Abstract
Epidemiological studies have revealed that caffeine consumption during pregnancy is associated with adverse gestational outcomes, yet the underlying mechanisms remain obscure. Recent animal studies with physiologically relevant dosages have begun to dissect adverse effects of caffeine during pregnancy with respect to oviduct contractility, embryo development, uterine receptivity, and placentation that jointly contribute to pregnancy complications. Interestingly, caffeine’s effects are highly variable between individual animals under well-controlled experimental settings, suggesting the possibility of epigenetic regulation of these phenotypes, in addition to genetic variants. Moreover, caffeine exposure during sensitive windows of pregnancy may induce epigenetic changes in the developing fetus or even the germ cells to cause adult-onset diseases in subsequent generations. We discuss these research frontiers in light of emerging data.
A Snapshot of Caffeine’s Impacts
Caffeine is the most widely consumed psychoactive substance throughout the world [1,2]. In the USA, approximately 70% of women continue to consume caffeine during pregnancy [3,4]. It has been reported that some women consume more than 300–500 mg of caffeine per day during pregnancy [5], which is the equivalent of approximately three to five 240 ml cups of coffee daily (https://www.fda.gov). Although caffeine consumption in adults has beneficial effects on neurological diseases (e.g., Parkinson’s and Alzheimer’s diseases), cardiovascular diseases (e.g., coronary heart disease, stroke), certain cancers (e.g., prostate cancer, melanoma, liver cancer, breast cancer), liver diseases (e.g., liver fibrosis, liver cirrhosis) and type 2 diabetes [6,7], it has also been well documented that maternal caffeine consumption during pregnancy increases the risk of pregnancy failure or gestational complications [6,7], the underlying mechanisms of which are just beginning to be revealed. Importantly, the effects of caffeine exposure on reproductive performance vary greatly from individual to individual [5,8–10], which could be a result of combined effects of genetic variants, epigenetic factors, and environmental inputs that jointly predispose individual sensitivity. The study of caffeine’s heterogeneous effects within a well-controlled animal cohort may provide an ideal model in which to study the genetic and epigenetic bases of phenotypic variation (see Glossary) that may account for the different individual sensitivity towards caffeine and thus may lead to future solutions in precision medicine. In addition, recent studies have begun to provide evidence showing that caffeine exposure during pregnancy can cause adverse effects to the offspring, or even subsequent generations [11], suggesting possible epigenetic regulation through early embryonic or fetal germ cells via the maternal environment; this has triggered great interest and warrants further in-depth mechanistic study. Here, in light of recent research findings, we discuss these emerging topics of gestational exposure to caffeine, especially focusing on animal and human studies with physiologically relevant dosages that may shed light on the impacts, of daily caffeine consumption.
Caffeine’s Negative Impacts on Pregnancy: Evidence from Humans
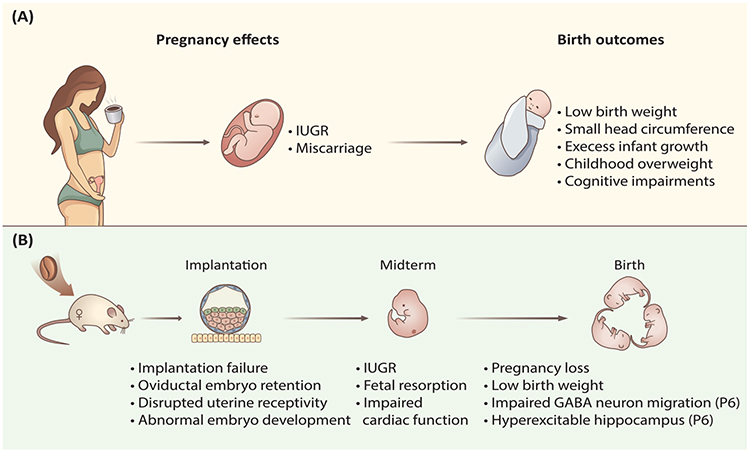
Caffeine’s effects on gestational health have received widespread attention since the 1980s [12]. During pregnancy, caffeine’s metabolic rate in mothers is significantly decreased, especially after the first trimester [13], and the half-life of caffeine increases from 2.5–4.5 hours to approximately 15 hours towards the end of pregnancy [2]. Moreover, caffeine is lipophilic enough to freely transfer across all biological membranes, including the blood–placental barrier, while neither fetus nor placenta has the enzymes for its metabolism [14]; caffeine absorbed by mothers may also accumulate in oviductal or uterine fluid environments [15], which potentially affects embryonic development and generates adult-onset diseases. Epidemiological studies showed that caffeine consumption during pregnancy was associated with intrauterine growth retardation (IUGR)/low birth weight [12], subfertility [16], and spontaneous abortion [17] (Figure 1A). A ′safe′ maximum caffeine dosage for gestational health has been previously claimed: daily intake of less than 300 mg caffeine (approximately three cups of coffee) during pregnancy was deemed unlikely to harm gestational health [10,18]. However, this ′safe′ dosage is being reevaluated based on increasing evidence, which has shown that even daily doses of less than 300 mg may increase the risk of pregnancy failure [17,19–21]. Furthermore, other studies found that even a daily intake as low as 100–200 mg during pregnancy is associated with an increased risk of miscarriage [4], fetal growth restriction [5], low birth weight [22–24], as well as increased risks to the offspring, including cognitive development impairments [25], overweight, and obesity [26,27]. These studies raise concern that there may be no one absolute ‘safe’ threshold of caffeine consumption during pregnancy [5,21,22,27,28].
Figure 1. Maternal Caffeine Intake Compromises Pregnancy and Birth Outcomes.
(A) In humans, caffeine intake during pregnancy may give rise to pregnancy complications, such as miscarriage [4,17], fetal growth restriction [5,12], and low birth weight [21–24], which would also increase the risk of overweight and impaired cognitive development in childhood [25–27]. (B) Potential mechanisms for the effects of caffeine as revealed by a rodent model. Current studies show that maternal caffeine exposure not only severely disrupts embryo implantation but also disrupts ongoing midterm fetal growth and development, resulting in pregnancy loss, low birth weight, and impaired brain development in offspring on postnatal day 6 (P6) [35–38,55]. Abbreviations: GABA, γ-aminobutyric acid; IUGR, intrauterine growth retardation.
Despite the differences in gestation and fetal development, caffeine absorption and bioavailability are generally similar between humans and rodents [29], which has allowed for the opportunity to understand caffeine’s physiological and molecular effects through the use of animal models. According to human studies, nearly 99% of caffeine is absorbed across the wall of the gastrointestinal (GI) tract within approximately 30–45 minutes [1,2], and the pharmacokinetics of caffeine are independent of the route of administration, since there is a negligible first-pass effect for caffeine metabolism [2,29]. Caffeine is primarily metabolized in the liver by the cytochrome-450 oxidase enzyme system in which CYP1A2 (cytochrome P450 1A2) is the rate-limiting enzyme that is responsible for the clearance of the absorbed caffeine (~ 95% in humans [29] and ~ 87% in mice [30]) and is the main isoform detected in the livers of both humans and rodents [31]. Under physiological conditions, caffeine generates its cellular effects by blocking adenosine receptors, mainly through ADORA1 (adenosine A1 receptor) and ADORA2A (adenosine A2A receptor) [2,32], which are conserved across species between humans and rodents [33]. Therefore, rodent studies in well-controlled experimental settings may provide insights into the cellular mechanisms underlying caffeine’s effects on human pregnancy.
Pathophysiological Impacts of Caffeine on Pregnancy: Insights from Rodent Studies
Consistent with the results from human epidemiological studies, caffeine exposure in rodents causes similar adverse effects on pregnancy outcomes and fetal development (Figure 1B). In the following discussion, caffeine dosages used in rodents are extrapolated to the equivalents of 240 ml cups of coffee consumption in humans, which is based on equal circulating levels of caffeine after consumption by rodents or humans [2,11,34–36]. Mice exposed to high dosages of caffeine, equivalent to about six or 12 cups of coffee in humans, 1 week prior to and during the entire period of gestation, resulted in delayed conception, decreased maternal bodyweight gain and placenta weight, as well as increased risks of IUGR, fetus resorption, and low birth weight [37]. Importantly, recent studies have further revealed that brief caffeine exposure during early pregnancy or even the preconception period can induce a ripple of adverse effects throughout the whole pregnancy. Female rats administrated with a dosage, equivalent to six cups of coffee in humans, for 4 consecutive days before conception had reduced fertility due to impaired embryo implantation [38]. Disrupted embryo implantation was also found when mice were treated with a dosage equivalent to nine cups of coffee or one to two cups of coffee per day in humans during preimplantation [35] (Figure 1B). These observations are closely related to human health and raise a red flag for caffeine consumption before embryo implantation and prior to any signs of pregnancy that can be clinically recognized. Moreover, a series of studies also demonstrated that maternal diet during this period can also affect long-term health of offspring [39]. Therefore, for women who are planning to conceive, restricted caffeine consumption should be considered for future clinical guidance.
Mechanistically, caffeine-induced implantation failure could be partially ascribed to delayed oviductal embryo transport, as preimplantation caffeine exposure can result in embryo retention in the isthmus of the fallopian tube [35]; this in vivo observation is consistent with previous ex vivo data showing that caffeine abolished the generation of electrical slow wave pacemaker activity and the underlying rhythmic contractility of the oviduct smooth muscle via a cAMP-dependent pathway [40]. Impaired oviductal embryo transport is detrimental to the ongoing fetal development, as it has been previously established that a brief delay in transport would cause the embryo to miss the implantation window and generate adverse ripple effects in future embryonic development [41]. Caffeine treatment during the preimplantation stage also disrupted early embryo development and compromised blastocyst quality, potentially through a direct effect on the developing embryo, as demonstrated by in vitro caffeine treatment, or through an in vivo secondary effect involving disruption of the oviductal/uterine environment, or both. In addition, dysregulation of steroid hormone-regulated genes, such as leukemia inhibitory factor (Lif), mucin 1 (Muc1), lactoferrin (Ltf), and amphiregulin (Areg), which are important determinants of uterine receptivity, was also found in preimplantation uterine epithelium after caffeine treatment, suggesting impaired uterine receptivity (Figure 1B). All of these factors may act together to cause abnormal embryo implantation and thus lead to further pregnancy complications and pregnancy loss [35].
In addition to early gestational exposure, caffeine exposure during the middle and late stages of pregnancy also leads to a variety of adverse effects. For example, a single administration of caffeine, with a dosage equivalent to two cups of coffee in humans, in pregnant mice on embryonic day 8.5 can impair embryonic cardiac development and reduce ventricular myocardial area as well as cardiac output and contractility, resulting in compromised cardiac function in adulthood [34,42]. When caffeine was administered daily to pregnant mice during days 9.5 to 18.5, with a modest dosage equivalent to one cup of coffee in humans, it was capable of disrupting embryonic cardiovascular growth and function [36] (Figure 1B). Using the adenosine A2A receptor antagonist [36] and an adenosine A1 receptor knockout mouse model [42], it was demonstrated that the effects of caffeine on cardiac function are mediated by blockade of adenosine receptor signaling [36,42]. Moreover, the effects of caffeine on embryo development could also be a result of impaired placental function, as demonstrated by decreased placental weight and abnormal structure after caffeine treatment during mid-to-late pregnancy in rats, which could be acting through chronic activation of maternal and placental renin–angiotensin system and induction of tumor protein p53-dependent trophoblast apoptosis [37]. In addition, a single administration of caffeine on day 12 of pregnancy in rats significantly reduced blood flow to the maternal ovary, uterus, and decidua [43], which may lead to vasoconstriction in the uteroplacental circulation and compromise the ability of the placenta to transfer nutrients to the developing embryo. Moreover, increased serum adrenaline concentration and significantly decreased (~ 25%) intervillous placental blood flow have been reported in pregnant women after ingesting two cups of coffee during the last trimester [44]. These findings suggest that middle and late gestational caffeine exposure can have a profound effect on both embryonic and placental development, and could explain the observed IUGR associated with gestational caffeine consumption in humans [5].
Interindividual Variation in Caffeine Response
Notably, caffeine’s effects on pregnancy outcomes have been shown to be highly variable between individuals in both rodents [35,38] and humans [5,8,9,45,46] (Figure 2A). Substantial interindividual phenotypic variation and the underling mechanism(s) in complex traits and diseases have become an area of significant scientific interest over the past two decades. It is now increasingly accepted that an individual’s disease susceptibility is a complex readout of combined effects from genetic, epigenetic, and environmental inputs as well as their dynamic interaction during the process of development [47,48]. However, the relative weights of these different factors in the contribution of interindividual variation and disease predisposition varies case by case and depends on specific conditions and sometimes may show significant synergism.
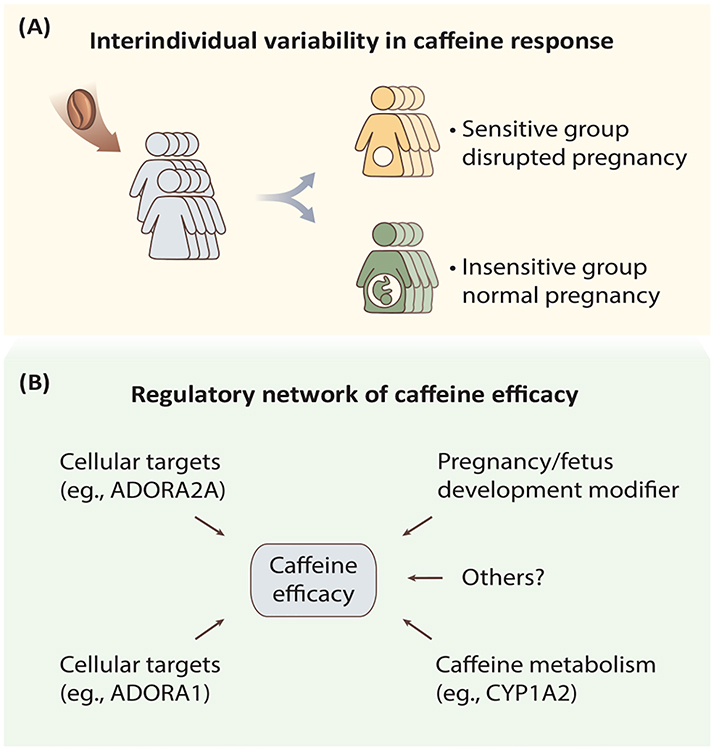
Figure 2. Interindividual Variability in Caffeine Response.
Postulated models underpinning the highly variable individual response to caffeine exposure. (A) Women showed different responses to caffeine exposure during pregnancy [5,8,9,45,46]. (B) The effects of caffeine might be regulated by multiple factors in vivo, including the ability to metabolize caffeine (determined by the rate-limiting enzyme CYP1A2) and cellular targets (mainly through ADORA1/A2A), or regulated through modulators associated with pregnancy and fetal development, all of which modify the outcome of pregnancy for individual women after caffeine exposure. Abbreviations: ADORA1, adenosine A1 receptor; ADORA2A, adenosine A2A receptor; CYP1A2, cytochrome P450 1A2.
The individual variation in caffeine response involves its metabolism as well as the sensitivity of adenosine receptors [29] (Figure 2B). In particular, regulation of CYP1A2, the rate-limiting enzyme in caffeine metabolism, represents a well-studied example (Figure 2B and Box 1). Epidemiological studies have found that when exposed to the same dosages of caffeine, women with higher CYP1A2 enzyme activity (rapid caffeine metabolism) have an increased risk of pregnancy disorders compared to those with lower CYP1A2 enzyme activity [5,8,9,45,46]. Human CYP1A2 mRNA levels represent more than 40-fold interindividual differences, and the in vivo activity of the CYP1A2 enzyme varies up to 60-fold, as probed by the 3-demethylation of caffeine [49]. In addition to the constitutive expression of hepatic CYP1A2, the activity of CYP1A2 can be regulated by a range of extrinsic and intrinsic factors, such as induced by cigarette smoking and heavy coffee consumption, inhibited by oral contraceptives [50], and coregulated by other liver-enriched transcription factors [51]. The regulation of CYP1A2 via environmental factors may also involve multiple layers of epigenetic mechanisms in addition to genetic variations (Box 1), which represents an exciting area for future research and may lead to personalized precision medicine.
Box 1. Genetic and Epigenetic Regulation of CYP1A2.
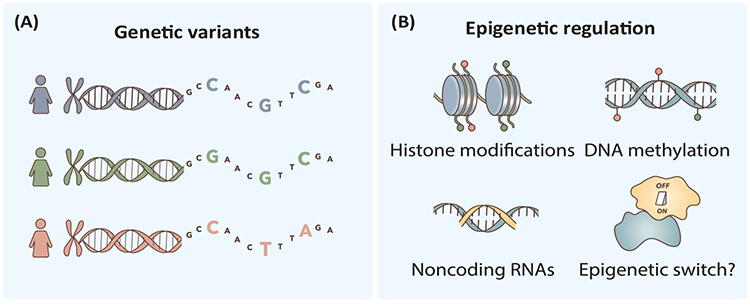
Two genetic variants in the CYP1A2 gene have been reported to cause different levels of CYP1A2 expression after exposure to smoking or coffee: the variant –163C>A (rs762551) in intron 1 of the CYP1A2 gene was associated with higher inducibility in Caucasian or Swedish smokers [71,72] and heavy coffee consumers in Serbia and Sweden [73], while the 5′-flanking variant –3860G>A (rs2069514) conferred a decreased CYP1A2 inducibility in Japanese smokers [74]. It is interesting to note that ten single-nucleotide polymorphisms in the genes of ARNT (aryl hydrocarbon receptor nuclear translocator), AhRR (aryl hydrocarbon receptor regulator), HNF1α (hepatocyte nuclear factor 1α), IL-1β (interleukin-1 β), SRC-1 (steroid receptor coactivator 1), and VDR (vitamin D receptor) were suggested to be correlated with variability in CYP1A2 enzyme activity, but none of them was located in the CYP1A locus [51], suggesting that genetic factors outside the CYP1A locus may play an important role in establishing the genotype–phenotype relationship of CYP1A2 (Figure I). However, genetic and environmental factors combined can explain only 30%–40% of the individual variability in CYP1A2 activity [75,76], suggesting the complexity of CYP1A2 regulation, which may involve a layer of epigenetic regulation.
Human hepatic CYP1A2 expression was found to display allele-specific expression [77], and it was found that environmental stimuli can directly affect CYP1A2 expression through epigenetic factors. Cigarette smoke condensate treatment in vitro could increase the levels of H3K4me3 and H4K16ac and decrease the level of H3K27me3 in the segments of CYP1A2 gene [78], and influence caffeine metabolism. CYP1A2 mRNA transcripts were also regulated by DNA methylation. For example, the DNA methylation extent of a CpG island (containing 17 CpG sites) close to its translation start site [77] and the CCGG site (bp-2579) in the 5′-flanking region showed an inverse association with CYP1A2 mRNA expression [79]. Moreover, small RNAs, such as hsa-miR-132–5p, were also found to directly target the 3′-untranslated regions of CYP1A2 mRNA and suppress the expression of CYP1A2 protein [80]. In addition to DNA methylation, histone modifications and small RNAs mediated epigenetic regulation of CYP1A2 expression, recent studies from monozygotic twins revealed that the causes of phenotypic discordance also contain gene–environment interaction(s), as well as other intangible stochastic factors [81] or epigenetic switch for bistable downstream gene expression, as shown in other systems [82] (Figure I).
Figure I.
Genetic Variation and Epigenetic Factors may Contribute to CYP1A2-Mediated Interindividual Variability in Caffeine Response.
Prenatal Caffeine Exposure: Significance to the Long-Term Health of the Offspring
In addition to the adverse effects on gestational outcomes, caffeine consumption during pregnancy also affects the long-term development of the fetus and elicits adult diseases, thus reflecting the theory of developmental origins of health and disease (DOHaD) [52] (Box 2). In humans, it has been found that exposure to high dosages of prenatal caffeine significantly increases the risk of childhood acute lymphoblastic leukemia [53]. If mothers ingested more than 200 mg of caffeine per day, their children showed a twofold higher risk of impaired cognitive development and low IQ (intelligence quotient) at age 5.5 years, compared with those whose mothers ingested less than 100 mg of caffeine per day [25]. Two additional independent studies both found that even low dosages (<150 mg daily) of maternal caffeine consumption during pregnancy was associated with increased risks of offspring’s excess growth in babyhood and overweight in early childhood [26,27]. This rapid ‘catch-up’ weight gain after low birth weight is a prime risk factor for subsequent developmental risks of adult cardiovascular or metabolic diseases [54]. Fetal development impairments and adult diseases caused by prenatal caffeine exposure were similarly found in mice, showing that maternal caffeine exposure from pregnancy until lactation could result in possible long-term neuronal and behavior impairments in offspring [55] (Figure 3).
Box 2. Developmental Origins of Health and Disease (DOHaD).
Although scientists investigating the Dutch Hunger Winter (1944–1945) in 1976 reported that in utero and early infantile famine exposure are linked to the offspring ′ s obesity risk [83], David Barker greatly expanded the concept of DOHaD from 1986 onwards, by finding high correlation between maternal starvation and increased risk of low birth weight as well as offspring cardiometabolic diseases in adulthood [84]. This concept was subsequently supported by additional reports from epidemiological and clinical studies, which found that early-life environmental exposure, such as maternal nutrition status and mental health during the conception or early infancy period, was strongly linked to future chronic diseases, especially noncommunicable diseases, including obesity, certain cancers, abnormal bone density, schizophrenia, atopic dermatitis, and asthma [52,85]. Although the mechanisms underlying DOHaD are still unclear, it is well accepted that epigenetic regulation is involved in embryonic and placental developmental disorders caused by the maternal environment perturbation [52,85]. For example, individuals periconceptionally exposed to famine during the Dutch Hunger Winter were associated with altered IGF2 (insulin-like growth factor II) DMR (differentially methylated region) methylation 6 decades later [86], reinforcing the great importance of early-life experience on lifelong health conditioning [39].
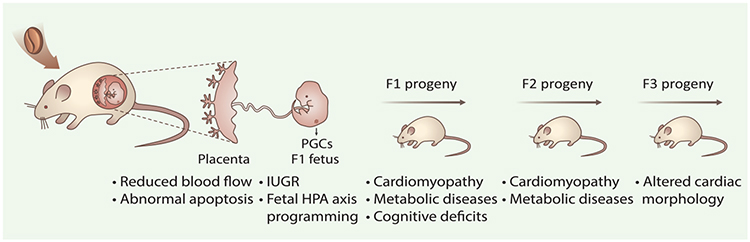
Figure 3. In Utero Caffeine Exposure Triggers Adult-Onset Diseases and Transmits Adverse Effects into Subsequent Generations.
Caffeine can freely cross the blood–placenta barrier and thus exposes the fetus. Exposure to caffeine during pregnancy can induce long-term health disorders in subsequent generations [11,55,59]. Compromised maternal caffeine metabolism, disrupted placenta development, and an abnormal uterine environment may affect fetal development as well as the offspring’s health (F1), which may also influence the primordial germ cells (PGCs) of the developing fetus and transfer diseases, such as cardiomyopathy, to the F2 descendants. Abbreviations: IUGR, intrauterine growth retardation; HPA axis, hypothalamic-pituitary-adrenal axis.
Mechanistically, the adverse effects of caffeine on F1 offspring could be due to early embryo caffeine exposure via oviductal or uterine fluid [15], or during later exposure that bypasses the blood–placenta barrier. In addition to the direct effect of caffeine exposure, recent studies also found that caffeine intake during mid-to-late pregnancy can cause an increase in maternal glucocorticoids [56]; fetus exposed to such an environment can result in long-term programming of fetal hypothalamic-pituitary-adrenal axis [57], which could disrupt neuroendocrine metabolism and increase susceptibility to metabolism syndrome, such as hypercholesterolemia, in adult offspring [56,58] (Figure 3).
Beyond the F1 offspring, disorders induced by maternal caffeine exposure may also be transferred to the second and/or third generations. Prenatal caffeine exposure in rats during mid-to-late pregnancy can increase susceptibility to metabolic syndrome in the F2 generation [59]. Interestingly, different cardiac phenotypes were developed in the succeeding generations, depending on the timing of in utero caffeine exposure: pregnant mice treated with caffeine once daily, equivalent to two cups of coffee in humans, during days 6.5–9.5 can induce dilated cardiomyopathy only in the F1 generation, but treatment during days 10.5–13.5 caused hypertrophic cardiomyopathy in the F2 generation and morphological changes in the F3 generation [11,60] (Figure 3). It is well known that during days 7.5–13.5, mouse primordial germ cells (PGCs) undergo a genome-wide epigenetic reset as they migrate and settle at genital ridges. Recent studies have found that environmental stimuli during this period, such as dietary treatment, affect PGCs development in the fetuses and induce specific epigenetic alterations in germ cells, such as disrupted DNA methylation [61]. Notably, caffeine exposure during days 6.5–10.5 can change the expression of DNA methyltransferases Dnmt1/3a/3b [11,62], and demethylases Tet1/2/3 in embryonic heart [62], which are critical in regulating DNA methylation landscape. Caffeine treatment during these important periods of early embryo and/or germ line development may also affect the epigenetic state that goes beyond one generation. Importantly, fetal PGCs regain DNA methylation in a sex-specific manner: male germ cells reestablish methylation after day 13.5 prenatally, whereas female germ cells undergo remethylation after birth [61,63]. These differences may lead to a sex-different response in offspring, which has been found in prenatal caffeine exposure induced metabolic phenotype in rat [59]. Moreover, a considerable fraction of genomic sequences have been found to bypass the removal of DNA methylation during PGC and preimplantation reprogramming [64], which also might contribute to caffeine exposure induced DOHaD. Besides, other epigenetic carriers, such as histone modifications, noncoding RNAs, and RNA modifications [65–67], may also be involved in caffeine-induced long-lasting effects across generations, as well as the gender-dependent outcomes, which deserve more in-depth examinations in the future.
Concluding Remarks and Future Perspectives
The World Health Organization and European Food Safety Authority recommend that daily caffeine consumption remain below 200–300 mg as a safe dosage for pregnant health [68,69]. It is prudent to note that the current recommended maximal dose may be too high for certain individuals. At the molecular level, the mechanisms of caffeine-induced disease susceptibility and heritability remain unclear, but represent an interesting model that may help us to reevaluate the effects of environmental exposure.
Studies have provided both epidemiological and experimental evidence suggesting that the further investigation of the mechanisms involved in responsiveness to caffeine might provide a new avenue in precision medicine. That said, developing a rapid and efficient method to evaluate individual susceptibility to caffeine will not only be beneficial for women in pregnancy health management, but also provide a basis in the guidelines of personalized drug usage and drug discovery [70]. For example, the key enzyme for caffeine metabolism, CYP1A2, could be pursued as a specific drug target and could be used as the basis of personalized caffeine sensitivity tests in daily life. Finally, by what mechanism and to what extent caffeine’s effect before or during pregnancy can affect offspring’s phenotype are currently intriguing questions that warrant in-depth investigations (see Outstanding Questions).
Outstanding Questions.
What mechanisms underlie the interindividual variation in caffeine responses? In what ways can future studies reveal the precise functions or interaction of genetic variations, epigenetic regulation, and the environment in this complex process? Are there any key molecules that control an individual’s response to caffeine?
How can one assess the personalized safe dosage of gestational caffeine intake? Is it possible to predict the potential effects of caffeine before pregnancy? If so, what is the best way to formulate a standard and personalized guideline for women who are preparing to conceive?
What are the long-term consequences, especially adult-onset disease, of in utero caffeine exposure in humans? Can future longitudinal studies corroborate and extend initial findings on the associations between caffeine exposure and certain chronic diseases in the clinic? Questions as to whether these adverse exposures and effects of caffeine can be transferred into subsequent generations need to be addressed.
To what extent, if at all, does caffeine affect the metabolism and efficacy of clinical drugs? Can a person’s response to caffeine reflect his/her potential responses to some drugs or treatments, allowing a preliminary prediction in the clinic?
What is the best way to balance the advantages and disadvantages of caffeine in pregnant women, especially for those who are suffering from neurological diseases, liver diseases, cardiovascular diseases, or certain cancers?
Highlights.
A growing, robust body of evidence from both epidemiological and animal studies unveils harmful effects of maternal gestational caffeine exposure, even from doses previously considered ′ safe. ′
Rodent studies revealed that caffeine exposure during specific stages of pregnancy may disrupt embryo transport, embryo development, embryo implantation, and placental function, leading to pregnancy complications.
Notably, caffeine sensitivity is highly variable from individual to individual. Genetic variations and epigenetic regulation, intermingling with intrinsic and environmental factors, might play pivotal roles in shaping the complex phenotypic variability. Exploring the underlying mechanism(s) will be helpful, not only for improving the guidelines of gestational caffeine consumption, but also for personalized dosing of drugs that interact with caffeine at the pharmacokinetic or pharmacodynamic level.
Evidence from rodent studies demonstrated that in utero caffeine exposure triggered cardiometabolic defects on both the immediate offspring and subsequent generations. Further studies are needed regarding caffeine’s long-term effects and multigenerational influence in humans.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2019YFA0802600 to Y.Z., 2017YFC1001401 to E.D., 2018YFC1004500 to Y.Z., 2016YFA0500903 to E.D., and 2015CB943003 to Y.Z.); the National Basic Research Program of China (81490742 to E.D.); the National Natural Science Foundation of China (31671201 to Y.Z. and 31671568 to E.D.); the Youth Innovation Promotion Association, CAS (grant number 2016081 to Y.Z.); and the NIH (R01HD092431 to Q.C.), and Nevada Women’s Health Initiative Fund to S.M.W.
Glossary
- Adenosine receptors
a family of G protein-coupled receptors, comprising subtypes A1, A2A, A2B, and A3, with adenosine as an endogenous ligand; common nonselective antagonists of these receptors include caffeine and theophylline
- Blood–placental barrier
a structure composed of layers of cells that prevents diffusion of substances between the mother and fetus; this barrier changes over the course of pregnancy
- Embryo implantation
a stage of pregnancy at which the activated blastocyst adheres to the receptive uterus
- First-pass effect
also known as first-pass metabolism; an orally administered substance absorbed from the GI tract first enters the liver through the portal vein, and a proportion of it, to what extent depends on the specific substance, must be metabolized in the GI tract or liver before reaches the systemic circulation
- Genital ridge
also known as gonadal ridge; a mesodermal primordium of the somatic gonads in the vertebrate embryo
- Hypothalamic-pituitary-adrenal axis
a complex neuroendocrine system composed of the hypothalamus, pituitary gland, and adrenal gland, acting through direct effects or feedback interactions among these components to control neuroendocrine functions, such as stress response and immunoregulation
- Implantation window
a limited stage in which the uterus is conducive to the activated blastocyst to initiate implantation, which is principally regulated by ovarian steroids estrogen and progesterone
- Intrauterine growth retardation (IUGR)
a condition in which the fetus is abnormally small while in the mother’s womb, causing pregnancy complications, including low birth weight, stillbirth, and long-term developmental defects
- Isthmus of the fallopian tube
the narrow part of the fallopian tube that links the ampulla (beginning of the fallopian tube near the ovary) to the uterotubal junction (connection between the fallopian tube and the uterus)
- Phenotypic variation
the differences in a given phenotype between individuals in a population, considered a prerequisite for adaptation and evolution
- Precision medicine
a developing approach to disease prevention and treatment that is tailored to variations in an individual’s genes, environment, and lifestyle, aiming to improve therapeutic efficacy and safety while alleviating adverse side effects
- Primordial germ cells (PGCs)
gamete precursors that develop into haploid germ cells, that is, sperm and eggs, which generate a new organism upon fertilization
- Spontaneous abortion
also termed miscarriage; induced embryonic or fetal death before the 20th week of pregnancy, when the embryo or fetus cannot survive independently
- Uteroplacental circulation
the connection between the uterus and placenta, which plays a pivotal role in nutrient, oxygen, and waste exchange between the mother and the fetus
References
- 1.Gonzalez de Mejia E and Ramirez-Mares MV (2014) Impact of caffeine and coffee on our health. Trends Endocrinol. Metab 25, 489–492 [DOI] [PubMed] [Google Scholar]
- 2.Fredholm BB et al. (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev 51, 83–133 [PubMed] [Google Scholar]
- 3.Frary CD et al. (2005) Food sources and intakes of caffeine in the diets of persons in the United States.J. Am. Diet. Assoc 105, 110–113 [DOI] [PubMed] [Google Scholar]
- 4.Weng X et al. (2008) Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am. J. Obstet. Gynecol 198, 279. [DOI] [PubMed] [Google Scholar]
- 5.Group CS (2008) Maternal caffeine intake during pregnancy and risk of fetal growth restriction: a large prospective observational study. BMJ 337, a2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poole R et al. (2017) Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ 359, j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grosso G et al. (2017) Coffee, caffeine, and health outcomes: an umbrella review. Annu. Rev. Nutr 37, 131–156 [DOI] [PubMed] [Google Scholar]
- 8.Signorello LB et al. (2001) Caffeine metabolism and the risk of spontaneous abortion of normal karyotype fetuses. Obstet. Gynecol 98, 1059–1066 [DOI] [PubMed] [Google Scholar]
- 9.Grosso LM et al. (2006) Caffeine metabolites in umbilical cord blood, cytochrome P-450 1A2 activity, and intrauterine growth restriction. Am. J. Epidemiol 163, 1035–1041 [DOI] [PubMed] [Google Scholar]
- 10.Doepker C et al. (2016) Caffeine: friend or foe? Annu. Rev. Food Sci. Technol 7, 117–137 [DOI] [PubMed] [Google Scholar]
- 11.Rivkees SA and Wendler CC (2017) Long-term consequences of disrupting adenosine signaling during embryonic development. Mol. Aspects Med 55, 110–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenster L et al. (1991) Caffeine consumption during pregnancy and fetal growth. Am. J. Public Health 81, 458–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu T et al. (2016) Pregnancy-induced changes in the pharmacokinetics of caffeine and its metabolites. J. Clin. Pharmacol 56, 590–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grosso LM and Bracken MB (2005) Caffeine metabolism, genetics, and perinatal outcomes: a review of exposure assessment considerations during pregnancy. Ann. Epidemiol 15, 460–466 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y et al. (2017) Uterine fluid in pregnancy: a biological and clinical outlook. Trends Mol. Med 23, 604–614 [DOI] [PubMed] [Google Scholar]
- 16.Wilcox A et al. (1988) Caffeinated beverages and decreased fertility. Lancet 2, 1453–1456 [DOI] [PubMed] [Google Scholar]
- 17.Cnattingius S et al. (2000) Caffeine intake and the risk of first-trimester spontaneous abortion. N. Engl. J. Med 343, 1839–1845 [DOI] [PubMed] [Google Scholar]
- 18.Wikoff D et al. (2017) Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem. Toxicol 109, 585–648 [DOI] [PubMed] [Google Scholar]
- 19.Hatch EE and Bracken MB (1993) Association of delayed conception with caffeine consumption. Am. J. Epidemiol 138, 1082–1092 [DOI] [PubMed] [Google Scholar]
- 20.Lyngso J et al. (2017) Association between coffee or caffeine consumption and fecundity and fertility: a systematic review and dose-response meta-analysis. Clin. Epidemiol 9, 699–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sengpiel V et al. (2013) Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: results from a large prospective observational cohort study. BMC Med. 11, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen LW et al. (2014) Maternal caffeine intake during pregnancy is associated with risk of low birth weight: a systematic review and dose-response meta-analysis. BMC Med. 12, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee J et al. (2015) Maternal caffeine consumption during pregnancy and risk of low birth weight: a dose-response meta-analysis of observational studies. PLoS One 10, e0132334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen LW et al. (2018) Associations of maternal caffeine intake with birth outcomes: results from the Lifeways cross generation cohort study. Am. J. Clin. Nutr 108, 1301–1308 [DOI] [PubMed] [Google Scholar]
- 25.Galera C et al. (2016) Prenatal caffeine exposure and child IQ at age 5.5 years: the EDEN mother–child cohort. Biol. Psychiatry 80, 720–726 [DOI] [PubMed] [Google Scholar]
- 26.Li DK et al. (2015) Maternal caffeine intake during pregnancy and risk of obesity in offspring: a prospective cohort study. Int. J. Obes. (Lond) 39, 658–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papadopoulou E et al. (2018) Maternal caffeine intake during pregnancy and childhood growth and overweight: results from a large Norwegian prospective observational cohort study. BMJ Open 8, e018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenwood DC et al. (2014) Caffeine intake during pregnancy and adverse birth outcomes: a systematic review and dose-response meta-analysis. Eur. J. Epidemiol 29, 725–734 [DOI] [PubMed] [Google Scholar]
- 29.Nehlig A (2018) Interindividual differences in caffeine metabolism and factors driving caffeine consumption. Pharmacol. Rev 70, 384–411 [DOI] [PubMed] [Google Scholar]
- 30.Buters JT et al. (1996) Role of CYP1A2 in caffeine pharmacokinetics and metabolism: studies using mice deficient in CYP1A2. Pharmacogenetics 6, 291–296 [DOI] [PubMed] [Google Scholar]
- 31.Berthou F et al. (1992) Interspecies variations in caffeine metabolism related to cytochrome P4501A enzymes. Xenobiotica 22, 671–680 [DOI] [PubMed] [Google Scholar]
- 32.Fredholm BB et al. (2017) Low, but not high, dose caffeine is a readily available probe for adenosine actions. Mol. Aspects Med 55, 20–25 [DOI] [PubMed] [Google Scholar]
- 33.Baraldi PG et al. (2008) Adenosine receptor antagonists: translating medicinal chemistry and pharmacology into clinical utility. Chem. Rev 108, 238–263 [DOI] [PubMed] [Google Scholar]
- 34.Wendler CC et al. (2009) Embryonic caffeine exposure induces adverse effects in adulthood. FASEB J. 23, 1272–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian J et al. (2018) Caffeine consumption during early pregnancy impairs oviductal embryo transport, embryonic development and uterine receptivity in mice. Biol. Reprod 99, 1266–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Momoi N et al. (2008) Modest maternal caffeine exposure affects developing embryonic cardiovascular function and growth. Am. J. Physiol. Heart Circ. Physiol 294, H2248–H2256 [DOI] [PubMed] [Google Scholar]
- 37.Huang J et al. (2012) Role of p53-dependent placental apoptosis in the reproductive and developmental toxicities of caffeine in rodents. Clin. Exp. Pharmacol. Physiol 39, 357–363 [DOI] [PubMed] [Google Scholar]
- 38.Pollard I et al. (1999) Effects of preconceptual caffeine exposure on pregnancy and progeny viability. J. Matern. Fetal Med 8, 220–224 [DOI] [PubMed] [Google Scholar]
- 39.Fleming TP et al. (2018) Origins of lifetime health around the time of conception: causes and consequences. Lancet 391, 1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dixon R et al. (2011) Inhibitory effect of caffeine on pacemaker activity in the oviduct is mediated by cAMP-regulated conductances. Br. J. Pharmacol 163, 745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S et al. (2013) Physiological and molecular determinants of embryo implantation. Mol. Aspects Med 34, 939–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buscariollo DL et al. (2014) Embryonic caffeine exposure acts via A1 adenosine receptors to alter adult cardiac function and DNA methylation in mice. PLoS One 9, e87547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimmel CA et al. (1984) Blood flow changes and conceptal development in pregnant rats in response to caffeine. Fundam. Appl. Toxicol 4, 240–247 [DOI] [PubMed] [Google Scholar]
- 44.Kirkinen P et al. (1983) The effect of caffeine on placental and fetal blood flow in human pregnancy. Am. J. Obstet. Gynecol 147, 939–942 [DOI] [PubMed] [Google Scholar]
- 45.Sata F et al. (2005) Caffeine intake, CYP1A2 polymorphism and the risk of recurrent pregnancy loss. Mol. Hum. Reprod 11, 357–360 [DOI] [PubMed] [Google Scholar]
- 46.Sasaki S et al. (2017) Interaction between maternal caffeine intake during pregnancy and CYP1A2 C164A polymorphism affects infant birth size in the Hokkaido study. Pediatr. Res 82, 19–28 [DOI] [PubMed] [Google Scholar]
- 47.Cavalli G and Heard E (2019) Advances in epigenetics link genetics to the environment and disease. Nature 571, 489–499 [DOI] [PubMed] [Google Scholar]
- 48.Panzeri I and Pospisilik JA (2018) Epigenetic control of variation and stochasticity in metabolic disease. Mol. Metab 14, 26–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunes A and Dahl ML (2008) Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics 9, 625–637 [DOI] [PubMed] [Google Scholar]
- 50.Zhou SF et al. (2010) Structure, function, regulation and polymorphism and the clinical significance of human cytochrome P450 1A2. Drug Metab. Rev 42, 268–354 [DOI] [PubMed] [Google Scholar]
- 51.Klein K et al. (2010) Pathway-targeted pharmacogenomics of CYP1A2 in human liver. Front. Pharmacol 1, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanson MA and Gluckman PD (2014) Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol. Rev 94, 1027–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng J et al. (2014) Maternal coffee consumption during pregnancy and risk of childhood acute leukemia: a metaanalysis. Am. J. Obstet. Gynecol 210, 151.e1–151.e10 [DOI] [PubMed] [Google Scholar]
- 54.Barker DJ et al. (2005) Trajectories of growth among children who have coronary events as adults.N. Engl. J. Med 353, 1802–1809 [DOI] [PubMed] [Google Scholar]
- 55.Silva CG et al. (2013) Adenosine receptor antagonists including caffeine alter fetal brain development in mice. Sci. Transl. Med 5, 197ra104. [DOI] [PubMed] [Google Scholar]
- 56.Xu D et al. (2018) Intrauterine programming mechanism for hypercholesterolemia in prenatal caffeine-exposed female adult rat offspring. FASEB J. 32, 5563–5576 [DOI] [PubMed] [Google Scholar]
- 57.Moisiadis VG and Matthews SG (2014) Glucocorticoids and fetal programming part 1: outcomes. Nat. Rev. Endocrinol 10, 391–402 [DOI] [PubMed] [Google Scholar]
- 58.Dearden L and Ozanne SE (2015) Early life origins of metabolic disease: developmental programming of hypothalamic pathways controlling energy homeostasis. Front. Neuroendocrinol 39, 3–16 [DOI] [PubMed] [Google Scholar]
- 59.Luo H et al. (2014) Prenatal caffeine ingestion induces transgenerational neuroendocrine metabolic programming alteration in second generation rats. Toxicol. Appl. Pharmacol 274, 383–392 [DOI] [PubMed] [Google Scholar]
- 60.Fang X et al. (2016) In utero caffeine exposure induces transgenerational effects on the adult heart. Sci. Rep 6, 34106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Radford EJ (2018) Exploring the extent and scope of epigenetic inheritance. Nat. Rev. Endocrinol 14, 345–355 [DOI] [PubMed] [Google Scholar]
- 62.Fang X et al. (2014) Caffeine exposure alters cardiac gene expression in embryonic cardiomyocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol 307, R1471–R1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang WW et al. (2016) Specification and epigenetic programming of the human germ line. Nat. Rev. Genet 17, 585–600 [DOI] [PubMed] [Google Scholar]
- 64.Skvortsova K et al. (2018) Functions and mechanisms of epigenetic inheritance in animals. Nat. Rev. Mol. Cell Biol 19, 774–790 [DOI] [PubMed] [Google Scholar]
- 65.Chen Q et al. (2016) Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat. Rev. Genet 17, 733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y et al. (2018) Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small noncoding RNAs. Nat. Cell Biol 20, 535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perez MF and Lehner B (2019) Intergenerational and transgenerational epigenetic inheritance in animals. Nat. Cell Biol 21, 143–151 [DOI] [PubMed] [Google Scholar]
- 68.World Health Organization. (2016) WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience, WHO; [PubMed] [Google Scholar]
- 69.Agostoni C et al. (2015) Scientific opinion on the safety of caffeine. EFSA J. 13, 4102 [Google Scholar]
- 70.Chen JF et al. (2013) Adenosine receptors as drug targets – what are the challenges? Nat. Rev. Drug Discov 12, 265–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sachse C et al. (1999) Functional significance of a C →A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br. J. Clin. Pharmacol 47, 445–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghotbi R et al. (2007) Comparisons of CYP1A2 genetic polymorphisms, enzyme activity and the genotype–phenotype relationship in Swedes and Koreans. Eur. J. Clin. Pharmacol 63, 537–546 [DOI] [PubMed] [Google Scholar]
- 73.Djordjevic N et al. (2010) Induction of CYP1A2 by heavy coffee consumption is associated with the CYP1A2–163C>A polymorphism. Eur. J. Clin. Pharmacol 66, 697–703 [DOI] [PubMed] [Google Scholar]
- 74.Nakajima M et al. (1999) Genetic polymorphism in the 5’-flanking region of human CYP1A2 gene: effect on the CYP1A2 inducibility in humans. J. Biochem 125, 803–808 [DOI] [PubMed] [Google Scholar]
- 75.Zanger UM and Schwab M (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther 138, 103–141 [DOI] [PubMed] [Google Scholar]
- 76.Perera V et al. (2012) Influence of environmental and genetic factors on CYP1A2 activity in individuals of South Asian and European ancestry. Clin. Pharmacol. Ther 92, 511–519 [DOI] [PubMed] [Google Scholar]
- 77.Ghotbi R et al. (2009) Allele-specific expression and gene methylation in the control of CYP1A2 mRNA level in human livers. Pharmacogenomics J. 9, 208–217 [DOI] [PubMed] [Google Scholar]
- 78.Xie C et al. (2017) In vitro analysis of factors influencing CYP1A2 expression as potential determinants of interindividual variation. Pharmacol. Res. Perspect 5, e00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hammons GJ et al. (2001) Specific site methylation in the 5’-flanking region of CYP1A2 interindividual differences in human livers. Life Sci. 69, 839–845 [DOI] [PubMed] [Google Scholar]
- 80.Chen Y et al. (2017) The expression, induction and pharmacological activity of CYP1A2 are post-transcriptionally regulated by microRNA hsa-miR-132–5p. Biochem. Pharmacol 145, 178–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Czyz W et al. (2012) Genetic, environmental and stochastic factors in monozygotic twin discordance with a focus on epigenetic differences. BMC Med. 10, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dalgaard K et al. (2016) Trim28 haploinsufficiency triggers bi-stable epigenetic obesity. Cell 164, 353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ravelli GP et al. (1976) Obesity in young men after famine exposure in utero and early infancy. N. Engl. J. Med 295, 349–353 [DOI] [PubMed] [Google Scholar]
- 84.Barker D et al. (2013) Developmental biology: support mothers to secure future public health. Nature 504, 209–211 [DOI] [PubMed] [Google Scholar]
- 85.Godfrey KM et al. (2010) Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol. Metab 21, 199–205 [DOI] [PubMed] [Google Scholar]
- 86.Heijmans BT et al. (2008) Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. U. S. A 105, 17046–17049 [DOI] [PMC free article] [PubMed] [Google Scholar]