Abstract
Objective
Bicuspid aortic valve is a common risk factor for thoracic aortic aneurysm and dissection. Guidelines for elective ascending aortic intervention (AAI) in bicuspid aortic valve are derived from limited evidence, and the extent of practice variation due to patient and provider characteristics is unknown. Using data from 2 large cardiovascular registries, we investigated factors that influence decisions for AAI.
Methods
All bicuspid aortic valve cases with known aortic diameters and surgical status were included. We used multivariable logistic regression to profile predictors of isolated aortic valve replacement (AVR) or AVR+AAI, stratified by patient characteristics, surgical indications, and institution.
Results
We studied 2861 subjects at 18 institutions from 1996 to 2015. The median aortic diameter of patients who underwent AVR+AAI varied widely across institutions (39–52 mm). Aortic diameters were <45 mm in 38% of patients undergoing AVR+AAI. Patients who underwent AAI at <45 mm, compared with those managed nonoperatively, were younger (54 ± 13 vs 61 ± 15 years; P < .001) with more frequent aortic stenosis (53% vs 28%; P < .001) and regurgitation (52% vs 18%; P < .001).
Conclusions
Clinical and institutional factors influence the timing of AAI and are associated with significant variability in ascending aortic diameter at AAI across institutions. More than one third of patients with a bicuspid aortic valve undergo AAI at aortic diameters <45 mm. Long-term outcomes of this subgroup of patients, who may manifest earlier and more severe disease, are needed to determine the risk–benefit ratio of routine aortic interventions at smaller diameters.
Keywords: bicuspid aortic valve, thoracic aortic aneurysm, thoracic aortic dissection, ascending aortic intervention
Bicuspid aortic valve (BAV) occurs in 1% to 2% of the general population and is associated with thoracic aortic aneurysms and acute aortic dissections.1 BAV is known to be associated with altered ascending aortic wall stress, medial degeneration, aneurysmal dilation, and dissection.2–4 The age-adjusted rate of aortic dissection in patients with BAV is 8-fold greater than patients with tricuspid aortic valves (TAV),5 and BAV remains an independent risk factor for new Stanford Type A dissections even after treatment of previous Type B dissections.6 Among young patients who experience acute aortic dissections, patients with BAV are disproportionately represented compared with their TAV peers.7,8
To prevent aortic dissections in patients with BAV, the American College of Cardiology and American Heart Association recently revised guidelines for elective ascending aortic interventions (AAI) in patients with BAV, considering aortic diameter and other patient factors.9–14 The American College of Cardiology and American Heart Association guidelines recommend elective AAI when aortic diameter exceeds 55 mm (Class I); 50 mm when other factors are present, such as family history, expansion rate (Class IIa), or low operative risk at a center with established expertise (Class IIa); or 45 mm if concomitant aortic valve replacement (AVR) is planned (Class IIa).12 The 2018 American Association for Thoracic Surgery guidelines10 downgraded recommendations for elective AAI when aortic diameter exceeds 50 mm from Class IIa to IIb, and decreased the threshold to consider expansion rate as an independent risk for dissection or rupture from 5 to 3 mm/year.
Even after these revisions, the optimal timing of elective AAI for BAV-related aortopathy continues to be debated and has been the focus of institutional case series.15–19 However, the real-world landscape of surgery for BAV-related aortopathy, and the degree to which patient and institution-specific factors influence decisions for aortic interventions, are not well characterized.
The International Bicuspid Aortic Valve Consortium (BAVCon) and the National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC) are observational research registries that include clinical data and images of participants with BAV.20–25 Using BAVCon and GenTAC data, we examined the patterns of surgical intervention for BAV and related aortopathy, seeking to identify factors associated with increased likelihood of elective combined AVR and AAI in patients with BAV, and relevant subgroups.
METHODS
Databases and Subjects
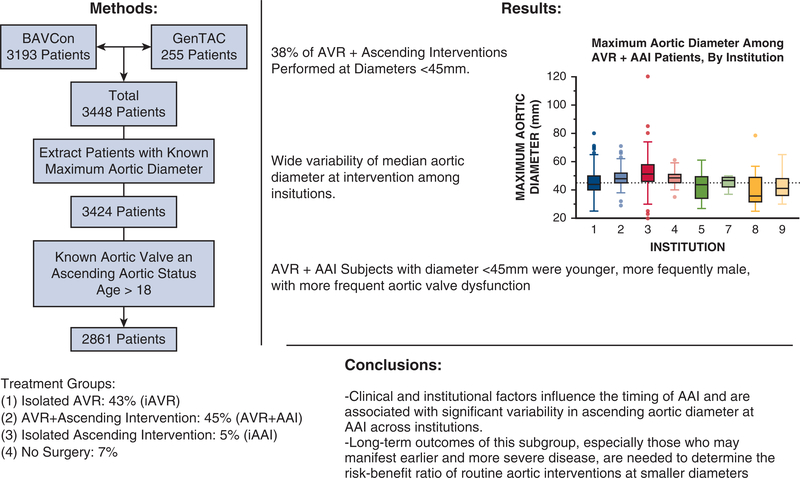
BAVCon is an international longitudinal registry that enrolls patients aged ≥8 years with BAV as well as TAV controls. Informed consent was obtained from patients at the individual sites. Abstracted data include demographic characteristics, medical history, serial images, and aortic operations.20 GenTAC was a longitudinal registry for patients with heritable thoracic aortic disease, including BAV with aortic enlargement.22 Clinical data and biologic specimens were collected as described.25 Protocols were approved by local institutional review boards at all participating sites. Appropriate data sharing agreements were established before transfer of protected health information. After patient data from all databases was collated, patients with known maximum aortic size, surgical status, and age ≥18 years were extracted for analysis (Figure 1).
FIGURE 1.
Consort diagram demonstrating patient data compilation and extraction to arrive at our final 2861 bicuspid aortic valve patient cohort for analysis. BAVCon, International Bicuspid Aortic Valve Consortium. GenTAC, National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions.
Definitions
Participants were grouped according to treatment strategy and aortic diameter. Treatment groups included those who underwent no surgery, isolated aortic valve replacement (iAVR), isolated ascending aortic intervention (iAAI), or AVR+AAI. Aortic diameter was recorded at several locations for each patient (eg, annulus, sinus of Valsalva, sinotubular junction, or ascending aorta). The reported maximum diameter is the largest of any recorded measurements from the aortic annulus to the aortic arch. Aortic measurements were obtained by echocardiography for 2176 patients, magnetic resonance imaging for 316 patients, computed tomography for 121 patients, and by unknown modality for the remaining 248 patients, including the operative record in many of these cases. For the nonsurgical group of patients, aortic measurements represent the largest recorded measurement available at the time of our BAVCon and GenTAC data requests. Aortic regurgitation and aortic stenosis were considered to be present if coded as moderate or severe, and to be absent if coded as none, trace, or mild. Aortic cross-sectional area to height ratio was calculated using previously published methodology.26
Statistical Analysis
Univariate analyses were performed using Student t, Wilcoxon rank sum, χ2, Fisher exact, or Kruskal-Wallis test with post-hoc Dunn test of honest statistical difference between individual groups, as appropriate. Assignment to iAVR or AVR+AAI was evaluated using multilevel logistic regression with random intercepts to account for institutional heterogeneity. The model included all variables that were significantly different between treatment groups (P < .05), variables that were independently associated with aortic valve dysfunction, and hypertension, while avoiding collinear variables to prevent destabilizing the model.27 We conducted multiple imputations28 by chained equations to impute missing data for aortic regurgitation, aortic stenosis, smoking, diabetes, and hypertension (13%, 15%, 21%, 24%, and 13%, respectively). We generated 10 imputed datasets and reported the combined adjusted odds ratios (ORs) with their 95% confidence intervals (CIs) and P values.29 To evaluate the potential impact of imputation, we profiled patients with extensive missing data to identify systematic differences in other variables (Tables E1 and E2) and performed sensitivity analyses after excluding the imputed variables or variables with missing data (Tables E3 through E5). All analysis was performed using Stata statistical software, version 15 (StataCorp, College Station, Tex).
TABLE E1.
Univariate comparison of variables with known values between patients with unknown smoking status versus those with known smoking status. Comparisons were done for only nonmissing variables, among patients who underwent isolated aortic valve replacement or combined aortic valve replacement and ascending aortic intervention (n = 2522)
| Variable | Missing smoking data (n = 587) | Known smoking status (n = 1935) | P value |
|---|---|---|---|
| Aortic diameter (mm) | 44.8 ± 10.5 | 41.0 ± 8.1 | <.001 |
| Age (y) | 52.0 ± 14.1 | 59.1 ± 12.1 | <.001 |
| Male sex | 454 (77.3) | 1463 (75.6) | .388 |
Values for continuous variables are presented as mean ± standard deviation, whereas values for categorical variables are presented as n (%). P values were calculated by Student t test for continuous variables and χ2 test for categorical variables.
TABLE E2.
Univariate comparison of variables with known values between patients with unknown diabetes status versus those with known diabetes status. Comparisons were done for only nonmissing variables, among patients who underwent isolated aortic valve replacement or combined aortic valve replacement and ascending aortic intervention (n = 2522)
| Variable | Missing diabetes data (n = 424) | Known diabetes status (n = 2098) | P value |
|---|---|---|---|
| Aortic Diameter (mm) | 46.7 ± 10.0 | 40.9 ± 8.3 | <.001 |
| Age (y) | 54.7 ± 13.4 | 58.0 ± 12.8 | <.001 |
| Male Sex | 337 (79.5) | 1580 (75.3) | .067 |
Values for continuous variables are presented as mean ± standard deviation, whereas values for categorical variables are presented as n (%). P value was calculated by Student t test for continuous variables and χ2 test for categorical variables.
TABLE E3.
Sensitivity analysis showing multilevel regression model, with random effects by institution, using imputed data for only variables with <10% missing data (aortic regurgitation, aortic stenosis, and hypertension) (n = 1821)
| Variable | Odds ratio* | P value | 95% Confidence interval |
|---|---|---|---|
| Diameter (mm) | 1.31 | <.001 | 1.28–1.35 |
| Age (y) | 0.98 | .002 | 0.97–0.99 |
| Male sex | 1.07 | .674 | 0.78–1.48 |
| Aortic regurgitation | 0.97 | .843 | 0.70–1.34 |
| Aortic stenosis | 0.47 | <.001 | 0.34–0.64 |
| Hypertension | 0.83 | .233 | 0.60–1.13 |
| Diabetes mellitus | 0.77 | .134 | 0.55–1.09 |
| Smoking | 0.69 | .208 | 0.38–1.23 |
Odds ratio >1 indicates increased likelihood of combined aortic valve replacement and ascending aortic intervention. Odds ratio <1 indicates increased likelihood of isolated aortic valve replacement.
TABLE E5.
Sensitivity analysis showing multilevel regression model, with random effects by institution, using only variables with no missing data (n = 2432)
| Variable | Odds ratio* | P value | 95% Confidence interval |
|---|---|---|---|
| Diameter (mm) | 1.25 | <.001 | 1.23–1.28 |
| Age (y) | 0.98 | <.001 | 0.97–0.98 |
| Male sex | 1.04 | .716 | 0.81–1.36 |
Odds ratio >1 indicates increased likelihood of combined aortic valve replacement and ascending aortic intervention. Odds ratio <1 indicates increased likelihood of isolated aortic valve replacement.
RESULTS
Characteristics of the Study Cohort
After extracting adult patients with available aortic measurements and surgical status, the study cohort included 2861 patients at 18 institutions in the United States, Canada, and Europe (Table 1). The median age was 59 years and 2153 participants (75%) were men. The maximum aortic diameter at the time of surgery was <45 mm in 1786 patients (62% ), 45 to 49 mm in 496 patients (17%), 50 to 54 mm in 363 patients (13%), and ≥55 mm in 216 patients (7.6%). One thousand eighty-three patients (43%) had moderate or severe aortic regurgitation, 1399 patients (57%) had moderate or severe aortic stenosis, and 511 patients (21%) had combined regurgitation and stenosis. Genetic syndromes, including Turner syndrome (3 patients; 0.4%), Marfan syndrome (17 patients; 1.1%), or a self-reported family history of aneurysms or dissections (32 patients; 3.0%), were infrequent in the study cohort.
TABLE 1.
Baseline characteristics of the study cohort (N = 2861)
| Variable | No surgery (n = 193) | iAVR (n = 1224) | iAAI (n = 146) | AVR+AAI (n = 1298) | P value |
|---|---|---|---|---|---|
| Aortic diameter (mm) | 40.6 ± 5.6 | 37.0 ± 5.9 | 50.3 ± 8.7 | 46.5 ± 8.7 | <.001 |
| Aortic diameter <45 mm | 153 (79.3) | 1117 (91.3) | 28 (19.2) | 488 (37.6) | <.001 |
| Aortic diameter 45–49 mm | 25 (13.0) | 78 (6.4) | 37 (25.3) | 356 (27.4) | <.001 |
| Aortic diameter 50–54 mm | 15 (7.8) | 25 (2.0) | 37 (25.3) | 286 (22.0) | <.001 |
| Aortic diameter ≥55 mm | 0 (0) | 4 (0.3) | 44 (30.1) | 168 (12.9) | <.001 |
| Age (y) | 62.2 ± 14.6 | 59.8 ± 13.0 | 49.4 ± 12.7 | 55.3 ± 12.6 | <.001 |
| Male sex | 127 (65.8) | 885 (72.3) | 109 (74.7) | 1032 (79.5) | <.001 |
| Height (cm)* | 173.0 ± 11.5 | 172.3 ± 10.1 | 175.2 ± 13.2 | 175.4 ± 10.0 | <.001 |
| Weight (kg)† | 85.6 ± 21 | 83.7 ± 17.9 | 86.8 ± 19.7 | 87.1 ± 18.6 | <.001 |
| Body surface area (m2)‡ | 1.99 ± 0.3 | 1.96 ± 0.23 | 2.02 ± 0.28 | 2.02 ± 0.24 | <.001 |
| Aortic cross-sectional area to height ratio* (cm2/m) | 7.5 ± 1.9 | 6.4 ± 2.0 | 10.8 ± 2.8 | 9.5 ± 3.1 | <.001 |
| Moderate or severe aortic insufficiency§ | 32 (17.9) | 438 (41.1) | 43 (30.1) | 570 (51.4) | <.001 |
| Moderate or severe aortic stenosis∥ | 39 (26.2) | 820 (76.6) | 14 (10.5) | 526 (47.9) | <.001 |
| Mixed aortic regurgitation and stenosis¶ | 6 (4.2) | 269 (25.4) | 3 (2.3) | 233 (21.5) | <.001 |
| Hypertension# | 118 (61.1) | 782 (67.3) | 35 (48.6) | 700 (66.3) | .006 |
| Smoking** | 82 (45.3) | 125 (11.8) | 12 (16.7) | 73 (8.3) | <.001 |
| Hyperlipidemia†† | 91 (49.7) | 428 (75.4) | 13 (72.2) | 352 (64.9) | <.001 |
| Diabetes mellitus‡‡ | 0 (0) | 237 (21.5) | 4 (5.7) | 150 (15.1) | <.001 |
| Coronary artery disease§§ | 15 (4.2) | 227 (30.1) | 8 (13.1) | 109 (18.2) | <.001 |
| Stroke or TIA∥∥ | 5 (6.2) | 48 (9.8) | 2 (7.1) | 48 (8.8) | .737 |
| Turner syndrome¶¶ | – | 0 (0) | 2 (4.8) | 1 (0.3) | .003 |
| Family history of aneurysm or dissection## | – | 10 (1.8) | 2 (4.9) | 20 (4.2) | .059 |
| Marfan syndrome*** | – | 4 (0.5) | 2 (4.6) | 11 (1.5) | .019 |
Values for continuous variables are presented as mean ± standard deviation, whereas values for categorical variables are presented as as n (%). P values were calculated by Kruskal-Wallis test for continuous variables and χ2 test for categorical variables with n >5 or Fisher exact test if n ≤5. iAVR, Isolated aortic valve replacement; iAAI, isolated ascending aortic intervention; AVR+AAI, aortic valve replacement and ascending aortic intervention; TIA, transient ischemic attack.
n = 2150.
n = 2138.
n = 2097.
n = 2497.
n = 2451.
n = 2417.
n = 2483.
n = 2188.
n = 1311.
n = 2174.
n = 1527.
n = 1144.
n = 847.
n = 1066.
n = 1584.
Most patients (88%) underwent AVR, either alone or in combination with AAI, whereas 50% of patients underwent AAI, either alone or in combination with AVR. When surgical interventions were further characterized, 193 patients (6.8%) underwent no surgery, 1224 patients (43%) underwent isolated AVR (iAVR), 146 patients (5.1%) underwent isolated ascending aortic intervention (iAAI), and 1298 patients (45%) underwent AVR+AAI.
Aortic Diameter at Surgery
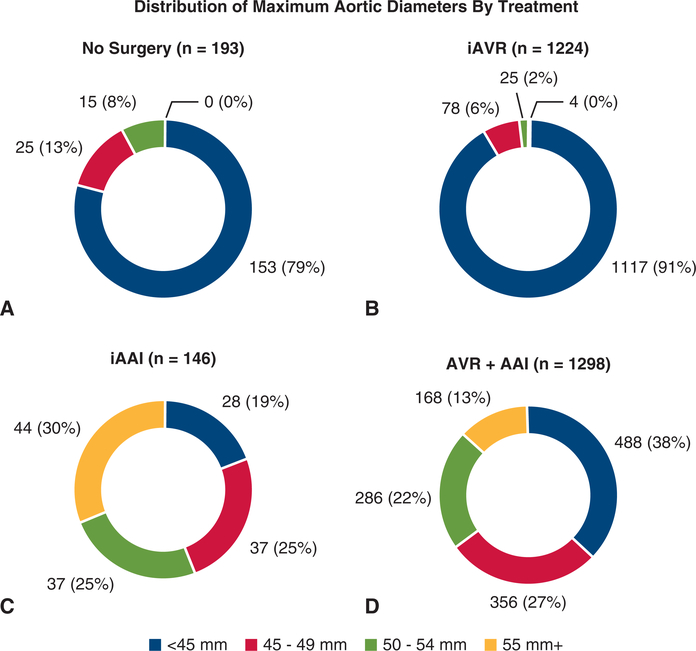
The mean aortic diameters of each surgical group were significantly different (No Surgery 40.6 ± 5.6 mm, Iavr 37.0 ± 5.9 mm, iAAI 50.3 ± 8.7 mm, AVR+AAI ± 8.7 mm; P < .001). These differences remained significant even after adjustment for body size using mean aortic cross-sectional area to height ratios (No Surgery ± 1.9 cm2/m, iAVR 6.4 ± 2.0 cm2/m, iAAI 10.8 ± 2.8 cm2/m, AVR+AAI 9.5 ± 3.1 cm2/m; P < .001 for all comparisons by Dunn test). Two hundred twelve of 216 patients with aortic diameter ≥55 mm underwent AAI, including 168 (78%) AVR+AAI and 44 (20%) iAAI operations. The remaining 4 (1.9%) underwent iAVR, and no patients in this subgroup were observed without surgery. 516 (29%) of 1786 patients with aortic diameter <45 mm underwent AAI, including 28 (1.6%) iAAI and 488 (27%) AVR+AAI operations. The 488 patients with aortic diameters <45 mm who underwent AVR+AAI represented 38% of the AVR+AAI group (Figure 2).
FIGURE 2.
Distribution of maximum aortic diameter according to treatment group. A, No Surgery group. B, Isolated aortic valve replacement (iAVR) group. C, Isolated ascending aortic intervention (iAAI) group. D, AVR+AAI group. AVR+AAI, Combined aortic valve replacement plus ascending aortic intervention.
Because 88% of patients (2522 out of 2861) underwent either iAVR or AVR+AAI, we focused our initial comparisons on these 2 groups (Table 1). Patients in the AVR+AAI group had larger mean aortic diameters (46.5 ± 8.7 mm vs 37.0 ± 5.9 mm; P < .001 by Dunn test) and were taller (175.4 ± 10.0 cm vs 172.3 ± 10.1 cm; P < .001 by Dunn test), with larger aortic cross-sectional area-to-height ratios (9.5 ± 3.1 cm2/m vs 6.4 ± 2.0 cm2/m; P < .001 by Dunn test), than patients undergoing iAVR. Aortic regurgitation was also significantly more prevalent in the AVR+AAI group (51% vs 41%; P < .001), but aortic stenosis (48% vs 77%; P < .001) or combined valve disease (21% vs 25%; P = .03) was less prevalent. Compared with patients undergoing AVR+AAI, cardiovascular comorbidities, including active smoking (12% vs 8.3%; P = .01), hyperlipidemia (75% vs 65%; P < .001), diabetes (21% vs 15%; P < .001), and coronary artery disease (30% vs 18%; P < .001), were more common in the iAVR group.
iAVR Versus AVR+AAI: Associated Clinical Characteristics
Patients who underwent iAVR or AVR+AAI comprised the largest study groups. Therefore, we performed multilevel regression assuming random effects by institution to identify distinguishing clinical characteristics (Table 2). We found that aortic diameter (OR, 1.24; 95% CI, 1.22–1.27; P < .001) was associated with AVR+AAI, whereas increased age in years (OR, 0.98; 95% CI, 0.97–0.99; P < .001), aortic stenosis (OR, 0.48; 95% CI, 0.37–0.63; P < .001), and smoking (OR, 0.56; 95% CI, 0.35–0.92; P = .02) were associated with iAVR. The presence of aortic regurgitation, hypertension, or diabetes was not independently associated with either operation. Despite differences among groups with and without missing data regarding smoking status or diabetes (Tables E1 and E2), which each required >20% imputation in our model, the direction and approximate magnitude of the effect size of each variable reported above was conserved in all sensitivity analyses (Tables E3–E5).
TABLE 2.
Multilevel regression model, with random effects by institution, using imputed data
| Variable | Odds ratio* | P value | 95% Confidence interval |
|---|---|---|---|
| Diameter (mm) | 1.24 | < .001 | 1.22–1.27 |
| Age (y) | 0.98 | <.001 | 0.97–0.99 |
| Male sex | 1.03 | .843 | 0.78–1.35 |
| Aortic regurgitation | 0.88 | .391 | 0.65–1.19 |
| Aortic stenosis | 0.48 | <.001 | 0.37–0.63 |
| Hypertension | 0.94 | .662 | 0.72–1.23 |
| Diabetes mellitus | 0.78 | .107 | 0.57–1.06 |
| Smoking | 0.56 | .023 | 0.35–0.92 |
Odds ratio >1 indicates increased likelihood of aortic valve replacement plus ascending aortic intervention. Odds ratio <1 indicates increased likelihood of isolated aortic valve replacement.
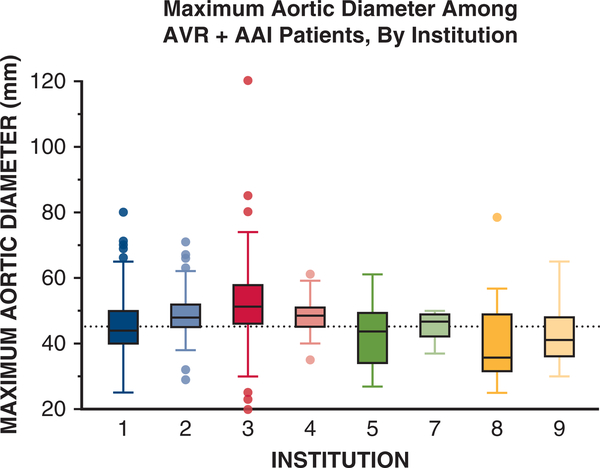
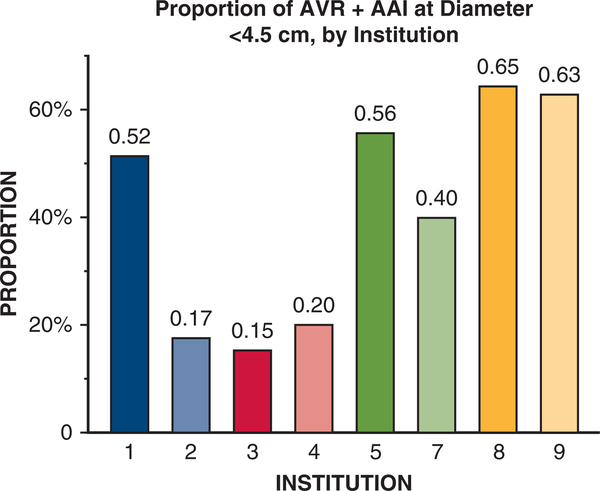
The institutions where subjects were evaluated also strongly influenced mean aortic diameters at elective AVR+AAI operations. We observed extensive variation of median aortic diameters (range, 39–52 mm) at the time of AVR+AAI across 9 referral centers that accounted for more than 95% (2767 out of 2861 patients) of the cohort (Figure 3). We found that at least 40% of AVR+AAI cases were performed when the maximum aortic diameter was <45 mm at 5 of the 9 largest contributing institutions (Figure 4). We observed similar trends in data from the other 9 participating institutions (data not shown). Across 3 different time periods (1996–2005, 2006–2009, and 2010–2015), mean aortic diameter at the time of AVR+AAI did not significantly change (46.8 ± 9.0 mm, 46.3 ± 7.9 mm, and 47.5 ± 8.1 mm; P = .23, all individual comparisons > .05 by Dunn test).
FIGURE 3.
Maximum aortic size at the time of aortic valve replacement plus ascending aortic intervention (AVR+AAT) by institution. Institutions are presented in decreasing order of patients treated. Boxes represent median and interquartile range for aortic diameter at the time of AVR+AAI by institution. Reference line represents 45 mm on the y-axis. For boxes, the midline represents the median value, upper and lower box edges represent the upper and lower interquartile range, respectively. Whiskers represent maximum and minimum values of nonoutliers and additional data points represent outliers. Nine of 18 contributing institutions were included because they contributed more than 95% of patient data (2767 out of 2861) for this study. Institution 6 is missing because all reported cases from this institution were either isolated AVR or isolated AAI.
FIGURE 4.
Frequency of aortic valve replacement plus ascending aortic intervention (AVR+AAI) for aortic diameter <45 mm among all cases of AVR+AAI, by institution. Institutions are presented in decreasing order of patients treated. Nine of 18 contributing institutions were included because they contributed more than 95% of patient data (2767 out of 2861) for this study. Institution 6 is missing because all reported cases from this institution were either isolated AVR or isolated AAI.
Characteristics of Patients With Aortic Diameters <45 mm at AAI
In the subgroup of patients with aortic diameters <45 mm, we compared patients who underwent any AAI (iAAI or AVR+AAI) and patients who underwent no surgery (Table 3). The mean aortic diameters of these subgroups were not significantly different (38.6 ± 4.3 mm vs 38.4 ± 5.2 mm; P = .79). Patients in the AAI group were younger (54.2 ± 13.2 years vs ± 15.0 years; P < .001), more frequently men (73.5% vs 62.1%; P = .007), with more prevalent aortic regurgitation (52% vs 18%; P < .001), aortic stenosis (53% vs 28%; P < .001), and mixed aortic valve dysfunction (26% vs 5%; P < .001), than patients who underwent no surgery. The prevalence of hyperlipidemia (67% vs 51%; P = .001) and smoking (7.7% vs 43%; P < .001), but not hypertension (66% vs 61%; P = .366) or coronary artery disease (15% vs 12%; P = .470), were also significantly different between groups.
TABLE 3.
Subgroup of patients with aortic diameter <45 mm who underwent no surgery versus those who underwent any ascending aortic intervention (isolated ascending aortic intervention or aortic valve replacement plus ascending aortic valve replacement) (n = 669)
| Variable | <45 mm and no surgery (n = 153) | <45 and any ascending intervention (n = 516) | P value |
|---|---|---|---|
| Aortic diameter (mm) | 38.6 ± 4.3 | 38.4 ± 5.2 | .788 |
| Age (y) | 61.5 ± 15.0 | 54.2 ± 13.2 | <.001 |
| Male sex | 95 (62.1) | 379 (73.5) | .007 |
| Height (cm)* | 171.4 ± 10.9 | 173.8 ± 10.4 | .055 |
| Weight (kg)† | 83.5 ± 21.2 | 84.1 ± 19.0 | .810 |
| Body surface area (m2)‡ | 1.95 ± 0.25 | 1.98 ± 0.25 | .369 |
| Aortic cross-sectional area to height ratio (cm2/m)* | 6.8 [±1.4] | 6.8 ± 1.7 | .789 |
| Aortic regurgitation§ | 25 (17.6) | 221 (51.9) | <.001 |
| Aortic stenosis∥ | 33 (28.0) | 218 (52.7) | <.001 |
| Mixed aortic regurgitation and stenosis¶ | 6 (5.3) | 106 (26.4) | <.001 |
| Hypertension# | 94 (61.4) | 296 (65.5) | .366 |
| Smoking** | 62 (43.1) | 28 (7.7) | <.001 |
| Hyperlipidemia†† | 73 (50.7) | 189 (67.0) | .001 |
| Diabetes mellitus‡‡ | 0 (0) | 76 (17.6) | .596 |
| Coronary artery disease§§ | 10 (11.5) | 32 (15.0) | .470 |
| Stroke or TIA∥∥ | 3 (4.6) | 32 (10.4) | .101 |
| Turner syndrome¶¶ | - | 2 (3.1) | N/A |
| Family history of aneurysm or dissection## | - | 9 (6.1) | N/A |
| Marfan syndrome*** | - | 6 (2.1%) | N/A |
Values for continuous variables are presented as mean ± standard deviation, whereas values for categorical variables are presented as n (%). P values calculated by Student t test for continuous variables and χ2 test for categorical variables with n >5 or Fisher exact test for n ≤5. TIA, Transient ischemic attack. N/A, not available.
n = 476.
n = 470.
n = 458.
n = 568.
n = 532.
n = 584.
n = 605.
n = 507.
n = 426.
n = 439.
n = 301.
n = 374.
n = 64.
n = 148.
n = 289.
DISCUSSION
In a large multinational cohort of BAVCon and GenTAC registry participants, we observed considerable variation in the demographic characteristics and aortic diameters of patients who underwent elective aortic procedures. Surprisingly, nearly two thirds of the entire cohort was operated on when their maximum aortic diameters were <45 mm, including 38% of patients in the AVR+AAI subgroup. Patients who received elective AVR+AAI were on average younger, with larger aortic diameters, and more frequent aortic regurgitation. Obvious high-risk characteristics that might predict aortic events, including Marfan syndrome, Turner syndrome, and a family history of aortic aneurysms or dissections, were infrequent and do not explain the preponderance of AAI at small diameters. These data demonstrate that early referral for AAI is more frequent than is currently recommended, based on prospective observations that the risk of acute aortic dissections or deaths is low in most BAV cohorts. These, and other key points of our research methodology, results, and conclusions are additionally summarized in Figure 5 and Video 1.
FIGURE 5.
Key points of our research methods, findings, and conclusions. After isolating 2861 patients with bicuspid aortic valve (BAV), treatment groups were defined. We subsequently examined both clinical and institutional factors associated with aortic intervention at diameters <45 mm in patients with BAV. BAVCon, International Bicuspid Aortic Valve Consortium; GenTAC, National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions; iAVR, Isolated aortic valve replacement; AVR+AAI, combined aortic valve replacement and ascending aortic intervention; iAAI, isolated ascending aortic replacement.
We also found extensive variation between institutions in the timing and extent of aortic operations for BAV aortopathy. At several study sites, almost half of elective AVR+AAI operations were performed when the maximum aortic diameter was <45 mm. This institutional effect was consistently strong and independent of patient-specific factors over the 20-year study period, even as guideline thresholds for elective AAI have increased. There are many potential explanations for this discrepancy. Most instructive data about surgical management of BAV aortopathy are derived from single-center experiences16,17,26,30 that may be compromised by significant bias.21,31 These types of studies live in the numerator8 because they frequently do not include the substantial proportion of patients with BAV who never require surgery and tend to reinforce the perception that most BAV patients are at increased risk for aortic events. In a recent survey about BAV aortopathy,31 78% of surgeons responded that they would opt for AAI at diameters <50 mm in patients who did not require AVR, and 31 % would operate at <40 mm when AVR is planned.31 These results underscore frequent observations that guidelines do not substitute for clinical judgment or capture the complexity of individualized decisions about surgery.
There is no conclusive evidence that elective AAI prevents acute aortic dissections in BAV aortopathy. In fact, observational studies found that aortic dilation progresses slowly in patients with BAV when baseline diameters <45 mm5 and may not progress at all after iAVR in patients with isolated aortic valve dysfunction and preoperative diameters of 40 to 50 mm.32 A recent meta-analysis found that patients with BAV without significant baseline aortic dilation demonstrated mean ascending dilation rates of 1 mm/year and had comparable aortic event rates to patients with TAV.33 Prophylactic aortic replacement exposes patients to additional risks that may not be adequately compensated by proportional reductions in deaths, aortic dissections, or second operations for progressive dilation.19 Patients who underwent AAI at smaller diameters (<45 mm) tended to be younger, presented with more severe aortic disease, and had a greater burden of cardiovascular risk factors. Unfortunately, there is little longitudinal data to assess the surgical complications and long-term outcomes of patients with BAV after early AAI. In the absence of relevant long-term data, surgical decisions for BAV patients may be considered uniformly, when only some may benefit from early AAI. To better discern the subpopulation most likely to benefit from early AAI, additional natural history data and prospective studies analyzing morbidity and late aortic events are necessary to identify high-risk patients who may benefit from early interventions.
The clinical assessment of patients with BAV before aortic surgery can be improved by the systematic application of currently available tools. Measurements based on double-oblique computed tomography or magnetic resonance images,34 or normalization of diameters to body size using cross-sectional ratios or z scores, demonstrate improved reproducibility and discriminatory value compared with unadjusted aortic diameters.17,26 Decision tools can also be expanded to account for factors other than aortic diameters and expansion rates, such as clinical and genetic variables, long-term outcomes, or plans for concomitant AVR, to allow for more individualization of surgical decision making. This will require a strategic shift from models based on single-center case series to multicenter registry data that may be more representative of the general BAV population. For prospective evaluation of prognostic variables to include in these models, future studies should also include patients with BAV who never require interventions to create evidence-based recommendations with realistic estimates of aortic risk.
Limitations
Patients who did not undergo aortic or valvular interventions were underrepresented in our study cohort. Some analyses were limited by missing data, which may collectively impart significant information bias. We were restricted to AAI or AVR as binary outcomes and could not specify the type or extent of aortic interventions (eg, root, supracoronary, ascending, hemi-arch, or total arch). The Sievers35 classification of valve morphology, which may influence the likelihood of valve or aortic interventions, was not available for analysis.36 Heterogeneity of diagnostic imaging modalities and image interpretation may decrease the reproducibility of measurements. The cross-sectional study design precluded longitudinal measurements of aortic enlargement, which might indicate additional risk.
CONCLUSIONS
In a multicenter study of 2861 registry participants with BAV, we describe clinical characteristics that vary among patients treated with iAVR, iAAI, AVR+AAI, or no surgery. A substantial proportion of subjects underwent AAI when the maximum aortic diameter was <45 mm. These patients were younger and had more significant valve dysfunction than peers who were managed nonoperatively. Long-term outcomes of this subgroup of patients who may manifest earlier and more severe disease are needed to determine the risk–benefit ratio of routine aortic interventions at smaller diameters. Because BAV affects more than 3 million people in the United States alone, evidence-based changes in clinical practice have the potential for substantial improvement of the morbidity and mortality associated with aortic disease.
Supplementary Material
VIDEO 1. Illustrative video reviewing key points of our manuscript. Video available at: https://www.jtcvs.org/article/S0022-5223(19)31583-1/fulltext.
TABLE E4.
Sensitivity analysis showing multilevel regression model, with random effects by institution, without imputed data (n = 1514)
| Variable | Odds ratio* | P value | 95% Confidence interval |
|---|---|---|---|
| Diameter (mm) | 1.36 | <.001 | 1.32–1.41 |
| Age (y) | 0.98 | .002 | 0.97–0.99 |
| Male sex | 1.10 | .604 | 0.76–1.60 |
| Aortic regurgitation | 0.92 | .634 | 0.67–1.28 |
| Aortic stenosis | 0.51 | <.001 | 0.37–0.71 |
| Hypertension | 0.87 | .460 | 0.62–1.24 |
| Diabetes mellitus | 0.73 | .123 | 0.49–1.09 |
| Smoking | 0.66 | .237 | 0.34–1.30 |
Odds ratio >1 indicates increased likelihood of combined aortic valve replacement and ascending aortic intervention. Odds ratio <1 indicates increased likelihood of isolated aortic valve replacement.
Acknowledgments
Dr Prakash is supported by grant No. R01HL137028 and Mr Body is supported by grant No. R01HL114823. The GenTAC Registry was supported by contracts HHSN268200648199C and HHSN268201000048C from the National Heart, Lung, and Blood Institute and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda.
The BAVCon Investigators and GenTAC Registry Investigators are listed in the Acknowledgments.
The authors thank Chris Akers for his assistance with the illustrations and figures.
BAVCon Investigators: Eduardo Bossone, MD, PhD (A. Cardarelli Hospital); Rodolfo Citro, MD, PhD; Simon Body, MB, ChB, MPH, J. Daniel Muehlschlegel, MD, MMSc, Jasmine T. Shahram, BS, Thy B. Nguyen, BA (Brigham and Women’s Hospital); Vicenza Stefano Nistri, MD (CMSR Veneto Medica); Dan Gilon, MD, Ronen Durst, MD (Hadassah Hebrew University); Carlo de Vincentiis, MD, Francesca R. Pluchinotta, MD (IRCCS Policlinico San Donato); Thoralf M. Sundt, MD (Massachusetts General Hospital); Hector I. Michelena, MD (Mayo Clinic); Giuseppe Limongelli, MD (Monaldi Hospital, Italy); Patrick M. McCarthy, MD, S. Chris Malaisrie, MD, Aakash Bavishi, MD (Northwestern University); Malenka M. Bissell, MD, DPhil (University of Leeds); Gordon S. Huggins, MD (Tufts Medical Center); Victor Dayan, MD (Universidad de la República, Uruguay); Francois Dagenais, MD (Université Laval); Alessandro Della Corte, MD, PhD (University of Campania); Evaldas Girdsaukas, MD, PhD (University Heart Center Hamburg); Bo Yang, MD, PhD, Kim Eagle, MD (University of Michigan); Siddharth K. Prakash, MD, PhD, Dianna M. Milewicz, MD, PhD, Tom C. Nguyen, MD, Harleen K. Sandhu, MD, MPH, Hazim J. Safi, MD (The University of Texas Health Science Center at Houston); Josh C. Denny, MD (Vanderbilt University); Arturo Evangelista, MD, Laura Galian-Gay, MD (Vall d’Hebron University Hospital).
GenTAC Registry Investigators: Kim A. Eagle MD (University of Michigan); Williams Ravekes, MD, Harry C. Dietz, MD, PhD, Kathryn W. Holmes, MD, Jennifer Habashi, MD (Johns Hopkins University); Dianna M. Milewicz, MD, PhD, Siddharth K. Prakash, MD, PhD (The University of Texas Health Science Center at Houston); Scott A. LeMaire, MD, Joseph S. Coselli MD, Shaine A. Morris, MD (Baylor College of Medicine); Cheryl L. Maslen, PhD, Howard K. Song, MD, PhD, G. Michael Silberbach, MD (Oregon Health and Science University); Reed E. Pyeritz, MD, PhD, Joseph E. Bavaria, MD, Karianna Milewski, MD, PhD (University of Pennsylvania); Richard B. Devereux, MD, PhD, Jonathan W. Weinsaft, MD, Mary J. Roman, MD (Weill Medical College of Cornell University); Ralph V. Shohet, MD; (The Queen’s Medical Center); Nazli McDonnell, MD (National Institute on Aging); Federico M. Asch, MD (MedStar Health Research Institute); H. Eser Tolunay, PhD, Patrice Desvigne-Nickens, MD (National Heart, Lung, and Blood Institute); Hung Tseng, PhD (National Institute of Arthritis, Musculoskeletal and Skin Diseases); Barbara L. Kroner, PhD (RTI International).
Abbreviations and Acronyms
- AAI
ascending aortic intervention
- AVR
aortic valve replacement
- AVR+AAI
combined aortic valve replacement and ascending aortic intervention
- BAV
bicuspid aortic valve
- BAVCon
International Bicuspid Aortic Valve Consortium
- GenTAC
National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions
- iAAI
isolated ascending aortic intervention
- iAVR
isolated aortic valve replacement
- TAV
tricuspid aortic valve
Biography

Dr Marjan Jahangiri (London, United Kingdom). I would like to thank the Association for the privilege to discuss this manuscript and thank the authors for having sent it to me in advance.
Dr Nissen and colleagues report on 2861 patients with bicuspid aortic valve (BAV) reported into 2 national and international registries. The cohort is reported over a 19-year period of 18 institutions; that makes fewer than 10 cases per year per institute. Patients had a variety of interventions for aortic valve and the aorta. It was of the nature of these registries that the authors do not know the exact type of these operations. Furthermore, the registries and the manuscript do not provide any results of surgery and follow-up. The authors report that up to 60% of patients had interventions below 4.5 cm, the majority of which were performed in high-volume centers. So it was difficult to draw any other conclusions due to the lack of data in the registries and the heterogeneous nature of the population. However, and I think very importantly, you have highlighted and drawn our attention to the lack of data and inconsistency in the management of a condition that affects 2% of the population.
I would like to make some comments and I have a few brief questions. I apologize if my comments include some of those of Dr Sundt and Dr Gleason.
The risk of aortic emergencies are higher in BAV patients. We are looking for the optimum time of intervention when the risk of conservative management becomes higher than the risk of surgery. In finding that optimum timing, I think we should consider a few things.
About one third of patients dissect below the diameter of the guideline. Mortality for dissection in the best hands is about 10% to 15%. However, mortality for even more extensive intervention in BAV aortopathy—be it valve-sparing root replacement or aortic root replacement—is less than 1% or 2%, as you have heard.
Current reports in the literature are inconsistent regarding measurement of the aorta, using echo in systole, in diastole, outer-to-outer edge, inner-to-inner edge, computed tomography imaging, magnetic resonance imaging, which axial view. Then with this inconsistency of measurement, we haggle over half a centimeter. Speaking of imaging, should we include wall shear stress analysis to find the more vulnerable part of the aorta in identifying high-risk patients and consistently include indexes like the Svensson index?
The morphology of the aorta and the aortic valve are very important. We heard it from Dr Carrel earlier. Supracoronary as opposed to root dilation? Is it symmetrical or is it asymmetrical? What about the bulging of the greater curve? Replacement of the ascending aorta is really not a high-risk operation.
Again, another point came up: What about the arch and the need for follow-up? A member of the audience asked if the arch continues to grow. I think so far with the studies reported and some of the work we have done, the arch doesn’t grow.
If I may, 3 questions for you: You mention that the mean diameter for intervention has not changed over the years. This is surprising, because the guidelines 20-odd years ago were recommending bigger aortas to operate on; we weren’t doing anything below 5.5 cm. So Ijust found it surprising that the diameter hasn’t changed, and if anything, much of it remains stable. Might you be able to explain it?

Dr Alexander P. Nissen (Houston, Tex). I think that’s a very important point. And to get back to an earlier comment, we did have the dates of these interventions and it was over a long time span, but we broke the timing of the operation down essentially to 3 time periods, and, as you mentioned, showed that the median aortic diameter at the time of ascending intervention did not change across the entire span from which the data were collected. Our interpretation of that is essentially that along with the institutional variation we showed that essentially these institutional practice patterns may be fairly entrenched and may more or less not change based on institutional results, which generally at these referral centers are very good for elective aortic replacement regardless of the fact that guidelines have kind of crept toward being a bit more conservative in terms of observing rather than replacing smaller aortas.
Dr Jahangiri. Thank you. Only 15% of the cohort had had computed tomography or magnetic resonance images. It seems a very low number. Do you think, again, this was institutional practice?
Dr Nissen. I think part of this is related to the registries we used. In general, when multiple imaging modalities were available, we tended to stick with echo, especially within the International BAV Consortium cohort because this allowed some uniformity in terms of the assessment of the patients. Rather than switching back and forth, it allowed as many patients as possible to be assessed with the same imaging modality.
Dr Jahangiri. Thank you. About 10% of patients didn’t have anything done, and we don’t have any follow-up on them. Do you think there is any value to keep this in the cohort?
Dr Nissen. Absolutely, yes, 100%. I guess the simple way that I think about it is that as surgeons we should stop looking for BAV patients in our clinics and start enrolling them in our cardiologists’ clinics, because that’s really I think where this observed or nonsurgical cohort exists.
Dr Jahangiri. But have follow-up on them.
Dr Nissen. Yes, we need to boost that denominator as much as possible, and I think that’s absolutely something we plan to address in our follow-up study in terms of the longitudinal outcomes of these patients, not only in terms of their aortic dilation but also acute aortic events and any prophylactic replacement and postoperative outcomes.
Dr Jahangiri. Thank you very much.
Dr Nissen. Thanks very much, ma’am.
Unidentified Speaker. Do you think your demonstration of the significant variability between institutions is evidence of a collective equipoise about management of the small aorta that would justify a prospectively randomized trial of patients in that section? The vascular surgeons did it with abdominal aortic aneurysms. Can we do it?
Dr Nissen. I think that’s a very good question. I think it would be really helpful to look at the numbers that would be required, because if we need to enroll 10,000 patients, that might be very hard to do. But the 8 or 9 institutions we show were all large referral centers, so that patient accrual could be possible.
In terms of true equipoise of what we are preventing versus between-center risk, those are different questions. I think with the between-center risks there might be equipoise, because these all are high-volume centers with low risk. Equipoise in terms of how are we actually helping patients and what are we actually preventing, I don’t think anyone knows the answer to that. Those are really 2 questions and I can really only answer 1 of them.

Dr Lars G. Svensson (Cleveland, Ohio). So the question is, what would be your groups? Would it be medical management/concomitant surgery, aortoplasty, and replacement of the tube graft in the 4 to 4.5? Is that what you are proposing, something like that?
Unidentified Speaker. Those are exactly the issues one has to hammer out when designing the study, because if you are messy with those groups, we have seen what happens. I have seen that in trials where they mixed in radials with internal thoracic arteries, with a second internal thoracic artery, and so on and so forth. Exactly how that’s tailored is the critical point. I would like to see nonintervention versus intervention, myself.
Dr Nissen. That would be very interesting, but if we use raw size as an inclusion criterion, we are only going to further entrench the bias of using a bad marker of prediction of dissection. If we base our trial on the most available but worst decision-making tool, then we are just going to kind of dig that trench deeper. We need to really refine how we define size. I think lots of surgeons say “size” and mean different things, and that’s very important.
Footnotes
Conflict of Interest Statement
Dr Estrera has received support as a consultant from W.L. Gore & Associates. All other authors have nothing to disclose with regard to commercial support.
References
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–900. [DOI] [PubMed] [Google Scholar]
- 2.Eleid MF, Forde I, Edwards WD, Maleszewski JJ, Suri RM, Schaff HV, et al. Type A aortic dissection in patients with bicuspid aortic valves: clinical and pathological comparison with tricuspid aortic valves. Heart. 2013;99:1668–74. [DOI] [PubMed] [Google Scholar]
- 3.Farag ES, van Ooij P, Planken RN, Dukker KCP, de Heer F, Bouma BJ, et al. Aortic valve stenosis and aortic diameters determine the extent of increased wall shear stress in bicuspid aortic valve disease. J Magn Reson Imaging. 2018;48:522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shan Y, Li J, Wang Y, Wu B, Barker AJ, Markl M, et al. Aortic shear stress in patients with bicuspid aortic valve with stenosis and insufficiency. J Thorac Cardiovasc Surg. 2017;153:1263–72.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–12. [DOI] [PubMed] [Google Scholar]
- 6.Charlton-Ouw KM, Sandhu HK, Leake SS, Miller CC III, Afifi RO, Azizzadeh A, et al. New type A dissection after acute type B aortic dissection. J Vasc Surg. 2018;67:85–92. [DOI] [PubMed] [Google Scholar]
- 7.Januzzi JL, Isselbacher EM, Fattori R, Cooper JV, Smith DE, Fang J, et al. Characterizing the young patient with aortic dissection: results from the international registry of aortic dissection (IRAD). J Am Coll Cardiol. 2004;43: 665–9. [DOI] [PubMed] [Google Scholar]
- 8.Sundt TM III. Replacement of the ascending aorta in bicuspid aortic valve disease: where do we draw the line? J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S41–4. [DOI] [PubMed] [Google Scholar]
- 9.Bonow RO, Carabello B, de Leon AC, Edmunds LH Jr, Fedderly BJ, Freed MD, et al. ACC/AHA guidelines for the management of patients with valvular heart disease. Executive summary. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on management of patients with valvular heart disease). J Heart Valve Dis. 1998; 7:672–707. [PubMed] [Google Scholar]
- 10.Borger MA, Fedak PWM, Stephens EH, Gleason TG, Girdauskas E, Ikonomidis JS, et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: executive summary. J Thorac Cardiovasc Surg. 2018;156:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Catheter Cardiovasc Interv. 2010;76:E43–86. [DOI] [PubMed] [Google Scholar]
- 12.Hiratzka LF, Creager MA, Isselbacher EM, Svensson LG, Nishimura RA, Bonow RO, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Thorac Cardiovasc Surg. 2016;151:959–66. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Thorac Cardiovasc Surg. 2014; 148:e1–132. [DOI] [PubMed] [Google Scholar]
- 14.Bonow RO, Carabello BA, Kanu C, de Leon AC Jr, Faxon DP, Freed MD, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–231. [DOI] [PubMed] [Google Scholar]
- 15.Masri A, Kalahasti V, Alkharabsheh S, Svensson LG, Sabik JF, Roselli EE, et al. Characteristics and long-term outcomes of contemporary patients with bicuspid aortic valves. J Thorac Cardiovasc Surg. 2016;151:1650–9.e1. [DOI] [PubMed] [Google Scholar]
- 16.Sievers HH, Stock S, Stierle U, Klotz S, Charitos EI, Diwoky M, et al. Longer-term results, z scores, and decision nomograms for treatment of the ascending aorta in 1693 bicuspid aortic valve operations. J Thorac Cardiovasc Surg. 2018;155:549–59.e2. [DOI] [PubMed] [Google Scholar]
- 17.Wojnarski CM, Svensson LG, Roselli EE, Idrees JJ, Lowry AM, Ehrlinger J, et al. Aortic dissection in patients with bicuspid aortic valve-associated aneurysms. Ann Thorac Surg. 2015;100:1666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rinewalt D, McCarthy PM, Malaisrie SC, Fedak PW, Andrei AC, Puthumana JJ, et al. Effect of aortic aneurysm replacement on outcomes after bicuspid aortic valve surgery: validation of contemporary guidelines. J Thorac Cardiovasc Surg. 2014;148:2060–9. [DOI] [PubMed] [Google Scholar]
- 19.Kaneko T, Shekar P, Ivkovic V, Longford NT, Huang CC, Sigurdsson MI, et al. Should the dilated ascending aorta be repaired at the time of bicuspid aortic valve replacement? Eur J Cardiothorac Surg. 2018;53:560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International Bicuspid Aortic Valve Consortium (BAVCon). Available at: https://clinicaltrials.gov/ct2/show/study/NCT01980797. Accessed November 11, 2018.
- 21.Della Corte A, Body SC, Booher AM, Schaefers HJ, Milewski RK, Michelena HI, et al. Surgical treatment of bicuspid aortic valve disease: knowledge gaps and research perspectives. J Thorac Cardiovasc Surg. 2014;147: 1749–57.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroner BL, Tolunay HE, Basson CT, Pyeritz RE, Holmes KW, Maslen CL, et al. The national registry of genetically triggered thoracic aortic aneurysms and cardiovascular conditions (GenTAC): results from phase I and scientific opportunities in phase II. Am Heart J. 2011;162:627–32.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michelena HI, Prakash SK, Della Corte A, Bissell MM, Anavekar N, Mathieu P, et al. Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the international bicuspid aortic valve consortium (BAVCon). Circulation. 2014;129:2691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prakash SK, Bosse Y, Muehlschlegel JD, Michelena HI, Limongelli G, Della Corte A, et al. A roadmap to investigate the genetic basis of bicuspid aortic valve and its complications: insights from the international BAVCon (bicuspid aortic valve consortium). J Am Coll Cardiol. 2014;64:832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eagle KA. Rationale and design of the national registry of genetically triggered thoracic aortic aneurysms and cardiovascular conditions (GenTAC). Am Heart J. 2009;157:319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg. 2003;126:892–3. [DOI] [PubMed] [Google Scholar]
- 27.Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. 2012;60:1854–63. [DOI] [PubMed] [Google Scholar]
- 28.Raghunathan T, Lepkowksi J, Van Hoewyk J, Solenbeger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27:85–95. [Google Scholar]
- 29.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10:585–98. [DOI] [PubMed] [Google Scholar]
- 30.Borger MA, Preston M, Ivanov J, Fedak PW, Davierwala P, Armstrong S, et al. Should the ascending aorta be replaced more frequently in patients with bicuspid aortic valve disease? J Thorac Cardiovasc Surg. 2004;128:677–83. [DOI] [PubMed] [Google Scholar]
- 31.Verma S, Yanagawa B, Kalra S, Ruel M, Peterson MD, Yamashita MH, et al. Knowledge, attitudes, and practice patterns in surgical management of bicuspid aortopathy: a survey of 100 cardiac surgeons. J Thorac Cardiovasc Surg. 2013; 146:1033–10340 e4. [DOI] [PubMed] [Google Scholar]
- 32.Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg. 2012;42:832–7. [DOI] [PubMed] [Google Scholar]
- 33.Hardikar AA, Marwick TH. Surgical thresholds for bicuspid aortic valve associated aortopathy. JACC Cardiovasc Imaging. 2013;6:1311–20. [DOI] [PubMed] [Google Scholar]
- 34.Mendoza DD, Kochar M, Devereux RB, Basson CT, Min JK, Holmes K, et al. Impact of image analysis methodology on diagnostic and surgical classification of patients with thoracic aortic aneurysms. Ann Thorac Surg. 2011;92:904–12. [DOI] [PubMed] [Google Scholar]
- 35.Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133: 1226–33. [DOI] [PubMed] [Google Scholar]
- 36.Schaefer BM, Lewin MB, Stout KK, Gill E, Prueitt A, Byers PH, et al. The bicuspid aortic valve: an integrated phenotypic classification of leaflet morphology and aortic root shape. Heart. 2008;94:1634–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
VIDEO 1. Illustrative video reviewing key points of our manuscript. Video available at: https://www.jtcvs.org/article/S0022-5223(19)31583-1/fulltext.