Abstract
Turner syndrome (TS) is a genetic condition characterized by partial or complete monosomy X. Alterations in hormonal function, height, and peer relationships, among other features and correlates of TS, appear to be risks for depressive illness. In order to summarize what is known about depression in Turner syndrome, with the aim of determining if individuals with TS are at increased risk for depression, a literature search and analysis was conducted. In total, 69 studies were identified and 35 met criteria of being peer-reviewed English language articles that collected original data on the experience of depression in individuals with TS. Most studies used patient or parent questionnaires to evaluate depressive symptoms. These studies, a majority of which examined adults and half that examined adolescents, found that individuals with TS experienced more frequent and severe depressive symptoms than individuals without TS diagnoses. Articles studying children with TS did not demonstrate a difference in their depressive experience compared to individuals without TS. Three articles used clinician administered scales, such as the Structured Clinical Interview for DSM-IV; all diagnosed depression in those with TS at higher rates than others. Five studies relied on expert opinion to evaluate depression. The remaining eight articles were case reports or case series that relied on expert opinion. From these data, we conclude that adolescents and adults with TS are at risk for depression and adulthood appears to be the period of highest risk. Studies in the last 12 years show consistently more severe depressive symptoms in individuals with TS than in previous years. Implications, risk factors, and recommendations for future research are discussed.
Keywords: Turner syndrome, depression, disorders of sex development (DSD), systematic review, mental health
INTRODUCTION
Turner syndrome (TS) is a condition involving complete or partial absence of one sex chromosome, leaving one intact X chromosome, present in 1 in 2,000–2,500 live female births (Cardoso et al., 2004; Saenger et al., 2001). TS is sometimes classified within a larger cluster of diagnoses involving atypical gonadal and pubertal development, currently referred to as disorders of sex development (DSD). The term DSD is most often associated with conditions in which there is disjunction between genital anatomy and sex chromosomes but can be extended to include conditions in which sex chromosomes are atypical, such as TS and Klinefelter Syndrome. In 2006, multiple American and European clinical and advocacy societies published consensus statements on the management of DSD (Consortium on the Management of Disorders of Sex Development, 2006; Hughes, Houk, Ahmed, & Lee, 2006). While promoting psychosocial evaluation and treatment “when needed,” the statements failed to identify relevant psychiatric conditions other than noting that patients with DSD may have gender identity concerns. A 2010 follow-up article indicated that the consensus statement resulted in the integration of psychiatrists and psychologists into DSD clinics (Pasterski, Prentice, & Hughes, 2010), yet little investigation has been conducted into their optimal role or utility. Clinical practice guidelines specific to the medical management of individuals with TS were published more recently by the International Turner Syndrome Consensus Group (Gravholt et al., 2017). These guidelines recommend integration of neuropsychological care, educational evaluations, and regular neuropsychological assessments during times of life and educational transition, although make limited reference to psychiatric diagnostic concerns specific to TS. Though recommendations are made regarding mental and behavioral health issues, optimal timing for such assessment is not provided and little is detailed about provider adherence to these guidelines. Due to the lack of knowledge about the prevalence of mental health issues in people with TS, we determined that a literature review was warranted to gain an understanding of the risk of depression in the population diagnosed with TS. This literature review, 1) summarizes research findings related to depression in individuals with TS, 2) comments on the methodological adequacy of the research investigating depression in this population, and 3) provides a critique of the strength of the evidence in the available studies.
The TS phenotype may occur due to a variety of genetic variations: a 45,X monosomy chromosome complement due to the total absence of the second sex chromosome on peripheral blood karyotype (approximately half of individuals), a functional monosomy chromosome complement involving one intact X chromosome with a structural anomaly of the second sex chromosome (such as a ring chromosome, isochromosome, or partial deletion of the X chromosome), or a mosaic karyotype. A mosaic TS karyotype describes multiple cell lines co-occurring within the same individual; 45,X may occur with one or more other cell lines such as 46,XX, 46,XY, trisomy X (47,XXX), or an intact X chromosome with an anomalous second sex chromosome (i.e., ring chromosome, isochromosome or partial deletion) (Saenger et al., 2001). Without two functional X chromosomes, ovarian follicles are atretic as early as in utero or in the first few months following birth (Abir et al., 2001; Ebrahimi & Akbari Asbagh, 2011; Weiss, 1971). The time course is variable. While some individuals with TS have evidence of gonadal failure in childhood, a minority, typically those with a mosaic chromosome complement, undergo spontaneous puberty. Approximately 2–5% have regular menstrual cycles and approximately 2% achieve spontaneous pregnancy. Ovarian insufficiency generally occurs at an early age, even in those who achieve menses (Abir et al., 2001; Visser et al., 2013; Williams, 2016). Premature ovarian insufficiency, regardless of when it occurs, results in decreased ovarian production of estrogen, progesterone, and inhibin. This, in turn, leads to elevation of luteinizing hormone (LH) and follicle stimulating hormone (FSH) due to lack of feedback from estrogen, inhibin, and progesterone produced by the ovaries. FSH is used as a screening test for ovarian insufficiency in this population and may determine the timing of exogenous estradiol therapy (Abir et al., 2001; Williams, 2016).
TS is also characterized by other endocrine abnormalities. In females, testosterone is derived from ovarian and adrenal androgens; as a result of ovarian insufficiency, individuals with TS have a relative testosterone deficiency (Williams, 2016). Within the population with TS, 10–31% of individuals have primary hypothyroidism and an even greater percentage have thyroid autoantibodies, though the significance of these antibodies in the absence of true hypothyroidism is unclear (Gawlik & Malecka-Tendera, 2014; Saenger et al., 2001). Due to the lack of duplicate copies of the short stature homeobox (SHOX) gene, which is present on the short arm of the X and Y chromosomes and is believed to regulate elements of bone development and growth, individuals with TS have growth impairment starting in utero and lasting through childhood (Clement-Jones et al., 2000). Short stature, a prominent physical feature of TS, is made worse by the lack of a pubertal growth spurt (Williams, 2016). Untreated individuals in the United States have an average height of 142–143 cm (~4’ 7”). With standard treatment, adult height is on average 149.3 ± 6.6cm (~4’10”−4’11”) (Williams, 2016). Of note, people with TS do not have growth hormone (GH) deficiency. However, to stimulate growth, children are typically treated with GH beginning at the onset of growth failure, often by the age of 5–6. Low dose oxandrolone, an androgen, can also be used in patients with TS to promote the action of insulin-like growth factor 1 (IGF-1), a hormone made in response to growth hormone, on the growth plate (Sheanon & Backeljauw, 2015). Previously, standard practice was to delay initiation of exogenous estrogen therapy for pubertal induction in order to prolong the period of growth and optimize height. However, more recent treatment guidelines suggest initiating estrogen replacement between the ages of 11 and 12 years and increasing to adult dosages over 2–3 years to better reflect the timing of typical puberty (Gravholt et al., 2017). Other common features of TS include cardiac and aortic defects, shield chest, webbed neck, and edema (Williams, 2016). Physical differences associated with TS may have mental health implications.
Understanding the mental health presentation and needs of individuals with TS is critical to providing empirically informed holistic care and to effectively respond to the TS treatment guidelines. The biological abnormalities of TS and the resulting psychosocial impacts appear to be significant risks for depression. Biologically, the hormonal profile of TS may create vulnerability for depression. Variability in estrogen and testosterone levels have been linked to depressed mood (Chaplin et al., 2011; Gordon et al., 2015; McHenry, Carrier, Hull, & Kabbaj, 2014; Thomsen, Kvist, Andersen, & Kessing, 2005). Multiple mechanisms have been proposed for estrogen’s effect on mood. These have included regulation of serotonin and norepinephrine, anti-inflammatory properties, neuroprotective effects, role in memory formation and processing of emotionally laden information, and downstream effects on the hypothalamic-pituitary-adrenal (HPA) axis (Gordon et al., 2015; Ostlund, Keller, & Hurd, 2003). While the exact mechanism of estrogen’s role in depression is not yet determined, the increased incidence in women compared to men and the higher frequency of depression during periods of hormonal flux when estrogen levels eventually fall, such as menopause and the peripartum period, highlights a potential correlation between estrogen and mood (Gordon et al., 2015; McHenry et al., 2014). Ovarian insufficiency, which leads to menopausal estrogen levels, progesterone levels, and elevated LH/FSH ratio due to lack of feedback, may put individuals with TS at higher risk for depression than others in their age cohort. Low testosterone levels in TS may be a risk factor for psychiatric illness, as testosterone supplementation has been shown to have anxiolytic and antidepressant effects in women (McHenry et al., 2014). Hypothyroidism is known to predispose patients to both neurovegetative symptoms and clinical depressive illness (Gawlik & Malecka-Tendera, 2014; Saenger et al., 2001; Thomsen et al., 2005; Williams, 2016). Exposure to growth hormone is also likely to be a depression modifying factor. In the idiopathic short stature population (those without growth hormone deficiency), treatment with GH to stimulate growth leads to improved energy levels, improved quality of life, improved cognition, and reduced depressive symptoms on clinical inventories (Chaplin et al., 2011). Taken together, these features specific to TS place individuals at biologically greater risk for depression than peers unaffected by TS.
Other features of TS can be associated with an increased risk of depression. Depression has been observed in populations with shared characteristics, such as women and those with chronic medical conditions (Deneke, Schultz, & Fluent, 2014; Gordon et al., 2015). More specifically for individuals with TS, overt physical appearance differences, such as short stature, shield chest, webbed neck, and edema, may confer risk due to self-perception and fears about the responses of others. It has been shown that a negative subjective evaluation of appearance in young adults is a predictor of depression (Ehlinger & Blashill, 2016). In the HIV population, shorter height for one’s age and dissatisfaction with physical appearance are risks for more depressive symptoms (M. H. Kim et al., 2015). In their review paper regarding the psychosocial impacts of short stature, Sandberg and Gardner (2015) reported that while people with short stature tend to have a negative self-perception about their height and to report a low quality of life, there are data to show that short height, in the absence of an underlying pathology, does not lead to psychosocial dysfunction or increased risk for anxiety or depression. Conversely, other research has demonstrated a 1.7 odds ratio of depression in children and adolescents with a diagnosis of short stature due to an endocrine disorder (Kostev, Teichgräber, Konrad, & Jacob, 2019). There is not a clear consensus about the psychosocial impacts of short stature, but there is a potential for this to be a depression risk factor for someone with TS. Specifically, investigations in the population with TS demonstrate that peer teasing about general body appearance is an even more significant factor affecting depression risk than the internalized stigma of poor self-image about physical appearance and height (Rickert, Hassed, Hendon, & Cunniff, 1996). Furthermore, the process of learning about and coping with infertility adds an additional risk for depression for individuals with TS. A high prevalence of depression is found in infertile women in international populations, either exceeding rates in controls or matching rates of other chronically ill patients (Burns, 2007). These psychological features interact with a patient’s biology to have social functioning impacts, which could heighten depression risk as well.
Social risk factors for depression revolve around impaired social functioning and peer relationships. This population can have difficulties with family and peer acceptance and familial distress (Suorsa et al., 2015). Multiple studies have demonstrated that women with TS have poorer quality of life, fewer sexual experiences and intimate partner relationship, and lower self-esteem in childhood and adulthood when compared to population norms and healthy age-matched controls (Carel et al., 2006; Kiliç, Ergür, & Ocal, 2005; Ros, Alobid, Balasch, Mullol, & Castelo-Branco, 2013; Sheaffer, Lange, & Bondy, 2008). Studies in adolescents without TS have demonstrated that body consciousness during physical intimacy leads to lower levels of sexual behavior and that body dissatisfaction is associated with increased depressive symptoms; thus, the physical characteristics of TS may be a significant risk for depressed mood and sexual impairment (Almeida, Severo, Araújo, Lopes, & Ramos, 2012; Rousseau, Beyens, Eggermont, & Vandenbosch, 2017). However, studies of cohorts with TS have determined that physical stigmata such as webbed neck did not impact the degree of impairment in sexual functioning. Other factors that did not impact sexual function were the use of estrogen, testosterone levels, and hearing loss (Sheaffer et al., 2008). A history of cardiac issues, delayed puberty, and dysfunction in sexual arousal were identified as major contributors to fewer sexual experiences in studies demonstrating poor quality of life in women age 18 to 50 with TS (Carel et al., 2006; Ros et al., 2013). Lower self-esteem was associated with fewer sexual experiences and high BMI. Karyotype, height, GH treatment, type of estrogen used, use of progestin, and genital malformations were not associated with variations in self-esteem (Carel et al., 2006). Women with TS have been documented to frequently have poorer than average facial affect recognition, difficulty with Theory of Mind, deficits in nonverbal processing and social cognition, and impairments with eye gaze, although neuropsychological profiles and interpersonal functioning are variable in this population (Anaki, Zadikov Mor, Gepstein, & Hochberg, 2016; McCauley, Kay, Ito, & Treder, 1987; Saenger et al., 2001). Such limitations may contribute to challenges in establishing and building relationships. Social deficits extend to non-peer arenas, as women with TS are less likely to live independently and tend to have low levels of professional achievement for their education level (Anaki et al., 2016). These challenges could increase depression vulnerability even without the consideration of the above mentioned biological and psychological factors.
Despite these risks, a limited amount of information is available regarding characteristic mental health issues for individuals with Turner syndrome. While primary research articles exist, there are no systematic reviews on this topic which could provide the data necessary to accurately comment on the recommendations made by the DSD organizing bodies and the TS consensus group (Consortium on the Management of Disorders of Sex Development, 2006; Gravholt et al., 2017; Hughes et al., 2006). El Abd, Turk and Hill (1995) reviewed publications about the psychological characteristics of TS, commenting on cognitive function, face recognition, affective discrimination, personality, and psychopathology but did not include a comprehensive literature search. Their review article identified a higher prevalence of attention deficit and hyperactivity. El Abd et al. stated that the literature is divided about the presence of other psychopathology, such as depression, but made no effort to understand this information further. A 2014 article by Mueller, Grissom, and Dohanich (2014) reviewed the influence of gonadal hormones on general psychopathology in animal and human models and referenced one study that found a high rate of depression in individuals with TS. This review also discussed a study describing poorer processing of affective states by women with TS, especially fear and anger, as well as poor recognition of the emotional valence of speech. A mouse model of TS demonstrated poorer attention and higher anxiety-like behavior than wild-type mice. These reviews present a consistent picture of attention issues and summarize work demonstrating impaired affective processing but are lacking the detail needed to comment on any other psychiatric symptoms or diagnoses.
In summary, many aspects of the TS phenotype would seem to increase risk for depression: hormone dysfunction, atypical physical appearance, neurocognitive variants, impaired social skills, and poorer quality of life. Yet, thus far, there has been no consensus as to whether depression is more prevalent in TS than in the general population. Due to these risk factors and the recommendations in the 2006 and 2017 consensus statements (Consortium on the Management of Disorders of Sex Development, 2006; Gravholt et al., 2017; Hughes et al., 2006), we aim to describe the current state of the literature regarding depression risk in the population with TS.
METHOD
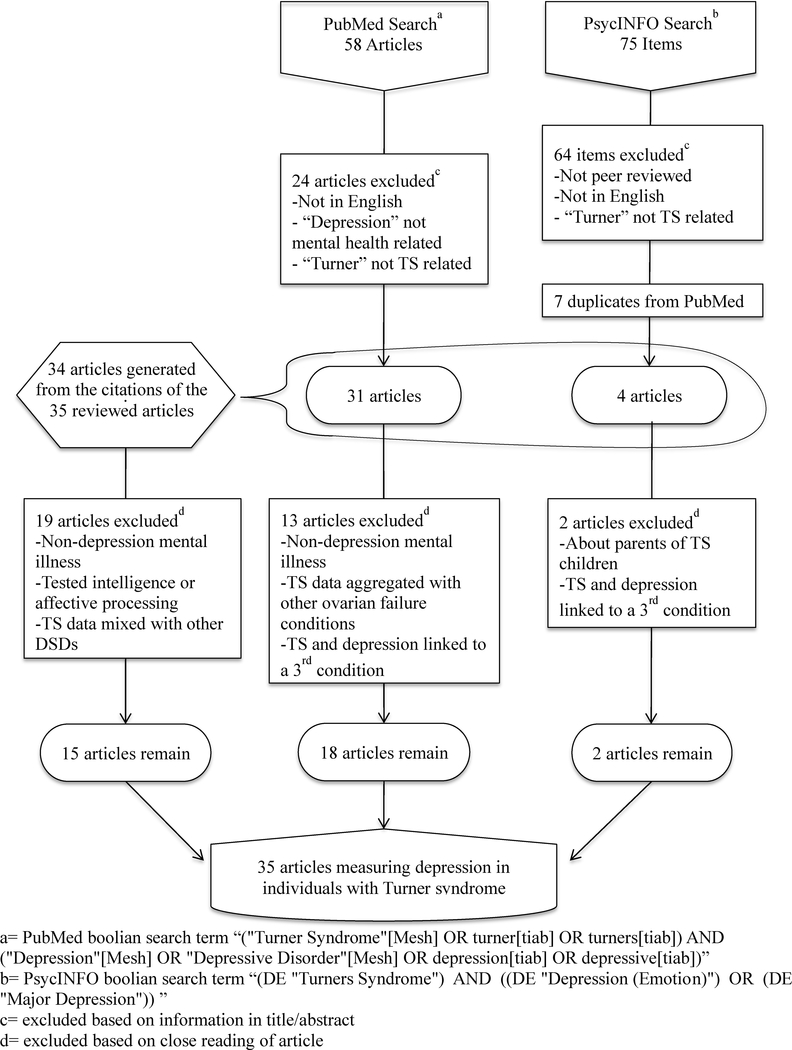
A systematic review was conducted by searching two databases, PubMed and PsycINFO, for articles that had the following keywords in the title or abstract: Turner syndrome, Turner, depression, and depressive. The search and exclusion procedures are summarized in Fig. 1. The initial search produced 58 articles (PubMed) and 75 items (PsycINFO). These results were subject to the following exclusion criteria: non-peer reviewed articles (i.e., book chapters or dissertations), articles not in the English language, studies performing intelligence or affective processing testing, or studies not generating data on the presence or absence of depressive symptoms in people with TS (i.e., review articles, articles mentioning TS but focusing on other DSDs, articles mentioning depression but focusing on other forms of psychiatric illness, articles commenting that TS and depression are co-morbidities of a third condition, or articles studying depression in the parents of children with TS). Book chapters and dissertations were excluded since they are not subject to a peer-review process to evaluate the appropriate use of scientific methods. The excluded dissertation is listed in Appendix 1. Non-English publications were excluded due to our lack of resources to accurately interpret those sources. These articles are listed in Appendix 2. Review articles were not included in the analysis portion of our study due to their lack of specific data on depression risk in the study population. Case reports and case series were included, however. Despite their potential for publication bias and their purely descriptive status, they have relevance in this review by presenting cases of co-occurring depression and TS from which we can obtain information on potential risk factors for depressed mood in this specific population. There was no restriction based on publishing date, and all articles published prior to 2018 were included. The sample size for this review was determined by the number of relevant studies. There was no minimum number of subjects per study nor were studies excluded based on age of subjects. For the purpose of this review, no articles were rejected based on TS diagnosis mechanism; it was assumed that each original study appropriately identified subjects as qualifying for a TS diagnosis.
Figure 1.
Flowchart of method of literature search
Twenty articles met these criteria. Additional articles were obtained from the references of the papers in the original literature search. These were selected based on their title or the reason for their citation in the article from the original search. After close reading, four of the additional 19 articles collected were excluded due to the secondary exclusion criteria of presenting TS data in aggregate with other forms of DSD or hypogonadism. In total, 35 articles were included in the analysis of this review.
Procedural Analysis
The results of each article were recorded as they pertain to the depression experience of individuals with TS. Graphical analysis was then conducted to determine which factors relate to the experience of depression in this population. Independent variables that were investigated included: mean subject age, method of depression evaluation, study design, and year of publication. Due to the great variability in study design, primary outcome measures, method of evaluating depression, and variability of data presentation, a meta-analysis of the surveyed studies was not possible.
The pool of studies was broken down into four major categories: studies that specifically diagnosed depression in individuals with TS, studies that evaluated the severity and frequency of depressive symptoms via validated measures, studies that used clinical examination to comment on presence of depressive symptoms, and the remaining case reports/series. One case report utilized the Hamilton Depression Rating Scale (HAM-D) to evaluate depressive symptoms; this study was analyzed alongside the full studies that relied on validated measures (Mao, Sun, Li, Zhao, & Yang, 2015). The methods of evaluating depression were ranked. Clinician administered scales (a structured validated evaluation scale completed by a trained professional) allow studies to diagnose depression; these were considered superior methods of evaluating depression. Studies that used patient self-report scales (validated questionnaire filled out by patient) or parent report scales (validated questionnaire filled out by parents) were able to comment on the severity of depressive symptoms. We identified patient and parent depression self-report scales as superior methods for ascertaining depressive symptoms. Although they comment less precisely on depression diagnosis, their emphasis on symptoms is highly relatable to clinical settings and they are well validated. Expert opinion (diagnosis made via clinical psychiatric exam) allows for commentary on the presence of depressive symptoms, but due to low inter-rater reliability, as compared to other available detection methods (Von Korff et al., 1987), it is not an objective or reproducible technique. The articles of lowest quality in their ability to comment on a population’s depression risk were the case reports/series which are not designed to make population-based commentary.
Multiple scales were used by the articles analyzed in this review. The clinician administered scales were: Structured Clinical Interview for DSM-IV (SCID) and Schedule for Affective Disorders and Schizophrenia-Lifetime version (SADS-L). The patient self-report questionnaires were: Beck Depression Inventory (BDI) 1st and 2nd edition, Carroll Rating Scale for Depression (CRS), Center for Epidemiologic Studies-Depression Scale (CESD), Childhood Depression Inventory (CDI), HAM-D, Hospital Anxiety and Depression Scale (HADS), Profile of Mood State (POMS), Reynolds Adolescent Depression Scale (RADS), Symptom Checklist 90-Revised (SCL-90-R), and Youth Self Report (YSR). The parent report questionnaires are: Child Behavior Checklist (CBCL) 1983 and 1991 edition.
In many of the articles, published data included a score on a depression symptom questionnaire or the average rate of depressive illness in the study group. We compared the depressive experience of the cohort with TS to the cohort without TS, looking at frequency and severity of depressive symptoms. Some publications only provided data on frequency or severity of symptoms, but not both. If a study had a control group, TS data were compared to the control group data. In the absence of a control group, if a study used a questionnaire to evaluate depression, we interpreted a mean score in the mild or ‘normal’ range to indicate average frequency and average severity. If the mean score was in the mild range but multiple individual subjects scored in the moderate-severe range, our interpretation was that depressed subjects had a greater severity of depression than average. For studies using expert opinion, we were only able to comment on frequency of depression unless the authors of the original paper made a comment on severity of symptoms. The results from each study were classified as greater, average, or lesser on frequency and severity. These two parameters were then consolidated into an “Overall depression burden” parameter and all data, even studies which included an internal control group, were compared to published average rates of depression matched by age group (child and adolescent: Thapar, Collishaw, Pine, & Thapar, 2012; adult: Kessler et al., 1994). We noted in the table if different results emerged when study data were compared to internal controls versus when study data were compared with the population average.
RESULTS
Table 1 summarizes the number of subjects, mean age, study design type, quality of data (described in detail below), depression assessment technique, and the study results about the depressive experience of the subjects with TS. The following study design types were used in the articles we collected: case report (the publication of clinical information about a single patient, usually presenting with a unique finding), case series (a publication documenting several patients with the notable finding), cross-sectional with or without controls (studies that collect data on a population at one moment in time), and prospective (studies that collect data on a group over a period of time). To compare relative depression rates and experiences, we divided the articles into four subgroups, designated as “quality of data.” These groups do not map directly to study design. These groups are below and represented in Table 2:
Quality of Data Level 1: Studies that report on rates of depression diagnosis in the study group by using clinician administered scales such as the SCID (N=three, 9% of the surveyed literature).
Quality of Data Level 2: Studies that report on depressive symptom severity by using validated patient or parent questionnaires, such as the BDI and CBCL respectively (N=eighteen, 51% of the surveyed literature).
Quality of Data Level 3: Studies that use expert opinion to report on the frequency of depressive symptomatology in the study population (N=five, 14% of the surveyed literature).
Quality of Data Level 4: Case reports and case series that document cases of depressive symptoms in individuals with Turner syndrome by using expert opinion (N=nine, 26% of the surveyed literature). Note, Mao’s (2015) case report was included in Quality of Data Level 2 due to its use of the HAM-D to evaluate depressive symptom severity.
Table 1.
Results of Literature Search
| Authors, year | Country | No. of TS subjects | Mean age in years | Study type | Details of control group | Quality of data | Depression assessment | Frequency of TS depression | Severity of TS depression | Overall depression burden |
|---|---|---|---|---|---|---|---|---|---|---|
| Downey et al., 1989 | USA | 23 | 27.1 SD 5.2 | Cross-sectional with controls | Women, age 18–40, with constitutional short stature who had undergone spontaneous puberty and healthy sisters. | 1 | SADS-L and SCL-90-R | Average* | Average* | =* |
| Delooz et al., 1993 | Belgium | 20 | 29 | Cross-sectional w/o controls | N/A | 1 | SADS-L | Greater | Unable to assess | ↑↑ |
| Cardoso et al., 2004 | USA | 100 | 34.7 SD 11.6 | Cross-sectional w/o controls | N/A | 1 | SCID | Greater | Unable to assess | ↑↑ |
| Saad et al., 2014 | Egypt | 53 | 6.9255a | Cross-sectional w/o controls | N/A | 2 | CDI | Greater | Greater | ↑ |
| Lagrou et al., 1998 | Belgium | 31 | 9.67 | Prospective w/o cohort | N/A | 2 | CBCL 1991 and YSR | Average | Average | = |
| McCauley et al., 1995 | USA | 97 | 10.2 SD 1.7 | Cross-sectional with controls | Age matched girls without known chronic illnesses with similar racial composition and verbal IQ to TS subjects. | 2 | CBCL 1983 | Average | Average | = |
| Rovet & Ireland, 1994 | Canada | 103 | 10.4 SD 1.7 | Cross-sectional with controls | Age-matched girls without known endocrine disorders. | 2 | CBCL 1991 | Average | Average | = |
| Rovet, 1993 | Canada | 67 | 12.2 | Cross-sectional with controls | Girls and boys with an average age of 12 without known endocrine disorders. | 2 | CBCL 1983 | Unable to assess | Greater | ↑ |
| Kiliç et al., 2005 | Turkey | 11 | 12.65 SD 2.6 | Cross-sectional with controls | Healthy girls, age 9–16.7, matched to the TS group in grade level and parental education level. | 2 | CDI | Greater | Average | ↑ |
| Mao et al., 2015 | China | 1 | 13 | Case report | N/A | 2 | HAM-D | N/A | Greater | ↑ |
| McCauley et al., 1986a | USA | 17 | 13.1 SD 2.43 | Cross-sectional with controls | Short stature girls of comparable age, height, verbal IQ and family socioeconomic status to TS subjects. | 2 | CBCL 1983 and CDI | Average | Average | = |
| Ross et al., 1996 | USA | 31 | 13.4 SD 0.3 | Prospective with cohort | Girls, age11–14, with heights and weights between the 5th and 95th percentile and similar racial composition to the TS subjects. | 2 | CBCL 1991 | Average | Average | = |
| Rickert et al., 1996 | USA | 59 | 14.8 | Cross-sectional w/o controls | N/A | 2 | RADS | Average | Greater | ↑ |
| McCauley et al., 2001 | USA | 122 | 14.8 SD 1.5 | Cross-sectional with controls | Girls, age 13–18, without known genetic disorder, growth impairment or chronic illness. | 2 | CDI | Lesser | Unable to assess | ↓ |
| van Pareren et al., 2005 | The Netherlands | 50 | 18.8 SD 0.3 | Cross-sectional with controls | Randomly selected Dutch females with average age of 17.1 years. | 2 | CDI and YSR | Average | Average | = |
| Lašaite et al., 2010 | Lithuania | 18 | 21.47 SD 4.09 | Cross-sectional with controls | Females with average age of 21.87 SD 0.89. | 2 | POMS | Greater | Unable to assess | ↑ |
| Freriks et al., 2015 | The Netherlands | 68 | 24 | Cross-sectional w/o controls | N/A | 2 | BDI - 2 | Average | Average | = |
| Cunniff et al., 1995 | USA | 131 | 25.89a | Cross-sectional w/o controls | N/A | 2 | BDI −1 (age >18) RADS (age <18) | Average | Greater | ↑ |
| McCauley et al., 1986b | USA | 30 | 28.7 SD 6.9 | Cross-sectional w/o controls | N/A | 2 | CRS | Greater | Average | ↑ |
| Chadwick et al., 2014 | UK | 30 | 34.5 SD 14.4 | Cross-sectional w/o controls | N/A | 2 | HADS | Greater | Average | ↑ |
| Schmidt et al., 2006 | USA | 100 | 34.7 SD 11.6 | Cross-sectional with controls | Healthy women, age 19–50. Women, age 19–42, with premature ovarian failure. | 2 | CESD | Greater | Unable to assess | ↑ |
| Money & Mittenthal, 1970 | USA | 73 | 17a | Cross-sectional w/o controls | N/A | 3 | Psychiatric evaluation | Average | Average | = |
| Sabbath et al., 1961 | USA | 7 | 17.4 SD 2.15 | Cross-sectional w/o controls | N/A | 3 | Psychiatric evaluation | Greater | Unable to assess | ↑ |
| Bender et al., 1995 | USA | 15 | 17.5 | Cross-sectional with controls | Female and male siblings, age 12–19. | 3 | DSM-IIIR Criteria | Average | Unable to assess | = |
| Nielsen et al., 1977 | Denmark | 45 | 20.4 SD 7.4 | Cross-sectional w/o controls | N/A | 3 | Psychiatric evaluation | Average | Average | = |
| Nielsen & Stradiot, 1987 | Denmark | 111 | 22.15a | Cross-sectional w/o controls | N/A | 3 | Psychiatric evaluation | Average | Unable to assess | = |
| Dickens, 1970 | Australia | 1 | 14 | Case report | N/A | 4 | Psychiatric evaluation | N/A | Unable to assess | + |
| Larocca, 1985 | USA | 1 | 17 | Case report | N/A | 4 | DSM-III Criteria | N/A | Unable to assess | + |
| Pitts & Guze, 1963 | USA | 1 | 18 | Case report | N/A | 4 | Psychiatric evaluation | N/A | Unable to assess | + |
| Nielsen, 1970 | Denmark | 13 | 27.15 SD 15.5 | Case series | N/A | 4 | Psychiatric evaluation | N/A | Unable to assess | + |
| Shea & Wilson, 2017 | Canada | 1 | 32 | Case report | N/A | 4 | Psychiatric evaluation | N/A | Unable to assess | + |
| Fishbain & Vilasuso, 1981 | USA | 1 | 33 | Case report | N/A | 4 | Psychiatric evaluation | N/A | Unable to assess | + |
| Panzer & Tandon, 1991 | USA | 1 | 36 | Case report | N/A | 4 | Psychiatric evaluation | N/A | Unable to assess | + |
| Christodorescu et al., 1970 | Romania | 3 | 38 SD 15 | Case series | N/A | 4 | Psychiatric evaluation | N/A | Unable to assess | + |
| Nielsen & Thomsen, 1968 | Denmark | 1 | 67 | Case report | N/A | 4 | Psychiatric evaluation | N/A | Unable to assess | + |
Studies were ordered by quality level and then mean subject age.
Abbreviations: Beck Depression Inventory, 1st edition (BDI-1), Beck Depression Inventory, 2nd edition (BDI-2), Carroll Rating Scale for Depression (CRS), Center for Epidemiologic Studies-Depression Scale (CESD), Child Behavior Checklist 1983 (CBCL 1983), Child Behavior Checklist 1991 (CBCL 1991), Childhood Depression Inventory (CDI), Hamilton Depression Rating Scale (HAM-D), Hospital Anxiety and Depression Scale (HADS), Profile of Mood State (POMS), Reynolds Adolescent Depression Scale (RADS), Schedule for Affective Disorders and Schizophrenia- Lifetime version (SADS-L), Structured Clinical Interview for DSM IV (SCID), Symptom Checklist 90 – Revised (SCL-90-R), and Youth Self Report (YSR).
Legend:
1= Research study using clinician administered scale
2= Research study using patient or parent questionnaire
3= Research study using expert opinion
4= Case report or case series using expert opinion
indicates median calculated from age range for studies where mean was not available
indicates that when compared to community averages instead of the study control group TS subjects had greater frequency and severity of depression.
+ indicates depression present but not able to compare against controls or community average
= indicates depressive symptoms frequency or severity is equivalent to controls or community average
↑ indicates depressive symptoms frequency or severity is greater than controls or community average
↓ indicates depressive symptoms frequency or severity is less than controls or community average
↑↑ indicates depression was diagnosed at a higher rate than controls or community average
Table 2.
Depression Evaluation Methods
| Quality of data | Method to evaluate depression | N (% of all articles) | Control group N (% of method subgroup) |
|---|---|---|---|
| Level 1 | Clinician administered metric | 3 (9) | Yes 1 (33) |
| No 2 (66) | |||
| Level 2 | Patient/parent questionnaires | 18 (51) | Yes 10 (56) |
| No 8 (44) | |||
| Level 3 | Expert opinion | 5 (14) | Yes 1 (20) |
| No 4 (80) | |||
| Level 4 | Non-study case report/series | 9 (26) | No 9 (100) |
In Table 1, study results are represented with directional arrows to signify a descriptive difference between the depressive experience of individuals with TS and the comparison data. While the data from the pool of articles have too much variability to analyze for statistical significance, we can observe general trends by collecting the study results and presenting them alongside each other.
All three studies (Cardoso et al., 2004; Delooz, Van den Berghe, Swillen, Kleczkowska, & Fryns, 1993; Downey, Ehrhardt, Gruen, Bell, & Morishima, 1989; Kessler et al., 1994) that reported on rates of depression diagnosis found that adults with TS had higher rates of depression than the community average. Downey et al. (1989) reported an equivalent rate (labeled as average frequency in Table 1) of depression diagnosis between people with TS and two internal control groups: women with constitutional short stature (a normal variety of growth characterized by delayed puberty and pubertal growth spurt) (Williams, 2016) and healthy sisters of those with TS. Downey et al. (1989) also used the SCL-90-R, which assessed symptom severity to be relatively equal between all study groups. However, when the data from Downey et al. (1989) were compared with community samples, individuals with TS had higher rates of depression diagnosis and more severe symptoms. The remaining two studies reporting rate of depression diagnosis (Quality of Data Level 1) could not comment on symptom severity (Cardoso et al., 2004; Delooz et al., 1993). Overall, these studies were classified as demonstrating that subjects with TS were more likely to be depressed than the average population.
Among those studies that evaluated depressive symptoms via a validated questionnaire (Quality of Data Level 2) results were as follows: regarding symptoms severity, five studies (28% of subgroup) reported that individuals with TS had high severity depressive symptoms (Cunniff, Hassed, Hendon, & Rickert, 1995; Mao et al., 2015; Rickert et al., 1996; Rovet, 1993; Saad et al., 2014), 10 studies (56%) reported average severity symptoms (Chadwick, Smyth, & Liao, 2014; Freriks et al., 2015; Kiliç et al., 2005; Lagrou et al., 1998; McCauley, Ito, & Kay, 1986a; McCauley, Ross, Kushner, & Cutler, 1995; McCauley, Sybert, & Ehrhardt, 1986b; Ross et al., 1996; Rovet & Ireland, 1994; van Pareren et al., 2005), and three (17%) studies did not report data that were interpretable with regard to symptom severity (Lasaite, Lasiene, & Lasas, 2010; McCauley, Feuillan, Kushner, & Ross, 2001; Schmidt et al., 2006). Regarding symptom frequency, six studies (33%) demonstrated that subjects with TS were more frequently experiencing depressive symptoms as compared to those without TS (Chadwick et al., 2014; Kiliç et al., 2005; Lasaite et al., 2010; McCauley et al., 1986b; Saad et al., 2014; Schmidt et al., 2006), nine (50%) demonstrated an average frequency of symptoms (Cunniff et al., 1995; Freriks et al., 2015; Lagrou et al., 1998; McCauley et al., 1986a, 1995; Rickert et al., 1996; Ross et al., 1996; Rovet & Ireland, 1994; van Pareren et al., 2005), one (6%) reported that individuals with TS were less frequently depressed than the internal control groups (McCauley et al., 2001), and in two studies (11%) an assessment of frequency was not possible based on published data (Mao et al., 2015; Rovet, 1993). Consolidating this information indicates that people with TS were more greatly depressed in 10 studies (56% of subgroup) (Chadwick et al., 2014; Cunniff et al., 1995; Kiliç et al., 2005; Lasaite et al., 2010; Mao et al., 2015; McCauley et al., 1986b; Rickert et al., 1996; Rovet, 1993; Saad et al., 2014; Schmidt et al., 2006), had average depressive symptoms in seven studies (39%) (Freriks et al., 2015; Lagrou et al., 1998; McCauley et al., 1986a, 1995; Ross et al., 1996; Rovet & Ireland, 1994; van Pareren et al., 2005), and fewer symptoms than the control group in one study (6%)(McCauley et al., 2001).
In those studies relying on expert opinion (Quality of Data Level 3) results were as follows: regarding symptom frequency, one of the five studies (20% of subgroup) reported that individuals with TS were depressed at a greater frequency than expected (Sabbath, Morris, Menzer-Benaron, & Sturgis, 1961), one of the five studies (20%) reported that subjects with TS has an equivalent frequency of depressive symptoms to the control group (Bender, Harmon, Linden, & Robinson, 1995), and three of the five studies (60%) reported that people with TS had a frequency of symptoms equivalent to the general population (Money & Mittenthal, 1970; Nielsen, Nyborg, & Dahl, 1977; Nielsen & Stradiot, 1987). Regarding symptom severity, two of these studies (Money & Mittenthal, 1970; Nielsen et al., 1977) reported an average severity of depressive illness, while the remaining three were not able to comment on severity (Bender et al., 1995; Nielsen & Stradiot, 1987; Sabbath et al., 1961). The remaining nine articles (26% of the surveyed literature) are case reports or case series that relied only on expert opinion to make the determination of depression (Quality of Data Level 4), thus by design they do not have a control group or present data that are comparable to other populations (Christodorescu, Collino, Zellingher, & Tăutu, 1970; Dickens, 1970; Fishbain & Vilasuso, 1981; Larocca, 1985; Nielsen, 1970; Nielsen & Thomsen, 1968; Panzer & Tandon, 1991; Pitts & Guze, 1963; Shea & Wolfman, 2017). Table 2 summarizes the studies discussed above, categorized by method of depression evaluation and presence/absence of control group. Twelve studies (34% of the surveyed literature) compared depression or depressive symptom severity in individuals with TS to depression or depressive symptoms in an internal control group (Bender et al., 1995; Downey et al., 1989; Kiliç et al., 2005; Lasaite et al., 2010; McCauley et al., 2001, 1986a, 1995; Ross et al., 1996; Rovet, 1993; Rovet & Ireland, 1994; Schmidt et al., 2006; van Pareren et al., 2005). Of those studies, four (33%) found that people with TS reported more severe depressive symptoms or greater rates of depression than the control group (Kiliç et al., 2005; Lasaite et al., 2010; Rovet, 1993; Schmidt et al., 2006). Seven studies (58%) found that depressive symptoms were equivalent between individuals with TS and controls (Bender et al., 1995; Downey et al., 1989; McCauley et al., 1986a, 1995; Ross et al., 1996; Rovet & Ireland, 1994; van Pareren et al., 2005). One study (8%) found that individuals with TS have less severe depressive symptoms (McCauley et al., 2001). Notably, the latter study found that the group with TS scored highly on a portion of an anxiety scale that indicates inaccurate self-report, which calls into question the validity of the self-report depression ratings (McCauley et al., 2001).
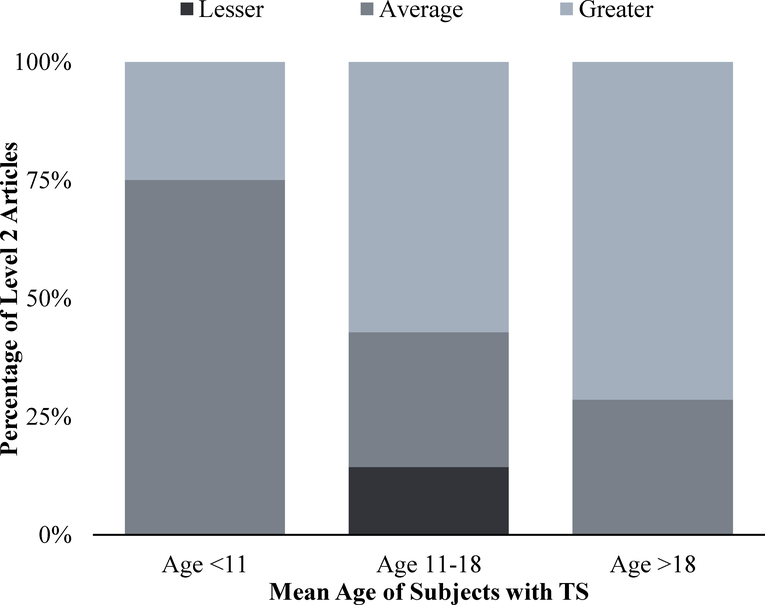
To facilitate comparisons within a heterogeneous pool of articles, we conducted sub-analysis by the mean age of study subjects breaking them into three categories: children, adolescents, and adults. From the total population of articles, four (11%) studied children under age 11 (Lagrou et al., 1998; McCauley et al., 1995; Rovet & Ireland, 1994; Saad et al., 2014), 13 (37%) examined adolescents with a mean age between 11 and 18 years old (Bender et al., 1995; Dickens, 1970; Kiliç et al., 2005; Larocca, 1985; Mao et al., 2015; McCauley et al., 2001, 1986a; Money & Mittenthal, 1970; Pitts & Guze, 1963; Rickert et al., 1996; Ross et al., 1996; Rovet, 1993; Sabbath et al., 1961), and 18 (51%) utilized adults with a mean age over 18 years old. The three studies (9%) using validated metrics to diagnose depression were conducted with adult women in the years 1989, 1993, and 2004 (Cardoso et al., 2004; Delooz et al., 1993; Downey et al., 1989). Of the 18 studies (51%) using patient or parent questionnaires to evaluate symptom severity, four studies (22% of subgroup) looked only at children under age 11 (Lagrou et al., 1998; McCauley et al., 1995; Rovet & Ireland, 1994; Saad et al., 2014), seven (39%) examined adolescents (Kiliç et al., 2005; Mao et al., 2015; McCauley et al., 2001, 1986a; Rickert et al., 1996; Ross et al., 1996; Rovet, 1993), and seven (39%) studied adults (Chadwick et al., 2014; Cunniff et al., 1995; Freriks et al., 2015; Lasaite et al., 2010; McCauley et al., 1986b; Schmidt et al., 2006; van Pareren et al., 2005). In the studies examining children, one (25% of subgroup) showed that youth with TS had more severe depressive symptoms than average (Saad et al., 2014) and three (75%) showed an equivalent severity of depressive symptoms to the control group (Lagrou et al., 1998; McCauley et al., 2001; Rovet & Ireland, 1994). In the adolescent age range, four studies (57% of subgroup) showed greater overall depressive experience (Kiliç et al., 2005; Mao et al., 2015; Rickert et al., 1996; Rovet, 1993) and two studies (29%) showed an average depressive experience when compared to individuals without a diagnosis of TS (McCauley et al., 1986a; Ross et al., 1996). In the case of adults, five of the seven articles (71%) found that women with TS had more severe or more frequent symptoms than controls or the average population (Chadwick et al., 2014; Cunniff et al., 1995; Lasaite et al., 2010; McCauley et al., 1986b; Schmidt et al., 2006). As shown in Fig. 2, for studies from quality of evidence Level 2, at least 50% of adolescents and adults had a greater depression burden than age matched peers. An examination by age shows that the adolescent and adult years may be times of higher risk for depression.
Figure 2.
Overall depression burden by age for quality of data level 2 articles
The data presented on the hormonal exposure of research participants with TS were variable and few articles had a study population with homogenous exposure to estrogen and/or growth hormone. Similarly, the karyotypes of subjects with TS were not documented in all studies and, when they were commented on, depression data for 45,X and other chromosome complements were grouped together.
DISCUSSION
The findings of this review suggest that individuals with TS may have an equal or greater risk for depression or depressive symptoms than those unaffected by TS. In addition, findings suggest that risk for depression may be more robust as an individual matures into adulthood. This research review found no significant evidence to suggest that TS is protective against depression. Although there are numerous potential risk factors for depression related to the biological and psychosocial characteristics of TS, we cannot conclude which are most impactful in this population. Our conclusions were limited by a small literature base, inconsistent and, at times, flawed study design, the necessity of extracting data on depression from research that was not designed to study this as a primary outcome measure, and the inability to run statistical measures to compare data across studies. Findings strongly suggest that clinical research on depression risk in girls and women with TS is lacking, which could translate to inadequate mental health care for this community.
Women with TS appear to have an elevated risk of depression compared to the general population and some medically ill patient populations, but not all. In the studies using research metrics that make a diagnosis of depression (Cardoso et al., 2004; Delooz et al., 1993; Downey et al., 1989), the adults with TS had a higher lifetime prevalence rate, 36%, 50%, and 65.2% respectively, than the community rate of 21% (Kessler et al., 1994). The prevalence of depression in women with TS in these studies was higher than in women with the chronic medical conditions of epilepsy and type 2 diabetes (26.54% and 34% respectively), which are medical conditions that are chronic and can lead to functional impairment (Khaledi, Haghighatdoost, Feizi, & Aminorroaya, 2019; M. Kim, Kim, Kim, Yang, & Kwon, 2018). In contrast, the lifetime rate of a major depressive diagnosis was roughly equivalent in the women with TS as compared to women with ovarian insufficiency (54.5%), a condition with some shared hormonal and psychosocial factors (Schmidt et al., 2011). Looking at a psychiatrically ill population, the rate of co-morbid depression for women with TS is fairly equivalent to people with an anxiety disorder (58%) but much lower in comparison to patients with anorexia and bulimia (46–92%) and people with schizophrenia (40–80%) (Al-Asadi, Klein, & Meyer, 2015; Upthegrove, Marwaha, & Birchwood, 2017). It is reasonable, and expected, that the co-morbidity of a second psychiatric condition along with depression would be more common than co-occurrence of depression with TS due to the overlapping biochemical processes and predisposing life events related to psychiatric ailments.
As noted, an interesting trend emerging from the reviewed literature is that the frequency and severity of depression seems to increase with a subject’s age. Regardless of the depression assessment method, studies examining children did not consistently demonstrate a difference between the depressive experience of girls with TS and their peers. Studies that used questionnaires to assess depressive symptoms in adolescents with TS demonstrated that about half of the adolescents with TS surveyed reported more severe depressive symptoms than peers. A majority of studies that used questionnaires to evaluate depressive symptoms in adults with TS reported that these symptoms occurred with greater frequency than in populations without TS. This is consistent with findings above regarding the occurrence rates of depression in women with epilepsy, women with diabetes and otherwise healthy women.
The results of this review suggest that adolescents with TS are more prone to depression than children and that depressive symptoms are likely more firmly established by adulthood. This may be because biological and psychosocial risk factors are more prominent after childhood. For example, the physical disparities between girls with TS and their peers are likely to become evident during adolescence due to differences in stature and timing of pubertal development. The additional increase in depressive symptoms in adulthood may reflect the impact of psychosocial variables at this stage of life. These include lower quality of life, low self-esteem, infertility, sexual function differences, and diminished rates of intimate partnerships. Additionally, interaction with medical providers has been shown to decrease in frequency as individuals with TS enter adolescence and adulthood, which may lead to decreased support for patients and families with TS (Bondy & Turner Syndrome Study Group, 2007; Gawlik & Malecka-Tendera, 2014; Gravholt et al., 2017; Saenger et al., 2001). Our study supports the emphasis on monitoring patients with TS after childhood and suggests that intervention in adolescence could protect from depression risk.
A limitation of our review was the necessity of breaking down the articles into subgroups for comparative analysis. This is important, however, because each method of assessing depression generated different results. Clinician administered scales, like the SCID, are structured interviews designed to make a diagnosis of depression in an organized, repeatable fashion but subjects experiencing symptoms below threshold will not be reported as cases. These types of studies are important because of their specificity but are difficult to administer and are dependent on the Diagnostic and Statistical Manual of Mental Disorders (DSM), which undergoes nosological updates between editions (Highlights of Changes from DSM-IV-TR to DSM-5, 2013). Questionnaires evaluate for symptom occurrence and/or severity, but they are not designed to render diagnoses. They are important due to their ease of use in the clinical domain and because symptoms (unlike diagnoses) do not change with DSM updates. As a result, even these two types of high-quality methods cannot simply be compared to each other. As previously mentioned, expert opinion is subjective and has low inter-rater reliability. Furthermore, case reports/series simply document the presence of depression and TS in the same individual but cannot be used to make population-based commentary. Most of the case reports/series used expert opinion for their depression evaluation, yielding results that were inconclusive. Increasing the quantity of articles that use related techniques will improve our ability to make population-based commentary.
Despite advances in the medical care of patients with TS, our research indicates that more recent studies have found a higher prevalence of depressive symptoms. Between 1980 and 2006, there were an equal number of articles demonstrating typical severity of depressive symptoms and high severity symptoms/higher rates of diagnosed depression. From the seven articles published since 2006, five studies (Chadwick et al., 2014; Lasaite et al., 2010; Mao et al., 2015; Saad et al., 2014; Schmidt et al., 2011) detected either more severe symptoms or more frequent symptoms in individuals with TS. One was a study of children (Saad et al., 2014), one was a study of adolescents (Mao et al., 2015), and three were studies of adults (Chadwick et al., 2014; Lasaite et al., 2010; Schmidt et al., 2011). Only two of the studies published since 2006 included control groups and, in both, the women with TS experienced more frequent symptoms than controls (Lasaite et al., 2010; Schmidt et al., 2011). This is the inverse of our expectation, given some improvements in TS intervention and treatment in recent years (Bondy & Turner Syndrome Study Group, 2007). During this time period, it has been more common to treat girls and women with TS with hormones and at younger ages. It is possible that hormone use may be an independent risk factor affecting depressed mood. It may be possible that this trend is an artifact of the increased prevalence of depressive symptoms noted in the general adolescent and adult populations since the start of the twenty-first century (Twenge, 2015). There may be other cultural or societal changes, perhaps related to the use of the internet and social media, that has more negatively impacted the psychosocial wellbeing of people with TS in recent decades.
Unfortunately, there was not enough information to determine causal factors in our study population, but some evidence is emerging to confirm certain depression risk factors. The combination of control groups and disease specific comparison groups can be used to highlight which biological and psychosocial features of TS contribute to depression risk. Comparing individuals with TS to girls and women with short stature, premature ovarian function, anatomical anomalies, primary amenorrhea, infertility, and chronic medical conditions would each provide commentary on a different potential risk factor for depression. For example, Schmidt et al.’s (2006) study found that individuals with TS and those with premature ovarian failure (without TS) had equivalent depression questionnaire scores; both groups’ scores, however, were significantly higher than that of healthy controls. This indicates that impaired ovarian function may be a significant contributor to increased symptoms and illustrates the importance of considering the make-up of a control group when designing and interpreting research.
The study by Downey et al. (1989) compared women with TS to two control groups: their healthy sisters and women with constitutional short stature. This study had equivocal results. An internal analysis demonstrated that the group with TS and both comparison groups had equivalent frequency and severity of depression. However, when data from study participants with TS were compared by the authors of this review to data from the general community, research participants with TS had higher rates of depression, as did the two comparison groups. The interpretation by Downey et al. (1989) that the women with TS in their study were not experiencing atypical depressive symptoms may have been confounded by the presence of short stature in one of their control groups since there is evidence that variations in appearance and height are a risk factor for depression (Ehlinger & Blashill, 2016; M. H. Kim et al., 2015; Rickert et al., 1996). The comparison to sisters also carries the potential to be confounding based on family history. By only comparing TS data to a group with a shared feature, and not a healthy control/general population, the vulnerability of people with TS to depression may not be highlighted appropriately. Another example of the impact of the control and comparison group is the work of Cardoso et al. (2004), which showed that women with TS had lifetime rates of depression that were equivalent to the elevated rates in primary care and gynecologic clinic populations. While less specific, this suggests that having a chronic illness that requires routine care increases risk for depression and that TS can be considered analogous to chronic illness in terms of depression risk. These findings highlight that patients with TS should be regularly screened for depression in the general medical setting like other medically ill groups (Deneke et al., 2014). Drawing these parallels between patient populations would allow for exploration of the many variables likely to act as depression risk factors in TS such as short stature, impaired ovarian function, diminished fertility, sexual dysfunction, and chronic illness. Additionally, demonstrating that patients with TS have rates of depression that are similar to groups with a well-documented need for screening and treating depression accrues further evidence in support of the TS and DSD treatment guidelines recommending attention to mental health (Consortium on the Management of Disorders of Sex Development, 2006; Gravholt et al., 2017; Hughes et al., 2006; Saenger et al., 2001).
Estrogen and growth hormone exposure have been shown to impact mood and quality of life in other groups (Chaplin et al., 2011; Gordon et al., 2015). As most studies in this literature review did not report whether subjects received hormonal therapy (growth hormone or estradiol), we were not able to complete an analysis of the role that these hormones play in conferring or mitigating risk for depression in people with TS. Similarly, we hoped to look at the results of TS 45,X in comparison to TS mosaic and ring karyotypes to see if there were any difference in risk but limited karyotype data were available. The results of this review highlight the need for more systematic research that can examine some of the potential risk variables while recognizing the diversity of individuals who receive a TS diagnosis.
Future research utilizing diagnostic and screening tools would allow for continued hypothesis generation regarding the intersection of TS and depression. Studies should be designed to examine systematically whether depression risk is elevated in girls and women with TS diagnoses, to examine risk variability by type of TS (e.g., mosaic vs, non-mosaic), and to use control groups such as non-diagnosed healthy populations and those with other medical conditions. Consistent use of control groups, large sample size, cross-sectional design, and longitudinal designs would boost the utility of the current body of literature and allow the study of age-related factors. Qualitative methodologies can also be relevant to understanding the nuances of experience in girls and women with TS. More stringent, high quality methodology would provide better reference for clinicians to evaluate the mental health needs of the population with TS, as is called for in the 2006 and 2017 treatment guidelines (Consortium on the Management of Disorders of Sex Development, 2006; Gravholt et al., 2017; Hughes et al., 2006). If psychiatric diagnoses are effectively recognized, then it follows that appropriate treatments will be made available. Effective intervention can alleviate distress, improve quality of life, and provide the ability to mitigate the risk of suicide. Additionally, untreated depression is linked to increased health care costs in the medically ill (Katon, 2008). Since a clear majority of patients with TS are not medically monitored in adulthood at the level of intensity that their chronic illness requires, the first priority of research must be justification for screening these patients for depression in a general medical setting (Gawlik & Malecka-Tendera, 2014). Recruiting more specific study populations, such as individuals with TS with and without a family history of depression, would continue to improve the precision of the outcome data. Studies that follow participants through multiple life stages (childhood, adolescence, and adulthood) would be valuable in examining the findings of this literature review, albeit labor intensive and costly. More studies focusing on adults would allow for stratification by age groupings instead of clustering all women who are over 18 years old. Collecting precise data on hormone exposure, such as estrogen, growth hormone, and thyroid hormone, as well as TS karyotype would allow for analysis of the role these may play in an individual’s risk for depression. Knowing the age at which ovarian failure occurred could highlight the relevance of estrogen exposure during brain and social development. Along with biological aspects, collecting more detailed information about positive social and psychological influences in the non-depressed TS groups may elucidate resilience and protective factors. This type of work, at a greater volume, would provide the statistical power needed to confirm the finding that there is an increased risk for depression in TS individuals.
This review demonstrates the importance of understanding the developmental risks for depression and the mitigating factors in the population diagnosed with TS. Intervention in the adolescent years may be transformative for the depression trajectory of individuals with TS as they develop into adults. Management in interdisciplinary teams may be the most appropriate setting for patients with TS at all ages. Education of mental health and other medical providers is needed about the intersection of depression with TS and DSD. It is prudent for clinicians to consider depression when working with patients who have TS. Continuing to involve mental health providers in interdisciplinary clinics that address DSD alongside endocrinology, urology, gynecology, genetics, nursing, social work, and primary care will be important. Much like the recommendations to screen for depression in primary care settings (Deneke et al., 2014), routine depression screening will be beneficial in clinics that treat adolescent and adult patients with DSD.
Appendix 1. Appendix of excluded dissertation
- Diamond DC, (2004). Depression and quality of life in women with Turner’s syndrome (Doctoral dissertation). Available from ProQuest Dissertations Publishing. (3184214) [Google Scholar]
Appendix 2. Appendix of excluded non-English articles
- Bricaire L, Laroche E, Bourcigaux N, Donadille B, & Christin-Maitre S (2013). [Premature ovarian failures]. Presse Medicale, 42, 1500–1507. 10.1016/j.lpm.2013.04.018 [DOI] [PubMed] [Google Scholar]
- Cristodorescu D, & Alexandru S (1968). [Pure gonadal dysgenesis from the psychiatric viewpoint]. Neurologia, Psihiatria, Neurochirurgia, 13, 125–130. [PubMed] [Google Scholar]
- Romani JD, Lôo H, Reyes F, Lôo P, Saba S, & Parry J (1967). [On a case of Turner’s syndrome]. Annales Medico-Psychologiques, 125, 102–105. [PubMed] [Google Scholar]
- Skałba P, Jez W, & Kabzińska M (2002). [Anorexia nervosa in woman with Turner’s syndrome]. Ginekologia Polska, 73, 540–542. [PubMed] [Google Scholar]
- Toublanc JE, Thibaud F, & Lecointre C (1997). [Psychosocial and sexual outcome in women with Turner syndrome]. Contraception, Fertilite, Sexualite, 25, 633–638. [PubMed] [Google Scholar]
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
REFERENCES
- Abir R, Fisch B, Nahum R, Orvieto R, Nitke S, & Ben Rafael Z (2001). Turner’s syndrome and fertility: Current status and possible putative prospects. Human Reproduction Update, 7, 603–610. [DOI] [PubMed] [Google Scholar]
- Al-Asadi AM, Klein B, & Meyer D (2015). Multiple comorbidities of 21 psychological disorders and relationships with psychosocial variables: A study of the online assessment and diagnostic system within a web-based population. Journal of Medical Internet Research, 17, e55 10.2196/jmir.4143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida S, Severo M, Araújo J, Lopes C, & Ramos E (2012). Body image and depressive symptoms in 13-year-old adolescents. Journal of Paediatrics and Child Health, 48, E165–171. 10.1111/j.1440-1754.2012.02576.x [DOI] [PubMed] [Google Scholar]
- Anaki D, Zadikov Mor T, Gepstein V, & Hochberg Z (2016). Face perception in women with Turner syndrome and its underlying factors. Neuropsychologia, 90, 274–285. 10.1016/j.neuropsychologia.2016.08.024 [DOI] [PubMed] [Google Scholar]
- Bender BG, Harmon RJ, Linden MG, & Robinson A (1995). Psychosocial adaptation of 39 adolescents with sex chromosome abnormalities. Pediatrics, 96, 302–308. [PubMed] [Google Scholar]
- Bondy CA, & Turner Syndrome Study Group. (2007). Care of girls and women with Turner syndrome: A guideline of the Turner Syndrome Study Group. Journal of Clinical Endocrinology and Metabolism, 92, 10–25. 10.1210/jc.2006-1374 [DOI] [PubMed] [Google Scholar]
- Burns LH (2007). Psychiatric aspects of infertility and infertility treatments. Psychiatric Clinics of North America, 30, 689–716. 10.1016/j.psc.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Cardoso G, Daly R, Haq NA, Hanton L, Rubinow DR, Bondy CA, & Schmidt P (2004). Current and lifetime psychiatric illness in women with Turner syndrome. Gynecological Endocrinology, 19, 313–319. [PubMed] [Google Scholar]
- Carel J-C, Elie C, Ecosse E, Tauber M, Léger J, Cabrol S, … Coste J (2006). Self-esteem and social adjustment in young women with Turner syndrome--influence of pubertal management and sexuality: Population-based cohort study. The Journal of Clinical Endocrinology and Metabolism, 91, 2972–2979. 10.1210/jc.2005-2652 [DOI] [PubMed] [Google Scholar]
- Chadwick PM, Smyth A, & Liao L-M (2014). Improving self-esteem in women diagnosed with Turner syndrome: Results of a pilot intervention. Journal of Pediatric and Adolescent Gynecology, 27, 129–132. 10.1016/j.jpag.2013.09.004 [DOI] [PubMed] [Google Scholar]
- Chaplin JE, Kriström B, Jonsson B, Hägglöf B, Tuvemo T, Aronson AS, … Albertsson-Wikland K (2011). Improvements in behaviour and self-esteem following growth hormone treatment in short prepubertal children. Hormone Research in Pædiatrics, 75, 291–303. 10.1159/000322937 [DOI] [PubMed] [Google Scholar]
- Christodorescu D, Collino S, Zellingher R, & Tăutu C (1970). Psychiatric disturbances in Turner syndrome: Report of three cases. Psychiatria Clinica, 114–124. [PubMed] [Google Scholar]
- Clement-Jones M, Schiller S, Rao E, Blaschke RJ, Zuniga A, Zeller R, … Rappold GA (2000). The short stature homeobox gene SHOX is involved in skeletal abnormalities in Turner syndrome. Human Molecular Genetics, 9, 695–702. [DOI] [PubMed] [Google Scholar]
- Consortium on the Management of Disorders of Sex Development. (2006). Clinical guidelines for the management of disorders of sex development in childhood (1st ed.). Retrieved from http://www.dsdguidelines.org/files/clinical.pdf
- Cunniff C, Hassed SJ, Hendon AE, & Rickert VI (1995). Health care utilization and perceptions of health among adolescents and adults with Turner syndrome. Clinical Genetics, 48, 17–22. [DOI] [PubMed] [Google Scholar]
- Delooz J, Van den Berghe H, Swillen A, Kleczkowska A, & Fryns JP (1993). Turner syndrome patients as adults: A study of their cognitive profile, psychosocial functioning and psychopathological findings. Genetic Counseling, 4, 169–179. [PubMed] [Google Scholar]
- Deneke DE, Schultz H, & Fluent TE (2014). Screening for depression in the primary care population. Primary Care, 41, 399–420. 10.1016/j.pop.2014.02.011 [DOI] [PubMed] [Google Scholar]
- Dickens JA (1970). Concurrence of Turner’s syndrome and anorexia nervosa. British Journal of Psychiatry, 117, 237. [PubMed] [Google Scholar]
- Downey J, Ehrhardt AA, Gruen R, Bell JJ, & Morishima A (1989). Psychopathology and social functioning in women with Turner syndrome. Journal of Nervous and Mental Disease, 177, 191–201. [DOI] [PubMed] [Google Scholar]
- Ebrahimi M, & Akbari Asbagh F (2011). Pathogenesis and causes of premature ovarian failure: An update. International Journal of Fertility & Sterility, 5, 54–65. [PMC free article] [PubMed] [Google Scholar]
- Ehlinger PP, & Blashill AJ (2016). Self-perceived vs. actual physical attractiveness: Associations with depression as a function of sexual orientation. Journal of Affective Disorders, 189, 70–76. 10.1016/j.jad.2015.08.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Abd S, Turk J, & Hill P (1995). Psychological characteristics of Turner syndrome. Journal of Child Psychology and Psychiatry, 36, 1109–1125. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, & Vilasuso A (1981). Manic-depressive illness associated with Turner’s syndrome mosaicism. Journal of Nervous and Mental Disease, 169, 459–461. [DOI] [PubMed] [Google Scholar]
- Freriks K, Verhaak CM, Sas TCJ, Menke LA, Wit JM, Otten BJ, … Timmers HJLM (2015). Long-term effects of oxandrolone treatment in childhood on neurocognition, quality of life and social-emotional functioning in young adults with Turner syndrome. Hormones and Behavior, 69, 59–67. 10.1016/j.yhbeh.2014.12.008 [DOI] [PubMed] [Google Scholar]
- Gawlik A, & Malecka-Tendera E (2014). Transitions in endocrinology: Treatment of Turner’s syndrome during transition. European Journal of Endocrinology, 170, R57–74. 10.1530/EJE-13-0900 [DOI] [PubMed] [Google Scholar]
- Gordon JL, Girdler SS, Meltzer-Brody SE, Stika CS, Thurston RC, Clark CT, … Wisner KL (2015). Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: A novel heuristic model. American Journal of Psychiatry, 172, 227–236. 10.1176/appi.ajp.2014.14070918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, … International Turner Syndrome Consensus Group. (2017). Clinical practice guidelines for the care of girls and women with Turner syndrome: Proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. European Journal of Endocrinology, 177, G1–G70. 10.1530/EJE-17-0430 [DOI] [PubMed] [Google Scholar]
- Highlights of Changes from DSM-IV-TR to DSM-5. (2013). Retrieved from https://www.psychiatry.org/File%20Library/Psychiatrists/Practice/DSM/APA_DSM_Changes_from_DSM-IV-TR_-to_DSM-5.pdf.
- Hughes IA, Houk C, Ahmed SF, & Lee PA (2006). Consensus statement on management of intersex disorders. Archives of Disease in Childhood, 91, 554–563. 10.1136/adc.2006.098319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon WJ (2008). The comorbidity of diabetes mellitus and depression. American Journal of Medicine, 121, S8–S15. 10.1016/j.amjmed.2008.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, … Kendler KS (1994). Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: Results from the National Comorbidity Survey. Archives of General Psychiatry, 51, 8–19. 10.1001/archpsyc.1994.03950010008002 [DOI] [PubMed] [Google Scholar]
- Khaledi M, Haghighatdoost F, Feizi A, & Aminorroaya A (2019). The prevalence of comorbid depression in patients with type 2 diabetes: An updated systematic review and meta-analysis on huge number of observational studies. Acta Diabetologica, 56, 631–650. 10.1007/s00592-019-01295-9 [DOI] [PubMed] [Google Scholar]
- Kiliç BG, Ergür AT, & Ocal G (2005). Depression, levels of anxiety and self-concept in girls with Turner’s syndrome. Journal of Pediatric Endocrinology & Metabolism, 18, 1111–1117. [DOI] [PubMed] [Google Scholar]
- Kim MH, Mazenga AC, Yu X, Devandra A, Nguyen C, Ahmed S, … Sharp C (2015). Factors associated with depression among adolescents living with HIV in Malawi. BMC Psychiatry, 15, 264 10.1186/s12888-015-0649-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Kim Y-S, Kim D-H, Yang T-W, & Kwon O-Y (2018). Major depressive disorder in epilepsy clinics: A meta-analysis. Epilepsy & Behavior, 84, 56–69. 10.1016/j.yebeh.2018.04.015 [DOI] [PubMed] [Google Scholar]
- Kostev K, Teichgräber F, Konrad M, & Jacob L (2019). Association between chronic somatic conditions and depression in children and adolescents: A retrospective study of 13,326 patients. Journal of Affective Disorders, 245, 697–701. 10.1016/j.jad.2018.11.014 [DOI] [PubMed] [Google Scholar]
- Lagrou K, Xhrouet-Heinrichs D, Heinrichs C, Craen M, Chanoine JP, Malvaux P, & Bourguignon JP (1998). Age-related perception of stature, acceptance of therapy, and psychosocial functioning in human growth hormone-treated girls with Turner’s syndrome. Journal of Clinical Endocrinology and Metabolism, 83, 1494–1501. 10.1210/jcem.83.5.4807 [DOI] [PubMed] [Google Scholar]
- Larocca FE (1985). Concurrence of Turner’s syndrome, anorexia nervosa, and mood disorders: Case report. Journal of Clinical Psychiatry, 46, 296–297. [PubMed] [Google Scholar]
- Lasaite L, Lasiene D, & Lasas L (2010). Cognition, emotions and quality of life in Lithuanian girls with Turner syndrome after growth hormone therapy discontinuation. Journal of Pediatric Endocrinology & Metabolism, 23, 443–450. [PubMed] [Google Scholar]
- Mao S, Sun L, Li R, Zhao Z, & Yang R (2015). Major depressive disorder in an adolescent with Turner syndrome: A case report. Gynecological Endocrinology, 32, 354–356. 10.3109/09513590.2015.1126710 [DOI] [PubMed] [Google Scholar]
- McCauley E, Feuillan P, Kushner H, & Ross JL (2001). Psychosocial development in adolescents with Turner syndrome. Journal of Developmental and Behavioral Pediatrics, 22, 360–365. [DOI] [PubMed] [Google Scholar]
- McCauley E, Ito J, & Kay T (1986a). Psychosocial functioning in girls with Turner’s syndrome and short stature: Social skills, behavior problems, and self-concept. Journal of the American Academy of Child Psychiatry, 25, 105–112. [DOI] [PubMed] [Google Scholar]
- McCauley E, Kay T, Ito J, & Treder R (1987). The Turner syndrome: Cognitive deficits, affective discrimination, and behavior problems. Child Development, 58, 464–473. [PubMed] [Google Scholar]
- McCauley E, Ross JL, Kushner H, & Cutler G (1995). Self-esteem and behavior in girls with Turner syndrome. Journal of Developmental and Behavioral Pediatrics, 16, 82–88. [PubMed] [Google Scholar]
- McCauley E, Sybert VP, & Ehrhardt AA (1986b). Psychosocial adjustment of adult women with Turner syndrome. Clinical Genetics, 29, 284–290. [DOI] [PubMed] [Google Scholar]
- McHenry J, Carrier N, Hull E, & Kabbaj M (2014). Sex differences in anxiety and depression: Role of testosterone. Frontiers in Neuroendocrinology, 35, 42–57. 10.1016/j.yfrne.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Money J, & Mittenthal S (1970). Lack of personality pathology in Turner’s syndrome: Relation to cytogenetics, hormones and physique. Behavior Genetics, 1, 43–56. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Grissom EM, & Dohanich GP (2014). Assessing gonadal hormone contributions to affective psychopathologies across humans and animal models. Psychoneuroendocrinology, 46, 114–128. 10.1016/j.psyneuen.2014.04.015 [DOI] [PubMed] [Google Scholar]
- Nielsen J (1970). Turner’s syndrome in medical, neurological and psychiatric wards: A psychiatric, cytogenetic and clinical study. Acta Psychiatrica Scandinavica, 46, 286–310. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Nyborg H, & Dahl G (1977). Turner’s syndrome: A psychiatric psychological study of 45 women with Turner’s syndrome, compared with their sisters and women with normal karyotypes, growth retardation and primary amenorrhoea. Århus: Laerde Selskab, Aarhus University. [Google Scholar]
- Nielsen J, & Stradiot M (1987). Transcultural study of Turner’s syndrome. Clinical Genetics, 32, 260–270. [DOI] [PubMed] [Google Scholar]
- Nielsen J, & Thomsen N (1968). A psychiatric-cytogenetic study of a female patient with 45–46-47 chromosomes and sex chromosomes XO-XX-XXX. Acta Psychiatrica Scandinavica, 44, 141–155. [DOI] [PubMed] [Google Scholar]
- Ostlund H, Keller E, & Hurd YL (2003). Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Sciences, 1007, 54–63. [DOI] [PubMed] [Google Scholar]
- Panzer MJ, & Tandon R (1991). Bipolar disorder associated with Turner’s syndrome. Journal of Nervous and Mental Disease, 179, 702. [DOI] [PubMed] [Google Scholar]
- Pasterski V, Prentice P, & Hughes IA (2010). Impact of the consensus statement and the new DSD classification system. Best Practice & Research Clinical Endocrinology & Metabolism, 24, 187–195. 10.1016/j.beem.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Pitts FN, & Guze SB (1963). Anorexia nervosa and gonadal dysgenesis (Turner’s syndrome). American Journal of Psychiatry, 119, 1100–1102. 10.1176/ajp.119.11.1100 [DOI] [Google Scholar]
- Rickert VI, Hassed SJ, Hendon AE, & Cunniff C (1996). The effects of peer ridicule on depression and self-image among adolescent females with Turner syndrome. Journal of Adolescent Health, 19, 34–38. 10.1016/1054-139X(95)00225-H [DOI] [PubMed] [Google Scholar]
- Ros C, Alobid I, Balasch J, Mullol J, & Castelo-Branco C (2013). Turner’s syndrome and other forms of congenital hypogonadism impair quality of life and sexual function. American Journal of Obstetrics and Gynecology, 208(6), 484.e1–6. 10.1016/j.ajog.2013.01.011 [DOI] [PubMed] [Google Scholar]
- Ross JL, McCauley E, Roeltgen D, Long L, Kushner H, Feuillan P, & Cutler GB (1996). Self-concept and behavior in adolescent girls with Turner syndrome: Potential estrogen effects. Journal of Clinical Endocrinology and Metabolism, 81, 926–931. 10.1210/jcem.81.3.8772552 [DOI] [PubMed] [Google Scholar]
- Rousseau A, Beyens I, Eggermont S, & Vandenbosch L (2017). The dual role of media internalization in adolescent sexual behavior. Archives of Sexual Behavior, 46, 1685–1697. 10.1007/s10508-016-0902-4 [DOI] [PubMed] [Google Scholar]
- Rovet J (1993). The psychoeducational characteristics of children with Turner syndrome. Journal of Learning Disabilities, 26, 333–341. [DOI] [PubMed] [Google Scholar]
- Rovet J, & Ireland L (1994). Behavioral phenotype in children with Turner syndrome. Journal of Pediatric Psychology, 19, 779–790. [DOI] [PubMed] [Google Scholar]
- Saad K, Abdelrahman AA, Abdel-Raheem YF, Othman ER, Badry R, Othman HAK, & Sobhy KM (2014). Turner syndrome: Review of clinical, neuropsychiatric, and EEG status: An experience of tertiary center. Acta Neurologica Belgica, 114, 1–9. 10.1007/s13760-013-0264-9 [DOI] [PubMed] [Google Scholar]
- Sabbath JC, Morris TA, Menzer-Benaron D, & Sturgis SH (1961). Psychiatric observations in adolescent girls lacking ovarian function. Psychosomatic Medicine, 23, 224–231. [DOI] [PubMed] [Google Scholar]
- Saenger P, Wikland KA, Conway GS, Davenport M, Gravholt CH, Hintz R, … Fifth International Symposium on Turner Syndrome. (2001). Recommendations for the diagnosis and management of Turner syndrome. Journal of Clinical Endocrinology and Metabolism, 86, 3061–3069. 10.1210/jcem.86.7.7683 [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Cardoso GMP, Ross JL, Haq N, Rubinow DR, & Bondy CA (2006). Shyness, social anxiety, and impaired self-esteem in Turner syndrome and premature ovarian failure. Journal of the American Medical Association, 295, 1374–1376. 10.1001/jama.295.12.1374 [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Luff JA, Haq NA, Vanderhoof VH, Koziol DE, Calis KA, … Nelson LM (2011). Depression in women with spontaneous 46, XX primary ovarian insufficiency. The Journal of Clinical Endocrinology and Metabolism, 96, E278–287. 10.1210/jc.2010-0613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea AK, & Wolfman W (2017). The role of hormone therapy in the management of severe postpartum depression in patients with Turner syndrome. Menopause, 24, 1309–1312. 10.1097/GME.0000000000000915 [DOI] [PubMed] [Google Scholar]
- Sheaffer AT, Lange E, & Bondy CA (2008). Sexual function in women with Turner syndrome. Journal of Women’s Health, 17, 27–33. 10.1089/jwh.2007.0488 [DOI] [PubMed] [Google Scholar]
- Sheanon NM, & Backeljauw PF (2015). Effect of oxandrolone therapy on adult height in Turner syndrome patients treated with growth hormone: A meta-analysis. International Journal of Pediatric Endocrinology, 18 10.1186/s13633-015-0013-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suorsa KI, Mullins AJ, Tackett AP, Reyes KJS, Austin P, Baskin L, … Mullins LL (2015). Characterizing early psychosocial functioning of parents of children with moderate to severe genital ambiguity due to disorders of sex development. Journal of Urology, 194, 1737–1742. 10.1016/j.juro.2015.06.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Collishaw S, Pine DS, & Thapar AK (2012). Depression in adolescence. Lancet, 379, 1056–1067. 10.1016/S0140-6736(11)60871-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen AF, Kvist TK, Andersen PK, & Kessing LV (2005). Increased risk of developing affective disorder in patients with hypothyroidism: A register-based study. Thyroid, 15, 700–707. 10.1089/thy.2005.15.700 [DOI] [PubMed] [Google Scholar]
- Twenge JM (2015). Time period and birth cohort differences in depressive symptoms in the U.S., 1982–2013. Social Indicators Research, 121, 437–454. 10.1007/s11205-014-0647-1 [DOI] [Google Scholar]
- Upthegrove R, Marwaha S, & Birchwood M (2017). Depression and schizophrenia: Cause, consequence, or trans-diagnostic Issue? Schizophrenia Bulletin, 43, 240–244. 10.1093/schbul/sbw097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pareren YK, Duivenvoorden HJ, Slijper FME, Koot HM, Drop SLS, & de Muinck Keizer-Schrama SMPF (2005). Psychosocial functioning after discontinuation of long-term growth hormone treatment in girls with Turner syndrome. Hormone Research, 63, 238–244. 10.1159/000085841 [DOI] [PubMed] [Google Scholar]
- Visser JA, Hokken-Koelega ACS, Zandwijken GRJ, Limacher A, Ranke MB, & Flück CE (2013). Anti-Müllerian hormone levels in girls and adolescents with Turner syndrome are related to karyotype, pubertal development and growth hormone treatment. Human Reproduction, 28, 1899–1907. 10.1093/humrep/det089 [DOI] [PubMed] [Google Scholar]
- Von Korff M, Shapiro S, Burke JD, Teitlebaum M, Skinner EA, German P, … Burns B (1987). Anxiety and depression in a primary care clinic: Comparison of Diagnostic Interview Schedule, General Health Questionnaire, and practitioner assessments. Archives of General Psychiatry, 44, 152–156. [DOI] [PubMed] [Google Scholar]
- Weiss L (1971). Additional evidence of gradual loss of germ cells in the pathogenesis of streak ovaries in Turner’s syndrome. Journal of Medical Genetics, 8, 540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R (2016). Williams textbook of endocrinology (13th ed.). Philadelphia, PA: Elsevier. [Google Scholar]