impaled ICC directly to obtain first-hand recordings of pacemaker activity. For example, cells were impaled in guinea pig gastric muscles, and the majority of cells were identified as SMCs by dye injection [34]. Occasionally, slow waves with much faster upstroke depolarizations and greater maximal levels of depolarization were observed in impaled cells and called driving potentials. Lucifer yellow or neurobiotin injection during recording showed that driving potentials originated in ICC. Recordings were also made by impalements of SMCs and ICC simultaneously [33, 34]. These recordings clearly showed that initiation of slow waves (aka driving potentials; with greater total amplitude and upstroke velocities) occurred in ICC, and lower amplitude events with reduced upstroke velocities occurred in SMCs (Fig. 1.2). Coupling between ICC-MY and SMCs in both the circular and longitudinal muscle layers was also investigated by simultaneous impalements, and these experiments showed strong coupling between cells of a given type, but far weaker coupling between ICC-MY and SMCs. A lower level of coupling between ICC-MY and SMCs is an important property, allowing conservation of current within an ICC-MY network to facilitate generation of slow waves and active propagation while still permitting enough current to pass to SMCs to depolarize these cells.
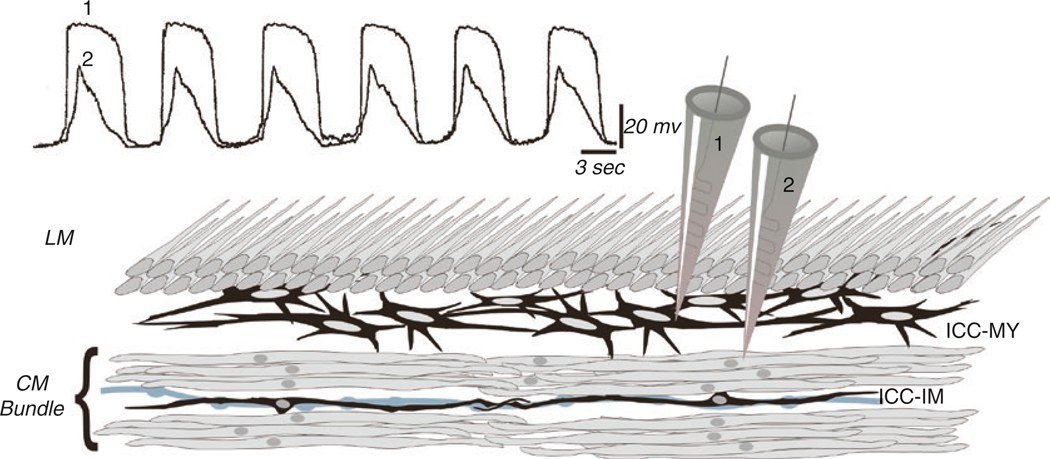
Fig. 1.2.
Simultaneous recording from ICC-MY and SMC. Recording from ICC-MY and SMCs simultaneously shows that the upstroke of slow waves originates in ICC-MY and conducts with decrement to electrically coupled SMCs. The conductances present in SMCs cannot support active propagation of slow waves in these cells; however, the depolarization can activate other voltage-dependent conductances that support contractions (L-type Ca2+ channels and shape the slow wave; various voltage-dependent K+ channels). The peak of the slow wave reaches about −10 mV (approximately the equilibrium potential for Cl− ions) and is relatively constant for durations of a second or more. Anatomical drawing depicts circular (CM) and longitudinal (LM) muscle layers, ICC-MY in a network between CM and LM, and ICC-IM, lying in close apposition to an enteric motor neurons (gray varicose process). Redrawn from [2], and original data was provided by Professor David Hirst
Direct recordings from ICC-MY in the small intestine from two species have also been reported [44–47]. These studies investigated the ionic conductances responsible for the upstroke and plateau phases of slow waves. As in studies of gastric cells, impalements were validated by injection of Lucifer Yellow during recording. Slow waves in ICC-MY of the murine small intestine also were found to be more robust than in SMCs, having upstroke velocities (dV/dt) of approximately 2 V/s, amplitudes of more than 60 mV, and maximal depolarizations to about −10 mV at their apexes. Nifedipine did not affect the upstrokes or frequency of slow waves, but Ni2+ slowed the rate of the upstroke depolarization and reduced slow wave frequency. Mibefradil, a T-type Ca2+ channel antagonist, also reduced dV/dt of the upstroke depolarization and reduced slow wave frequency. In the presence of mibefradil, the period of the inter-slow wave interval increased and a greater depolarization level was required before the threshold for slow wave generation was reached. During the slow depolarization, small oscillations in membrane potential were noted. Pinacidil, an ATP-dependent K channel (KATP) agonist that elicits strong hyperpolarization of GI SMCs [48], also hyperpolarized ICC-MY, but failed to inhibit slow wave activity. In fact the maximum amplitude of slow waves in ICC-MY increased in the presence of pinacidil, and depolarization to approximately the same maximum point (about −10 mV) occurred. DIDS, a Cl− conductance antagonist, and reduced extracellular [Cl−] reduced the plateau phase. High enough concentrations of DIDS or T-type Ca2+ channel antagonists or membrane-permeable Ca2+ chelators (BAPTA-AM or MAPTA-AM) blocked slow wave activity [31, 45]. The actions of the Ca2+ chelators suggest that the Cl− conductance may be due to Ca2+-activated Cl− channels (CaCCs). Taken together, these observations suggested that the upstroke potential depends upon a T-type Ca2+ conductance and the plateau potentials depend upon a Cl− conductance.
As in mice, the upstroke depolarization of slow waves in ICC-MY of the rabbit small intestine were unaffected by nifedipine, but there were also interesting differences in the electrical events recorded from rabbit ICC-MY. The upstroke velocities of rabbit slow waves reached 10 V/s, and the events were reduced somewhat by Ni2+ and by leaving Ca2+ out of the extracellular solution [49]. These data suggest that the upstroke depolarization in rabbit slow waves is only partially mediated by Ca2+ influx, and the inward current is much less sensitive to block by Ni2+ than in mouse. Replacement of [Ca2+]o with [Sr2+]o in rabbits enhanced upstroke depolarization velocity but reduced the amplitude and duration of the plateau phase. Thus, Sr2+ appears to be an effective charge carrier for the upstroke conductance, but less effective in activating CaCCs. DIDS, cyclopiazonic acid and bumetanide, an inhibitor of the Na+K+Cl− co-transporter (NKCC1), also reduced the amplitude and duration of plateau potentials. In both rabbit and mouse Ca2+ free solution and replacement of Ca2+ with Sr2+ reduced the frequency of slow waves dramatically. These data generally supported the mechanism proposed for slow waves in the mouse small intestine, but the differences observed suggest that conductances involved in slow waves may vary from species to species.
1.2.3. Origination of Pacemaker Activity
Discussions about pacemaker sites in the GI tract often refer to the location of the dominant pacemaker in GI organs. For example, the dominant, organ-level pacemaker region in the stomach drives gastric peristalsis, resulting in propagation of slow waves and contractions from the proximal corpus to the pyloric sphincter [50]. A more specific question about pacemaker regions in GI muscles relates to the local source of pacemaker activity. Muscles removed from phasic regions of the GI track display intrinsic pacemaker activity. Within segments of muscle no specific pacemaker site appears to be dominant and the point of origin of slow waves shifts from cycle to cycle [51]. Dissection experiments, in which muscle strips are split in various ways, reveal dominant planes of activity through the thickness of the tunica muscularis. With this approach the dominant pacemaker in canine gastric antral muscles was found to reside in the myenteric region. Even in small muscle strips containing the myenteric plexus region, pacemaker activity shifted from moment to moment along the length of the muscle strip [52]. In the stomach slow waves persisted in muscle tissues separated from the myenteric region, but these events occurred at reduced frequencies [53]. This observation suggests that cells within the thickness of the gastric tunica muscularis, such as the ICC that line septa (ICC-SEP) between muscle bundles or ICC-IM, are capable of generating pacemaker activity, albeit at a lower frequency.
Studies of animal models have shown that the organ-level dominant pacemaker in the stomach exists in the orad corpus where the frequency of slow waves is greatest [16, 54, 55]. The dominant pacemaker in the human stomach is also likely to reside in the proximal corpus; however, comparison of slow waves recorded from gastric antral and corpus muscles does not clearly resolve a dominant slow wave frequency gradient [56]. It should also be noted that an anatomical region was found in the guinea pig corpus where intramuscular ICC (ICC-IM) are plentiful, but ICC-MY are absent. Slow waves were generated from this region at a frequency matching the frequency of slow waves in the intact stomach [55]. Thus, dominant pacemaker activity may originate from ICC-IM or ICC-SEP in these animals.
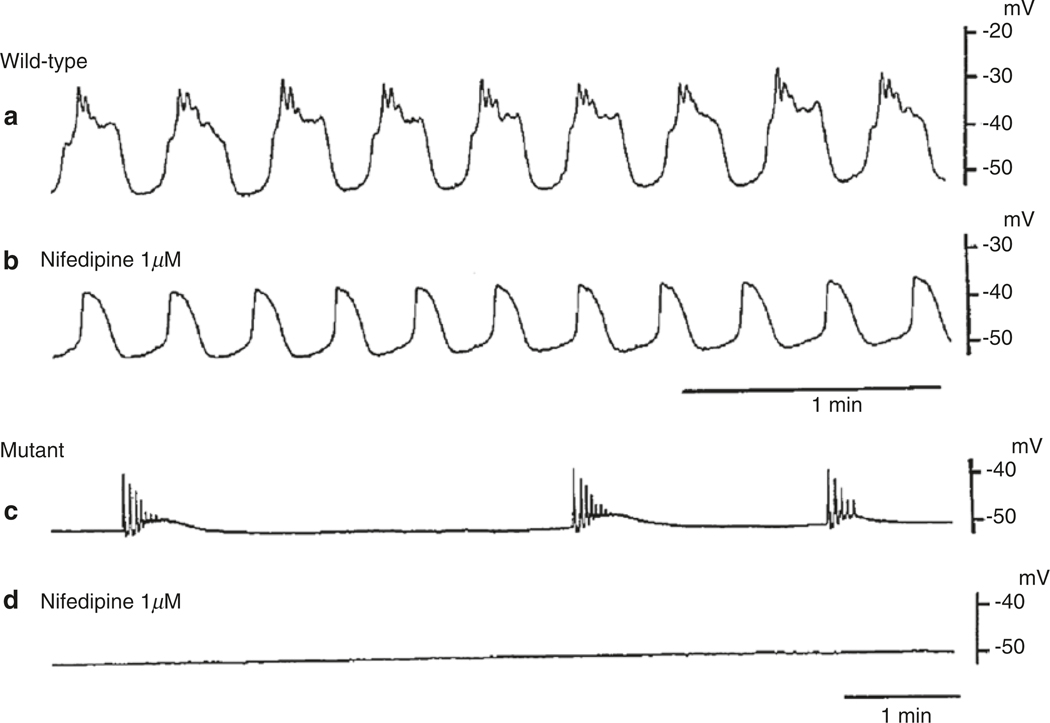
Organ-level pacemaker dominance would be difficult in the small intestine because slow wave frequency is higher than in the stomach, and the propagation velocity of slow waves is relatively slow. Therefore, slow wave propagation is normally limited to rather small segments of tissue, because when an event is initiated at any point along the intestine it tends to soon collide with slow waves generated at more distal or more proximal pacemakers. Older studies suggested that slow waves originated in the longitudinal muscle layer in the small intestine [12], but that concept has been refuted by more recent work. Dissection experiments from several species and experiments on mutant animals in which ICC-MY fail to develop show that dominant pacemaker activity emerges from the myenteric region in the small bowel [5, 57]. The muscle wall of the mouse small bowel is devoid of slow waves in the absence of ICC-MY, suggesting that only these cells are pacemakers in this region of the murine GI tract. However, in dogs, isolated circular muscle strips from the region of muscle near the deep muscular plexus displayed rhythmic activity, suggesting that ICC-DMP may also be capable of pacemaker activity in this species [58].
The colon has two discrete regions of pacemaker activity, one lying along the submucosal border of the circular muscle layer that produces slow waves [59–62] and another pacemaker area, producing higher frequency activity known as myenteric potential oscillations (MPOs) is located in the myenteric region. The two colonic pacemakers occur at such different frequencies, that one cannot not drive or dominate the other. Therefore, these events summate in the circular muscle layer [60, 61].
When morphology and ultrastructure investigations were performed on the regions of tissue from which dominant frequency pacemaker activity was recorded, networks of ICC were identified (Fig. 1.3) [53, 63–66]. Early structural descriptions of ICC included the suggestion that ICC might serve as pacemakers in the GI tract because gap junctions between ICC and SMCs were observed [67–71]. ICC within pacemaker regions are electrically coupled to each other via numerous gap junctions, forming the basis of these syncytial networks. Gap junctions between ICC and SMCs are less abundant (e.g., [53]) but capable of conveying slow waves from ICC-MY to SMCs.
Fig. 1.3.
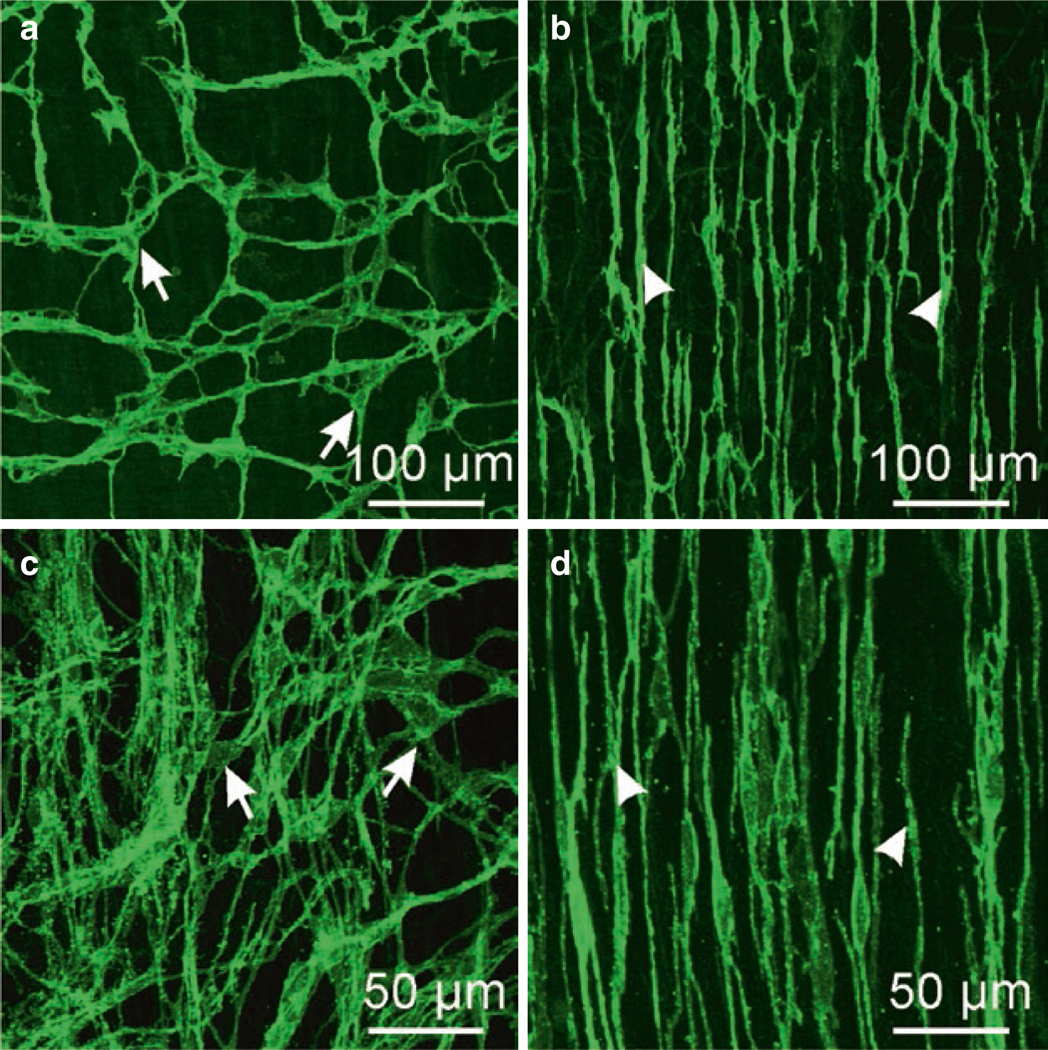
ICC in murine and monkey small intestine. (a, b) are whole mounts imaged by confocal microscopy of ICC-MY (a) labeled with anti-c-Kit antibody (arrows) and ICC-DMP (b; arrowheads) in murine small intestine. ICC-MY have multiple processes and form an extensive interconnected network via gap junction coupling between ICC and with adjacent SMCs. ICC-DMP run in parallel with the circular muscle fibers and are concentrated very close to the submucosal edge of the circular muscle layer in the mouse. ICC-DMP are closely associated with the processes of enteric motor neurons (not shown) and PDGFRα+ cells (not shown). (c, d) are images from the small intestine of Macaca fascicularis (cynomolgus monkey). ICC-MY (c; arrows) in this species also display a network of cells between the circular and longitudinal muscle layers and ICC-DMP (d; arrowheads) are also present near the submucosal surface of the circular muscle layer. Redrawn from [206]
1.2.4. Propagation of Slow Waves
Slow waves propagate actively within GI muscles, and this is why long distance coordination and sequencing of contractions, such as in gastric peristalsis, is possible. Dissection experiments showed that continuous structural integrity of tissues determined to be pacemaker areas is necessary for active propagation. For example, experiments on dog colon showed that slow waves propagate actively, as long as the ICC-SM network along the submucosal surface of the circular muscle layer remains intact. If a thin section of tissue at the submucosal surface (pacemaker area) is damaged or removed, slow waves decay in amplitude within a few mm from an active area [72]. In contrast, when the myenteric pacemaker region was separated from gastric muscles, active propagation occurred at approximately the same velocity [53]. Complete removal of the myenteric pacemaker region caused a decrease in the frequency of slow waves, but pacemaker activity persisted and slow waves propagated within isolated strips of circular muscle. These data indicate that cells capable of active propagation penetrate into the circular muscle layer in some regions. The cells capable of slow wave regeneration within the circular muscle in the stomach are not known but could be either ICC-SEP between muscle bundles or the ICC-IM within muscle bundles. ICC-SEP have not been isolated for study of their specific properties.
Active propagation of electrical events is a common property of excitable cells when the cells are longer in length than a few space constants or when the cells are arranged into a syncytium connected by gap junctions. The SIP syncytium is an example of the latter case, and SMCs and ICC are electrically coupled to other cells of the same type and to each other. Many studies of propagation velocity were performed on whole organs and tissues in vitro with extracellular electrodes [50, 73, 74]. However, data from studies of this type are misleading because the recordings are likely to be contaminated by mechanical artifacts, making it impossible to know with precision when an electrical event passes a recording point [75, 76]. Measurements of slow wave propagation velocity in strips and sheets of muscle have also been made using intracellular microelectrodes. This approach provides more precise determination of the point in time when events pass a recording site [77]. With this approach, anisotropic propagation was observed in muscles of the canine antrum. The propagation velocity in the axis of the circular muscle was 23 mm/s but only 11 mm/s perpendicular to the circular muscle axis. The cause of the anisotropy in slow wave propagation is not fully understood but may be due to relatively lower cell-to-cell resistance in the circular axis than in the longitudinal axis.
The mechanism of slow wave propagation in tissues has been explored using muscle strips and partitioned recording chambers. Muscle strips pulled through latex partitions provide the opportunity to make intracellular recordings from parts of the tissue exposed to different external solutions (Fig. 1.4). One chamber serves as the site of unfettered slow wave generation, and the second chamber provides a site to record the effects of various test solutions on slow wave propagation. Slow waves recorded from two well-spaced cells in canine colonic muscles were of equal amplitude under control conditions, but drugs to block IP3 receptors (IP3 Rs), reduced [Ca2+]o and antagonists of T-type voltage-dependent Ca2+ channels (Ni2+ and Mn2+) inhibited slow wave propagation. The amplitudes of propagating slow waves decayed to the resting potential in less than 3 mm from the partition [78].
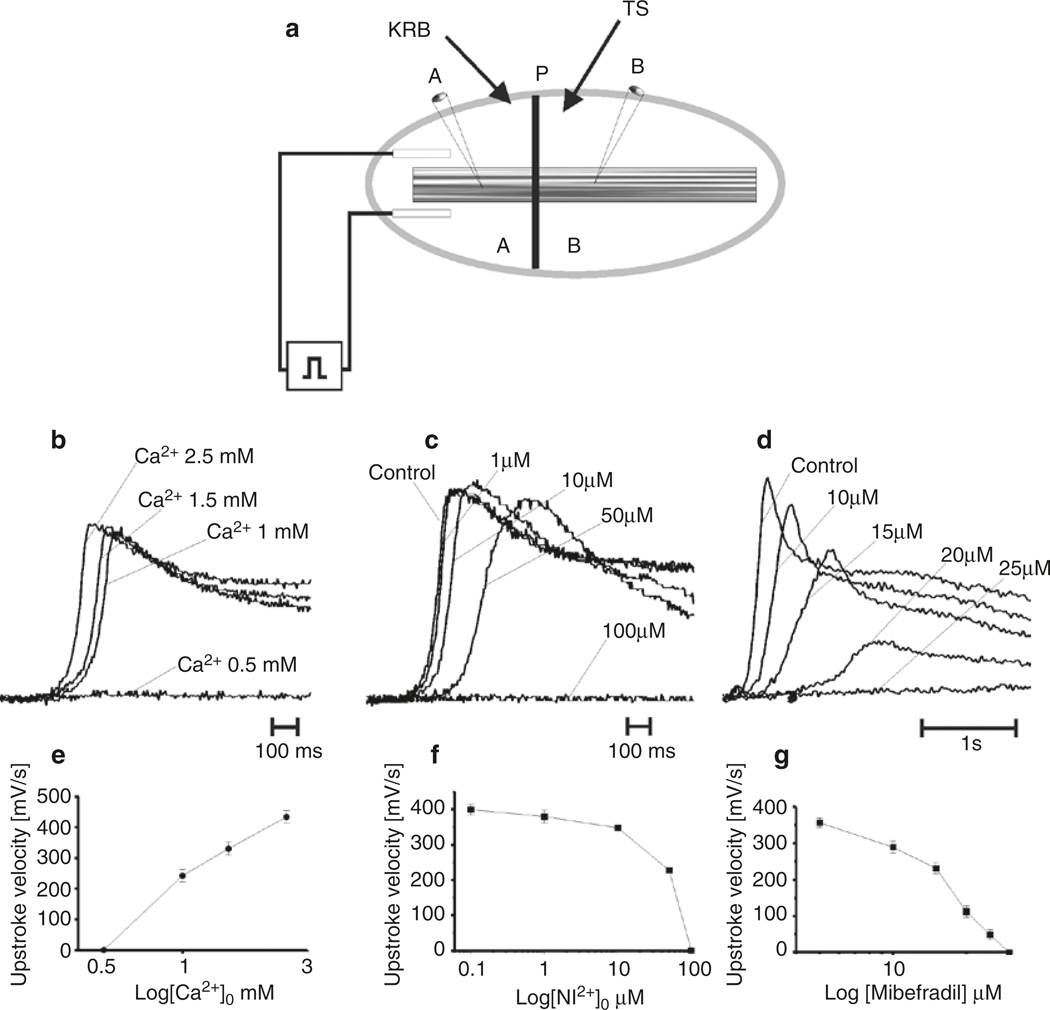
Fig. 1.4.
Role of Ca2+ entry in slow wave propagation. (a) Shows a partitioned chamber apparatus used to study slow wave propagation. Slow waves can be reliably generated in Chamber A perfused with Krebs solution (KRB). Slow waves are initiated by passing a current pulse through electrodes placed on either side of the muscle strip. The muscle is pulled through a latex partition into Chamber B that can be independently perfused with Test Solutions (TS). Cells in Chambers A and B record control slow waves, and propagating slow waves, as modified by the Test Solution. (b–d) Show control slow waves (as recorded in Chamber A) and slow waves exposed to Test Solutions containing reduced [Ca2+]o (b), extracellular Ni2+ (c), or mibefradil (d). Each Test Solution caused a concentration-dependent decrease in propagation velocity (not shown in this example) and decreased upstroke velocity, as shown by superimposed slow waves. [Ca2+]o of 0.5 mM, Ni2+ at 100 μM, and mibefradil at 25 μM did not support active propagation, and slow waves decayed in amplitude before reaching the impaled cell in Chamber B. Graphs in (e–g) summarize this series of experiments. Redrawn from [80]
Other experiments tested the propagation in canine antral muscles and used a triple partitioned chamber [79]. In these experiments the central chamber served as the test chamber and simultaneous intracellular recordings were made in the chambers to the left and right (Chambers A&C) of the central chamber (Chamber B). Coupling of slow waves recorded in chambers A&C was 1:1 under control conditions and the propagation velocity of slow waves was 22 mm/min, confirming previous studies [77]. Propagation velocity decreased and coupling between chambers A&C broke down when temperature was decreased in Chamber B. For example, propagation velocity fell from 19 mm/s at 37 °C to 3.6 mm/s at 27 °C. Slow waves failed to propagate from chamber A to chamber C below 21 °C. The upstroke velocity of slow waves also decreased from 720 mV/s at 37 °C to 522 mV/s at 24 °C. Depolarization or hyperpolarization of the central test chamber also inhibited propagation and coupling of slow waves in chambers A&C. Finally, reduced extracellular Ca2+ or antagonists of T-type voltage-dependent Ca2+ channels (e.g., Ni2+ or mibefradil) inhibited slow wave upstroke depolarization velocity and propagation. These experiments suggested that voltage-dependent Ca2+ entry, possibly due to T-type Ca2+ channels, is required for active propagation of slow waves.
The slow wave upstroke depolarization is the leading edge of propagating slow waves, and the rate-of-rise (dV/dt) of the upstroke is an indication of the inward current density depolarizing the SIP syncytium. Various Ca2+ channel antagonists have been tested on dV/dt of canine antral slow waves. Nicardipine, an L-type Ca2+ channel antagonist, had no effect on upstroke velocity or propagation velocity [80]. However, Ni2+ and mibefradil, both T-type Ca2+ channel antagonists, reduced dV/dt and propagation velocity in a concentration-dependent manner. Reduction in extracellular Ca2+ also reduced upstroke and propagation velocities such that slow waves failed to propagate actively when [Ca2+]o was reduced below 0.5 mM. These observations are consistent with the idea that dihydropyridine-insensitive, T-type voltage-dependent Ca2+ channels are required for slow wave propagation (Fig. 1.4). Another interesting observation from this study was that cyclopiazonic acid, which depletes Ca2+ stores in cells, significantly blocked the plateau phase of slow waves without affecting the upstroke or propagation velocity [80].
1.2.5. Electrical Pacing of GI Muscles
Gastrointestinal muscles can be paced electrically by applying current pulses, 1–2 s in duration [81]; however, the degree to which slow wave frequency can be enhanced is limited by the duration and refractory properties of slow waves. Pacing has been suggested as a therapy for motility disorders, such as gastroparesis; however, the power required for direct pacing of slow waves has restricted the usefulness of this technique in patients [82]. The effects of pacing were investigated in muscles of the canine antrum [83]. At pacing frequencies of 3.5 cycles per minute nearly identical slow waves were elicited by each pulse, but as frequency increased, an alternating pattern developed, in which every other slow wave displayed a greatly attenuated plateau phase. Complete slow wave block occurred when the interval between repolarization and the next stimulus was less than about 2 s. The muscarinic agonist reduced the refractory period. Similar properties of slow wave refractoriness were observed in the guinea pig stomach, and about 6 s were required between slow waves for full restoration of amplitude [84]. As in other excitable cells, the refractory period was inversely related to the amplitude and duration of the depolarization. Acetylcholine also reduced the refractory period in guinea pig stomach, and this was attributed to the activation of protein kinase C (PKC), because the effects of acetylcholine were mimicked by phorbol-12-myristate-acetate and blocked by an inhibitor of protein kinase C.
1.3. Ca 2+ Action Potentials
Depolarization of SMCs elicits action potentials that occur by activation of inward current carried by L-type voltage-dependent Ca2+ channels [85–88]. Ca2+ entry into SMCs through L-type (dihydropyridine-sensitive) Ca2+ channels during action potentials is substantial, as indicated by the rapid upstroke velocities of action potentials (up to approximately 20 V/s), [89]. Activation of L-type Ca2+ channels is a major mechanism through which GI SMCs achieve excitation-contraction coupling [90, 91]. We know that SMCs are the source of Ca2+ action potentials in the SIP syncytium, because isolated cells generate these events (Fig. 1.5) [92] and muscles devoid of slow waves through loss of ICC persist in generating action potentials [93, 94]. When action potentials occur in phasic GI muscles, they are superimposed upon the slow wave depolarizations [36]. This is an example of the integration achieved by contributions of different cells in the SIP syncytium: ICC generate slow waves, these events conduct into SMCs, and the depolarization of SMCs elicits action potentials. In taenia coli action potentials occur in the absence of slow waves [14]. Single action potentials couple to twitch-like contractions and trains of action potential firing creates tetanic-like contractions [95–97]. While action potentials are not a common behavior in most regions in the stomach, they do occur in the terminal antrum and pyloric sphincter [16, 98].
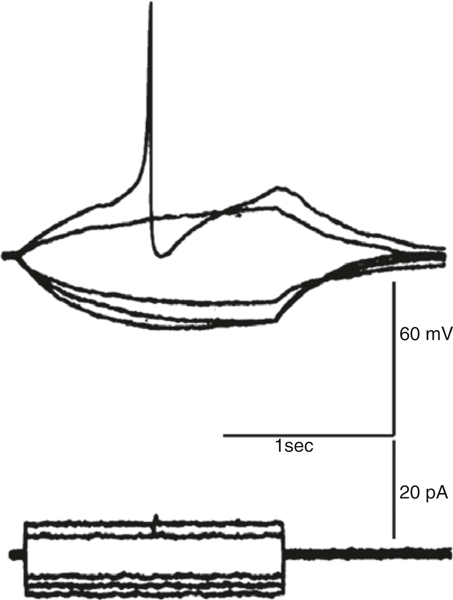
Fig. 1.5.
Ca2+ action potential in an isolated smooth muscle cell from rabbit jejunum. Cell held under current clamp conditions and hyperpolarized or depolarized by passing constant current pulses. Depolarization activated Ca2+ action potential. Redrawn from [92]
1.4. Relationship Between Electrical and Contractile Behaviors
Depolarization of smooth muscle cells increases the open-probability of L-type Ca2+ channels, initiating Ca2+ entry and contraction. As shown in Fig. 1.1, slow waves typically depolarize cells into the range of potentials in which L-type Ca2+ channels are activated in SMCs. In some muscles, such as the corpus and antrum of the stomach, slow wave depolarizations of SMCs are sufficient to activate enough Ca2+ entry to elicit contractions, but in other regions of the gut slow waves elicit only small amplitude contractions and major contraction requires generation of Ca2+ action potentials [99]. The relationship between slow waves and contractions was studied in detail in canine gastric muscles [100]. A one-to-one correlation between slow waves and phasic contractions occurs in gastric corpus and antrum. Two components of contraction are apparent, one appears to correlate with the upstroke depolarization and a second depends upon the amplitude and duration of the plateau phase of the slow wave. A mechanical threshold was observed in which the increase in the force of phasic contractions correlated with the amplitude of the plateau potential. The curve describing the increase in force as a function of voltage is similar to the activation curve for L-type Ca2+ channels. This relationship was revisited when it became possible to record slow waves, Ca2+ transients and contractions simultaneously in strips of canine gastric antral muscle [90]. These experiments utilized muscles loaded with the ratiometric Ca2+ sensor, indo-1. A sequence of activation occurred that was initiated by the upstroke depolarizations of slow wave, followed by a rise in the fluorescence ratio indicating an increase in [Ca2+]i in SMCs and then development of contraction (Fig. 1.6). If the amplitude of the plateau phase of the slow wave was increased by an excitatory agonist, a secondary phase of the Ca2+ transient developed, and this was associated with a secondary phase of contraction. In the same manner, decreasing the plateau with a dihydropyridine to block L-type Ca2+ channels reduced the amplitude of Ca2+ transients and diminished contractile force.
Fig. 1.6.
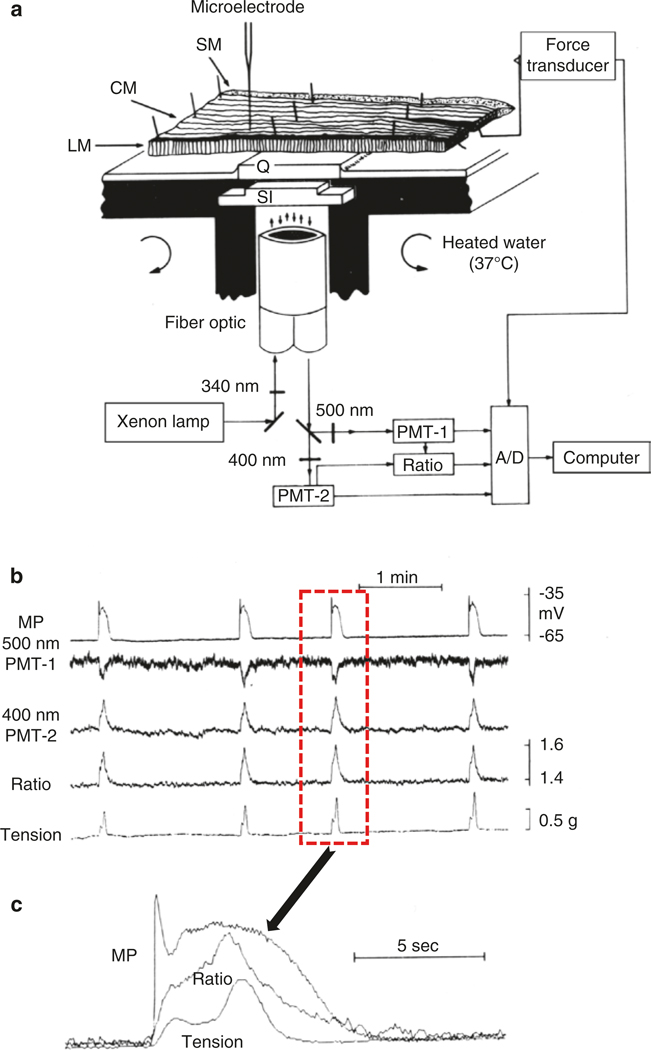
Measurement of membrane potential, fluorescence of a Ca2+ indicator and contraction in muscles of canine antrum. (a) Apparatus to make simultaneous measurements that includes illumination of the muscle strip in selected areas with 340 nm light and collection of 400 and 500 nm signals and analogue determination of the F400/F500 ratio. After determination of the continuous ratio, signals were digitized, along with tension and membrane potential (MP), and recorded on a computer. Antral muscles were cut in cross-section through the thickness of the tunica muscularis and pinned over a quartz window (Q). Measurements were made on longitudinal muscle (LM) or areas of muscle near the submucosal (SM) surface of the circular muscle (CM) or from CM close to the myenteric plexus. A microelectrode was used to impale SMCs near the field of view. (b) Recordings of MP, 500 nm signal, 400 nm signal, the F400/F500 ratio, and tension. Note the correlation between these signals. (c) One slow wave cycle is shown at higher resolution from the events outlined by the dotted line box in b, and the traces are superimposed. Note the initiation of the signal complex by the upstroke depolarization of the slow wave, followed by initiation of a Ca2+ transient and then initiation of contraction. Figure is redrawn from [90]
Loss of slow waves and/or propagation of slow waves disrupts normal motility patterns in the small intestine. Observation of intestinal motility with radiological contrast fluid showed peristaltic waves that moved contents through the proximal small intestine [101]. Movements of this sort were not observed in the intestines of W/WV mice. Loss of ICC-MY leads to aberrant motility initiated by sporadic Ca2+ action potentials in clusters of cells that do not propagate very far in tissues. The motor activity in W/WV muscles was blocked by nifedipine, showing it was unrelated to slow wave activity. The ability of ICC-MY to coordinate contractions was further studied in sheets of intestinal muscle and intact loops of exteriorized small intestine [102]. Impalements of cells in circular and longitudinal muscle layers, validated by filling of cells with propidium iodide during recording, showed that both layers of muscle in the small intestine are paced by ICC-MY. Slow waves of equal amplitude and frequency were present in both layers in wild-type mice and absent in tissues from W/WV mice that have only a few ICC-MY. Movements of the longitudinal muscles were tabulated by an imaging technique called motility mapping in which changes in the distances between points in surface marker arrays are used to describe motor patterns. Ileal contractions occurred in wildtype mice and propagated along segments of bowel at 5.6 mm/s with little variation in waveto-wave period or velocity. W/WV tissues displayed forceful contractions, but these were uncoordinated and unstable in terms of site of initiation or propagation pattern. The velocity of spread of contractile movements was difficult to determine because contractions were abrupt and spread for short distances. The major motility defect noted in animals with reduced ICC-MY was loss of organization and propagation of coherent phasic contractions. An interesting point illustrated by these studies is the integrated behavior of ICC and SMCs in intestinal muscles. Each type of cell has its own intrinsic ability to influence excitation-contraction coupling. SMCs have the ability to generate action potentials, and this can produce a form of phasic contractions in muscles lacking ICC. However, in small intestinal tissues with a normal ICC-MY network, slow waves override the intrinsic behavior of SMCs, organize excitation-contraction coupling into a contractile pattern driven by the frequency and duration of slow waves, and convey this information to large numbers of cells through slow wave propagation.
Studies to better understand the correlation between electrical activity and contractions have also been performed on colonic muscles. As described previously there are regional differences in the electrical activity of the colon [62], and the relationship between electrical and mechanical activities were evaluated in mouse colon [103]. Slow waves with plateau potentials were recorded from cells near the submucosal border of the CM, and some of these recordings were made from ICC-SM. These events generate smaller amplitude and higher frequency (12–21 cycles per minute) contractions. When recordings were made from the serosal surface in either LM or CM, clusters of membrane potential oscillations or action potentials were recorded at about 3.4 cycles per minute. These events coupled to phasic contraction of large amplitude. These contractions were superimposed when contractions in the CM axis were recorded, but only the low frequency contractions were apparent in the LM recordings. Similar types of patterns are displayed in recordings from human colon; however, the slow wave frequency is only 3 cycles per minute, and this frequency appears to be the dominant frequency of phasic contractions [104]. Thus, the dominant phasic contractile pattern appears to emanate from the pacemaker cells along the submucosal surface of the CM in human colon. How these basic patterns are integrated to form colonic motility is poorly understood.
1.5. The Pacemaker Cells
As above, the dominant pacemaker cells in stomach and small intestine are ICC-MY. These cells are stellate in shape with a prominent nuclear region and multiple processes (Fig. 1.3). Gap junctions are plentiful, connecting ICC-MY into a network running in the space between the circular and longitudinal muscle layers. ICC-MY are distributed on both the longitudinal and circular muscle sides of myenteric ganglia. Labeling of ICC was accomplished in early studies with methylene blue [70, 105]; however, this histological label is not specific. Antibodies against vimentin have also been used, but again this protein is not specific for ICC [106]. Antibodies to c-Kit were found to label cells in the gut wall [107], and these cells were later identified as ICC [3, 5, 108]. Labeling with c-Kit antibodies has been the standard for identifying ICC for the past 20 years, and it was also used to suggest that gastrointestinal stromal tumors arise from ICC [109]. Labeling with c-Kit antibodies in some species, such as primates or humans, can be hampered by issues of non-specificity, because mast cells also express KIT and these cells are present in the tunica muscularis. However, the shape and size of mast cells and ICC are different, making it possible to distinguish the two cell types. Better discrimination is possible in whole mounts than in cryosections, because of the better resolution of cell shape in the former. More recently additional immunolabels for ICC have emerged, such as antibodies for CaCCs, encoded by Ano1 [110, 111], or Na+K+ Cl− cotransporter 1 (NKCC1), encoded by Slc12a2 [47, 112, 113]. Both of these genes are highly expressed in ICC and not resolved in other cells in GI muscles, making them useful for studies of the tissue distribution of ICC.
Unfortunately, enzymes utilized to disperse tissues often damage extracellular epitopes of c-Kit, making it difficult to label ICC with c-Kit antibodies after dispersing cells. Thus, it is difficult to identify ICC unequivocally in mixtures of cells resulting from enzymatic dispersion of GI muscles. This problem was solved, at least for mice, by development of a reporter strain in which a bright green fluorescent protein (copGFP) was knocked-in to Kit, making use of endogenous, cell-specific promoters to accomplish constitutive labeling of ICC [114, 115]. These mice have been useful for molecular and functional studies of pacemaker activity in ICC because cells can be identified by their constitutive fluorescence. Fluorescence activated cell sorting (FACS) has also been used to purify ICC [116], and this made it possible to collect enough cells for gene array studies and deep sequencing of ICC transcriptomes [117, 118]. Genome-wide expression data has generated many new ideas about the nature and functions of ICC, such as expression of additional receptors that might regulate the functions of ICC and participate in regulation of motility, potential interactions of ICC with the extracellular matrix, connectivity with enteric motor neurons and mediation of neurotransmitter effects, additional ion channels that might have function, and a possible role for ICC in generating bioactive molecules (e.g., paracrine mediators).
1.5.1. Studies of Cultured ICC
Due to the difficulties in identifying ICC for physiological studies, investigators developed cell cultures from enzymatically dispersed cells, and identified cells as ICC by c-Kit labeling or by morphological criteria (e.g., multipolar cells with a prominent nucleus that may or may not be integrated into a network). Cell cultures were promising because electrical rhythmicity is preserved [25, 119], and many studies used these cultures to evaluate the expression and function of ion channels, the basis for electrical rhythmicity, and responses to drugs. However, several confounding factors hamper interpretations of the results from these studies: (i) ICC exhibit significant plasticity in culture, and the native phenotype changes rapidly; (ii) it is hard to know whether the ionic conductances found in these cells are native or develop as cells remodel. (iii) ICC, or the cells they become in culture, form gap junctions with other cells. Thus, it is difficult to know whether the electrical activity recorded from cells in networks is intrinsic to ICC or to another type of cell that is electrically coupled to ICC. A plethora of ion channels have been attributed to ICC and reported as functional from experiments on cultured cells, but studies on freshly dispersed cells have failed to resolve many of the conductances described. Expression of Ano1, a conductance of primary importance to the functions of ICC (see below), appears to be suppressed in cultured cells, as this conductance has not been described in studies of these cells. Other unrecognized conductances may also contribute to slow waves in some organs or species, so additional studies to analyze these conductances are still needed.
1.5.2. Specialized Conductances and Transporters in ICC that Contribute to Pacemaker Activity
The rapid decline in the native phenotype in cell culture underscores the importance of studying ICC in situ or developing techniques to investigate these cells soon after they are dispersed from tissues. A clever approach using partial dispersion of the myenteric region of the mouse small intestine was developed to record from cells soon after disruption of the extracellular matrix [120]. Slow wave-like activity was recorded from cells identified as ICC-MY. Slow wave-like events occurred at 16 cycles per minute with durations of 489 ms. The dV/dt of these events was 7 V/s. Under voltage clamp these cells produced unique currents that were not subject to the duration of the depolarizing stimulus and persisted for what appeared to be a fixed duration (~500 ms) even after repolarization. Because of this property, the currents were termed “autonomous currents,” and reversal potential of the current was found to be +3 mV. Removal of [Ca2+]o caused a gradual decline in the autonomous current, and this was judged to be due to depletion of internal Ca2+ stores. Acetylcholine increased the duration of the autonomous currents, consistent with the effects of muscarinic stimulation on slow waves. The authors concluded that the autonomous current in ICC-MY was likely due to a nonselective cation conductance.
Cells from the reporter strain of mice expressing copGFP in ICC can be voltage-clamped shortly after enzymatic dispersion [115]. Molecular evaluation showed that these cells display robust expression of Ano1 CaCCs, and voltage-clamp revealed a current with properties similar to the autonomous current discussed above. Depolarization activated an inward current with a duration that was independent of the duration of the depolarizing pulse. In contrast to the autonomous current, the inward current in ICC of copGFP mice reversed at the equilibrium potential for Cl− ions (ECl). Another interesting property was that the latency for activation after depolarization varied significantly with the strength of the depolarization, which suggested a secondary process might be involved in activating the Cl− conductance. It was reasoned, based on many experiments performed on whole muscles, that Ca2+ might be a factor in activating the Cl− conductance in ICC. Ni2+, reduced [Ca2+]o, and replacement of [Ca2+]o with Ba2+ all blocked activation of the inward current. ICC also express CaCCs with a single channel conductance of 8 pS, consistent with the expression of Ano1. The inward currents in ICC, termed slow wave currents in this study, were blocked by niflumic acid, a well-known, however not highly specific, antagonist of CaCCs. Slow wave-like events in single ICC under current clamp were also blocked by niflumic acid.
Expression of Ano1 (aka Tmem16a) in ICC was first revealed by a microarray study of gene expression in ICC isolated from the murine intestine [118]. After development of antibodies to Ano1 protein (aka Dog1) and discovery that Tmem16a encodes CaCCs [121–123], expression of Ano1 protein in ICC was found throughout the GI tracts of several species including humans [6, 110, 111, 124, 125] (Fig. 1.7). Several Ano1 splice variants are expressed in ICC [111], and this diversity may convey different Ca2+ sensitivities or pharmacology [126, 127] and be of clinical interest since the complement of splice variants changes in diabetes [128]. Several CaCC antagonists inhibited slow waves in gastric and small intestinal muscles [111]. Probably of greatest importance was the concentration-dependent reduction in slow wave frequency caused by CaCC antagonists. This suggests that CaCC are a key conductance in the basic pacemaker mechanism. It is of interest to note that gastric slow waves are far more sensitive to niflumic acid (IC50 = 5.4 μM) and DIDS (IC50 = 150 μM) than small intestinal slow waves (IC50s = 150 μM and 1368 μM for niflumic acid and DIDS, respectively). While these rather nonspecific CaCC antagonists were investigated in the original study, the potency of so-called third generation CaCC antagonists (CaCCinh-A01, T16Ainh-A01, benzbromarone, hexachlorophene, and dichlorophene) were recently compared for their ability to block slow waves [129]. Sensitivities to these antagonists varied significantly, and again their potency for blocking gastric slow waves exceeded their potency in the small intestine. For example, one of the more potent antagonists, CaCCinh-A01, blocked slow waves in the murine stomach at 5 μM, but more than 30 μM was needed to inhibit slow waves in the small intestine. The reasons for these differences are not entirely understood, but possibilities are: (i) channels in addition to Ano1 contribute to slow waves in small intestinal ICC; (ii) splice variants with different sensitivities to CaCC antagonists may be expressed in the two regions; (iii) local Ca2+ concentrations activating Ano1 channels differ in gastric and small intestinal ICC. The latter points arises from the observation that the inhibitory effects of Ano1 antagonists decrease as intracellular Ca2+ increases [127]. It is possible that Ca2+ reaches higher levels in the nanodomains created by junctions between endoplasmic reticulum (ER) and the plasma membranes in small intestinal ICC than in gastric ICC, and therefore Ano1 channels display reduced sensitivity to Ano1 antagonists in intestinal ICC.
Fig. 1.7.
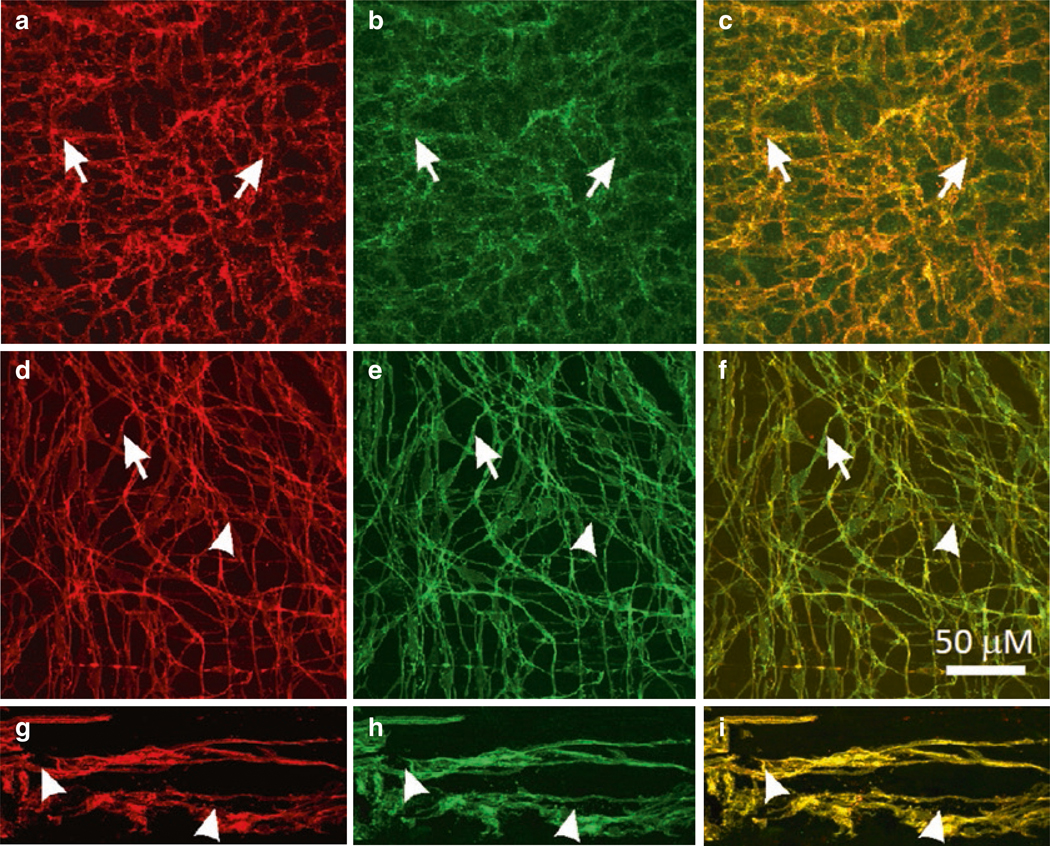
Expression of Ano1 in ICC. (a–c) c-Kit-LI (a, red) and ANO1-LI (b, green) in ICC-MY (arrows) of the murine small intestine. (c) Shows merged file demonstrating co-localization of c-Kit-LI and ANO1-LI (yellow). (d–f) Co-localization of c-Kit-LI and ANO1-LI in ICC of the monkey small intestine. ICC-MY (d; arrowheads) and ICCDMP (d; arrows) are labeled by c-Kit antibody (red) and the same cells display ANO1-LI (e; green) in the small intestine. (f) Co-localization of Kit-LI and ANO1-LI (yellow) in ICC-MY and ICC-DMP. (g–i) c-Kit-LI (arrowheads; g; red) and ANO1-LI (arrowheads; h; green) are expressed in ICC-MY in the human small intestine. Merged images demonstrate co-localization of these proteins in ICC-MY (i; yellow). Scale bar in F applies to all panels
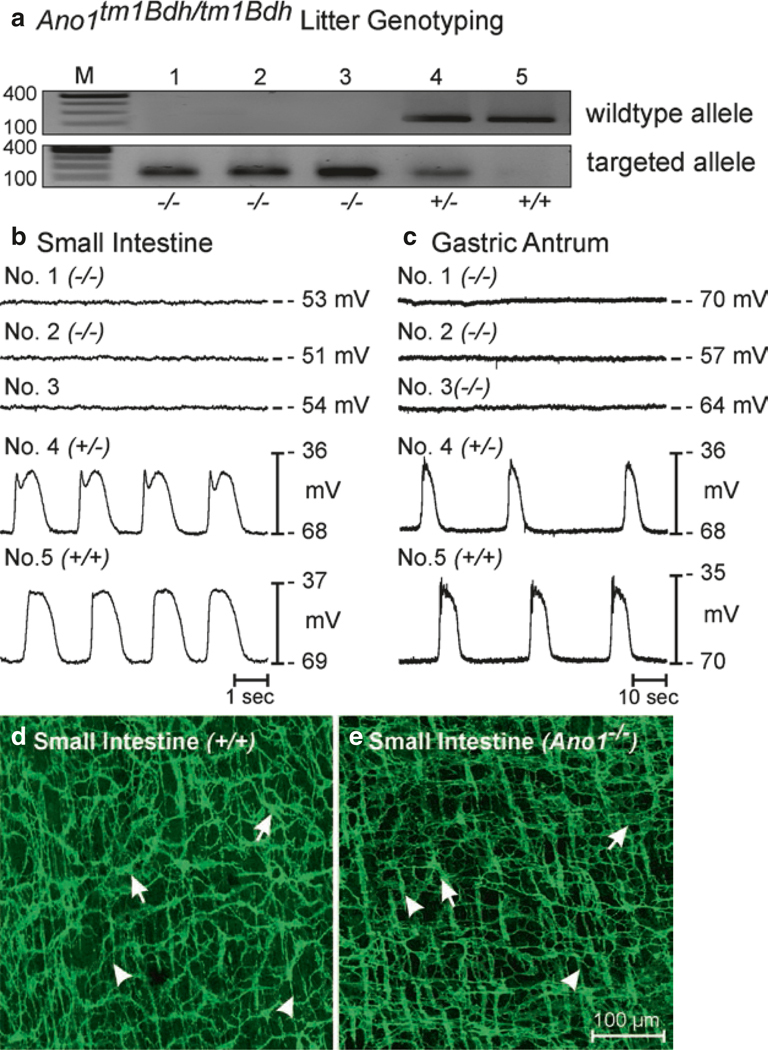
The role of Ano1 channels in pacemaker activity was more clearly defined by studies on intact GI muscles from mice with genetically deactivated Ano1 channels [111]. Unfortunately, Ano1−/− mice have a short life span, and most animals die before 20 days of age [130]. Therefore, it was necessary to study animals shortly after birth. In one experiment, intracellular recordings were made from ten newborn siblings; slow waves were observed in all Ano1+/+ and Ano1+/− muscles and were absent in three Ano1−/− siblings [111]. After 6 days in organotypic culture, which avoids the changes in ICC phenotype observed in cell cultures, slow waves increased in amplitude in homozygotes and heterozygotes, but were still absent in the Ano1−/− muscles. Several mice in one litter survived and were studied 23 days after birth. Mice from Ano1+/+ and Ano1+/− mice again displayed normal slow wave activity in the small intestine and stomach, but slow waves were absent in Ano1−/− muscles (Fig. 1.8).
Fig. 1.8.
Loss of slow waves in small intestinal and gastric muscles in Ano1−/− mice. (a) Genotypes of Ano1−/− mice. The wild-type allele was absent in animals 1–3 in this litter, demonstrating that these animals were Ano1−/−. Animal 4 was a heterozygote and animal 5 was a wild-type homozygote. (b, c) Electrical recording from jejunal and antral circular muscles from each animal with intracellular electrodes. Slow waves were absent in Ano1−/− mice, and normal in animals with wild-type alleles. (d, e) ICC-MY (arrows) and ICC-DMP (arrowheads), with an apparently normal distribution and density, were present in tissues of Ano1−/− mice (small intestine shown). Scale bar for d and e is shown in e. Figure is redrawn from [206] with permission
Use of constitutive genetic knockouts provided strong evidence for the role of Ano1 in slow waves, but inducible deactivation of Ano1, which avoids some of the shortcomings of constitutive knockouts, has also been performed on adult mice using Cre-loxP technology [131]. Theoretically, combining cell-specific iCre mice with mice with a floxed gene should result in knockout of the gene after treatment with tamoxifen. However, this does not typically result in a 100% knockout [132, 133], and Ano1 was knocked down by only 50% in intestinal muscles. Ano1 knockout mice showed differences in relative expression with some having dramatic reduction in Ano1 protein and others displaying only moderate knockdown of Ano1. Consistent with the expression patterns, intracellular recordings from jejunal muscles displayed various slow wave behaviors, ranging from complete loss of slow waves to irregular amplitude slow waves to normal amplitude slow waves. The normal amplitude slow waves were of shorter duration, and the irregular amplitude slow waves often occurred at higher than normal frequencies. By comparing expression levels of Ano1 with slow wave behavior, the authors concluded that as the expression of Ano1 in small intestinal ICC-MY decreases the duration of slow waves and the regularity of the slow wave pattern also decreases. Reduced Ano1 in ICC decreases slow wave entrainment, and cells in different clusters of ICC establish independent firing behaviors (uncoordinated pacemaker activity). Similar slow wave patterns have been observed after treatment of intestinal and gastric muscles with the gap junction blockers, heptanol and 18-β glycyrrhetinic acid [134, 135].
The study by Malysz and colleagues [131] is an important contribution because it is likely to predict some of the behaviors manifest in human motility disorders associated with ICC loss or ICC dysfunction. For example, a primary defect observed in the slow waves recorded from mice with partial reduction in Ano1 was reduction in the duration of the plateau phase. This phase is important for the induction of action potentials in small intestinal SMCs, so shortening of the plateau phase would tend to predispose the small intestine to weakened contractions and possibly cause conditions akin to pseudo-obstruction. It is unlikely that all ICC are lost in most human disease, but as the study by Malysz and colleagues suggests, abnormal slow wave patterns can develop far before ICC are lost or become totally dysfunctional. Abnormal slow wave patterns are likely to translate to abnormal and less effective motility patterns.
A fundamental need for the pacemaker class of ICC is a mechanism to facilitate active propagation and entrainment of slow waves. Such behavior is evident in the propagation studies discussed earlier and suggested that voltage-dependent Ca2+ entry may be a key property of pacemaker ICC. Lack of effects to dihydropyridines, significant reduction in the upstroke and propagation velocities in response to T-type Ca2+ channel antagonists [79, 80] and inhibition of slow wave currents in isolated ICC by these antagonists [115] strongly suggest a role for T-type Ca2+ channels, but, as discussed previously, experiments on rabbit small intestinal ICC suggest there may be some variability among species in the channels responsible for upstroke depolarization. Cacna1h (α1H isoform of T-type channels and primary subunit of CaV3.2) is expressed in ICC-MY of the small intestine, as determined by genome-wide gene array study [118]. ICC-DMP, the other class of ICC in the small intestine that do not generate slow waves [5], displayed relatively low expression of Cacna1h in the same screen [118]. Expression of Cacna1h was confirmed by quantitative PCR [136]. Isolated ICC also express Cacna1h, and lower levels of Cacna1g were also detected. ICC displayed two phases of voltage-dependent inward current in response to depolarization [137]. A small component of the inward current was blocked by nicardipine, and the second component was blocked by Ni2+ (30 μM) and mibefradil (1 μM). Replacement of Ca2+ with Ba2+ did not affect the current amplitude, suggesting either ion was a suitable charge carrier and equally permeable to the conductance present in ICC, a well-known property of T-type Ca2+ channels [138]. Consistent with the properties of channels encoded by Cacna1h [139], half-inactivation of the dihydropyridine-resistant conductance in ICC occurred at −59 mV and half activation occurred at −36 mV. The T-type conductance in ICC is also highly temperature sensitive, which is consistent with CaV3.2 channels [139]. Increasing temperature from 20 to 30 °C increased the amplitude of the current from −7 to −19 pA and decreased the activation time constant by more than half. Lowering temperature or addition of Ni2+ (30 μM) reduced dV/dt of the slow wave upstroke in intact muscles, but the effects of temperature were reduced after addition of Ni2+ or in Cacna1h−/− mice. The upstroke of slow waves recorded directly from ICC-MY in situ was also highly temperature sensitive [140]. Taken together, these observations suggest that a T-type Ca2+ current is present in murine ICC (from expression of Cacna1h and/or Cacna1g), such a conductance is functional and a key initiator of slow wave upstroke depolarization, and blocking this conductance interferes with propagation of slow waves.
T-type Ca2+current density is not extraordinary in ICC, in murine small intestinal ICC it averaged 6.6 pA/pF at −20 mV in the presence of nicardipine [137]. Actually, the maximum current density for the dihydropyridine-sensitive component of the inward current in jejunal ICC is similar. Thus, why is T-current necessary for slow wave propagation and dihydropyridine-sensitive current minimally important? The answer is likely to lie in the resting potentials of ICC in situ. In the small intestine the resting potentials of ICC-MY are −69 mV [46], where T-current is available (in fact this potential is near the activation threshold for this conductance in ICC; [137]), but activation of L-type channels occurs nearly 20 mV positive to the resting potential. Therefore, T-type Ca2+ channel activation is likely to establish the threshold for activation of slow waves, and L-type Ca2+ currents may contribute more during the plateau phase, possibly due to the sustained activation of L-type current in the voltage-range near the peaks of slow waves (i.e., window current [91]). The resting potential of the SIP syncytium is very important for the generation of slow waves, potentially in determining the frequency of slow waves, and in predetermining the availability of the voltage-dependent channels responsible for slow wave propagation. T-type Ca2+ currents are clearly important for slow wave propagation in stomach and small intestine (where resting membarne potentials (RMPs) are typically in the range of −60 to −70 mV), but may not be as important in the colon (where RMP in most species is in the range of −50 mV).
Regulation of membrane potential results from integration of inputs from the three cell types in the SIP syncytium. Through generation of spontaneous transient inward currents (STICs) and slow wave currents, ICC provide depolarizing influences by activation of CaCCs [2]. However, between periods of inward current activation, ICC may aid in restoration of negative membrane potentials to insure periods of reduced Ca2+ channel open probability, reduced Ca2+ entry and relaxation. Such behavior may be necessary to maintain the phasic contractile nature of the muscles. ICC also express genes encoding several inward rectifier K+ channels, including Kcnj2 (Kir2.1), Kcnj4 (Kir2.3), Kcnj14 (Kir2.4) and Kcnj5 (Kir3.4), Kcnj8 (Kir 6.1) and Kcnj11 (Kir6.2), that might contribute to regulation of the membrane potential [141]. Voltage clamp of mouse colonic ICC causes activation of an inward current when extracellular K+ ([K+]o) is made equal to [K+]i. This current is blocked by Ba2+ (10 μM) or ML-133 (10 μM). Expression of Kcnj8 (Kir 6.1) and Kcnj11 (Kir6.2) suggests the presence of a KATP conduction. However, no evidence was obtained for functional KATP, and no responses were observed upon application of KATP agonists or antagonists. This is another significant difference in the phenotypes of freshly dispersed and cultured ICC, as pinacidil causes hyperpolarization of cultured murine colonic ICC and this response is blocked by glyben-clamide [142]. Expression of Kcnj5 (Kir3.4) also suggests the presence of G protein-regulated inward rectifiers; however, no current was elicited by dialysis of ICC with Gβγ and none of the current blocked by Ba2+ was sensitive to tertiapin Q [141]. ML-133 caused depolarization of isolated colonic ICC under current clamp and depolarization of cells in intact colonic muscle strips. These experiments suggest that Kir2 family channels participate in regulation of membrane potentials in murine ICC, and this influences the resting membrane potentials of intact muscles.
The effects of bumetanide on slow waves [47, 112] suggest an important role for NKCC1 in pacemaker activity. Cl− channels provide inward current during slow waves, so a transmembrane gradient supporting efflux of Cl− must be maintained in spite of ongoing slow wave activity. This appears to occur through active accumulation of Cl− via NKCC1, a secondary active transporter, which utilizes the Na+ gradient to transport Cl− against its concentration gradient [143]. Slc12a2 and the encoded protein, NKCC1, are expressed robustly in ICC-MY in the small intestine [47, 112, 113]. In contrast, NKCC1 immunoreactivity was not resolved in ICC-DMP, suggesting that antibodies against NKCC1 with extracellular epitopes might provide an effective means of labeling ICC-MY vs. ICC-DMP selectively, facilitating separation of these two classes of ICC from mouse small intestine. The gramicidin-permeabilized patch technique, which has been reported not to disturb [Cl−]i [144], was used to measure the reversal potentials of STICs in ICC [113]. STICs reversed at −9 mV, and the reversal potential shifted as the Cl− equilibrium potential (ECl) was adjusted to more negative or more positive values. Thus, ESTICs may approximate ECl in ICC-MY. Treatment of cells with bumetanide shifted ESTICs to −56 mV within 5 min, suggesting that when active accumulation of Cl− is inhibited, [Cl−]i is decreased, causing a negative shift in ECl and decreasing the driving force for STICs and slow wave currents that are carried by Cl− ions. This was in fact the result, and both STICs and slow wave currents were inhibited by bumetanide [113].
Another consequence of cotransport of ions by NKCC1 is that the transporter brings half as much Na+ into cells as Cl−. Therefore, a means must also be available to rid the cells of excess Na+ and this is mostly likely accomplished by the Na+K+ ATPase (Na+ pump). A role for the Na+ pump in pacemaker activity was proposed many years ago by Ladd Prosser and colleagues [12]. In their model slow wave depolarization occurred by turning off the electrogenic Na+ pump (depolarization) and repolarization was the result of activating the Na+ pump. At the heart of this idea was the observation that ouabain caused depolarization to approximately the same level of depolarization as the peaks of slow waves. During the plateau of the slow wave recovery of Cl− by NKCC1 may cause accumulation of Na+ in a restricted volume that activates the Na+ pump. Activation of the pump could possibly contribute to repolarization of slow waves, but the precise role of the Na+ pump in the pacemaker mechanism is still awaiting clarification.
A schematic showing the stepwise integration of the conductances and transporters described above to accomplish the slow wave upstroke depolarization, cell-to-cell propagation, sustained depolarization during the plateau phase, and repolarization is shown in Fig. 1.9. The information incorporated into Fig. 1.9 has benefited from the experimental advantages provided by the use of transgenic mice. It is possible that such a fundamental mechanism is conserved among species, and there is evidence that c-Kit+ ICC-like networks, Ano1 expression and a role for these cells in GI motility is present even in non-mammalian vertebrates, such as Danio rerio (zebra fish) [145, 146] and Myoxocephalus scorpius (shorthorn sculpin) [147]. However, it must also be recognized that the driver for electrical rhythmicity in GI muscles may vary among organs and species and may have developed different or extended attributes in humans. At the present time physiological studies on species other than mice have depended largely on a pharmacological approach, and there have been few experiments reported on freshly dispersed ICC from other species. As discussed above, the pharmacology of Ano1 channels, for example, can be difficult to interpret, as block of these channels depends upon [Ca2+]i and expression and combination of splice variants [127]. Thus, inability of a CaCC antagonist to block slow waves in a given tissue may not be proof that Ano1 is not involved. While Ano1 is expressed by ICC in a variety of mammalian and non-mammalian species (mouse, monkey, fish, and human; [110, 111, 118, 125, 147]), its universal function in slow waves is yet to be determined. A caveat for universal acceptance of Ca2+ entry through T-type Ca2+ channels is also warranted because these channels are highly dependent upon the resting potentials upon which slow waves are superimposed. Even if isoforms of T-type channels are expressed, depolarized regions of the GI tract cannot rely on the availability of these channels due to their properties of voltage-dependent inactivation. In cells resting at more depolarized levels (e.g., ~−50 mV), it is likely that L-type Ca2+ channels, with nearly full availability in this potential range, provide the voltage-dependent Ca2+ entry mechanism that initiates Ca2+ release (see next section), activates Ano1, and coordinates activity in the SIP syncytium. Such a mechanism is apparent in the slow wave activity present in the internal anal sphincter [6], for example.
Fig. 1.9.
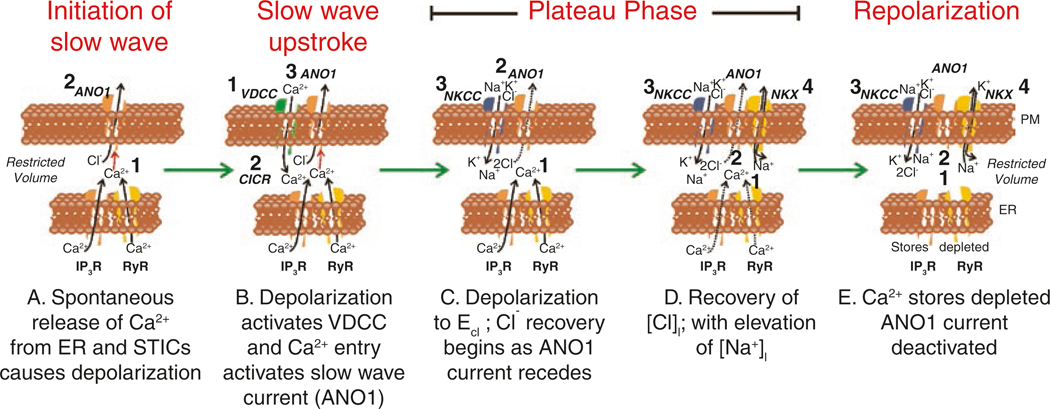
Proposed contributions of ion channels and transporters during the slow wave cycle corresponding to experimental evidence from the murine small intestine. Each panel represents the restricted volumes of nanodomains formed by close contacts between the plasma membrane (PM) and the endoplasmic reticulum (ER). Images are idealized membrane regions with ion channels and transporters that appear to be functional depicted in each snapshot through the slow wave cycle. Numbers in each panel show sequence of events. (A) Ca2+ release occurs spontaneously from ER through Ca2+ release channels (IP3R and RyR) (1). Due to the close apposition of the PM, Ca2+ transients activate Ano1 channels (2). Efflux of Cl− ions causes STICs. (B) Depolarization from STICs activates voltage-dependent Ca2+channels (T-Type) (1) initiating upstroke of the slow wave. Entry of Ca2+ into nanodomains initiates Ca2+-induced Ca2+ release (CICR; 2). Ca2+ release activates Ano1 channels in PM (3). (C) Asynchronous release of Ca2+ from stores (1) in different cellular locations (not shown) sustains activation of Ano1 channels causing membrane potential to linger near ECl and creating the plateau potential (2). Because membrane potential is near ECl there is little efflux of Cl− during the plateau (dotted arrow through Ano1 channel). However, loss of Cl− during the depolarization initiates recovery via NKCC1 (3). (D) As long as Ca2+ is sustained (1), Ano1 channels are activated (2) and membrane potential remains in the plateau phase. Recovery of Cl− proceeds and this is associated with influx of Na+, as NKCC1 uses the energy of the Na+ gradient to cause accumulation of [Cl−]i against its electrochemical gradient. Removal of excess Na+ is accomplished by the Na+K+ ATPase (NKX) (4). (E) When available Ca2+ stores are depleted (1), Ca2+ recovery by SERCA or extrusion by the plasmalemmal Ca2+ ATPase (PMCA) (pumps not shown) causes reduction in Ca2+ in nanodomains and deactivation of Ano1 channels (2). Recovery of gradients may extend into the period between slow waves via the actions of NKCC1 (3) and NKX (4)
1.6. Ca2+ Signaling in ICC
As described in the previous section, major ionic conductances expressed in ICC are either Ca2+ dependent (CaCCs) or result in Ca2+ entry (voltage-dependent Ca2+ channels). Pharmacological and gene knockout studies suggest that these conductances play a prominent role in the generation and propagation of pacemaker activity in ICC. Thus, Ca2+ handling mechanisms are of central importance to GI rhythmicity. This realization and the development of sensitive techniques to monitor intracellular Ca2+ signals prompted investigators to characterize the mechanisms involved in Ca2+ waves and transients in ICC. Ca2+ waves occur in networks of ICC, as observed through the use of membrane-permeable Ca2+ indicators [148–155]. These studies, conducted mainly on gastric and small intestinal muscles, revealed many important features of Ca2+ handling in ICC networks. Newer studies have utilized optogenetic sensors and added to our understanding of Ca2+ dynamics [156, 157].
Loading of mouse ileum with Fluo3-AM allowed visualization of Ca2+ transients in ICC and SMCs simultaneously [155]. SMCs displayed whole-cell Ca2+ events that rapidly shoot through the lengths of cells, and between the whole-cell events, localized Ca2+ transients were observed. Cyclopiazonic acid (CPA, 3–5 μM) or thapsigargin (1 μM) blocked the local responses, and no effect was observed with ryanodine (30 μM). ICC were also loaded with Fluo-3, and these cells were identified with methylene blue or c-Kit antibodies. A strong temporal relationship between Ca2+ events in SMCs and ICC was observed in only one-third of muscle preparations. In another third of the muscles, Ca2+ waves in ICC and SMC were not synchronized. It was concluded from this study that all ICC-MY may not be pacemakers, and they may have additional roles in intestinal motor activity.
ICC network behavior was further investigated in whole mount preparations of guinea pig stomach [149] and mouse jejunum [153] loaded with Fluo-4. Visualization of Ca2+ waves was facilitated in these studies by careful removal of longitudinal muscle fibers. Ca2+ waves at an average frequency of 4.9 cycles per minute spread through ICC networks in gastric muscles (verified by c-Kit immunolabeling) with an average velocity of 3.2 mm/s. Thus, the frequency and velocity of spread of Ca2+ waves matched the frequency [34] and propagation velocities of slow waves in the gastric antrum [77]. An interesting difference was that the anisotropic propagation of slow waves occurring in intact muscles was not observed in the propagation of Ca2+ waves in ICC networks. Thus, the order of magnitude greater slow wave propagation velocity in the circular vs. longitudinal axis in intact muscles must be a property of the interactions between ICC and SMCs. The Ca2+ waves exhibited either sharp upstrokes with somewhat slower return to baseline or events with more sustained, plateau-like maintenance of elevated fluorescence before return to baseline. The direction of propagation was variable, suggesting that the site from which primary pacemaker activity occurred shifted from event to event, as seen in electrical recording [51, 77]. Consistent with the idea that ICC are pacemaker cells, Ca2+ waves in ICC-MY preceded events in adjacent SMCs by about 50 ms. Tetrodotoxin (TTX) did not block Ca2+ waves in ICC-MY, but in some cases it reduced the frequency of Ca2+ waves, suggesting that nerves don’t initiate the Ca2+ waves but basal neural inputs have chronotropic influences on the generation of pacemaker activity. Nicardipine blocked Ca2+ waves in SMCs, but did not affect events in ICC-MY.
In the mouse jejunum loaded with Fluo4-AM, regular Ca2+ waves were observed in ICC-MY at the slow wave frequency, and a 1:1 relationship between slow waves and Ca2+ waves was observed directly by intracellular electrical recording from cells near the field of view [153]. This study also showed a lag between Ca2+ waves in ICC-MY and SMCs, supporting the idea that ICC are pacemaker cells. While most cells fired along a linear wave-front (high level of coherence) with each slow wave cycle, there were also periods in which ectopic pacemaker activity emerged and small clusters of cells escaped from the dominant pattern of propagation. The sequence of activation of ICC-MY within networks showed significant variability from event to event, and the rates at which ICC-MY activated during propagated events also varied. Electrical coupling is obviously important for the cell-to-cell spread of Ca2+ waves, as propagation was disrupted by the gap junction uncoupler 18β-glycyrrhetinic acid (β-GA). After treatment with β-GA, single or small clusters of ICC-MY persisted in generating Ca2+ waves, albeit at disparate frequencies. These observations suggest that individual ICC-MY are intrinsically active as pacemaker cells, but active propagation entrains slow waves into coherent waves of activation. The Ca2+ waves were blocked by mibefradil and upon depletion of intracellular Ca2+ stores with inhibitors of the sarcoplasmic reticulum Ca2+-ATPase (SERCA).
Loading of cells with Fluo-4 and imaging Ca2+ waves in ICC-MY were also performed on muscles of the human small intestine [151]. Biphasic Ca2+ waves (6 cycles per minute) were observed in jejunal ICC-MY, identified by c-Kit immunolabeling (Fig. 1.10). The Ca2+ waves corresponded to electrical slow waves, recorded simultaneously in some experiments, in both frequency and duration. The upstroke phase of Ca2+ waves was a discreet event, occurring at least 2 s before the peak of the plateau phase of the Ca2+ waves. The Ca2+ waves observed in human jejunum ICC-MY exhibited similar pharmacological responses as those observed in mice. The Ca2+ waves were blocked by CPA, disrupted by 2-APB and caffeine, and the frequencies and amplitudes were decreased or disrupted by Ni2+ or mibefradil. As in murine ICC-MY, treatment with β-GA also disrupted coherent propagation.
Fig. 1.10.
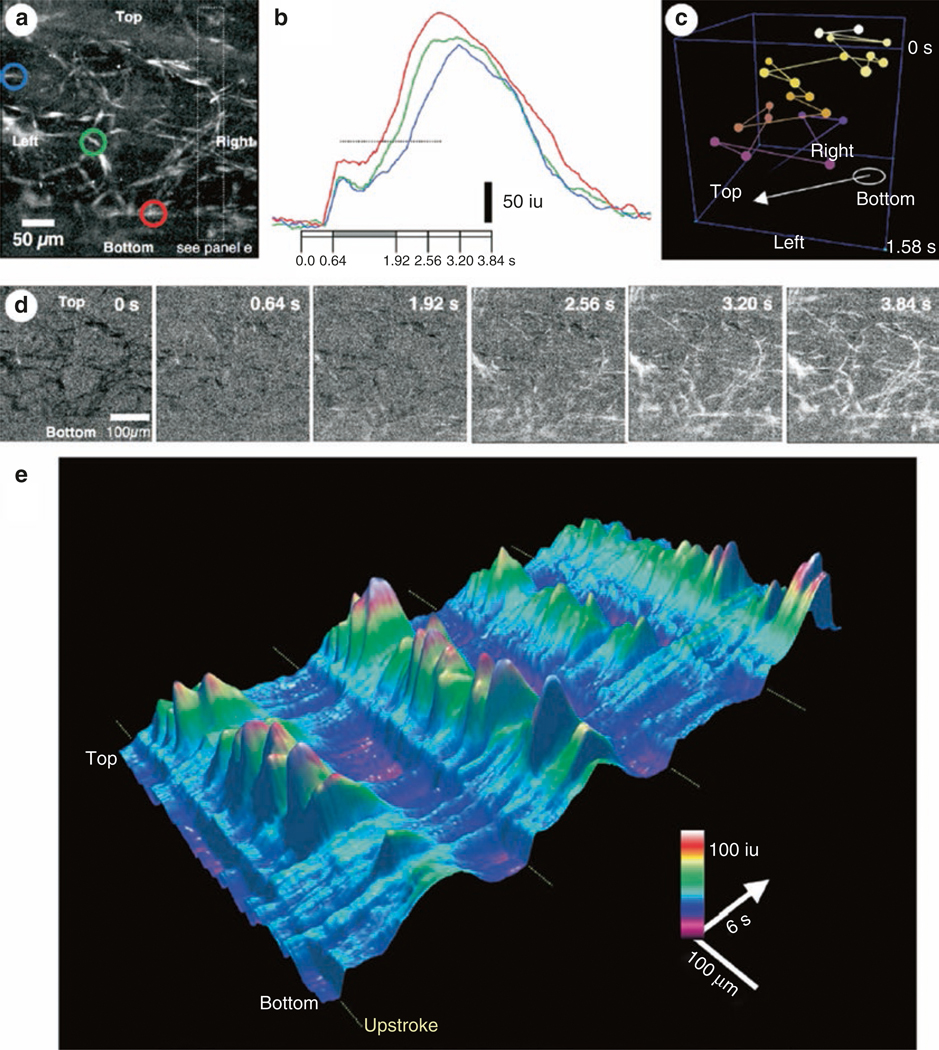
Propagation of Ca2+ waves in ICC-MY network in human jejunum. (a) Averaged Ca2+ waves show active ICC-MY. Colored circles represent regions (ROIs) of interest that have temporal changes in fluorescence plotted as colored traces in (b). Note the two phases of the Ca2+ transients and delay in second phase from the red to the blue ROI. (c) Shows an ST cube depicting the propagation of waves (white to mauve lines) from the bottom to the top of the field of view (FOV) in a (balls represent individual ICC-MY observed in a). Note that the Ca2+ traverses the FOV in a zigzag pattern. (d) Image sequence propagation of Ca2+ at different times during one slow wave cycle. The frames correspond to changes in fluorescence as a function of time in (b). The upstroke phase occurs at 0.64 s, and the plateau phase occurred between 1.92 and 3.84 s. (e) Shows a 3-dimensional ST map of 4 slow wave cycles in ICC-MY within the FOV shown in (a). The upstroke phase and plateau phase were constructed from 1 or 2 adjacent ICC-MY. The upstroke phase (small initial hump in fluorescence) occurs before each plateau (major peaks in fluorescence in which highest amplitude of fluorescence occurs during each cycle). Plateau phase in cells varies in rate-of-rise and amplitude from cycle to cycle across the FOV. Copied with permission from [150]
A contrasting view regarding the role of T-type Ca2+ current in Ca2+ waves in ICC-MY of the small intestine was reported in another study utilizing Fluo-4 AM loaded cells [158]. Mibefradil had no effect on these responses, but Ca2+ waves were reduced by the sodium-calcium exchange (NCX) inhibitor, KB-R7943. It was suggested that NCX might provide a mechanism for refilling Ca2+ stores. A significant decrease in Ca2+ waves was also observed with 2-APB or the phospholipase C inhibitor, U73122. Double immunolabeling showed that c-Kit+ cells express IP3 receptor 1 (IP3R1). These authors concluded that IP3 synthesis is ongoing in ICC-MY, and IP3 has a stimulatory effect on Ca2+ release, providing the clock mechanism responsible for slow wave generation and setting pacemaker frequency. Although voltage-dependent Ca2+ entry was discounted in this study, no explanation was provided to explain coordination and propagation of slow waves in ICC networks.
As described previously, the colon is more complex than small bowel and stomach in that there are two pacemaker zones with different frequencies. Slow waves are generated in ICC at the submucosal border of the circular muscle layer (ICC-SM) [61, 63, 134, 159]. Ca2+ waves were recorded from ICC-SM in the canine colon at the same frequency as slow waves [152]. These waves were highly coherent, propagating through the ICC network as seen in small bowel and stomach. Another pacemaker frequency was found in ICC-MY, that generated Ca2+ waves at the frequency of the electrical activity known as myenteric potential oscillations (MPOs) [60, 152]. In contrast to ICC-SM, the ICC-MY Ca2+ waves were unsynchronized, demonstrating characteristic differences in Ca2+ handling mechanisms or electrical coupling in these two populations of colonic ICC. ICC-MY in mouse colons were synchronized by neural inputs, and this response was blocked by atropine, suggesting innervation of these cells by cholinergic motor neurons [160]. Spontaneous or evoked colonic migrating motor complexes (CMMC) increased the frequency and amplitude of Ca2+ waves in ICC-MY. N(ω)-nitro-l-arginine and MRS2500, a P2Y1 antagonist, increased the Ca2+ transients ICC-MY and appeared to increase coupling between cells. Sodium nitroprusside inhibited Ca2+ waves in ICC-MY. These data show that ICC-MY are under tonic inhibition from inhibitory motor neurons. Inhibitory neural inputs inhibit release of Ca2+ and possibly reduce coupling between ICC-MY. Excitatory neural inputs activate Ca2+ waves and synchronization between cells and aid in coordination of contractions of circular and longitudinal muscles during the CMMC.
Human and dog GI muscles are far thicker than laboratory rodents, and therefore simple conduction of slow waves into SMCs is unlikely to activate the full thickness of the muscle layers because the amplitude of slow wave decays to nothing within a few mm without active propagation [72]. A mechanism of active electrical propagation into the thickness of the circular muscle layer was noted in canine colon, utilizing the ICC in septa between muscle bundles (ICC-SEP). ICC-SEP are active spontaneously when ICC-SM are removed [66]. The question of active propagation into the circular muscle was further investigated using imaging to monitor Ca2+ wave propagation into the thick circular muscle layer of canine jejunum, which also has a population of ICC-SEP [151]. ICC-MY and ICC-SEP form a three-dimensional network in which Ca2+ waves, and presumably slow waves, propagate from ICC-MY to ICC-SEP deep into the circular muscle layer. Thus, ICC-MY appear to be electrically coupled to ICC-SEP, based on the propagation of Ca2+ waves; however, gap junctions between ICC-MY and ICC-SEP have not been demonstrated definitively.
Fluo4-AM loading and whole tissue imaging was also used to evaluate the role of Ano1 channels in Ca2+ transients and waves [148]. Ano1−/− mice display an absence of slow waves in the small intestine and stomach [111]. These mice were utilized to evaluate whether Ca2+ transients could organize and propagate as coherent waves in the absence of Ano1 [148]. Potential developmental defects that might occur in constitutive knockout mice were controlled for by also testing the pharmacological blockade of Ano1 channels or acute deactivation via shRNA treatments of organotypic cultures. Synchronicity of Ca2+ transients was significantly depressed in Ano1−/− mice, and this behavior was also observed after treatment of wild-type muscles with Ano1 channel antagonists. Ano1 antagonists had no effect on Ca2+ transients in Ano1−/− tissues. Pretreatments with shRNA reduced Ano1 by 75%, and the synchronicity of Ca2+ transients and presence of slow waves (i.e., slow waves were absent in 80% of cells impaled) were reduced to a similar extent. Morphological examination showed that ICC networks were intact and appeared normal in shRNA-treated and Ano1−/− muscles. The reductions in Ano1 expression, coherent Ca2+ waves in ICC-MY networks, and slow wave activity were accompanied by weakened contractile activity with abnormal amplitudes and frequencies in Ano1−/− muscles.
Studies using membrane-permeable Ca2+ indicators have clearly demonstrated a link between Ca2+ release and Ca2+ entry events in ICC and pacemaker activity. These experiments, however, have limits and lack resolution due to only moderate signal-to-noise ratio, contaminating signals from other cells also loaded with dye, and relatively rapid photobleaching, especially with higher power imaging requiring stronger illumination. More recently, optogenetic sensors have been used, making it possible to selectively image Ca2+ transients in ICC by utilization of Cre-loxP technology. The bright, high signal-to-noise images produced with this technique allows dynamic resolution of subcellular Ca2+ transients and greater fluorophore longevity during sustained illumination. Cellular Ca2+ dynamics can also be resolved in ICC in situ at high magnification and at relatively high frame rates (e.g., 100 frames per second). Use of this technique, coupled with imaging by confocal microscopy, allows visualization of specific types of ICC because these cells are arranged in a fairly planar fashion within the tunica muscularis (e.g., ICC-MY of all organs, ICC-DMP in the small intestine, and ICC-SS and ICC-SM in the colon). GCaMPs are foreign proteins and Ca2+ buffers, but mice expressing GCaMP3 in ICC appeared to have normal slow waves and responses to neural inputs [156].
Optogenetic techniques have been used to study cellular mechanisms in the intramuscular class of ICC in the small intestine (ICC-DMP) which are located near the submucosal surface of the circular muscle layer [156]. ICC-DMP were investigated because they lack a voltage-dependent mechanism that allows development of slow waves but still manifest localized Ca2+ transients and activation of Ano1 channels to generate STICs. Under control conditions, ICCDMP fire Ca2+ transients from multiple locations, and these events are independent and stochastic in nature. There was little correlation between events at different sites within single cells or within adjacent cells, suggesting the lack of a voltage-dependent mechanism insulates the activity of these cells from events occurring in adjacent cells. There are neural inputs to ICC-DMP, with tonic inhibition of Ca2+ transients resulting from nitrergic input [156]. Intracellular Ca2+ stores were judged to be the source of Ca2+ in the spontaneous Ca2+ transients in ICC-DMP because: (i) exposing muscles to Ca2+ free solution for many minutes did not significantly affect the amplitude, duration, or spatial spread of Ca2+ transients, and (ii) Ca2+ transients were reduced or blocked by CPA, thapsigargin, ryanodine, and xestospongin C [156].
Ca2+ handling in ICC-MY has also been investigated in muscles with cell-specific expression of GCaMP3. Instead of Ca2+ waves spreading smoothly through ICC networks, as reported in previous studies using membrane-permeable Ca2+ indicators, higher resolution allowed observation of temporal clusters of discrete and localized Ca2+ transients in each cell as Ca2+ waves traversed ICC-MY networks [157]. Simultaneous recording of slow waves within fields of view showed that Ca2+ transients are clustered temporally within each slow wave cycle, and the clusters of Ca2+ transients developed shortly (~175 ms) after each slow wave upstroke depolarization (Fig. 1.11). Firing of Ca2+ transients persisted during each cluster for approximately the duration of the plateau phase of the slow waves. Multiple unique Ca2+ release sites were detected in ICC-MY, and local events did not spread to become global increases in [Ca2+]cyt. Activity in cell soma and processes was distinguished and analyzed by use of masking techniques and found to be equivalent in both regions of ICC-MY. Although the basic organization of the Ca2+ transient clusters (CTCs) was rhythmic and persistent in all recordings, Ca2+ transients at each site of initiation appeared to be stochastic in nature and characterized by irregular firing from CTC-to-CTC, occasional multiple transients firing from what appeared to be the same site during a single CTC, and transients of varying durations and spatial spread. CTCs were dependent upon Ca2+ entry and were abolished when [Ca2+]o was reduced below 0.5 mM or when cells were treated with T-type Ca2+ channel antagonists (NNC 55–0396 and TTA-A2; [161, 162]). CTCs were resistant to either 1 μM nicardipine or isradipine (antagonists of L-type channels encoded by Cacna1c and Cacna1d). This study led to the conclusion that CTCs are due to Ca2+ release from internal stores, and organization of Ca2+ release into CTCs is initiated by Ca2+ entry through T-type Ca2+ channels and initiation of Ca2+-induced Ca2+ release (CICR). The specific mechanisms of CICR are discussed in the section below. It is still a question whether the longer durations of slow waves observed in other regions of the GI tract or in other species (see Fig. 1.1) are due to longer duration CTCs in pacemaker ICC in these muscles. This level of resolution of Ca2+ transients underlying GI pacemaker activity might become an assay for disease-dependent changes in ICC that develop and compromise the normal functions of these cells. Changes in basic Ca2+ signaling may affect motility behaviors long before disruption of ICC networks occur, but future studies will be required to test this hypothesis.
Fig. 1.11.
Ca2+ clusters in ICC-MY during slow wave activity. (a) Slow waves and Ca2+ waves in ICC-MY networks recorded simultaneously from jejunum muscle from a mouse expressing GCaMP3 exclusively in ICC. Imaging performed with a confocal microscope to restrict view to ICC-MY. Note the one-to-one relationship between the slow waves and Ca2+ transients. If fact the waves were not smooth changes in global Ca2+ observed in lower resolution monitoring. When higher resolution was used, facilitated by the superior performance of GCaMP3 as a sensor and low background from restricting GCaMP3 expression to ICC, Ca2+ waves were seen to be composed of many localized Ca2+ transients occurring in different sites within cell soma and processes. Each firing site was assigned a color and these are plotted in the occurrence map (third panel in a). Mapping Ca2+ transients in this manner shows that the transients are temporally clustered together at the frequency of the slow waves. (b) Increased sweep speed of simultaneous Ca2+ transient and slow wave outlined by gray box in (a). The occurrence map from this slow wave cycle is shown beneath the traces. Each Ca2+ firing site in the FOV was assigned a different color, and this analysis shows that Ca2+ transients occurred with different delays after the initiation of the slow wave upstroke, the durations of Ca2+ transients was highly variable, and multiple events occurred from the same site on occasion. Note that the Ca2+ clusters begin with a slight delay after the upstroke of the slow wave, and all occur within the duration of the slow wave. These observations suggest that the duration of the slow wave plateau depolarization is determined by the spread in time of Ca2+ transients, as the open probability of Ano1 channels (causing depolarization) will continue to be elevated as long as Ca2+ release events persist. (c) Shows ICC-MY network and site of recording within the FOV and within a few μM from the cells being imaged. Copied with permission from [157]
1.6.1. Role of Ca2+ Stores in Pacemaker Activity
The role of Ca2+ stores in pacemaker activity is presented in a separate section because it is of such fundamental importance in GI pacemaker activity and responses to enteric motor neurotransmitters. As described in the section above, electrical rhythmicity is linked mechanistically to the Ca2+ waves arising from and propagating through pacemaker cells, and the frequency of these events may be determined by the rates at which Ca2+ stores release and are refilled. Ca2+ release from stores is accomplished by two types of ER Ca2+ channels, IP3 receptors (IP3Rs) or ryanodine receptors (RyRs). Descriptions of a few representative studies on different types of preparations are provided to illustrate major features of Ca2+ release and the types of Ca2+ release channels expressed and utilized in ICC pacemaker activity. A seminal study, performed on murine gastric muscles investigated global knockouts of Itpr1, showed that slow waves are independent of L-type Ca2+ channels in wildtype mice, and slow waves are absent in Itpr1−/− mice (Fig. 1.12) [163]. Responses to nitrergic and cholinergic neural inputs were also compromised in these mice. These results demonstrate that IP3 receptors play a fundamental role in the functions of ICC, and this conclusion has been supported by many studies of intact muscles. In pyloric smooth muscle, for example, slow waves were blocked by CPA, caffeine, and heparin loading [164]. However, no role for ryanodine-sensitive receptors was observed, as 10–20 μM ryanodine failed to inhibit slow waves. A combination of low Ca2+ and Cd2+ also failed to inhibit slow wave activity, and these authors concluded that Ca2+ entry was not involved in entrainment of slow waves or electrical rhythmicity. Slow waves can be initiated by depolarization, or in some cases by anode break (i.e., at termination of a hyperpolarization). Slow waves initiated in pyloric muscle in response to depolarization or anode break were also blocked by heparin loading but not by ryanodine. It was concluded that Ca2+ release from the ER via IP3 receptors is fundamental to electrical rhythmicity and proposed that responses to depolarization are due to voltage-dependent enhancement in IP3 synthesis or responsiveness of IP3R, as observed in SMCs [165]. This idea has also been proposed by other authors [31, 166], but no verification of voltage-dependent IP3 production or voltage-dependent sensitization of IP3R in ICC has been provided to date.
Fig. 1.12.
Loss of slow waves in Itpr1−/− mice. (a) Slow wave activity recorded by intracellular microelectrode from murine antral smooth muscle from a mouse 20 days after birth. (b) Slow waves are decreased in duration and amplitude by nifedipine. (c) Electrical activity recorded from mouse antrum from a 19-day-old Itpr1−/− mouse. Slow waves are absent and activity is spike-like complexes occurring without a constant frequency. (d) Electrical activity is abolished by nifedipine. Redrawn with permission from [163]
Another study of intact muscles characterized the effects of several Ca2+ store-active drugs on electrical rhythmicity in the murine small intestine [167]. CPA (1–3 μM) depolarized muscles and decreased slow wave frequency by 46%, but when compared with elevated [K+]o, which also caused depolarization and decreased slow wave frequency, the effects of CPA were significantly greater and could not be explained by depolarization alone. Thapsigargin (1 μM), another SERCA pump inhibitor, blocked slow waves after relatively long periods of exposure. Similar effects were produced by xestospongin C, an inhibitor of IP3R, caffeine and inhibitors of phospholipase C (U-73122, neomycin, and 2-nitro-4carboxyphenyl-N,N-diphenylcarbamate).
In contrast ryanodine (50 μM) depolarized muscles and caused minor effects on slow waves that were judged to be the result of the depolarization. Ca2+ stores are also important in slow wave propagation, as investigated with partitioned chambers, where slow waves could be reliably induced in a chamber perfused with normal Krebs solution and effects of Ca2+ store-active drugs could be evaluated in another chamber. Slow wave propagation was blocked by 2-APB in canine colonic muscles, and CPA greatly inhibited the plateau potentials of propagated slow waves in the canine gastric antrum [78, 80]. These studies all concluded that Ca2+ release from stores occurs via IP3R, and ryanodine receptors are not a significant factor in slow wave generation and propagation.
IP3Rs and RyRs are broadly expressed in cells of the SIP syncytium, making studies on intact GI muscles complicated because drugs blocking these receptors might have a multiplicity of effects. Attempts to avoid these problems have utilized cultured ICC, in which phenotypic changes might also be a confounding factor. Nevertheless, rhythmic inward currents were blocked by xestospongin C, thapsigargin, and heparin dialysis of cells [168]. Another study of this type needs to be discussed because of the uniqueness of its conclusion that ryanodine receptors are involved and fundamental to Ca2+ signaling in ICC. Cell cluster preparations were developed from partially digested murine gastric muscles containing circular and longitudinal muscles and myenteric plexus [169]. ICC were present in the cell clusters and identified by c-Kit labeling. After 2–4 days in culture, the clusters were loaded with Fluo-3 and Ca2+ oscillations were monitored. Nifedipine blocked Ca2+ transients in SMCs, and under these conditions remaining Ca2+ transients were viewed to be oscillations in pacemaker ICC. RyR subtype 3 was found to be expressed in c-Kit+ cells, and FKBP12 and FKBP12.6 (modulators of the Ca2+ conductance of ryanodine receptors) were expressed in the cell clusters. Recent focused expression studies and transcriptome analysis show that RyR1–3 receptors are expressed in small intestinal ICC, and the predominant paralog is RyR2 [156]. Fkbp1a is expressed in all of the SIP cells, not just SMCs [117]. Both ryanodine and FK506, an immunosuppressant drug, inhibited Ca2+ oscillations in c-Kit+ cells in cell clusters [169]. These were the first experiments identifying a potential role for RyRs in the generation of rhythmic activity. More recently, the role of Ca2+ stores in generating Ca2+ transients was investigated by imaging ICC of the murine small intestine in situ [156, 157]. These studies showed that Ca2+ transients in ICC-DMP were blocked by CPA and thapsigargin and by ryanodine (100 μM). Xestospongin C (10 μM) also blocked Ca2+ transients in ICC-DMP. Different effects were noted in pacemaker ICC-MY, where ryanodine (100 μM) blocked Ca2+ transients completely, but these events were far less sensitive to xestospongin C. In both types of ICC, interplay or amplification of Ca2+ release events appears to occur between IP3Rs and RyRs. The sequence of activation (i.e., RyR releasing Ca2+ and being amplified by release from IP3R or in reverse) is unknown at present, but in small intestinal ICC-MY the dominant release channels appear to be RyRs. Finding that Ca2+ release can be organized into CTCs (Fig. 1.11) and this depends upon Ca2+ entry via T-type Ca2+ channels suggests that CICR may be a fundamental factor in linking the voltage-dependent mechanism of propagation to regeneration of slow waves, as depicted in Fig. 1.9.
While not assaying Ca2+ release events directly, another study monitored activation of CaCCs and development of STICs and slow wave currents in isolated murine small intestinal ICC [170]. STICs and slow wave currents activated by depolarization were inhibited significantly by CPA and thapsigargin, showing the need for functional Ca2+ stores to generate these basic ICC behaviors. Tetracaine (also an inhibitor of RyRs) blocked STICs and reduced slow wave currents, and ryanodine at 50 μM had an initial small increase in STICs, but with a longer exposure inhibited these events. The same was true for slow wave currents when exposure time was increased to several minutes. Xestospongin C also had inhibitory effects on STICs and slow wave currents. The possibility that some of the store-active drugs might have nonselective effects and inhibit Ano1 was also tested. Ryanodine, CPA, and thapsigargin all had little or no effect on Ano1 directly.
The representative studies above suggest there is diversity in the Ca2+ release apparatus in the ICC found in different anatomical niches within a given GI muscle, and diversity between organs also occurs. More studies will be needed to verify this idea more broadly and to better understand how this diversity facilitates specific functions, determines the frequency of Ca2+ release events, or shapes the waveforms of electrical responses. There are also important structural questions about the relationship between the plasma membrane and Ca2+ release sites and channels, and these will be addressed in the section below on nanodomains in ICC.
1.6.2. Role of Mitochondria in Pacemaker Activity
A typical observation in ultrastructural studies of ICC is that these cells display an abundance of mitochondria, particularly in the perinuclear spaces [64, 171, 172]. Studies of cultured ICC and intact GI muscles have suggested a role for mitochondria in the Ca2+ handling required for pacemaker activity and propagation of slow waves. Several drugs used commonly to block key mitochondrial ion channels and transporters and to deactivate the electron transport chain reduce electrical rhythmicity in cultured ICC and slow waves in intact GI muscles of several species [168]. Oligomycin, a compound that blocks synthesis of ATP, was not effective in blocking slow waves either in the cultured cells or in intact muscles. Thus, compromising the metabolic role of mitochondria did not seem to be responsible for the effects of mitochondrial drugs on pacemaker activity. Many of the same compounds have been shown to inhibit slow waves in strips of muscles from several species [62, 166, 173], propagation of slow waves [78, 79], spread of Ca2+ waves in ICC networks [151, 153], and spontaneous transient depolarizations (aka unitary potentials; [19, 174]). Mitochondrial involvement was thought to involve uptake or regulation of Ca2+ release during the slow wave cycle [168], and a biophysically based model was developed, incorporating mitochondrial Ca2+ uptake into a slow wave mechanism [175]. However, more recent experiments have suggested more direct Ca2+ regulation of the pacemaker mechanism in ICC (i.e., via activation of Ano1 CaCCs), so the role of mitochondria in the pacemaker mechanism and the effects of mitochondrial agents on slow waves were revisited. All of the mitochondrial agents used in prior studies of slow wave generation and propagation reduced or blocked Ca2+ transients in ICC-MY, but had far less effect on Ca2+ transients in ICC-DMP [176]. The effects of mitochondrial drugs share similarities with the effects of T-type channel and Ano1 antagonists on Ca2+ transients, and mitochondrial drugs were found to be effective blockers of CaV3.2 and/or Ano1 channels expressed in HEK293 cells. Thus, the role of mitochondria in the pacemaker activity of ICC is questionable at present, as most of the effects noted in past studies can be explained by the nonspecific effects on key ion channels involved in pacemaker activity.
1.6.3. Nanodomains as the Basis for Functional Pacemaker Units in ICC
We have proposed that signaling in microdomains (aka pacemaker units, but the current preferred terminology is nanodomains due to size and volume of these junctions) is important for the pacemaker mechanism because the concentrations of key ions involved in transmembrane currents and transport are far more likely to reach effective concentrations in these small volumes than in the cytoplasm. A striking finding is that Ano1 channels, which are activated tonically in HEK293 cells bearing homologous expression of Ano1 splice variants when cells are dialyzed with elevated internal Ca2+ (100–500 nM), depending upon splice variant [121–123, 126–128], are not activated by cytosolic [Ca2+]i of 1 μM in ICC [170]. This observation suggests that Ano1 channels are clustered into areas of plasma membrane that are serviced by Ca2+ released from stores, but protected from changes in [Ca2+]cyt. Clustering of Ano1 channels also favors generation of STICs that occur through the opening of many channels in response to localized Ca2+ release. Clustered Ano1 channels are likely to occur in nanodomains, but questions remain whether other key proteins involved in pacemaker activity (e.g., CaV3.2, NKCC1, IP3R1, RYR, the sodium pump and possibly Na+Ca2+ exchange proteins) are also co-localized in these nanodomains. The high probability for Ca2+ release events to occur repetitively from specific sites in the cell soma and processes [157] favors the idea that specialized ER domains, containing elevated numbers of Ca2+ release channels, are present. Depolarization of network ICC leads to activation of T-type Ca2+ channels in ICC which appears to be the voltage-dependent mechanism allowing active propagation of slow waves through the network [2]. As T-type Ca2+ channels are activated, Ca2+ entry occurs, but the relatively low current density due to T-type Ca2+ channels in ICC [137] suggests that Ca2+ entry is focused into nanodomains where a rise in [Ca2+] is effective in activating CICR and CaCCs. A schematic showing the key proteins and their arrangement within nanodomains to facilitate CICR in ICC is shown in Fig. 1.9.
1.7. Dynamic Modulation of Intrinsic Electrical Rhythmicity
Until now, we have concentrated on spontaneous slow wave generation in ICC and propagation in ICC networks in GI muscles, but these cells do not operate in isolation, and many factors, too numerous to describe comprehensively in this chapter, influence slow waves and thus motility patterns in the gut. Transcriptome data indicate that ICC express myriad receptors for neurotransmitters, hormones, paracrine substances, and inflammatory mediators, and they also express effector proteins that facilitate reactions to a variety of physical factors such as stretch, compression (as might occur in muscle contraction), temperature, and pH [117]. ICC may also be secretory cells, and, as an example of this idea, they express a reactome consistent with production of PGE2 or PGI2 (e.g., Pla2g4a, Ptgs1, Ptges3, Ptgis). It should also be understood that due to the electrical coupling between SMCs, ICC, and PDGFRα+ cells (SIP syncytium), electrophysiological responses of any SIP cell can potentially affect the functions of ICC. For example, neurotransmitters or hormones linked to enhanced production of cAMP and activation of protein kinase A in SMCs tend to activate KATP channels and cause hyperpolarization of SMCs [46, 177–179]. Hyperpolarization of SMCs conducts to ICC and can influence voltage-dependent mechanisms in these cells (e.g., Ca2+ entry mechanisms and slow wave propagation). Similarly, P2Y1 receptor agonists (e.g., purine neurotransmitters) cause activation of small-conductance Ca2+-activated K+ channels in PDGFRα+ cells, causing profound hyperpolarization of these cells and other SIP cells [22, 49, 180]. Integration of inputs to the SIP syncytium are illustrated in Fig. 1.13.
Fig. 1.13.
Schematic showing integration of inputs in the SIP syncytium. Schematic is developed from experimental evidence obtained from murine small intestine. SIP syncytium consists of SMCs, PDGFRα+ cells, and ICC (in small intestine the ICC are ICC-MY and ICC-IM; see Table 1.1 for additional details). ICC-MY are pacemaker cells and generate electrical slow waves by processes described in detail in Fig. 1.8 and depicted here as Ca2+ release from ER and activation of Ano1 channels in plasma membrane. Slow wave conduct through gap junctions (GJ) and depolarize SMCs. Depolarization activates Ca2+ entry through L-type Ca2+ channels and initiates cross bridge cycling of contractile elements (CE). Slow waves are omnipresent, but their impact on SMC contraction is modulated by inputs from enteric excitatory (EEN) and inhibitory (EIN) neurons that are intermingled and closely associated with PDGFRα+ cells and ICC-IM. EENs release ACh and tachykinins (substance P and possibly neurokinin A). These neurotransmitters bind to muscarinic type 3 (M3) receptors and neurokinin 1 (NK1) receptors expressed by ICC-IM. Both receptors couple through Gq/11 and activate phospholipase Cβ (PLCβ) and production of IP3 and diacylglycerol (not shown). IP3 binds to IP3Rs in the ER (not shown) and causes intense release of Ca2+ (depicted as multiple black arrows from ER) and activation of Ano1 channels in the plasma membrane. This is a depolarizing response that conducts to SMCs via GJs and summates with the slow waves that are ongoing in the syncytium. EINs release NO, purines (β-NAD), and peptides (depicted here as VIP but also likely to include PACAP). NO binds to soluble guanylyl cyclase (GCα/GCβ dimer) in ICC, SMCs, and possibly in PDGFRα+ cells (not shown) that also express these subunits but no response has been documented. GCα/GCβ dimers in ICC generate cGMP and activate protein kinase G (PKG) or inhibit phosphodiesterases (PDE). The mechanisms coupled to these effectors are not entirely understood. But PKG is linked to reduction in Ca2+ release from ER (dotted arrow), and this decreases or halts activation of Ano1 currents (X). This is a hyperpolarizing trend that can also conduct to SMCs via GJs. In SMCs GCα/GCβ dimers can also be activated by NO, causing reduced Ca2+ sensitization of the CE and reduction in the force of contraction. β-NAD (and possibly other purines) is/are released from EINs and bind to P2Y1 receptors on PDGFRα+ cells. This activates PLCβ, generates IP3, and causes Ca2+ release from ER. Ca2+ release activates small-conductance Ca2+-activated K+ channels in PDGFRα+ cells. This pathway generates strong hyperpolarization that conducts to SMCs via GJ. Hyperpolarization of SMCs tends to reduce the open probability of L-type Ca2+ channels, restricting Ca2+ entry and reducing the force of contractions. Functions and responses of SIP cells to inhibitory peptides are not yet clarified
Neural inputs have chronotropic effects on gastric slow waves [54] and affect coupling between slow waves and generation of action potentials by SMCs in other regions of the GI tract [99, 181]. Studies of W/WV mutants, in which ICC fail to develop in some regions of the GI tract [3, 5, 108], show that cholinergic and nitrergic neural inputs to gastric muscles are reduced in the absence of the intramuscular class of ICC [182, 183]. The extent and significance of ICC in enteric motor neurotransmission has been hotly debated in the literature [184–187], but it seems beyond debate at this point that ICC are innervated. Therefore, post-junctional responses that alter membrane conductances in ICC will influence the integrated motor output of the SIP syncytium.
The discussion above describes how Ca2+ transients are fundamental to ICC behavior. Recent studies have investigated how neural inputs influence Ca2+ transients in ICC-DMP in the murine small intestine. ICC-DMP represent the intramuscular class of ICC in the small intestine and varicosities of motor neurons are closely associated with these cells and form synaptic-like connections of less than 20 nM between nerves and ICC [106, 188, 189]. Receptors for major enteric motor neurotransmitters are expressed by ICC-DMP [118, 190, 191], and the cells bind neurotransmitters, undergo receptor internalization, and translocate signaling molecules in response to nerve stimulation [192, 193]. Muscles expressing optogenetic Ca2+ sensors in ICC showed that stimulation of enteric motor neurons by electrical field stimulation (EFS) causes initial inhibition of Ca2+ transients followed by intense activation that persists well after termination of the stimulus [194]. Atropine decreased the frequency of Ca2+ transients somewhat, but a larger decrease was caused by RP 67580 or SR 140333, both NK1 receptor antagonists. The brief inhibitory period at the initiation of EFS was due to nitrergic neural regulation [195].
SMCs express nonselective cation channels that respond to muscarinic stimulation [196–198]. Ano1 channels, which are not expressed by GI SMCs, mediate muscarinic responses in ICC [23]. To address the controversy about the role of ICC in excitatory neurotransmission experiments were performed on Ano1−/− mice. When inhibitory responses are blocked, EFS of gastric muscles elicits EJPs in post-junctional cells. EJPs are absent in mice lacking Ano1, and contractile responses are also diminished. Pharmacological block of Ano1 channels also reduced EJPs and contractile responses. Inhibiting acetylcholinesterase caused development of a slow depolarization that followed the normal fast EJP. This event was retained in the Ano1−/− mice and was likely due to overflow of neurotransmitter to extrajunctional muscarinic receptors on SMCs. These experiments demonstrate primary innervation and transduction of cholinergic responses by ICC, and the results are consistent with previous evaluations of cholinergic innervation of ICC-IM using a variety of experimental tests and approaches [182, 199].
A study using a membrane-permeable Ca2+ indicator reported that the oscillatory activities of ICC-MY and ICC-DMP in the small intestine undergo phase-amplitude coupling [154], and the output of this coupling generates a slow wave pattern known as waxing and waning [200, 201]. ICC-MY and ICC-DMP are distinct populations of cells in the murine small intestine and separated by most of the thickness of the circular muscle layer. The only means of coupling between these cells seems to be via electrical coupling through SMCs. Thus, electrical events in one type of ICC might “couple” or interact with the electrical events in the other population of cells. Such an interaction seems unlikely, however, because the electrical events generated by ICC-DMP are STICs [202] due to activation of Ano1 CaCCs and generated by transient bursts of Ca2+ release from intracellular stores [156]. Neither the Ca2+ transients nor the STICs [170] are oscillatory in nature. These events occur in a stochastic manner with little or no correlation in the timing between events in the same cell or between the events in adjacent cells [156]. Recordings of Ca2+ transients for several minutes in adjacent ICC-DMP in full thickness small intestinal sheets of muscle (in which ICC-MY are present and slow waves are omnipresent) revealed no cyclical influences on Ca2+ transients in ICC-DMP. High resolution observations of ICC-DMP showed that Ca2+ transients in ICC-DMP are not regulated by membrane potential. Depolarizations of the SIP syncytium occurring through slow wave activity exert no apparent influence on the stochastic nature of Ca2+ release events in ICC-DMP [156]. Hyperpolarization of tissues upon the opening of K+ channels in other SIP cells also has no influence on Ca2+ transients in ICC-DMP. Lack of voltage-dependent regulation is likely to result from the fact that ICC-DMP do not express functional voltage-dependent Ca2+ channels [118, 170].
As discussed previously in this chapter, Ca2+ entry facilitates coordinated release of Ca2+, due to Ca2+-induced Ca2+ release in ICC-MY, but lacking such a mechanism in ICC-DMP frees these cells to fire Ca2+ transients independently. This is an important characteristic of intramuscular ICC that are innervated by enteric motor neurons, express receptors and post-receptor effector mechanisms. As discussed previously, neurotransmitters released from excitatory or inhibitory motor neurons enhance or suppress Ca2+ transients, respectively. If Ca2+ transients were regulated by voltage, then their responses to neural inputs may be unpredictable due to net hyperpolarizing or depolarizing influences that might originate elsewhere in the SIP syncytium. For example, if depolarization enhanced Ca2+ transients, then periods of depolarization (peaks of slow waves or responses to paracrine stimuli, etc.) would tend to predetermine neural responses. If hyperpolarization shut down Ca2+ release, then such a trend could render inhibitory neurotransmission ineffective and perhaps limit excitatory responses. Being unaffected by membrane potential allows neural responses of ICC-DMP to be independent, and therefore neural influences can be superimposed upon (i.e., summate with) the intrinsic activities of the SIP syncytium. In normal GI organs enteric motor neurons determine motility patterns, so it is important that their commands not be circumvented by the intrinsic behaviors of SIP cells.
Stretch is another factor that affects electrical rhythmicity; however, there is less known about this stimulus than regulation by nerves and hormones. Stretch of tissues making up the walls of hollow organs is inevitable as the organs are filled or as boluses of luminal contents are shifted from one segment to another. In one study murine gastric antral muscles were stretched by a computer-driven apparatus that allowed precise selection of ramp speed and maximum tension [203]. Stretch of muscles caused rate-dependent changes in membrane potential and slow wave frequency. Responses to stretch were mediated by ICC-IM, as the responses did not occur in W/WV mice that have reduced numbers of ICC-IM in the stomach. Indomethacin treatment also blocked the responses to stretch, and the responses were absent in Ptgs2−/− mice. These findings suggest that stretch-dependent activation of prostaglandin synthesis may be a chronotropic mechanism affecting pacemaker activity in the gastric antrum.
1.8. Areas in Need of Future Investigation
There are currently many shortcomings in our understanding of electrical rhythmicity in GI muscles. A goal of biomedical research is to understand human physiology and pathophysiology such that pharmaceuticals appropriate for the treatment of human disorders can be designed. Few treatments for common GI motility disorders exist, and this may be because understanding of human disease is based on animal models that do not translate to human physiology and pathophysiology with fidelity. Comprehensive study of electrical rhythmicity in human GI muscles is lacking, and it was surprising, for example, to learn recently that the frequency of electrical slow waves in the stomach may be higher than the literature has reported confidently for several decades [56]. Studies of human gastric muscles in vitro revealed many discrepancies from textbook descriptions of human gastric electrophysiology, including the presence of slow wave activity in the gastric fundus, a slow wave frequency in antral muscles about twice that reported from surface electrode recording, and no clear gradient in slow wave frequency from proximal to distal stomach. A more definitive understanding of human electrical rhythmicity is needed to understand what actually drives changes in slow wave activity, gastric contractions, and gastric emptying in motility disorders, such as gastroparesis. As mentioned previously, most recent work investigating the mechanisms of pacemaker activity have been performed on mice, and it is unclear whether these mechanisms are common to other species and humans. Better means of identifying ICC in mixed cell populations after tissue dispersion are needed to facilitate comparative physiological and molecular studies of these cells in isolation of the other cells in whole muscles. Work toward understanding the molecular phenotypes of ICC has started [117, 118], and the data from these studies suggest a broader role for ICC than previously suspected, including responses to hormones and paracrine substances and possibility a role as secretory cells. Transcriptomic data may also provide insights on how to recover the phenotype of ICC when cells are lost or damaged in disease. It would be valuable to develop a cell culture or organoid model of ICC that could be used for high-throughput screening of inflammatory mediators and/or drugs. Some progress has been made in this regard [169, 204], but it will be necessary to optimize conditions to maximize retention of the native phenotype in these in vitro preparations. Of many challenges remaining in the study of ICC, the largest are to understand what happens to ICC in a variety of human motility disorders and develop the means, possibly through manipulation of tissue stem cells or reseeding of progenitor cells [205], to reestablish functional networks of cells. Motility disorders involving loss or damage to ICC networks will be very difficult to treat effectively until these challenges are met.
Acknowledgments
The author is grateful for the editorial assistance of Dr. Bernard Drumm, whose careful reading of the manuscript and many suggestions improved the text and figures. I would also like to acknowledge my long-term collaborations with Profs. Sean Ward and Sang Don Koh for countless discussions, contributions, and data without which my knowledge and ability to write a chapter on GI rhythmicity would have been impossible. I would also like to acknowledge many astute contributions from Prof. David Hirst who, through yearly working trips to Reno, challenged many of the concepts we had developed from studies of cultured ICC and forced us to seek better techniques and preparations in which to investigate the mechanism of electrical slow waves in ICC. I am also extremely grateful to Nancy Horowitz, Yulia Bayguinov, Lauren O’Kane, Dr. Doug Redelman, and Byoung Koh for excellent and consistent technical support for investigations of ICC. As always, I am extremely grateful to the NIDDK for the support received through a Program Project Grant, P01 DK41315–29 and a MERIT Award, R37 DK40569.
References
- 1.Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility-insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9(11):633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. 2014;94(3):859–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373(6512):347–9. [DOI] [PubMed] [Google Scholar]
- 4.Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci U S A. 1989;86(18):7280–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480 (Pt 1:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobine CA, Hannah EE, Zhu MH, Lyle HE, Rock JR, Sanders KM, et al. ANO1 in intramuscular interstitial cells of Cajal plays a key role in the generation of slow waves and tone in the internal anal sphincter. J Physiol. 2017;595(6):2021–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez WCaM LJ. Action current in stomach and intestine. Am J Phys. 1922;58:476–93. [Google Scholar]
- 8.Richter CP. Action currents from the stomach. Am J Phys. 1924;67:612–33. [Google Scholar]
- 9.Sanders KM, Ward SM, Hennig GW. Extracellular gastrointestinal electrical recordings: movement not electrophysiology. Nat Rev Gastroenterol Hepatol. 2017;14(6):372. [DOI] [PubMed] [Google Scholar]
- 10.Kuriyama H, Tomita T. The action potential in the smooth muscle of the guinea pig taenia coli and ureter studied by the double sucrose-gap method. J Gen Physiol. 1970;55(2):147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szurszewski JH. Mechanism of action of pentagastrin and acetylcholine on the longitudinal muscle of the canine antrum. J Physiol. 1975;252(2):335–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor JA, Prosser CL, Weems WA. A study of pacemaker activity in intestinal smooth muscle. J Physiol. 1974;240(3):671–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohba M, Sakamoto Y, Tomita T. The slow wave in the circular muscle of the guinea-pig stomach. J Physiol. 1975;253(2):505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulbring E Smooth muscle potentials recorded in the taenia coli of the guineapig. J Physiol. 1954;123(3):55P–6P. [PubMed] [Google Scholar]
- 15.El-Sharkaway TY, Daniel EE. Ionic mechanisms of intestinal electrical control activity. Am J Phys. 1975;229(5):1287–98. [DOI] [PubMed] [Google Scholar]
- 16.el-Sharkawy TY, Morgan KG, Szurszewski JH. Intracellular electrical activity of canine and human gastric smooth muscle. J Physiol. 1978;279:291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniel EE, Honour AJ, Bogoch A. Electrical activity of the longitudinal muscle of dog small intestine studied in vivo using microelectrodes. Am J Phys. 1960;198:113–8. [DOI] [PubMed] [Google Scholar]
- 18.Koh SD, Ward SM, Ordog T, Sanders KM, Horowitz B. Conductances responsible for slow wave generation and propagation in interstitial cells of Cajal. Curr Opin Pharmacol. 2003;3(6):579–82. [DOI] [PubMed] [Google Scholar]
- 19.Beckett EA, Bayguinov YR, Sanders KM, Ward SM, Hirst GD. Properties of unitary potentials generated by intramuscular interstitial cells of Cajal in the murine and guinea-pig gastric fundus. J Physiol. 2004;559(Pt 1:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirst GD, Dickens EJ, Edwards FR. Pacemaker shift in the gastric antrum of guinea-pigs produced by excitatory vagal stimulation involves intramuscular interstitial cells. J Physiol. 2002;541(Pt 3: 917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirst GD, Silverberg GD, van Helden DF. The action potential and underlying ionic currents in proximal rat middle cerebral arterioles. J Physiol. 1986;371:289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurahashi M, Zheng H, Dwyer L, Ward SM, Don Koh S, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589(Pt 3:697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung TS, Hwang SJ, Koh SD, Bayguinov Y, Peri LE, Blair PJ, et al. The cells and conductance mediating cholinergic neurotransmission in the murine proximal stomach. J Physiol. 2018;596:1549–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Code CF, Szurszewski JH. The effect of duodenal and mid small bowel transection on the frequency gradient of the pacesetter potential in the canine small intestine. J Physiol. 1970;207(2):281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, et al. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 1998;4(7):848–51. [DOI] [PubMed] [Google Scholar]
- 26.Daniel EE, Robinson K, Duchon G, Henderson RM. The possible role of close contacts (nexuses) in the propagation of control electrical activity in the stomach and small intestine. Am J Dig Dis. 1971;16(7):611–22. [DOI] [PubMed] [Google Scholar]
- 27.Christensen J, Schedl HP, Clifton JA. The small intestinal basic electrical rhythm (slow wave) frequency gradient in normal men and in patients with variety of diseases. Gastroenterology. 1966;50(3):309–15. [PubMed] [Google Scholar]
- 28.Bortoff A Electrical transmission of slow waves from longitudinal to circular intestinal muscle. Am J Phys. 1965;209(6):1254–60. [DOI] [PubMed] [Google Scholar]
- 29.Bozler E The relation of the action potentials to mechanical activity in intestinal muscle. Am J Phys. 1946;146:496–501. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki H, Hirst GD. Regenerative potentials evoked in circular smooth muscle of the antral region of guineapig stomach. J Physiol. 1999;517(Pt 2):563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirst GD, Bramich NJ, Teramoto N, Suzuki H, Edwards FR. Regenerative component of slow waves in the guinea-pig gastric antrum involves a delayed increase in [Ca(2+)](i) and Cl(−) channels. J Physiol. 2002;540(Pt 3:907–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirst GD, Edwards FR. Generation of slow waves in the antral region of guinea-pig stomach—a stochastic process. J Physiol. 2001;535(Pt 1:165–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cousins HM, Edwards FR, Hickey H, Hill CE, Hirst GD. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J Physiol. 2003;550(Pt 3:829–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickens EJ, Hirst GD, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol. 1999;514(Pt 2):515–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ordog T, Ward SM, Sanders KM. Interstitial cells of Cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518 (Pt 1:257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szurszewski JH. Electrical basis for gastrointestinal motility In: Johnson LR, editor. Physiology of the gastrointestinal tract. 2nd ed. New York: Raven Press; 1981. p. 1435–66. [Google Scholar]
- 37.Tomita T Electrical activity (spikes and slow waves) in gastrointestinal smooth muscles In: Bulbring E, Brading AF, Tomita T, editors. Smooth muscle: An assessment of current knowledge. London: Edward Arnold; 1981. p. 127–56. [Google Scholar]
- 38.Dickens EJ, Edwards FR, Hirst GD. Selective knockout of intramuscular interstitial cells reveals their role in the generation of slow waves in mouse stomach. J Physiol. 2001;531(Pt 3:827–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horner MJ, Ward SM, Gerthoffer WT, Sanders KM, Horowitz B. Maintenance of morphology and function of canine proximal colon smooth muscle in organ culture. Am J Phys. 1997;272(3 Pt 1):G669–80. [DOI] [PubMed] [Google Scholar]
- 40.Hall KA, Ward SM, Cobine CA, Keef KD. Spatial organization and coordination of slow waves in the mouse anorectum. J Physiol. 2014;592(17):3813–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forster J, Damjanov I, Lin Z, Sarosiek I, Wetzel P, McCallum RW. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9(1):102–8. [DOI] [PubMed] [Google Scholar]
- 42.Grover M, Bernard CE, Pasricha PJ, Lurken MS, Faussone-Pellegrini MS, Smyrk TC, et al. Clinical-histological associations in gastroparesis: results from the Gastroparesis Clinical Research Consortium. Neurogastroenterol Motil. 2012;24(6):531–9. e249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ordog T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49(10):1731–9. [DOI] [PubMed] [Google Scholar]
- 44.Kito Y, Sanders KM, Ward SM, Suzuki H. Interstitial cells of Cajal generate spontaneous transient depolarizations in the rat gastric fundus. Am J Physiol Gastrointest Liver Physiol. 2009;297(4):G814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol. 2003;553(Pt 3:803–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kito Y, Ward SM, Sanders KM. Pacemaker potentials generated by interstitial cells of Cajal in the murine intestine. Am J Phys Cell Phys. 2005;288(3): C710–20. [DOI] [PubMed] [Google Scholar]
- 47.Kito Y, Mitsui R, Ward SM, Sanders KM. Characterization of slow waves generated by myenteric interstitial cells of Cajal of the rabbit small intestine. Am J Physiol Gastrointest Liver Physiol. 2015;308(5):G378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jun JY, Kong ID, Koh SD, Wang XY, Perrino BA, Ward SM, et al. Regulation of ATP-sensitive K(+) channels by protein kinase C in murine colonic myocytes. Am J Phys Cell Phys. 2001;281(3):C857–64. [DOI] [PubMed] [Google Scholar]
- 49.Kito Y, Kurahashi M, Mitsui R, Ward SM, Sanders KM. Spontaneous transient hyperpolarizations in the rabbit small intestine. J Physiol. 2014;592(Pt 21:4733–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelly KA, Code CF, Elveback LR. Patterns of canine gastric electrical activity. Am J Phys. 1969;217(2):461–70. [DOI] [PubMed] [Google Scholar]
- 51.Publicover NG, Sanders KM. A technique to locate the pacemaker in smooth muscles. J Appl Physiol. 1984;57(5):1586–90. [DOI] [PubMed] [Google Scholar]
- 52.Bauer AJ, Reed JB, Sanders KM. Slow wave heterogeneity within the circular muscle of the canine gastric antrum. J Physiol. 1985;366:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horiguchi K, Semple GS, Sanders KM, Ward SM. Distribution of pacemaker function through the tunica muscularis of the canine gastric antrum. J Physiol. 2001;537(Pt 1:237–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forrest AS, Ordog T, Sanders KM. Neural regulation of slow-wave frequency in the murine gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2006;290(3):G486–95. [DOI] [PubMed] [Google Scholar]
- 55.Hashitani H, Garcia-Londono AP, Hirst GD, Edwards FR. Atypical slow waves generated in gastric corpus provide dominant pacemaker activity in guinea pig stomach. J Physiol. 2005;569(Pt 2):459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rhee PL, Lee JY, Son HJ, Kim JJ, Rhee JC, Kim S, et al. Analysis of pacemaker activity in the human stomach. J Physiol. 2011;589(Pt 24:6105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hara Y, Kubota M, Szurszewski JH. Electrophysiology of smooth muscle of the small intestine of some mammals. J Physiol. 1986;372:501–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jimenez M, Cayabyab FS, Vergara P, Daniel EE. Heterogeneity in electrical activity of the canine ileal circular muscle: interaction of two pacemakers. Neurogastroenterol Motil. 1996;8(4):339–49. [DOI] [PubMed] [Google Scholar]
- 59.Durdle NG, Kingma YJ, Bowes KL, Chambers MM. Origin of slow waves in the canine colon. Gastroenterology. 1983;84(2):375–82. [PubMed] [Google Scholar]
- 60.Smith TK, Reed JB, Sanders KM. Interaction of two electrical pacemakers in muscularis of canine proximal colon. Am J Phys. 1987;252(3 Pt 1):C290–9. [DOI] [PubMed] [Google Scholar]
- 61.Smith TK, Reed JB, Sanders KM. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am J Phys. 1987;252(2 Pt 1):C215–24. [DOI] [PubMed] [Google Scholar]
- 62.Yoneda S, Takano H, Takaki M, Suzuki H. Properties of spontaneously active cells distributed in the submucosal layer of mouse proximal colon. J Physiol. 2002;542(Pt 3:887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berezin I, Huizinga JD, Daniel EE. Interstitial cells of Cajal in the canine colon: a special communication network at the inner border of the circular muscle. J Comp Neurol. 1988;273(1):42–51. [DOI] [PubMed] [Google Scholar]
- 64.Berezin I, Huizinga JD, Daniel EE. Structural characterization of interstitial cells of Cajal in myenteric plexus and muscle layers of canine colon. Can J Physiol Pharmacol. 1990;68(11):1419–31. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki N, Prosser CL, Dahms V. Boundary cells between longitudinal and circular layers: essential for electrical slow waves in cat intestine. Am J Phys. 1986;250(3 Pt 1):G287–94. [DOI] [PubMed] [Google Scholar]
- 66.Ward SM, Sanders KM. Pacemaker activity in septal structures of canine colonic circular muscle. Am J Phys. 1990;259(2 Pt 1):G264–73. [DOI] [PubMed] [Google Scholar]
- 67.Faussone Pellegrini MS. Ultrastructure and topography of Cajal interstitial cells in the circular muscle layer of the ileum and the colon in the rat. Boll Soc Ital Biol Sper. 1982;58(19):1260–5. [PubMed] [Google Scholar]
- 68.Rumessen JJ, Thuneberg L. Pacemaker cells in the gastrointestinal tract: interstitial cells of Cajal. Scand J Gastroenterol Suppl. 1996;216:82–94. [DOI] [PubMed] [Google Scholar]
- 69.Rumessen JJ, Vanderwinden JM, Rasmussen H, Hansen A, Horn T. Ultrastructure of interstitial cells of Cajal in myenteric plexus of human colon. Cell Tissue Res. 2009;337(2):197–212. [DOI] [PubMed] [Google Scholar]
- 70.Thuneberg L Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- 71.Komuro T, Seki K, Horiguchi K. Ultrastructural characterization of the interstitial cells of Cajal. Arch Histol Cytol. 1999;62(4):295–316. [DOI] [PubMed] [Google Scholar]
- 72.Sanders KM, Stevens R, Burke E, Ward SW. Slow waves actively propagate at submucosal surface of circular layer in canine colon. Am J Phys. 1990;259(2 Pt 1):G258–63. [DOI] [PubMed] [Google Scholar]
- 73.Carlson HC, Code CF, Nelson RA. Motor action of the canine gastroduodenal junction: a cineradiographic, pressure, and electric study. Am J Dig Dis. 1966;11(2):155–72. [DOI] [PubMed] [Google Scholar]
- 74.Sarna SK, Daniel EE. Electrical stimulation of gastric electrical control activity. Am J Phys. 1973;225(1):125–31. [DOI] [PubMed] [Google Scholar]
- 75.Bayguinov O, Hennig GW, Sanders KM. Movement based artifacts may contaminate extracellular electrical recordings from GI muscles. Neurogastroenterol Motil. 2011;23(11):1029–42. e498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanders KM, Ward SM, Hennig GW. Problems with extracellular recording of electrical activity in gastrointestinal muscle. Nat Rev Gastroenterol Hepatol. 2016;13(12):731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bauer AJ, Publicover NG, Sanders KM. Origin and spread of slow waves in canine gastric antral circular muscle. Am J Phys. 1985;249(6 Pt 1):G800–6. [DOI] [PubMed] [Google Scholar]
- 78.Ward SM, Baker SA, de Faoite A, Sanders KM. Propagation of slow waves requires IP3 receptors and mitochondrial Ca2+ uptake in canine colonic muscles. J Physiol. 2003;549(Pt 1):207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ward SM, Dixon RE, de Faoite A, Sanders KM. Voltage-dependent calcium entry underlies propagation of slow waves in canine gastric antrum. J Physiol. 2004;561(Pt 3):793–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bayguinov O, Ward SM, Kenyon JL, Sanders KM. Voltage-gated Ca2+ currents are necessary for slow-wave propagation in the canine gastric antrum. Am J Phys Cell Phys. 2007;293(5):C1645–59. [DOI] [PubMed] [Google Scholar]
- 81.Kelly KA, La Force RC. Pacing the canine stomach with electric stimulation. Am J Phys. 1972;222(3):588–94. [DOI] [PubMed] [Google Scholar]
- 82.Soffer EE. Gastric electrical stimulation for gastroparesis. J Neurogastroenterol Motil. 2012;18(2):131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Publicover NG, Sanders KM. Effects of frequency on the wave form of propagated slow waves in canine gastric antral muscle. J Physiol. 1986;371: 179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kito Y, Fukuta H, Yamamoto Y, Suzuki H. Excitation of smooth muscles isolated from the guinea-pig gastric antrum in response to depolarization. J Physiol. 2002;543(Pt 1):155–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walsh JV Jr, Singer JJ. Voltage clamp of single freshly dissociated smooth muscle cells: current-voltage relationships for three currents. Pflugers Arch. 1981;390(2):207–10. [DOI] [PubMed] [Google Scholar]
- 86.Yoshino M, Wang SY, Kao CY. Sodium and calcium inward currents in freshly dissociated smooth myocytes of rat uterus. J Gen Physiol. 1997;110(5): 565–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mitra R, Morad M. Ca2+ and Ca2+-activated K+ currents in mammalian gastric smooth muscle cells. Science. 1985;229(4710):269–72. [DOI] [PubMed] [Google Scholar]
- 88.Langton PD, Burke EP, Sanders KM. Participation of Ca currents in colonic electrical activity. Am J Phys. 1989;257(3 Pt 1):C451–60. [DOI] [PubMed] [Google Scholar]
- 89.Kuriyama H, Osa T, Toida N. Electrophysiological study of the intestinal smooth muscle of the guinea-pig. J Physiol. 1967;191(2):239–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ozaki H, Stevens RJ, Blondfield DP, Publicover NG, Sanders KM. Simultaneous measurement of membrane potential, cytosolic Ca2+, and tension in intact smooth muscles. Am J Phys. 1991;260(5 Pt 1):C917–25. [DOI] [PubMed] [Google Scholar]
- 91.Vogalis F, Publicover NG, Hume JR, Sanders KM. Relationship between calcium current and cytosolic calcium in canine gastric smooth muscle cells. Am J Phys. 1991;260(5 Pt 1):C1012–8. [DOI] [PubMed] [Google Scholar]
- 92.Benham CD, Bolton TB, Denbigh JS, Lang RJ. Inward rectification in freshly isolated single smooth muscle cells of the rabbit jejunum. J Physiol. 1987;383:461–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Malysz J, Thuneberg L, Mikkelsen HB, Huizinga JD. Action potential generation in the small intestine of W mutant mice that lack interstitial cells of Cajal. Am J Phys. 1996;271(3 Pt 1):G387–99. [DOI] [PubMed] [Google Scholar]
- 94.Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Phys. 1995;269(6Pt 1):C1577–85. [DOI] [PubMed] [Google Scholar]
- 95.Tashiro N, Tomita T. The effects of papaverine on the electrical and mechanical activity of the guinea-pig taenia coli. Br J Pharmacol. 1970;39(3):608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ward SM, Dalziel HH, Khoyi MA, Westfall AS, Sanders KM, Westfall DP. Hyperpolarization and inhibition of contraction mediated by nitric oxide released from enteric inhibitory neurones in guinea-pig taenia coli. Br J Pharmacol. 1996;118(1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duthie HL, Kirk D. Electrical activity of human colonic smooth muscle in vitro. J Physiol. 1978;283:319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vogalis F, Ward SM, Sanders KM. Correlation between electrical and morphological properties of canine pyloric circular muscle. Am J Phys. 1991;260(3 Pt 1):G390–8. [DOI] [PubMed] [Google Scholar]
- 99.Sanders KM. Excitation-contraction coupling without Ca2+ action potentials in small intestine. Am J Phys. 1983;244(5):C356–61. [DOI] [PubMed] [Google Scholar]
- 100.Morgan KG, Szurszewski JH. Mechanisms of phasic and tonic actions of pentagastrin on canine gastric smooth muscle. J Physiol. 1980;301:229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Der-Silaphet T, Malysz J, Hagel S, Larry Arsenault A, Huizinga JD. Interstitial cells of cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology. 1998;114(4):724–36. [DOI] [PubMed] [Google Scholar]
- 102.Hennig GW, Spencer NJ, Jokela-Willis S, Bayguinov PO, Lee HT, Ritchie LA, et al. ICC-MY coordinate smooth muscle electrical and mechanical activity in the murine small intestine. Neurogastroenterol Motil. 2010;22(5):e138–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yoneda S, Fukui H, Takaki M. Pacemaker activity from submucosal interstitial cells of Cajal drives high-frequency and low-amplitude circular muscle contractions in the mouse proximal colon. Neurogastroenterol Motil. 2004;16(5):621–7. [DOI] [PubMed] [Google Scholar]
- 104.Rae MG, Fleming N, McGregor DB, Sanders KM, Keef KD. Control of motility patterns in the human colonic circular muscle layer by pacemaker activity. J Physiol. 1998;510(Pt 1):309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thuneberg L, Johansen V, Rumessen JJ, Anderson BG. Interstitial cells of Cajal: selective uptake of methylene blue inhibits slow wave activity In: Roman C, editor. Gastrointestinal motility. Lancaster: Mtp Press Limited; 1984. p. 495–502. [Google Scholar]
- 106.Torihashi S, Kobayashi S, Gerthoffer WT, Sanders KM. Interstitial cells in deep muscular plexus of canine small intestine may be specialized smooth muscle cells. Am J Phys. 1993;265(4 Pt 1):G638–45. [DOI] [PubMed] [Google Scholar]
- 107.Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, et al. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116(2):369–75. [DOI] [PubMed] [Google Scholar]
- 108.Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280(1):97–111. [DOI] [PubMed] [Google Scholar]
- 109.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–80. [DOI] [PubMed] [Google Scholar]
- 110.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296(6): G1370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hwang SJ, Blair PJ, Britton FC, O’Driscoll KE, Hennig G, Bayguinov YR, et al. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587(Pt 20):4887–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wouters M, De Laet A, Donck LV, Delpire E, van Bogaert PP, Timmermans JP, et al. Subtractive hybridization unravels a role for the ion cotransporter NKCC1 in the murine intestinal pacemaker. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1219–27. [DOI] [PubMed] [Google Scholar]
- 113.Zhu MH, Sung TS, Kurahashi M, O’Kane LE, O’Driscoll K, Koh SD, et al. Na+-K+-Cl− cotransporter (NKCC) maintains the chloride gradient to sustain pacemaker activity in interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol. 2016;311(6):G1037–G46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ro S, Park C, Jin J, Zheng H, Blair PJ, Redelman D, et al. A model to study the phenotypic changes of interstitial cells of Cajal in gastrointestinal diseases. Gastroenterology. 2010;138(3):1068–78.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, et al. A Ca(2+)-activated Cl(−) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009;587(Pt 20):4905–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ordog T, Redelman D, Miller LJ, Horvath VJ, Zhong Q, Almeida-Porada G, et al. Purification of interstitial cells of Cajal by fluorescence-activated cell sorting. Am J Phys Cell Phys. 2004;286(2):C448–56. [DOI] [PubMed] [Google Scholar]
- 117.Lee MY, Ha SE, Park C, Park PJ, Fuchs R, Wei L, et al. Transcriptome of interstitial cells of Cajal reveals unique and selective gene signatures. PLoS One. 2017;12(4):e0176031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen H, Ordog T, Chen J, Young DL, Bardsley MR, Redelman D, et al. Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine. Physiol Genomics. 2007;31(3):492–509. [DOI] [PubMed] [Google Scholar]
- 119.Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of cajal from the murine small intestine. J Physiol. 1998;513(Pt 1):203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goto K, Matsuoka S, Noma A. Two types of spontaneous depolarizations in the interstitial cells freshly prepared from the murine small intestine. J Physiol. 2004;559(Pt 2):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322(5901):590–4. [DOI] [PubMed] [Google Scholar]
- 122.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134(6):1019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455(7217):1210–5. [DOI] [PubMed] [Google Scholar]
- 124.Kashyap P, Gomez-Pinilla PJ, Pozo MJ, Cima RR, Dozois EJ, Larson DW, et al. Immunoreactivity for Ano1 detects depletion of Kit-positive interstitial cells of Cajal in patients with slow transit constipation. Neurogastroenterol Motil. 2011;23(8):760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang F, Rock JR, Harfe BD, Cheng T, Huang X, Jan YN, et al. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc Natl Acad Sci U S A. 2009;106(50): 21413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Strege PR, Bernard CE, Mazzone A, Linden DR, Beyder A, Gibbons SJ, et al. A novel exon in the human Ca2+-activated Cl− channel Ano1 imparts greater sensitivity to intracellular Ca2. Am J Physiol Gastrointest Liver Physiol. 2015;309(9):G743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sung TS, O’Driscoll K, Zheng H, Yapp NJ, Leblanc N, Koh SD, et al. Influence of intracellular Ca2+ and alternative splicing on the pharmacological profile of ANO1 channels. Am J Phys Cell Phys. 2016;311(3):C437–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mazzone A, Bernard CE, Strege PR, Beyder A, Galietta LJ, Pasricha PJ, et al. Altered expression of Ano1 variants in human diabetic gastroparesis. J Biol Chem. 2011;286(15):13393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hwang SJ, Basma N, Sanders KM, Ward SM. Effects of new-generation inhibitors of the calcium-activated chloride channel anoctamin 1 on slow waves in the gastrointestinal tract. Br J Pharmacol. 2016;173(8):1339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rock JR, Futtner CR, Harfe BD. The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol. 2008;321(1):141–9. [DOI] [PubMed] [Google Scholar]
- 131.Malysz J, Gibbons SJ, Saravanaperumal SA, Du P, Eisenman ST, Cao C, et al. Conditional genetic deletion of Ano1 in interstitial cells of Cajal impairs Ca2+ transients and slow waves in adult mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2017;312(3):G228–G45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heinze C, Seniuk A, Sokolov MV, Huebner AK, Klementowicz AE, Szijarto IA, et al. Disruption of vascular Ca2+-activated chloride currents lowers blood pressure. J Clin Invest. 2014;124(2):675–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vooijs M, Jonkers J, Berns A. A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep. 2001;2(4):292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huizinga JD, Shin A, Chow E. Electrical coupling and pacemaker activity in colonic smooth muscle. Am J Phys. 1988;255(5 Pt 1):C653–60. [DOI] [PubMed] [Google Scholar]
- 135.van Helden DF, Imtiaz MS. Ca2+ phase waves: a basis for cellular pacemaking and long-range synchronicity in the guinea-pig gastric pylorus. J Physiol. 2003;548(Pt 1):271–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gibbons SJ, Strege PR, Lei S, Roeder JL, Mazzone A, Ou Y, et al. The alpha1H Ca2+ channel subunit is expressed in mouse jejunal interstitial cells of Cajal and myocytes. J Cell Mol Med. 2009;13(11–12):4422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zheng H, Park KS, Koh SD, Sanders KM. Expression and function of a T-type Ca2+ conductance in interstitial cells of Cajal of the murine small intestine. Am J Phys Cell Phys. 2014;306(7):C705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57(4):411–25. [DOI] [PubMed] [Google Scholar]
- 139.Iftinca M, McKay BE, Snutch TP, McRory JE, Turner RW, Zamponi GW. Temperature dependence of T-type calcium channel gating. Neuroscience. 2006;142(4):1031–42. [DOI] [PubMed] [Google Scholar]
- 140.Kito Y, Suzuki H. Effects of temperature on pacemaker potentials in the mouse small intestine. Pflugers Arch. 2007;454(2):263–75. [DOI] [PubMed] [Google Scholar]
- 141.Huang X, Lee SH, Lu H, Sanders KM, Koh SD. Molecular and functional characterization of inwardly rectifying K(+) currents in murine proximal colon. J Physiol. 2018;596(3):379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Na JS, Hong C, Kim MW, Park CG, Kang HG, Wu MJ, et al. ATP-sensitive K(+) channels maintain resting membrane potential in interstitial cells of Cajal from the mouse colon. Eur J Pharmacol. 2017;809:98–104. [DOI] [PubMed] [Google Scholar]
- 143.Markadieu N, Delpire E. Physiology and pathophysiology of SLC12A1/2 transporters. Pflugers Arch. 2014;466(1):91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ebihara S, Shirato K, Harata N, Akaike N. Gramicidin-perforated patch recording: GABA response in mammalian neurones with intact intracellular chloride. J Physiol. 1995;484(Pt 1):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ball ER, Matsuda MM, Dye L, Hoffmann V, Zerfas PM, Szarek E, et al. Ultra-structural identification of interstitial cells of Cajal in the zebrafish Danio rerio. Cell Tissue Res. 2012;349(2):483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rich A, Gordon S, Brown C, Gibbons SJ, Schaefer K, Hennig G, et al. Kit signaling is required for development of coordinated motility patterns in zebrafish gastrointestinal tract. Zebrafish. 2013;10:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brijs J, Hennig GW, Kellermann AM, Axelsson M, Olsson C. The presence and role of interstitial cells of Cajal in the proximal intestine of shorthorn sculpin (Myoxocephalus scorpius). J Exp Biol. 2017;220(Pt 3):347–57. [DOI] [PubMed] [Google Scholar]
- 148.Singh RD, Gibbons SJ, Saravanaperumal SA, Du P, Hennig GW, Eisenman ST, et al. Ano1, a Ca2+-activated Cl− channel, coordinates contractility in mouse intestine by Ca2+ transient coordination between interstitial cells of Cajal. J Physiol. 2014;592(Pt 18):4051–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hennig GW, Hirst GD, Park KJ, Smith CB, Sanders KM, Ward SM, et al. Propagation of pacemaker activity in the guinea-pig antrum. J Physiol. 2004;556(Pt 2):585–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, et al. The mechanism and spread of pacemaker activity through myenteric interstitial cells of Cajal in human small intestine. Gastroenterology. 2007;132(5):1852–65. [DOI] [PubMed] [Google Scholar]
- 151.Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, et al. Septal interstitial cells of Cajal conduct pacemaker activity to excite muscle bundles in human jejunum. Gastroenterology. 2007;133(3):907–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee HT, Hennig GW, Park KJ, Bayguinov PO, Ward SM, Sanders KM, et al. Heterogeneities in ICC Ca2+ activity within canine large intestine. Gastroenterology. 2009;136(7):2226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Park KJ, Hennig GW, Lee HT, Spencer NJ, Ward SM, Smith TK, et al. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Phys Cell Phys. 2006;290(5):C1411–27. [DOI] [PubMed] [Google Scholar]
- 154.Huizinga JD, Chen JH, Zhu YF, Pawelka A, McGinn RJ, Bardakjian BL, et al. The origin of segmentation motor activity in the intestine. Nat Commun. 2014;5:3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yamazawa T, Iino M. Simultaneous imaging of Ca2+ signals in interstitial cells of Cajal and longitudinal smooth muscle cells during rhythmic activity in mouse ileum. J Physiol. 2002;538(Pt 3):823–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Baker SA, Drumm BT, Saur D, Hennig GW, Ward SM, Sanders KM. Spontaneous Ca(2+) transients in interstitial cells of Cajal located within the deep muscular plexus of the murine small intestine. J Physiol. 2016;594(12):3317–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Drumm BT, Hennig GW, Battersby MJ, Cunningham EK, Sung TS, Ward SM, et al. Clustering of Ca2+ transients in interstitial cells of Cajal defines slow wave duration. J Gen Physiol. 2017;149(7): 703–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lowie BJ, Wang XY, White EJ, Huizinga JD. On the origin of rhythmic calcium transients in the ICC-MP of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2011;301(5):G835–45. [DOI] [PubMed] [Google Scholar]
- 159.Serio R, Barajas-Lopez C, Daniel EE, Berezin I, Huizinga JD. Slow-wave activity in colon: role of network of submucosal interstitial cells of Cajal. Am J Phys. 1991;260(4 Pt 1):G636–45. [DOI] [PubMed] [Google Scholar]
- 160.Bayguinov PO, Hennig GW, Smith TK. Ca2+ imaging of activity in ICC-MY during local mucosal reflexes and the colonic migrating motor complex in the murine large intestine. J Physiol. 2010;588(Pt 22):4453–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Huang L, Keyser BM, Tagmose TM, Hansen JB, Taylor JT, Zhuang H, et al. NNC 55–0396 [(1S,2S)-2-(2-(N-[(3-benzimidazol-2-yl) propyl]-N-methylamino)ethyl)-6-fluoro-1,2, 3,4-tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride]: a new selective inhibitor of T-type calcium channels. J Pharmacol Exp Ther. 2004;309(1):193–9. [DOI] [PubMed] [Google Scholar]
- 162.Kraus RL, Li Y, Gregan Y, Gotter AL, Uebele VN, Fox SV, et al. In vitro characterization of T-type calcium channel antagonist TTA-A2 and in vivo effects on arousal in mice. J Pharmacol Exp Ther. 2010;335(2):409–17. [DOI] [PubMed] [Google Scholar]
- 163.Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, et al. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J Physiol. 2000;525(Pt 1):105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van Helden DF, Imtiaz MS, Nurgaliyeva K, von der Weid P, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol. 2000;524(Pt 1):245–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ganitkevich V, Isenberg G. Membrane potential modulates inositol 1,4,5-trisphosphate-mediated Ca2+ transients in guinea-pig coronary myocytes. J Physiol. 1993;470:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fukuta H, Kito Y, Suzuki H. Spontaneous electrical activity and associated changes in calcium concentration in guinea-pig gastric smooth muscle. J Physiol. 2002;540(Pt 1):249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Malysz J, Donnelly G, Huizinga JD. Regulation of slow wave frequency by IP(3)-sensitive calcium release in the murine small intestine. Am J Physiol Gastrointest Liver Physiol. 2001;280(3):G439–48. [DOI] [PubMed] [Google Scholar]
- 168.Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, et al. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525(Pt 2):355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu HN, Ohya S, Wang J, Imaizumi Y, Nakayama S. Involvement of ryanodine receptors in pacemaker Ca2+ oscillation in murine gastric ICC. Biochem Biophys Res Commun. 2005;328(2):640–6. [DOI] [PubMed] [Google Scholar]
- 170.Zhu MH, Sung TS, O’Driscoll K, Koh SD, Sanders KM. Intracellular Ca2+ release from endoplasmic reticulum regulates slow wave currents and pacemaker activity of interstitial cells of Cajal. Am J Phys Cell Phys. 2015;308(8):C608–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Faussone-Pellegrini MS. Cytodifferentiation of the interstitial cells of Cajal related to the myenteric plexus of mouse intestinal muscle coat. An E.M. study from foetal to adult life. Anat Embryol. 1985;171(2):163–9. [DOI] [PubMed] [Google Scholar]
- 172.Rumessen JJ, Thuneberg L. Interstitial cells of Cajal in human small intestine. Ultrastructural identification and organization between the main smooth muscle layers. Gastroenterology. 1991;100(5 Pt 1):1417–31. [PubMed] [Google Scholar]
- 173.Kito Y, Suzuki H. Electrophysiological properties of gastric pacemaker potentials. J Smooth Muscle Res. 2003;39(5):163–73. [DOI] [PubMed] [Google Scholar]
- 174.Kito Y, Fukuta H, Suzuki H. Components of pacemaker potentials recorded from the guinea pig stomach antrum. Pflugers Arch. 2002;445(2):202–17. [DOI] [PubMed] [Google Scholar]
- 175.Faville RA, Pullan AJ, Sanders KM, Koh SD, Lloyd CM, Smith NP. Biophysically based mathematical modeling of interstitial cells of Cajal slow wave activity generated from a discrete unitary potential basis. Biophys J. 2009;96(12):4834–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Drumm BT, Sung TS, Zheng H, Baker SA, Koh SD, Sanders KM. The effects of mitochondrial inhibitors on Ca2+ signalling and electrical conductances required for pacemaking in interstitial cells of Cajal in the mouse small intestine. Cell Calcium. 2018;72(June):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kito Y, Suzuki H. Modulation of slow waves by hyperpolarization with potassium channel openers in antral smooth muscle of the guinea-pig stomach. J Physiol. 2003;548(Pt 1):175–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee JY, Ko EJ, Ahn KD, Kim S, Rhee PL. The role of K(+) conductances in regulating membrane excitability in human gastric corpus smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2015;308(7):G625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Koh SD, Bradley KK, Rae MG, Keef KD, Horowitz B, Sanders KM. Basal activation of ATP-sensitive potassium channels in murine colonic smooth muscle cell. Biophys J. 1998;75(4):1793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kurahashi M, Mutafova-Yambolieva V, Koh SD, Sanders KM. Platelet-derived growth factor receptor-alpha-positive cells and not smooth muscle cells mediate purinergic hyperpolarization in murine colonic muscles. Am J Phys Cell Phys. 2014;307(6):C561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chow E, Huizinga JD. Myogenic electrical control activity in longitudinal muscle of human and dog colon. J Physiol. 1987;392:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93(21):12008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20(4):1393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Goyal RK. CrossTalk opposing view: interstitial cells are not involved and physiologically important in neuromuscular transmission in the gut. J Physiol. 2016;594(6):1511–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Goyal RK, Chaudhury A. Mounting evidence against the role of ICC in neurotransmission to smooth muscle in the gut. Am J Physiol Gastrointest Liver Physiol. 2010;298(1):G10–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol. 2010;588(Pt 23):4621–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sanders KM, Ward SM, Friebe A. CrossTalk proposal: interstitial cells are involved and physiologically important in neuromuscular transmission in the gut. J Physiol. 2016;594(6):1507–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rumessen JJ, Mikkelsen HB, Thuneberg L. Ultrastructure of interstitial cells of Cajal associated with deep muscular plexus of human small intestine. Gastroenterology. 1992;102(1): 56–68. [DOI] [PubMed] [Google Scholar]
- 189.Zhou DS, Komuro T. Ultrastructure of the zinc iodide-osmic acid stained cells in guinea pig small intestine. J Anat. 1995;187(Pt 2):481–5. [PMC free article] [PubMed] [Google Scholar]
- 190.Sternini C, Su D, Gamp PD, Bunnett NW. Cellular sites of expression of the neurokinin-1 receptor in the rat gastrointestinal tract. J Comp Neurol. 1995;358(4):531–40. [DOI] [PubMed] [Google Scholar]
- 191.Vannucchi MG, De Giorgio R, Faussone-Pellegrini MS. NK1 receptor expression in the interstitial cells of Cajal and neurons and tachykinins distribution in rat ileum during development. J Comp Neurol. 1997;383(2):153–62. [DOI] [PubMed] [Google Scholar]
- 192.Iino S, Ward SM, Sanders KM. Interstitial cells of Cajal are functionally innervated by excitatory motor neurones in the murine intestine. J Physiol. 2004;556(Pt 2):521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang XY, Ward SM, Gerthoffer WT, Sanders KM. PKC-epsilon translocation in enteric neurons and interstitial cells of Cajal in response to muscarinic stimulation. Am J Physiol Gastrointest Liver Physiol. 2003;285(3):G593–601. [DOI] [PubMed] [Google Scholar]
- 194.Baker SA, Drumm BT, Skowronek KE, Rembetski BE, Peri LE, Hennig GW, Perrino BA, Sanders KM. Excitatory neuronal responses of Ca(2+) transients in interstitial cells of Cajal in the small intestine. eNeuro. 2018;5(2). pii: ENEURO.0080–18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Baker SA, Drumm BT, et al. Inhibitory neural regulation of the Ca2+ transients in intramuscular interstitial cells of Cajal in the small intestine. Front Physiol. 2018;9:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Inoue R, Isenberg G. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. J Physiol. 1990;424:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gordienko DV, Zholos AV. Regulation of muscarinic cationic current in myocytes from guinea-pig ileum by intracellular Ca2+ release: a central role of inositol 1,4,5-trisphosphate receptors. Cell Calcium. 2004;36(5):367–86. [DOI] [PubMed] [Google Scholar]
- 198.Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, et al. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 2009;137(4):1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bhetwal BP, Sanders KM, An C, Trappanese DM, Moreland RS, Perrino BA. Ca2+ sensitization pathways accessed by cholinergic neurotransmission in the murine gastric fundus. J Physiol. 2013;591(Pt 12): 2971–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Diamant NE, Bortoff A. Nature of the intestinal slow-wave frequency gradient. Am J Phys. 1969;216(2):301–7. [DOI] [PubMed] [Google Scholar]
- 201.Suzuki N, Prosser CL, DeVos W. Waxing and waning of slow waves in intestinal musculature. Am J Phys. 1986;250(1 Pt 1):G28–34. [DOI] [PubMed] [Google Scholar]
- 202.Zhu MH, Sung IK, Zheng H, Sung TS, Britton FC, O’Driscoll K, et al. Muscarinic activation of Ca2+-activated Cl− current in interstitial cells of Cajal. J Physiol. 2011;589(Pt 18):4565–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci U S A. 2005;102(41):14913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kaji N, Horiguchi K, Iino S, Nakayama S, Ohwada T, Otani Y, et al. Nitric oxide-induced oxidative stress impairs pacemaker function of murine interstitial cells of Cajal during inflammation. Pharmacol Res. 2016;111:838–48. [DOI] [PubMed] [Google Scholar]
- 205.Joddar B, Tasnim N, Thakur V, Kumar A, McCallum RW, Chattopadhyay M. Delivery of mesenchymal stem cells from gelatin-alginate hydrogels to stomach lumen for treatment of gastroparesis. Bioengineering (Basel). 2018;5(1):E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sanders KM, Kito Y, Hwang SJ, Ward SM. Regulation of gastrointestinal smooth muscle function by interstitial cells. Physiology (Bethesda). 2016;31(5):316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]