Abstract
Dopamine and serotonin in the basal ganglia interact in a bidirectional manner. On the one hand, serotonin (5-HT) receptors regulate the effects of dopamine agonists on several levels, ranging from molecular signaling to behavior. These interactions include 5-HT receptor-mediated facilitation of dopamine receptor-induced gene regulation in striatal output pathways, which involves the 5-HT1B receptor and others. Conversely, there is evidence that dopamine action by psychostimulants regulates 5-HT1B receptor expression in the striatum. To further investigate the effects of dopamine and agonists on 5-HT receptors, we assessed the expression of 5-HT1B and other serotonin receptor subtypes in the striatum after unilateral dopamine depletion by 6-OHDA and subsequent treatment with L-DOPA (5 mg/kg; 4 weeks). Neither dopamine depletion nor L-DOPA treatment produced significant changes in 5-HT2C, 5-HT4 or 5-HT6 receptor expression in the striatum. In contrast, the 6-OHDA lesion caused a (modest) increase in 5-HT1B mRNA levels throughout the striatum. Moreover, repeated L-DOPA treatment markedly further elevated 5-HT1B expression in the dopamine-depleted striatum, an effect that was most robust in the sensorimotor striatum. A minor L-DOPA-induced increase in 5-HT1B expression was also seen in the intact striatum. These changes in 5-HT1B expression mimicked changes in the expression of neuropeptide markers (dynorphin, enkephalin mRNA) in striatal projection neurons. After repeated L-DOPA treatment, the severity of L-DOPA-induced dyskinesias and turning behavior was positively correlated with the increase in 5-HT1B expression in the associative, but not sensorimotor, striatum ipsilateral to the lesion, suggesting that associative striatal 5-HT1B receptors may play a role in L-DOPA-induced behavioral abnormalities.
Keywords: dopamine, serotonin, gene expression, L-DOPA, striatum, Parkinson’s disease
Introduction
The ascending serotonin system modulates basal ganglia function in a complex manner, with serotonin input influencing neuronal activity in all basal ganglia nuclei. Serotonin effects are mediated by at least 14 serotonin (5-HT) receptor subtypes, many of which are present in the basal ganglia [1, 2]. For example, the striatum contains moderate to relatively high levels of 5-HT1B, 5-HT2C, 5-HT4 and 5-HT6, among others [1, 2]. These receptors impact striatal function by affecting intrinsic neurons directly and by altering striatal inputs. Thus, studies show that serotonin modifies dopamine input via 5-HT receptors located in the somatodendritic area of dopamine neurons in the midbrain and in dopamine terminal fields [2].
Among the dopamine actions regulated by serotonin are effects of psychostimulants (indirect dopamine agonists) such as cocaine and others on striatal gene regulation. This has been shown for several genes [3–6], including the neuropeptide dynorphin (e.g., [6]), a marker for D1 dopamine receptor-expressing direct pathway (striatonigral) neurons [7]. Several 5-HT receptors appear to be involved, but the 5-HT1B receptor plays a prominent role in this effect. For example, cocaine-induced gene regulation in striatal projection neurons, while principally mediated by D1 receptors [8–10], is facilitated by co-activation of 5-HT1B receptors [4, 11]. Our recent studies show that serotonin agonists (i.e., selective serotonin reuptake inhibitors, SSRIs) co-administered together with the psychostimulant methylphenidate, a dopamine reuptake blocker, potentiate methylphenidate-induced gene regulation in striatal projection neurons [12, 13]. This effect is also mediated at least in part by the 5-HT1B receptor [14].
The 5-HT1B receptor in turn is dynamically regulated by various factors, including stress (e.g., [15, 16]), drug withdrawal (e.g., [17]) and dopamine agonist treatments [18]. For example, upregulated 5-HT1B expression in the striatum has been shown after cocaine administration [19, 20]. Our studies recently demonstrated that repeated treatment with methylphenidate also increases the expression of 5-HT1B in striatal projection neurons [12]. This effect was selective in as far as another 5-HT receptor subtype that modifies dopamine and psychostimulant effects, 5-HT2C, was unaltered [12].
Serotonin receptors, including 5-HT1B, are also implicated in Parkinson’s disease and its treatment with L-DOPA [21, 22]. For example, recent studies reported upregulated 5-HT1B mRNA expression [23] and 5-HT1B receptor binding [24, 25] in the basal ganglia after dopamine depletion followed by repeated L-DOPA treatment. These findings raised the prospect that pharmacological targeting of serotonin receptors may provide an approach to control L-DOPA-induced dyskinesias, a problematic side effect of long-term L-DOPA treatment [21, 22].
In the present study, we used an animal model for Parkinson’s disease, dopamine depletion by 6-OHDA in combination with repeated L-DOPA treatment, to further investigated how dopamine and dopaminergic drugs impact the expression of 5-HT1B and other 5-HT receptor subtypes relatively highly expressed in striatal neurons (5-HT2C, 5-HT4, 5-HT6). We mapped the effects of these treatments on 5-HT receptor mRNA expression throughout the striatum in order to assess their impact on the different functional domains and compared the effects with the well-established changes in expression of neuropeptide markers in direct (dynorphin) and indirect pathway neurons (enkephalin) after a 6-OHDA lesion and L-DOPA treatment. Our findings demonstrate that dopamine depletion produces a minor increase in 5-HT1B mRNA levels and that repeated L-DOPA treatment causes a pronounced further increase in 5-HT1B expression, which mimics the regional distribution of increased dynorphin expression in direct pathway neurons (maximal in the sensorimotor striatum). Moreover, further analysis revealed that the severity of L-DOPA-induced dyskinesias was positively correlated with the magnitude of increases in 5-HT1B expression selectively in the associative striatum.
Materials and Methods
Animals
Adult male Sprague–Dawley rats (240–260 g at the beginning of the study; Harlan, Madison, WI, USA) were housed 2–3 per cage under standard laboratory conditions (12:12h light/dark cycle, lights on at 07:00h; with food and water available ad libitum). Experiments were performed between 13:00 and 17:00h. All procedures met the NIH guidelines for the care and use of laboratory animals and were approved by the Rosalind Franklin University Animal Care and Use Committee.
6-OHDA Lesions
Rats received an injection of desipramine (20 mg/kg, i.p.; in 0.9% saline; Sigma-Aldrich, St Louis, MO, USA) 30 minutes prior to surgery. After being deeply anesthetized with isoflurane vapors (2–5%), they received an infusion of either 6-OHDA (6-OHDA HBr, Sigma-Aldrich; 8 μg/4 μl, in saline containing 0.1% ascorbic acid) or saline/ascorbic acid into the right medial forebrain bundle as previously described [26]. The coordinates used were (in mm): anterior: 4.3 (from bregma), lateral: 1.6, ventral: 8.3 (from dura) [27]. 6-OHDA/vehicle were slowly infused at a rate of 0.4 μl/min, and the cannula was left in place for an additional 10 min before being removed.
The effectiveness of the 6-OHDA lesion was evaluated 4 weeks post-lesion by assessing deficits in forelimb movements using a stepping test. In this test, the number of adjusting steps with the forepaw contralateral to the lesion drops to 3 steps or fewer in animals with a >90% dopamine cell loss in the substantia nigra (normal response, 9–12 adjusting steps in our setting; [26, 28]). The present study included only 6-OHDA-infused rats that exhibited 3 or fewer adjusting steps (20 rats). The lesion was further characterized by measuring tyrosine hydroxylase (TH) immunoreactivity, and enkephalin and dynorphin expression in the striatum (Fig. 1).
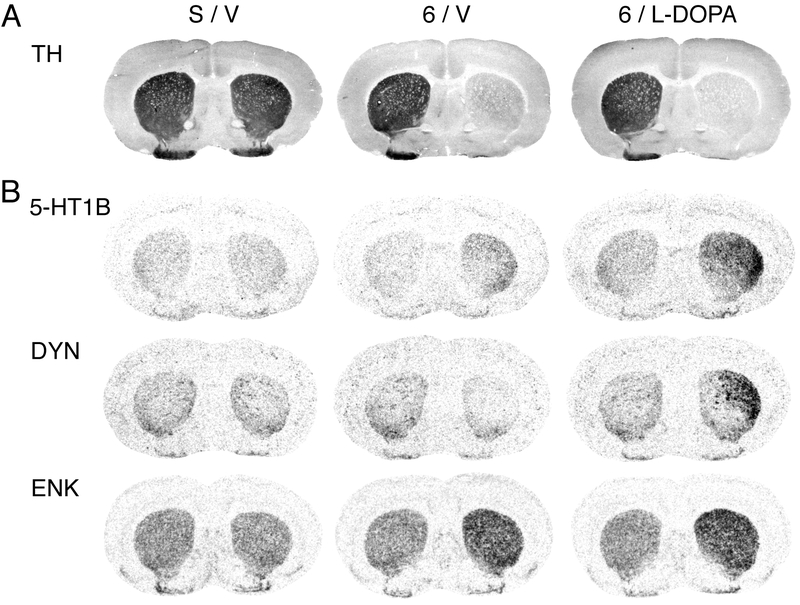
Figure 1.
Dopamine depletion by 6-OHDA and repeated L-DOPA treatment produce increased 5-HT1B expression in the striatum. (A) Coronal sections through the mid-level striatum labeled with tyrosine hydroxylase (TH) immunohistochemistry are shown for rats that received a sham lesion (S/V), a 6-OHDA infusion into the right medial forebrain bundle followed by vehicle treatment (6/V), or a 6-OHDA lesion followed by repeated treatment with L-DOPA (5 mg/kg + benserazide, 12.5 mg/kg; 4 weeks; 6/L-DOPA). (B) Illustrations of film autoradiograms depict expression of 5-HT1B, dynorphin (DYN), and enkephalin (ENK) mRNA in sections from the mid-level striatum in sham controls (S/V), after a 6-OHDA lesion only (6/V), or after dopamine depletion followed by repeated L-DOPA treatment (6/L-DOPA). Animals were killed 60 min after the last injection. The maximal hybridization signal is in black. Dopamine depletion alone (6/V) produced changes in gene expression with a fairly even distribution throughout the striatum (5-HT1B, DYN, ENK), matching the loss of dopamine terminals (TH signal).
In contrast, repeated L-DOPA treatment (6/L-DOPA) produced increases in gene expression (5-HT1B, DYN) with a distinct medial-lateral gradient (maximal increases in lateral, sensorimotor sectors).
Drug Treatment
Rats were randomly assigned to the different treatments. A sham/partial lesion group (S/V; n=9; five saline-infused rats plus four 6-OHDA-infused rats without behavioral deficits) received repeated injections of vehicle (saline, 1 ml/kg, i.p.). The 6-OHDA-infused rats with stepping deficits were treated with either vehicle (6/V; n=11) or L-DOPA (5 mg/kg, Alfa Aesar, Tewksbury, MA, USA; plus 12.5 mg/kg benserazide hydrochloride, Sigma-Aldrich) (6/L-DOPA; n=9). These two groups did not differ in their stepping deficits (p>0.05; data not shown). Animals received these drug treatments once daily on 5 days (Mon-Fri) for three weeks. In week 4, rats were treated on 3 days. Following the last injection, the rat was placed in an open-field apparatus (43 × 43 cm), and turning behavior was recorded with a video camera for 40 min. Rats were then killed 1 h after the final treatment.
Behavioral Analysis
L-DOPA-induced dyskinesias (abnormal involuntary movements, AIMs) were evaluated once daily on three days per week (Wed-Fri) during the first 3 weeks by a rater unaware of the treatment [29]. Behavior was videotaped, and AIMs were rated using a time-sampling procedure, i.e., during 2-min periods at 30 min intervals 30–180 min after the L-DOPA injection. Three AIMs subtypes were assessed, “axial”, “limb” (forelimb) and “orolingual” (see [30], for exact definitions). The frequency of these AIMs subtypes was rated using a standard scale (0 = absent; 1 = occasional; 2 = frequent with many interruptions; 3 = continuous but interrupted by external sensory stimuli; and 4 = continuous, not interrupted by strong sensory stimuli) [30–32]. In addition, the amplitude of AIMs was scored as follows [30]: axial AIMs (1 = consistent lateral deviation of head and neck at approximately 30° angle; 2 = lateral deviation of head and neck, 30° < angle ≤ 60°; 3 = lateral deviation of head, neck and upper trunk, 60° < angle ≤ 90°; 4 = torsion of head, neck and trunk at > 90° angle, often causing rat to lose balance), forelimb AIMs (1 = small involuntary movements of the distal forelimb; 2 = movements of low amplitude causing translocation of both distal and proximal forelimb; 3 = involuntary movements of the whole limb including shoulder muscles; 4 = strong limb and shoulder movements, often similar to ballism), and orolingual AIMs (1 = small involuntary movements of the orofacial muscles; 2 = orofacial movements of high amplitude with tongue protrusion). Partial scores such as 0.5, 1.5, 2.5, and 3.5 were given to increase the sensitivity of the rating. Frequency and amplitude scores were multiplied for each monitoring period (i.e., 30, 60, 90, 120, 150 and 180 min) and the values added up, giving a total AIMs score for each subtype and an overall total for each testing day. For the correlation analyses reported here, the average AIMs scores for Wed, Thu and Fri of week 3 were used.
Turning behavior in the open-field test on the last day (week 4) was assessed during 4 sampling periods, at 5–10, 15–20, 25–30, 35–40 min after drug injection. Tight circling contraversive to the lesion (i.e., towards the left side) (see [33]) was measured (counted as number of half-turns). In these animals, L-DOPA-induced turning behavior emerged between 11 and 18 min after L-DOPA injection. Thus, the total number of half-turns during the periods 25–30 and 35–40 min (“20–40 min”) were used for correlation analysis.
Tissue Preparation and In Situ Hybridization Histochemistry
The rats were killed with CO2, and the brain was rapidly removed, frozen in isopentane cooled on dry ice and then stored at −30 °C until cryostat sectioning. Coronal sections (12 μm) were thaw-mounted onto glass slides (Superfrost/Plus, Daigger, Wheeling, IL, USA), dried on a slide warmer and stored at −30 °C. In situ hybridization histochemistry was performed as described before [12, 34]. Oligonucleotide probes (48-mers; Invitrogen, Rockville, MD, USA) were labeled with [33P]-dATP. The probes had the following sequence: enkephalin, complementary to bases 436–483, GenBank accession number M28263; dynorphin, bases 862–909, M10088; 5-HT1B (Htr1b), bases 62–109, NM 022225; 5-HT2C (Htr2c), bases 363–410, NM 012765; 5-HT4 (Htr4), bases 683–730, NM 012853; and 5-HT6 (Htr6), bases 841–888, L41146. Hybridization and washing procedures were as reported [12, 35]. The sections were apposed to X-ray film (BioMax MR-2, Kodak) for 3–11 days.
Tyrosine Hydroxylase Immunohistochemistry
Striatal sections were processed for TH immunohistochemistry to determine the extent of the dopamine depletion, following previously published procedures [34, 36]. In short, fresh-frozen, thaw-mounted 12 μm sections were first fixed in 4% paraformaldehyde/saline for 10 min. TH immunolabeling was then revealed with a rabbit peroxidase–antiperoxidase (1:500) reaction, followed by a standard 3,3’-diaminobenzidine protocol with nickel intensification. The signal was measured by densitometry.
Analysis of Autoradiograms
Gene expression in the striatum was assessed in sections from 3 rostrocaudal levels [rostral, approximately at +1.6 mm relative to bregma [27]; middle, +0.4 (Fig. 1); caudal, −0.8] in a total of 23 sectors. These sectors are mostly defined by their predominant cortical inputs and thus reflect different functional domains (see [35, 37]). Eighteen of these sectors represent the caudate-putamen, and 5 the nucleus accumbens.
Hybridization signals on film autoradiograms were measured by densitometry (NIH Image; Wayne Rasband, NIMH, Bethesda, MD, USA), as described [12]. Mean densities (Fig. 2) were corrected for background by subtracting mean density values measured over white matter (corpus callosum). The illustrations of film autoradiograms displayed in Figure 1 are computer-generated images, and are contrast-enhanced where necessary.
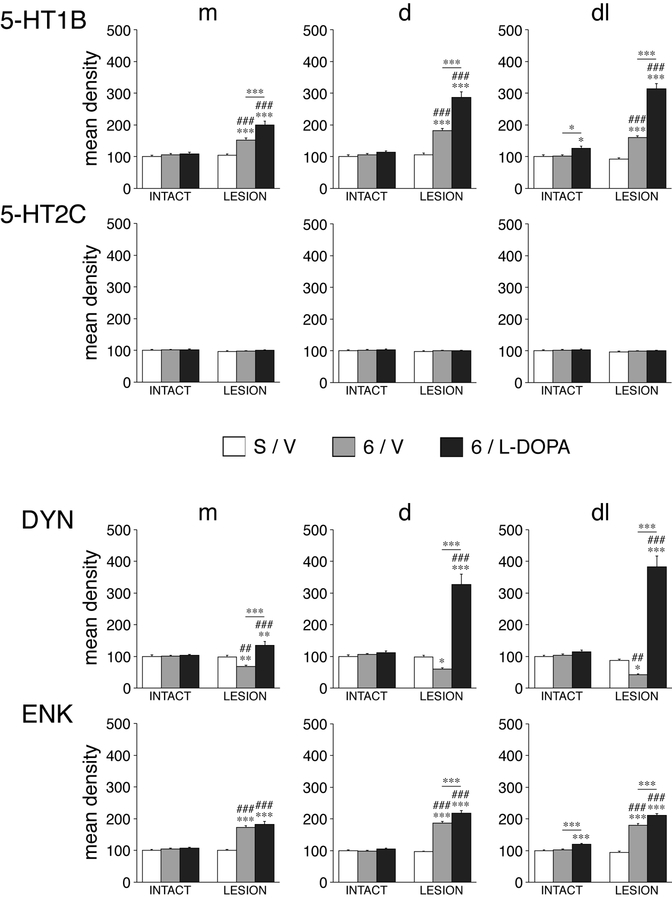
Figure 2.
Effects of unilateral dopamine depletion by 6-OHDA and repeated L-DOPA treatment on gene expression in the striatum. Mean density values (mean ± SEM) for 5-HT1B, 5-HT2C, dynorphin (DYN), and enkephalin (ENK) in rats with sham/partial lesions (S/V), rats with a 6-OHDA lesion only (6/V), and rats with a dopamine lesion followed by repeated L-DOPA treatment (6/L-DOPA) are given for the medial (m), dorsal (d), and dorsolateral (dl) sectors on the side ipsilateral (LESION) and contralateral (INTACT) to the lesion, on the middle striatal level (see Fig. 3 for locations of these sectors). In contrast to the robust changes in gene expression for 5-HT1B, dynorphin and enkephalin, no changes were found for 5-HT2C. *p<0.05, **p<0.01, ***p<0.001 vs. S/V or as indicated; #p<0.05, ##p<0.01, ###p<0.001 vs. same group on intact side.
Statistics
Treatment effects were determined by two-factor ANOVA. Newman-Keuls post hoc tests were used to describe differences between individual groups (Statistica, StatSoft, Tulsa, OK, USA). The distribution of changes in gene expression throughout the 23 striatal sectors was illustrated by maps (Fig. 3). For these maps, the difference in gene expression (vs. S/V or as indicated) in a given sector was expressed as the percentage of the values in S/V on the intact side (“basal” expression) and then normalized (percentage of maximal difference observed) for either gene (% max.). Gene regulation effects for different genes were compared by Pearson correlations. Changes in gene expression (values ipsilateral to the lesion expressed relative to those on the intact side) were also correlated with the behavioral outcomes for the L-DOPA-treated group.
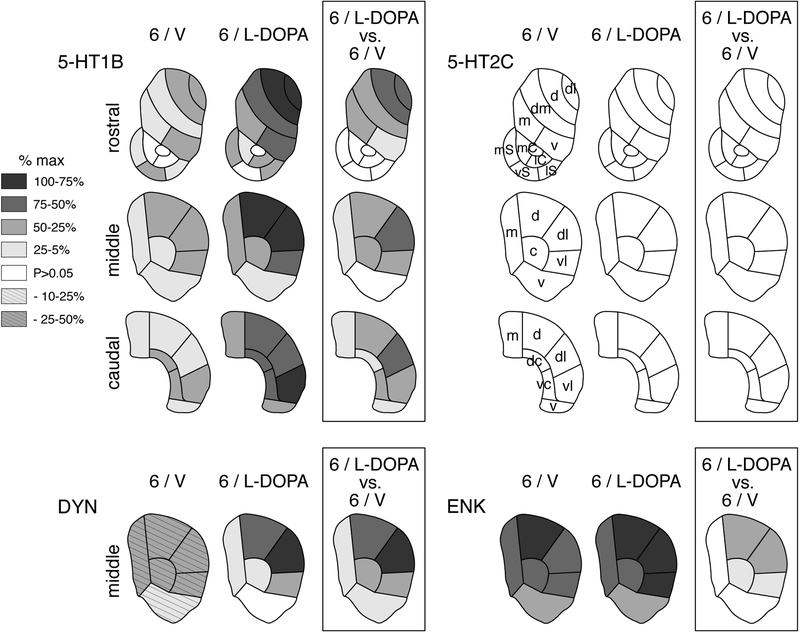
Figure 3.
Topography of gene regulation induced the 6-OHDA lesion and L-DOPA treatment in the striatum. Maps depict the distribution of changes in gene expression (i.e., the differences vs. sham/partial lesion controls, S/V) for 5-HT1B and 5-HT2C across the 23 sectors of the rostral, middle and caudal striatum, and for dynorphin (DYN) and enkephalin (ENK) in the 6 sectors of the middle striatum, on the side of the lesion in rats with 6-OHDA lesions only (6/V) and in rats with 6-OHDA lesions followed by repeated L-DOPA treatment (6/L-DOPA). The differences between 6/L-DOPA and 6/V groups are also shown (box). The data are normalized relative to the maximal increase observed (% of max.) for each gene. Sectors with a statistically significant difference (p<0.05) are shaded as indicated. Sectors with a decrease are hatched (DYN). Sectors without significant difference (vs. S/V) are in white. Note the distinct medial-lateral gradient in L-DOPA-induced gene regulation (6/L-DOPA) for 5-HT1B and dynorphin. No significant changes were seen for 5-HT2C. Sectors are based on corticostriatal inputs (see [35]): Limbic sectors (nucleus accumbens), rostral: medial shell (mS), ventral shell (vS), lateral shell (lS), medial core (mC), lateral core (lC); associative sectors, rostral: dorsomedial (dm), medial (m), ventral (v); middle: medial (m), central (c), ventral (v); caudal: medial (m), dorsal central (dc), ventral central (vc), ventral (v); sensorimotor sectors, rostral: dorsolateral (dl), dorsal (d); middle: dorsal (d), dorsolateral (dl), ventrolateral (vl); caudal: dorsal (d), dorsolateral (dl), ventrolateral (vl).
Results
Characterization of the Dopamine Depletion by 6-OHDA
Animals that met the inclusion criterion of 3 or less contralateral adjusting steps (groups 6/V and 6/L-DOPA) showed a loss of TH immunoreactivity in the striatum ipsilateral to the 6-OHDA infusion with a range of 73.5–100% (mean ± SEM, 90.0±1.5; % of intact side; total measured on the middle level; Figs. 1A, 4). In the S/V group, the four 6-OHDA-infused rats without behavioral deficits had partial lesions with a loss of striatal TH labeling of 11–45% (Fig. 4). None of these rats showed a reduced number of adjusting steps (not shown), in agreement with previous findings [38].
Figure 4.
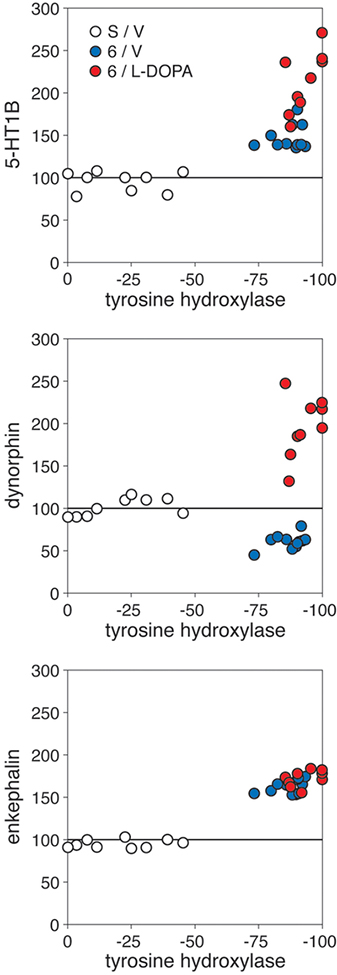
Relationship between the degree of dopamine depletion, as indicated by the loss of tyrosine hydroxylase (TH) immunoreactivity in the striatum, and changes in striatal gene expression. Scatter plots compare the TH signal with levels of 5-HT1B (top), dynorphin (middle) and enkephalin expression (bottom) (values in % of intact side) measured in the total striatal area on the middle level for individual animals in the sham/partial lesion group (S/V), after the 6-OHDA lesion only (6/V) and after the 6-OHDA lesion plus L-DOPA treatment (6/L-DOPA). Note that half of the S/V animals had partial lesions without behavioral deficits (see text) or effects on striatal gene expression.
Effects of 6-OHDA Lesion on Dynorphin and Enkephalin Expression
Consistent with previous findings (e.g., [39]), animals with partial dopamine depletion (S/V; <45% loss of striatal TH signal) displayed unchanged neuropeptide expression in the striatum ipsilateral to the 6-OHDA infusion (Fig. 4). In contrast, greater lesions (>73% loss of TH signal) produced decreased or increased expression of dynorphin or enkephalin, respectively, on the side of the lesion (6/V; Figs. 1, 2, 3, 4) [7]. Dopamine depletion (i.e., loss of TH signal) and these changes in neuropeptide expression were fairly uniform throughout the striatum (Figs. 1, 2, 3), with decreases in dynorphin expression present in 18 striatal sectors [no statistically significant decreases were seen in 5 caudal sectors that display very low basal dynorphin expression (i.e., likely “floor” effect); not shown] and increases in enkephalin expression in 23 of the 23 striatal sectors.
Effects of L-DOPA Treatment on Dynorphin and Enkephalin Expression
Repeated treatment with L-DOPA (6/L-DOPA) produced a dramatic increase in dynorphin expression and a statistically significant (but modest) further increase in enkephalin expression in the dopamine-depleted striatum (Figs. 1, 2). Overall, after L-DOPA treatment, significantly increased dynorphin expression (6/L-DOPA vs. S/V) was present in 16 of the 23 sectors and greater expression than in lesion-only animals (6/L-DOPA vs. 6/V) in all 23 sectors, on all three rostrocaudal levels (Figs. 1, 2, 3; only effects on middle level shown). However, in marked contrast to the uniform changes seen after dopamine depletion alone (6/V; see above), these increases displayed a distinct medial-lateral gradient. The increases were minimal in the medial striatum and were most pronounced in the dorsal and lateral (sensorimotor) striatum (Figs. 1, 2, 3).
Similarly, L-DOPA treatment produced significantly increased enkephalin expression (6/L-DOPA vs. S/V) in 22 of the 23 sectors and greater expression than in lesion-only animals (6/L-DOPA vs. 6/V) in 17 sectors, on all three rostrocaudal levels (only middle effects shown).
Repeated L-DOPA treatment also produced minor increases in enkephalin expression in the intact striatum (contralateral to lesion). These increases were restricted to dorsolateral (Fig. 2) and ventrolateral (sensorimotor) sectors on all rostrocaudal levels (6/L-DOPA vs. S/V, 6 of 23 sectors; 6/L-DOPA vs. 6/V, 4 of 23) (data not shown).
Effects of 6-OHDA Lesion and L-DOPA Treatment on 5-HT Receptors: Selective Impact on 5-HT1B Expression
Both dopamine depletion and repeated L-DOPA treatment produced differential changes in striatal 5-HT receptor expression. Marked effects were seen for 5-HT1B mRNA expression in the striatum ipsilateral to the lesion. These effects were widespread, present on all 3 rostrocaudal levels (Figs. 1, 2, 3, 4). After dopamine depletion alone (6/V) there was a statistically significant increase in 5-HT1B expression in 21 of the 23 sectors (Figs. 2, 3). Although again fairly uniform throughout the striatum, lateral sectors showed a somewhat more robust increase (Figs. 1, 3). Repeated L-DOPA treatment produced a more pronounced increase in 5-HT1B expression (Figs. 1, 2). This increase was seen in comparison to sham/partial lesion controls (6/L-DOPA vs. S/V, in 22 of the 23 sectors) and in comparison to lesion-only animals (6/L-DOPA vs. 6/V, in 17 sectors), on all three rostrocaudal levels (Fig. 3). Regionally, these effects were again strongest in the dorsal and lateral striatum and weakest in the medial and ventral striatum, including the nucleus accumbens (Figs. 1, 2, 3).
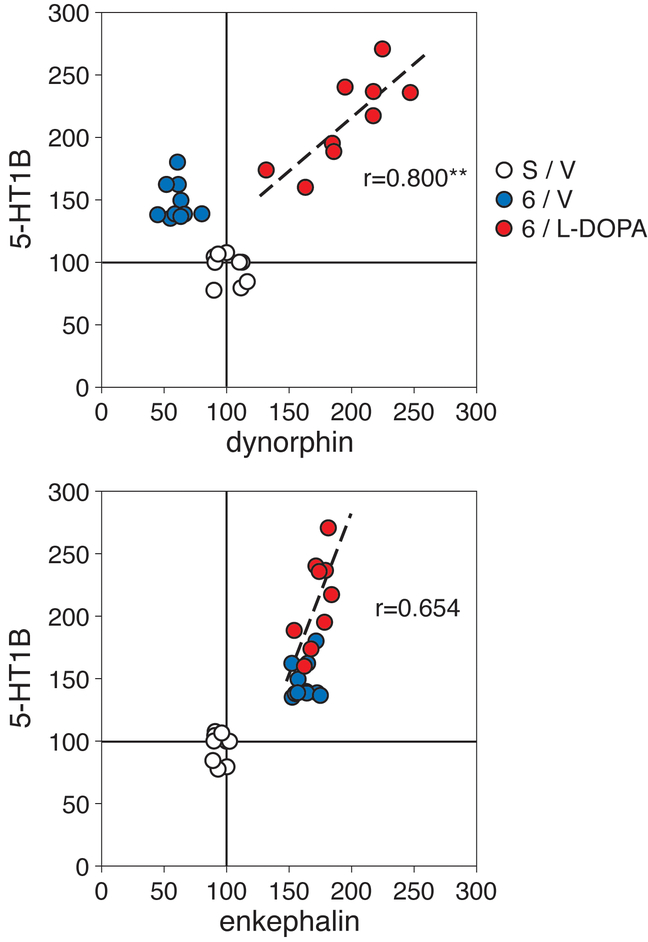
The effects of L-DOPA treatment on 5-HT1B expression in the dopamine-depleted striatum were also compared with those on dynorphin and enkephalin expression (middle level, total area) by correlation analysis (Fig. 5). Thus, in the 6/L-DOPA group, there was a significant positive correlation between the increases in dynorphin expression and those in 5-HT1B expression (r=0.800, p<0.01), and a strong trend towards a positive correlation between enkephalin and 5-HT1B expression (r=0.654, p<0.06), for individual animals (Fig. 5).
Figure 5.
Relationship between changes in the expression of 5-HT1B and those in dynorphin (top) or enkephalin expression (bottom) in the striatum. Scatter plots show levels of gene expression (in % of intact side) measured in the total striatal area on the middle level for individual animals in the sham/partial lesion group (S/V), after the 6-OHDA lesion only (6/V), and after the 6-OHDA lesion plus L-DOPA treatment (6/L-DOPA). For animals in the 6/L-DOPA group (red), there was a significant positive correlation between 5-HT1B and dynorphin expression (r=0.800, p<0.01) and a strong tendency between 5-HT1B and enkephalin expression (r=0.654, p<0.06). **p<0.01.
L-DOPA treatment also affected 5-HT1B expression in the intact striatum of animals with 6-OHDA lesions. Although minor, these effects were statistically significant on the middle level and maximal in the lateral striatum (6/L-DOPA vs. S/V, 3 of 23 sectors; 6/L-DOPA vs. 6/V, 2 of 23) (Figs. 1, 2).
In contrast to these prominent changes in 5-HT1B expression, no effects of dopamine depletion with or without L-DOPA treatment were found on the expression of striatal 5-HT2C (Figs. 2, 3), 5-HT4 or 5-HT6 receptors (data not shown).
Relationship Between L-DOPA-Induced Behavioral Effects and Striatal 5-HT1B Expression
Detailed behavioral results are presented elsewhere (Padovan-Neto et al., submitted).
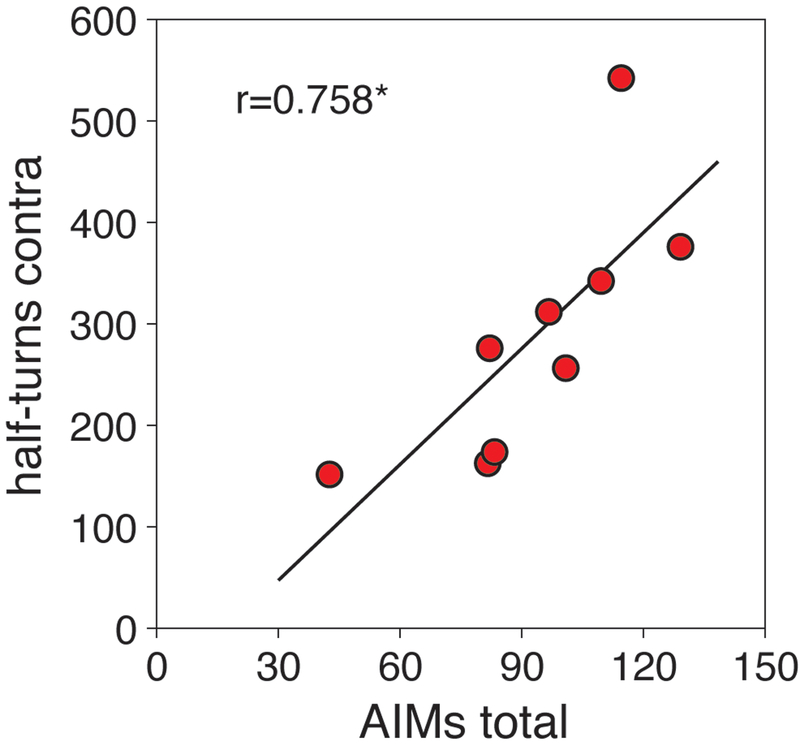
For the present analysis, dyskinesia (AIMs) scores and turning rates were considered (Fig. 6). All L-DOPA-treated rats (6/L-DOPA group) developed axial, limb and orolingual AIMs during the repeated L-DOPA treatment. Total AIMs score averages for Wed-Fri of week 3 are presented in Figure 6. These rats also displayed contraversive turning behavior in the open-field test in week 4. These turning rates (half-turns) in week 4 were positively correlated with the averaged total AIMs scores in week 3 (r=0.758, p<0.05; Fig. 6).
Figure 6.
Behavioral effects induced by repeated L-DOPA treatment (6/L-DOPA group). The scatter plot shows the positive correlation (r=0.758, p<0.05) between total AIMs scores in week 3 (3-day averages) and turning rates in week 4 (number of half-turns contraversive to the lesion) for these animals. *p<0.05.
We used correlation analysis to compare the increases in gene expression in the dopamine-depleted striatum with the 3-day averaged scores for total AIMs, orolingual, limb and axial AIMs, and turning rates in individual L-DOPA-treated rats. A correlation matrix for these behavior x gene expression correlations for all 23 striatal sectors showed that predominantly sectors of the associative striatum displayed positive correlations between the increases in 5-HT1B expression and behavioral changes. For example, Figure 7 displays the r values for total AIMs scores x gene expression correlations in the 23 sectors for 5-HT1B expression (in comparison to those for dynorphin expression); these individual-sector correlations were statistically significant (r>0.67, p<0.05) in the following sectors (see Fig. 3, for sector location): rostral: ventral; middle: medial, central, ventral; caudal: ventral (Fig. 7), all associative sectors.
Figure 7.
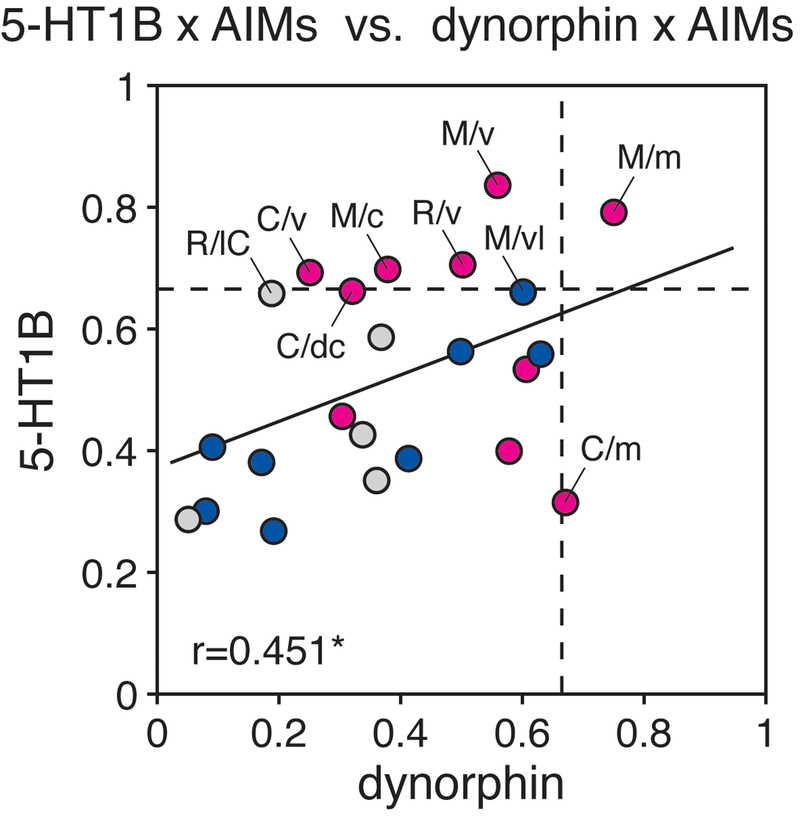
Relationship between L-DOPA-induced dyskinesias (AIMs) and increases in gene expression in the striatum. The scatter plot shows the relationship between the r values for gene expression x AIMs (total AIMs, 3-day averages, week 3) correlations for 5-HT1B expression and those for dynorphin expression, in the 23 striatal sectors of the 6/L-DOPA group [r=0.451, p<0.05; without outlier (C/m), r=0.575, p<0.005]. The broken lines indicate the significance threshold for the single-sector correlations (r=0.67, p=0.05). Note that 5 of the 10 associative sectors (red dots) showed significant positive correlations between 5-HT1B expression and total AIMs scores (r>0.67, p<0.05), and 2 of the 10 associative sectors showed significant positive correlations between dynorphin expression and total AIMs scores. In contrast, none of the 8 sensorimotor sectors (dark blue dots) or 5 limbic sectors (light gray dots) displayed significant gene expression x AIMs correlations (r<0.67, p>0.05). R/lC, rostral/lateral core; R/v, rostral/ventral; M/c, middle/central; M/m, middle/medial; M/v, middle/ventral; C/dc, caudal/dorsal central; C/m, caudal/medial; C/v, caudal/ventral. *p<0.05.
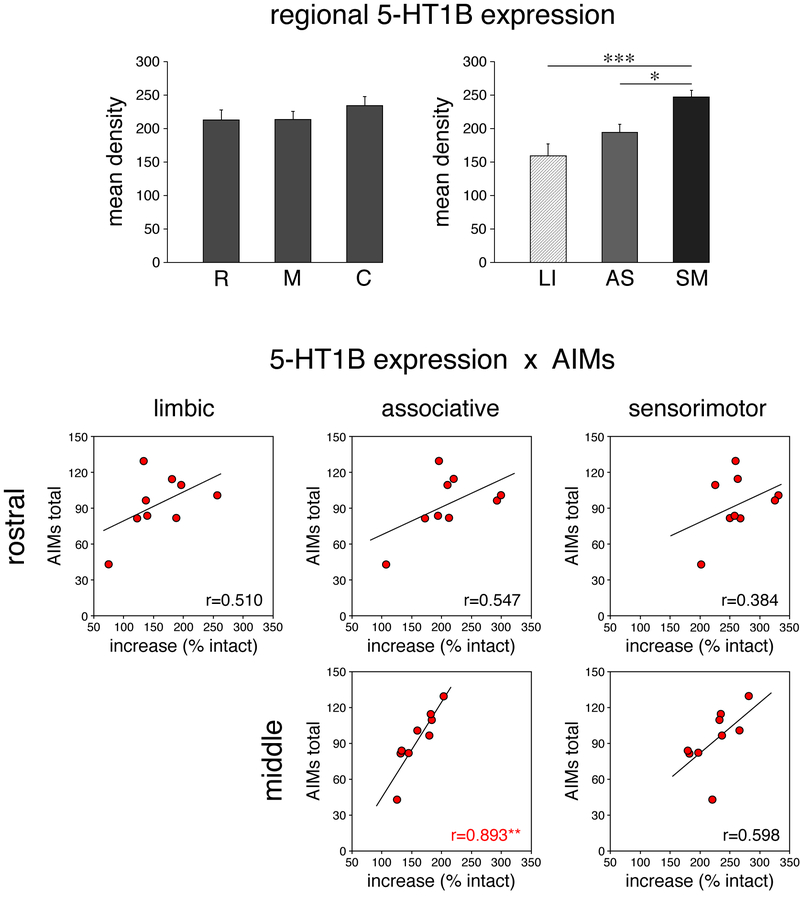
To increase statistical power, we thus averaged gene expression values from sensorimotor, associative or limbic sectors, respectively, for rostral, middle and caudal striatal levels and also for the total striatum (rostral, middle, caudal values pooled; Fig. 8, top). This analysis confirmed that the pooled sensorimotor sectors displayed a significantly greater increase in 5-HT1B expression than associative or limbic sectors (Fig. 8).
Figure 8.
Regional expression of 5-HT1B in the dopamine-depleted striatum and its relationship to L-DOPA-induced dyskinesias (total AIMs) in 6/L-DOPA group. (Top) Expression of 5-HT1B (mean density, mean ± SEM, in % of intact side) in total rostral (R), middle (M) and caudal (C) striatum (left), and in averaged limbic (LI), associative (AS) and sensorimotor (SM) sectors, on the side of the lesion is depicted. (Bottom) Scatter plots show the relationship between the total AIMs scores (3-day averages, week 3) and the increases in 5-HT1B expression in averaged limbic, associative or sensorimotor sectors on the rostral and middle striatal levels for individual animals of the 6/L-DOPA group. There was a significant positive correlation for total AIMs vs. increases in 5-HT1B expression in the associative striatum on the middle level (r=0.893, p<0.01). *p<0.05, **p<0.01, ***p<0.001.
For values from all 3 rostrocaudal levels pooled, there were positive correlations between increases in 5-HT1B expression and behavior, as follows: associative sectors (total of 10 sectors pooled): x total AIMs (r=0.788, p<0.05), x orolingual (r=0.869, p<0.01), x axial (r=0.715, p<0.05), and x turning (r=0.684, p<0.05); sensorimotor sectors (total of 8 sectors pooled): x orolingual (r=0.798, p<0.05); limbic sectors (rostral level, 5 nucleus accumbens sectors pooled): none.
Our regional analysis showed that these effects were not evenly distributed along the rostral-caudal axis, but were most robust in middle striatum (Figs. 8, 9). The scatter plots in Figure 8 (bottom) contrast these correlations between rostral and middle levels for total AIMs. There was a significant positive correlation for associative sectors x total AIMs (r=0.893, p<0.01) on the middle level, but no other significant correlations (p>0.05) on the rostral and middle levels (Fig. 8), or caudal levels (not shown).
Figure 9.
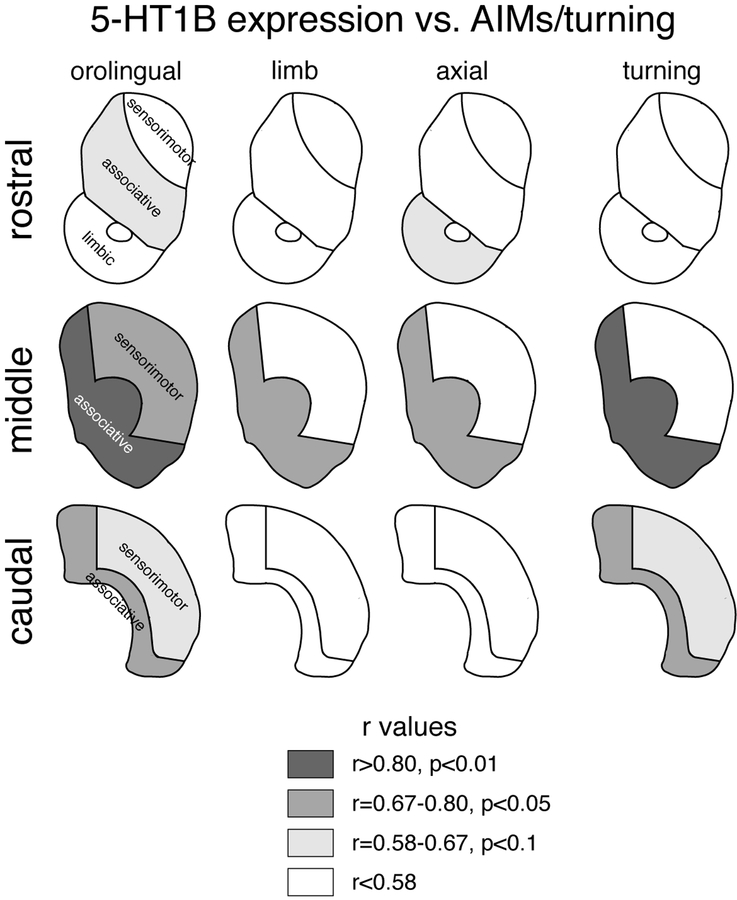
Relationship between L-DOPA-induced behavior and increases in 5-HT1B expression in the associative striatum. Correlation maps for rostral, middle and caudal striatal levels display the strength of correlations (r values) between AIMs/turning and increases in 5-HT1B expression in averaged limbic, associative or sensorimotor sectors for orolingual, limb and axial AIMs, and turning behavior.
Figure 9 displays correlation maps that summarize these effects for changes in 5-HT1B expression x specific AIMs scores (orolingual, limb, axial) and turning rates. Note that these maps also show tendencies (r=0.58–0.67, 0.1>p>0.05), as these confirm the overall regional patterns (i.e., associative > sensorimotor effects). There were no significant correlations on the rostral level (Fig. 9): associative, sensorimotor, limbic sectors, all p>0.05. In contrast, there were significant correlations for the middle level (Fig. 9): associative: x orolingual (r=0.871, p<0.01), x limb (r=0.790, p<0.05), x axial (r=0.773, p<0.05), and x turning (r=0.810, p<0.01); sensorimotor: x orolingual (r=0.797, p<0.05); and for the caudal level: associative: x orolingual (r=0.770, p<0.05), and x turning (r=0.679, p<0.05); sensorimotor: none.
We further assessed a potential relationship between these behavior (total AIMs) x gene expression correlations in the 23 striatal sectors for 5-HT1B expression and those for neuropeptide expression. Figure 7 shows that there was a significant positive correlation between the correlations (r values) for 5-HT1B vs. dynorphin expression (r=0.451, p<0.05; Fig. 7), but not enkephalin expression (r=0.361, p>0.05; not shown).
Discussion
The present study investigated the role of dopamine in the regulation of serotonin receptors in the basal ganglia in an animal model of Parkinson’s disease. Our main findings are summarized as follows. Dopamine depletion by 6-OHDA and dopamine replacement by L-DOPA had differential effects on the different serotonin receptor subtypes expressed in the striatum. Both dopamine depletion and repeated L-DOPA treatment produced increased expression of the 5-HT1B receptor, whereas no effects of these treatments on the expression of 5-HT2C, 5-HT4 or 5-HT6 receptors in the striatum were found. There were markedly differential effects on gene expression in the different functional domains of the striatum. After dopamine depletion alone, the increases in 5-HT1B mRNA expression, similar to changes in neuropeptide expression (dynorphin, enkephalin), were fairly uniform across the striatum and mirrored the loss of dopamine terminals (TH immunoreactivity). In contrast, L-DOPA-induced increases in 5-HT1B expression (as well as dynorphin and enkephalin) were most pronounced in the lateral (sensorimotor) striatum, with lesser or no effects in the medial (associative) and ventral (limbic) striatum. However, a comparison with L-DOPA-induced dyskinesias (AIMs) in these animals revealed significant positive correlations between AIMs scores and increases in 5-HT1B expression in the associative, but not sensorimotor, striatum, predominantly on the middle striatal level.
Characterization of the Dopamine Depletion: Effects on Stepping and Dynorphin and Enkephalin Expression
Degree and distribution of dopamine depletion were characterized by mapping the loss of TH immunoreactivity (dopamine terminals) throughout the striatum. Our results show that the 6-OHDA-infused rats that were included based on their significant deficit in contralateral forepaw stepping (3 or fewer adjusting steps; [38]) had a uniform TH loss throughout the striatum. This TH loss had a range of 73–100% (average 90%). Overall, these results are consistent with previous findings showing deficient stepping with a 90% or greater loss of dopamine cell bodies in the substantia nigra [26, 28] or 80–100% loss of dopamine tissue content in the striatum [38]. The somewhat smaller loss of striatal TH immunoreactivity seen in some of the severely deficient animals observed here, compared with the previous cell counts [26, 28], may reflect methodological differences between these two studies. It is conceivable that the terminal TH immunocytochemical signal was somewhat less reduced, perhaps due to terminal sprouting or upregulated TH expression in residual dopamine terminals [40].
Previous work also compared the lesion size with changes in striatal neuropeptide expression after 6-OHDA lesions. Similar to our findings, large dopamine loss was found to be necessary for changes in neuropeptide expression in the striatum, whereas partial lesions had no effect. Nisenbaum et al. [39] assessed the effects of dopamine depletion on the expression of substance P and enkephalin (markers for neurons of the direct and indirect, respectively, striatal output pathways [7]). Those results showed decreased substance P expression in animals with a dopamine depletion (i.e., loss of striatal dopamine tissue content) of 40–100% and increased enkephalin expression with a dopamine depletion of >90% [39]. Our study found decreased dynorphin expression (direct pathway) and increased enkephalin expression (indirect pathway) with 73–100% loss of TH immunoreactivity (and no changes with <45% loss, S/V group). The effects on enkephalin expression (increase with >73% TH loss) again suggest that the present TH signal (as compared to dopamine tissue content and cell counts) may slightly underestimate the lesion size.
Overall, our results on the regulation of dynorphin and enkephalin expression by near-complete dopamine depletion followed by L-DOPA treatment are also in agreement with previous work (e.g., [31, 41–47]).
Effects on 5-HT Receptor Expression: Robust Regulation of 5-HT1B by Dopamine Depletion and Repeated L-DOPA Treatment
Previous studies demonstrated that dopamine depletion followed by repeated L-DOPA treatment produces changes in the expression of thousands of genes in the striatum (e.g., [18, 48, 49]). Although interactions between the dopamine and serotonin systems are well established (e.g., [50, 51]), only a few previous studies have assessed the impact of these treatments on serotonin receptors in the basal ganglia. Zhang et al. [23] first reported increased expression of the 5-HT1B receptor (and the functionally related adaptor protein p11) in striatal neurons after 6-OHDA lesions followed by repeated L-DOPA treatment in rats and mice. Consistent with this finding, other studies found increased 5-HT1B receptor binding in basal ganglia nuclei following L-DOPA treatment in MPTP-lesioned monkeys and Parkinson’s disease patients [24, 25]. However, these latter binding studies did not resolve the localization (afferent terminals vs. intrinsic neurons) of these receptor changes, while our study and that by Zhang et al. [23] demonstrate increased receptor expression in striatal neurons.
Our study can be directly compared with that of Zhang et al. [23], as we used a similar approach (6-OHDA lesions in rats, in situ hybridization histochemistry to localize 5-HT1B mRNA). Our findings are for the most part in agreement with and extend these earlier results, although some different outcomes were also obtained. For example, in contrast to our finding of (moderately) increased 5-HT1B expression after dopamine depletion alone, that study found no statistically significant effects of the dopamine lesion (92–97% loss of TH protein as determined by Western blotting) on 5-HT1B (and p11) mRNA or protein levels, although trends for increases were discernible for 5-HT1B mRNA [23].
These discrepancies may be related in part to the striatal subregions examined. The earlier investigation [23] appeared to be limited to a part of the striatum that corresponded to our caudal striatal level. Our study mapped changes in 5-HT1B mRNA levels after dopamine depletion throughout the striatum and demonstrated statistically significant increases in most striatal sectors on rostral, middle and caudal levels, but with somewhat more robust increases in lateral (sensorimotor) sectors, especially on the middle level. We also found regionally differential effects on the rostral level, with modest increases in 5-HT1B expression in the nucleus accumbens shell, but not core. In summary, in our study, the dopamine depletion produced widespread increases in 5-HT1B expression throughout the striatum, but these effects were to some degree region-specific, with different functional domains of the striatum being differentially affected: limbic regions displayed minimal and sensorimotor regions the most robust impact.
Cell type-specific effects may also contribute to variable outcomes. Another study using a more sensitive approach - translating ribosome affinity purification (TRAP) analysis - determined changes in 5-HT1B and other 5-HT receptor mRNA expression in identified striatal projection neurons in mice (whole striatum) [18]. Results showed that dopamine depletion alone produced a modest increase in 5-HT1B expression preferentially in D2 receptor-expressing (indirect pathway) neurons. This is consistent with our findings showing, in rats, increases in 5-HT1B expression after the 6-OHDA lesion alone with dynamics similar to those for enkephalin expression (marker for indirect pathway).
The above study by Zhang et al. [23] also probed the impact of L-DOPA treatment on 5-HT1B expression by assessing the effects of high doses of L-DOPA (100 mg/kg, twice daily, 5 days, or 10 mg/kg, once daily, 28 days) after the 6-OHDA lesion in rats. Both high-dose treatments produced significantly increased 5-HT1B (and p11) mRNA or protein levels (measured in the caudal striatum) [23]. Our study demonstrates that a more moderate repeated L-DOPA treatment (5 mg/kg, 18 doses over 4 weeks) following dopamine depletion is sufficient to produce a pronounced increase in 5-HT1B expression.
In our study, this impact of L-DOPA on 5-HT1B expression mimicked the L-DOPA-induced increase in dynorphin expression, which is a well-established effect on this marker of direct pathway neurons (e.g., [18, 31, 52]). Indeed, TRAP analysis confirmed robust upregulation of 5-HT1B expression in D1 receptor-expressing (direct pathway) neurons, with no further changes in expression in D2 receptor-expressing (indirect pathway) neurons, by L-DOPA treatment in dopamine-depleted mice [18]. This effect resembles that on another striatal receptor involved in regulating striatal output pathways, the D3 dopamine receptor. The D3 receptor was also found to be preferentially upregulated in direct pathway neurons after dopamine depletion and repeated L-DOPA treatment (see [53]). Antagonism of these striatal receptors may be useful for attenuating L-DOPA-induced dyskinesias [53].
Our regional mapping analysis demonstrated L-DOPA-induced upregulation of 5-HT1B expression again preferentially in the sensorimotor striatum, with lesser effects in medial and ventral striatal sectors, including the nucleus accumbens. Furthermore, our regional analysis also revealed a modest increase in 5-HT1B expression in some sensorimotor sectors of the intact striatum after repeated L-DOPA treatment, an effect that would likely be missed with whole striatum approaches. This effect on the intact side is reminiscent of the upregulated 5-HT1B expression in the normal striatum after repeated treatment with other dopamine agonists/psychostimulants (e.g., [12]).
Together, these findings demonstrate a powerful regulation of 5-HT1B expression by dopamine that appears to be opposite in the two striatal output ways, that is, an increase in indirect pathway neurons after dopamine depletion vs. an increase in direct pathway neurons after dopamine replacement therapy with L-DOPA. These changes parallel established changes for neuropeptide markers in these neurons (enkephalin, dynorphin), which are thought to reflect changes in “neuronal activity” in these pathways [7].
The Robust 5-HT1B Effects Contrast with Limited or No Effects on 5-HT2C, 5-HT4 or 5-HT6 Receptors
In contrast to the striking increases in striatal 5-HT1B expression, we did not see significant effects of dopamine depletion or L-DOPA treatment on 5-HT2C, 5-HT4 or 5-HT6 expression. This finding appears to conflict with results in the previous TRAP study [18], which reported minor decreases in 5-HT2C and 5-HT4 expression selectively in indirect pathway neurons after dopamine depletion (5-HT6 was not assessed). On the other hand, the TRAP analysis revealed that L-DOPA treatment produced some increases in indirect pathway neurons for both 5-HT2C and 5-HT4, while at the same time 5-HT2C was decreased and 5-HT4 unchanged in direct pathway neurons [18]. Such differential cell type-specific changes would be expected to diminish or even cancel each other with approaches using whole tissue measurements such as the in situ hybridization analysis used in our study (see [18], for discussion). On the other hand, these considerations underscore the robustness of the observed changes in 5-HT1B receptor expression in our study.
In summary, our findings demonstrate a differential impact of dopamine depletion and subsequent L-DOPA treatment on 5-HT1B vs. 5-HT2C, 5-HT4 or 5-HT6 receptors. This is consistent with our previous results showing increased expression of 5-HT1B, but no changes for 5-HT2C, after repeated psychostimulant treatment [12]. Together, our findings highlight the sensitivity of 5-HT1B to variations in striatal dopamine tone induced by lesions or pharmacological treatments.
Mechanisms Underlying Increased 5-HT1B Expression
The markedly increased gene induction by dopamine agonists (L-DOPA) after dopamine depletion is considered to reflect a “supersensitive” response to stimulation of D1 receptors in direct pathway neurons, as a consequence of altered second messenger signaling after loss of dopamine input [54, 55]. However, our results show that dopamine (or loss thereof) is not the only factor contributing to such abnormal gene induction. Regionally, there was a clear mismatch between the observed L-DOPA-induced increases in 5-HT1B and dynorphin expression, which were strikingly predominant in lateral striatum, and the loss of dopamine input (TH signal), which was uniform throughout the striatum. This mismatch indicates that loss of dopamine input is not sufficient to account for the magnitude of L-DOPA-induced gene regulation.
D1 receptor stimulation is critical for dopamine agonist-induced increases in gene regulation in the striatum, but other inputs participate as well (for review, see [56]). For example, it has been shown that dopamine agonist-induced increases in striatal gene expression are also dependent on glutamate receptor stimulation/cortical input both in the normal striatum and after dopamine depletion (e.g., [57–59]). Therefore, the striking upregulation of gene expression predominantly in the lateral, sensorimotor striatum may, to some degree, reflect differential cortical (or other) inputs in interaction with supersensitive D1 receptors, during repeated L-DOPA treatment. We speculate that L-DOPA-induced enhanced output in the supersensitive, D1 receptor-regulated direct pathway and resulting abnormally enhanced activity in sensorimotor cortex (e.g., [60, 61]), followed by abnormal re-entrant corticostriatal activity to the sensorimotor striatum [28], sets up aberrant feedforward activation that facilitates and then reinforces these regionally restricted molecular changes, ultimately leading to abnormal sensorimotor function.
Functional significance and conclusions
Enhanced molecular signaling in D1 receptor-regulated direct pathway neurons is critically important for L-DOPA-induced dyskinesia (AIMs) after dopamine depletion (for reviews, see [49, 62]). Moreover, aberrant activation of the direct pathway arising in the sensorimotor striatum is sufficient for AIMs [63–67]. Consistent with these earlier findings (e.g., [31]), our present mapping results demonstrate that the most pronounced gene regulation effects of L-DOPA occur in the sensorimotor striatum. However, unexpectedly, our findings also show that AIMs scores after repeated L-DOPA treatment best correlated with upregulated gene (5-HT1B) expression in the associative, not the sensorimotor, striatum. Our findings are correlative, and future studies will have to determine how molecular changes in the associative striatum could contribute to L-DOPA-induced dyskinesias.
Our associative striatal sectors [35] receive inputs predominantly from various frontal cortical areas (orbital frontal, prefrontal/anterior cingulate, insular cortex; reviewed in [68, 69]). These frontal cortical areas play an important role in monitoring and adapting behavioral responses related to changing environmental or internal demands (“executive functions”), and are thus critical for goal-directed behavior [69]. How could changes in the associative striatum influence sensorimotor processes? The influential “parallel circuit” model of the basal ganglia [70] posits that cortico-basal ganglia-cortical circuits are organized in distinct anatomical channels connecting limbic, associative or motor domains in all basal ganglia nuclei. However, it is recognized that cross-talk between these channels is necessary and occurs on several levels (e.g., [69, 71]). For example, anatomical studies showed between-channel-connections for direct pathway projections to the substantia nigra, providing a mechanism for functional flow from limbic to associative to motor domains [69, 72]. While these “nonreciprocal” connections [69] to neighboring channels are established for targeting dopamine neurons [72], they also innervate surrounding GABAergic output neurons in the substantia nigra pars reticulata [69], thus providing a mechanism for cross-talk in the absence of dopamine neurons (Parkinson’s disease). This connectivity may forward effects of molecular changes in associative channels to motor channels and may thereby influence motor processes underlying L-DOPA-induced behavior.
How could striatal 5-HT1B receptors modify L-DOPA-induced behavior? 5-HT1B receptors have recently attracted attention for their potential utility in the treatment of L-DOPA-induced dyskinesia. In advanced Parkinson’s disease a certain portion of L-DOPA seems to be converted to dopamine in serotonin terminals and then released therefrom, thus contributing to overstimulation of dopamine receptors and direct pathway neurons, and promoting L-DOPA-induced dyskinesia [21, 22, 73, 74]. 5-HT1B receptors, in addition to being postsynaptic (heteroreceptors), are also autoreceptors on serotonin neurons, and, together with 5-HT1A receptors, regulate (inhibit) transmitter release from serotonin neurons [1, 2]. There is good evidence that agonists for these receptors can attenuate L-DOPA-induced dyskinesia presumably via inhibition of abnormal dopamine release from serotonin terminals (at higher doses the antiparkinsonian effects of L-DOPA may also be diminished) (e.g., [73, 75–77]; for reviews, see [21, 74]).
However, our finding of a significant positive correlation between the magnitude of increases in 5-HT1B mRNA expression in the associative striatum and the severity of AIMs suggests that stimulation of these 5-HT1B receptors (heteroreceptors) may facilitate rather than attenuate AIMs. 5-HT1B receptors expressed in direct pathway neurons are predominantly located presynaptically on axon terminals [1, 2] to regulate (inhibit) GABA release from these neurons [78, 79]. Increased 5-HT1B receptor levels on terminals of “nonreciprocal” connections from associative to sensorimotor circuits (see above) would thus be expected to increase inhibition of GABA release from these neurons [80, 81], thereby releasing sensorimotor circuits from associative modulation, and facilitating L-DOPA-induced dyskinesia.
It is noteworthy that one criterion for rating the severity of AIMs is whether or not AIMs can be interrupted by sensory input: the most severe AIMs are unlikely to be interrupted by ambient noise [30]. Such sensory input will likely engage associative striatal circuits [69] and would normally modify/interrupt ongoing behavior, perhaps by facilitating more appropriate motor programs. Deficiency in this associative striatal input, due to increasing 5-HT1B receptor function, would thus increasingly fail to interfere with ongoing activity in sensorimotor circuits, resulting in increased expression of L-DOPA-induced dyskinesia.
Future studies will have to determine how abnormal 5-HT1B signaling in striatal output pathways impacts L-DOPA-induced dyskinesia and whether pharmacological interventions could alleviate these effects.
Acknowledgements
This work was supported in part by National Institutes of Health Grants (DA011261 and DA046794 to H.S.; NS088554 to A.R.W.) and Rosalind Franklin University of Medicine and Science.
References
- 1.Barnes NM, Sharp T (1999) A review of central 5-HT receptors and their function. Neuropharmacology 38:1083–1152. [DOI] [PubMed] [Google Scholar]
- 2.De Deurwaerdère P, Di Giovanni G (2017) Serotonergic modulation of the activity of mesencephalic dopaminergic systems: Therapeutic implications. Prog Neurobiol 151:175–236. [DOI] [PubMed] [Google Scholar]
- 3.Bhat RV, Baraban JM (1993) Activation of transcription factor genes in striatum by cocaine: role of both serotonin and dopamine systems. J Pharmacol Exp Ther 267:496–505. [PubMed] [Google Scholar]
- 4.Lucas JJ, Segu L, Hen R (1997) 5-Hydroxytryptamine1B receptors modulate the effect of cocaine on c-fos expression: converging evidence using 5-hydroxytryptamine1B knockout mice and the 5-hydroxytryptamine1B/1D antagonist GR127935. Mol Pharmacol 51:755–763. [DOI] [PubMed] [Google Scholar]
- 5.Szucs RP, Frankel PS, McMahon LR, Cunningham KA (2005) Relationship of cocaine-induced c-Fos expression to behaviors and the role of serotonin 5-HT2A receptors in cocaine-induced c-Fos expression. Behav Neurosci 119:1173–1183. [DOI] [PubMed] [Google Scholar]
- 6.Horner KA, Adams DH, Hanson GR, Keefe KA (2005) Blockade of stimulant-induced preprodynorphin mRNA expression in the striatal matrix by serotonin depletion. Neuroscience 131:67–77. [DOI] [PubMed] [Google Scholar]
- 7.Steiner H, Gerfen CR (1998) Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res 123:60–76. [DOI] [PubMed] [Google Scholar]
- 8.Young ST, Porrino LJ, Iadarola MJ (1991) Cocaine induces striatal c-Fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci USA 88:1291–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole AJ, Bhat RV, Patt C, Worley PF, Baraban JM (1992) D1 dopamine receptor activation of multiple transcription factor genes in rat striatum. J Neurochem 58:1420–1426. [DOI] [PubMed] [Google Scholar]
- 10.Steiner H, Gerfen CR (1995) Dynorphin opioid inhibition of cocaine-induced, D1 dopamine receptor-mediated immediate-early gene expression in the striatum. J Comp Neurol 353:200–212. [DOI] [PubMed] [Google Scholar]
- 11.Castanon N, Scearce-Levie K, Lucas JJ, Rocha B, Hen R (2000) Modulation of the effects of cocaine by 5-HT1B receptors: a comparison of knockouts and antagonists. Pharmacol Biochem Behav 67:559–566. [DOI] [PubMed] [Google Scholar]
- 12.Van Waes V, Ehrlich S, Beverley JA, Steiner H (2015) Fluoxetine potentiation of methylphenidate-induced gene regulation in striatal output pathways: Potential role for 5-HT1B receptor. Neuropharmacology 89:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Waes V, Steiner H (2015) Fluoxetine and other SSRI antidepressants potentiate addiction-related gene regulation by psychostimulant medications In: Pinna G (ed) Fluoxetine: Pharmacology, Mechanisms of Action and Potential Side Effects. Nova Science Publishers, Hauppauge, NY, pp 207–225. [Google Scholar]
- 14.Alter D, Beverley JA, Patel R, Bolaños-Guzmán CA, Steiner H (2017) The 5-HT1B serotonin receptor regulates methylphenidate-induced gene expression in the striatum: Differential effects on immediate-early genes. J Psychopharmacol 31:1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furay AR, McDevitt RA, Miczek KA, Neumaier JF (2011) 5-HT1B mRNA expression after chronic social stress. Behav Brain Res 224:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murrough JW, Czermak C, Henry S, Nabulsi N, Gallezot JD, Gueorguieva R, Planeta-Wilson B, Krystal JH, Neumaier JF, Huang Y, Ding YS, Carson RE, Neumeister A (2011) The effect of early trauma exposure on serotonin type 1B receptor expression revealed by reduced selective radioligand binding. Arch Gen Psychiatry 68:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pentkowski NS, Cheung TH, Toy WA, Adams MD, Neumaier JF, Neisewander JL (2012) Protracted withdrawal from cocaine self-administration flips the switch on 5-HT(1B) receptor modulation of cocaine abuse-related behaviors. Biol Psychiatry 72:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heiman M, Heilbut A, Francardo V, Kulicke R, Fenster RJ, Kolaczyk ED, Mesirov JP, Surmeier DJ, Cenci MA, Greengard P (2014) Molecular adaptations of striatal spiny projection neurons during levodopa-induced dyskinesia. Proc Natl Acad Sci USA 111:4578–4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoplight BJ, Vincow ES, Neumaier JF (2007) Cocaine increases 5-HT1B mRNA in rat nucleus accumbens shell neurons. Neuropharmacology 52:444–449. [DOI] [PubMed] [Google Scholar]
- 20.Neumaier JF, McDevitt RA, Polis IY, Parsons LH (2009) Acquisition of and withdrawal from cocaine self-administration regulates 5-HT mRNA expression in rat striatum. J Neurochem 111:217–227. [DOI] [PubMed] [Google Scholar]
- 21.Carta M, Tronci E (2014) Serotonin system implication in l-DOPA-induced dyskinesia: From animal models to clinical investigations. Front Neurol 5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanza K, Bishop C (2018) Serotonergic targets for the treatment of L-DOPA-induced dyskinesia. J Neural Transm 125:1203–1216. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Andren PE, Greengard P, Svenningsson P (2008) Evidence for a role of the 5-HT1B receptor and its adaptor protein, p11, in L-DOPA treatment of an animal model of Parkinsonism. Proc Natl Acad Sci USA 105:2163–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riahi G, Morissette M, Samadi P, Parent M, Di Paolo T (2013) Basal ganglia serotonin 1B receptors in parkinsonian monkeys with L-DOPA-induced dyskinesia. Biochem Pharmacol 86:970–978. [DOI] [PubMed] [Google Scholar]
- 25.Morin N, Morissette M, Grégoire L, Rajput A, Rajput AH, Di Paolo T (2015) Contribution of brain serotonin subtype 1B receptors in levodopa-induced motor complications. Neuropharmacology 99:356–368. [DOI] [PubMed] [Google Scholar]
- 26.Tseng KY, Caballero A, Dec A, Cass DK, Simak N, Sunu E, Park MJ, Blume SR, Sammut S, Park DJ, West AR (2011) Inhibition of striatal soluble guanylyl cyclase-cGMP signaling reverses basal ganglia dysfunction and akinesia in experimental parkinsonism. PloS One 6:e27187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C (1998) The Rat Brain in Stereotaxic Coordinates. Academic Press, New York. [Google Scholar]
- 28.Jayasinghe VR, Flores-Barrera E, West AR, Tseng KY (2017) Frequency-dependent corticostriatal disinhibition resulting from chronic dopamine depletion: Role of local striatal cGMP and GABA-AR signaling. Cereb Cortex 27:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Padovan-Neto FE, Cavalcanti-Kiwiatkoviski R, Carolino RO, Anselmo-Franci J, Del Bel E (2015) Effects of prolonged neuronal nitric oxide synthase inhibition on the development and expression of L-DOPA-induced dyskinesia in 6-OHDA-lesioned rats. Neuropharmacology 89:87–99. [DOI] [PubMed] [Google Scholar]
- 30.Winkler C, Kirik D, Björklund A, Cenci MA (2002) L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of parkinson’s disease: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis 10:165–186. [DOI] [PubMed] [Google Scholar]
- 31.Cenci MA, Lee CS, Björklund A (1998) L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci 10:2694–2708. [PubMed] [Google Scholar]
- 32.Padovan-Neto FE, Echeverry MB, Tumas V, Del-Bel EA (2009) Nitric oxide synthase inhibition attenuates L-DOPA-induced dyskinesias in a rodent model of Parkinson’s disease. Neuroscience 159:927–935. [DOI] [PubMed] [Google Scholar]
- 33.Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA (2002) Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci 15:120–132. [DOI] [PubMed] [Google Scholar]
- 34.Steiner H, Kitai ST (2001) Unilateral striatal dopamine depletion: time-dependent effects on cortical function and behavioural correlates. Eur J Neurosci 14:1390–1404. [DOI] [PubMed] [Google Scholar]
- 35.Willuhn I, Sun W, Steiner H (2003) Topography of cocaine-induced gene regulation in the rat striatum: Relationship to cortical inputs and role of behavioural context. Eur J Neurosci 17:1053–1066. [DOI] [PubMed] [Google Scholar]
- 36.Steiner H, Blum M, Kitai ST, Fedi P (1999) Differential expression of ErbB3 and ErbB4 neuregulin receptors in dopamine neurons and forebrain areas of the adult rat. Exp Neurol 159:494–503. [DOI] [PubMed] [Google Scholar]
- 37.Yano M, Steiner H (2005) Methylphenidate (Ritalin) induces Homer 1a and zif 268 expression in specific corticostriatal circuits. Neuroscience 132:855–865. [DOI] [PubMed] [Google Scholar]
- 38.Chang JW, Wachtel SR, Young D, Kang UJ (1999) Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson’s disease: studies on medial forebrain bundle and striatal lesions. Neuroscience 88:617–628. [DOI] [PubMed] [Google Scholar]
- 39.Nisenbaum LK, Crowley WR, Kitai ST (1996) Partial striatal dopamine depletion differentially affects striatal substance P and enkephalin messenger RNA expression. Mol Brain Res 37:209–216. [DOI] [PubMed] [Google Scholar]
- 40.Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM (1990) Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci 13:290–296. [DOI] [PubMed] [Google Scholar]
- 41.Henry B, Crossman AR, Brotchie JM (1999) Effect of repeated L-DOPA, bromocriptine, or lisuride administration on preproenkephalin-A and preproenkephalin-B mRNA levels in the striatum of the 6-hydroxydopamine-lesioned rat. Exp Neurol 155:204–220. [DOI] [PubMed] [Google Scholar]
- 42.Pirker W, Tedroff J, Pontén H, Gunne L, Andrén PE, Hurd YL (2001) Coadministration of (−)-OSU6162 with l-DOPA normalizes preproenkephalin mRNA expression in the sensorimotor striatum of primates with unilateral 6-OHDA lesions. Exp Neurol 169:122–134. [DOI] [PubMed] [Google Scholar]
- 43.Calon F, Birdi S, Rajput AH, Hornykiewicz O, Bédard PJ, Di Paolo T (2002) Increase of preproenkephalin mRNA levels in the putamen of Parkinson disease patients with levodopa-induced dyskinesias. J Neuropathol Exp Neurol 61:186–196. [DOI] [PubMed] [Google Scholar]
- 44.Pavón N, Martín AB, Mendialdua A, Moratalla R (2006) ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry 59:64–74. [DOI] [PubMed] [Google Scholar]
- 45.Darmopil S, Martín AB, De Diego IR, Ares S, Moratalla R (2009) Genetic inactivation of dopamine D1 but not D2 receptors inhibits L-DOPA-induced dyskinesia and histone activation. Biol Psychiatry 66:603–613. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz-DeDiego I, Mellstrom B, Vallejo M, Naranjo JR, Moratalla R (2015) Activation of DREAM (downstream regulatory element antagonistic modulator), a calcium-binding protein, reduces L-DOPA-induced dyskinesias in mice. Biol Psychiatry 77:95–105. [DOI] [PubMed] [Google Scholar]
- 47.Sgroi S, Capper-Loup C, Paganetti P, Kaelin-Lang A (2016) Enkephalin and dynorphin neuropeptides are differently correlated with locomotor hypersensitivity and levodopa-induced dyskinesia in parkinsonian rats. Exp Neurol 280:80–88. [DOI] [PubMed] [Google Scholar]
- 48.Konradi C, Westin JE, Carta M, Eaton ME, Kuter K, Dekundy A, Lundblad M, Cenci MA (2004) Transcriptome analysis in a rat model of L-DOPA-induced dyskinesia. Neurobiol Dis 17:219–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cenci MA, Konradi C (2010) Maladaptive striatal plasticity in L-DOPA-induced dyskinesia. Prog Brain Res 183:209–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muller CP, Huston JP (2006) Determining the region-specific contributions of 5-HT receptors to the psychostimulant effects of cocaine. Trends Pharmacol Sci 27:105–112. [DOI] [PubMed] [Google Scholar]
- 51.Cunningham KA, Anastasio NC (2014) Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology 76:460–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andersson M, Hilbertson A, Cenci MA (1999) Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson’s disease. Neurobiol Dis 6:461–474. [DOI] [PubMed] [Google Scholar]
- 53.Solis O, Garcia-Montes JR, González-Granillo A, Xu M, Moratalla R (2017) Dopamine D3 receptor modulates l-DOPA-induced dyskinesia by targeting D1 receptor-mediated striatal signaling. Cereb Cortex 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerfen CR, Miyachi S, Paletzki R, Brown P (2002) D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci 22:5042–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerfen CR (2010) D1 dopamine receptor supersensitivity in the dopamine-depleted striatum: Aberrant ERK1/2 signaling In: Steiner H, Tseng KY (eds) Handbook of Basal Ganglia Structure and Function. Academic Press/Elsevier, London, pp 491–500. [Google Scholar]
- 56.Steiner H (2017) Psychostimulant-induced gene regulation in striatal circuits In: Steiner H, Tseng KY (eds) Handbook of Basal Ganglia Structure and Function, Second Edition Academic Press/Elsevier, London, pp 639–672. [Google Scholar]
- 57.Cenci MA, Björklund A (1993) Transection of corticostriatal afferents reduces amphetamine- and apomorphine-induced striatal Fos expression and turning behaviour in unilaterally 6-hydroxydopamine-lesioned rats. Eur J Neurosci 5:1062–1070. [DOI] [PubMed] [Google Scholar]
- 58.Wang JQ, Daunais JB, McGinty JF (1994) NMDA receptors mediate amphetamine-induced upregulation of zif/268 and preprodynorphin mRNA expression in rat striatum. Synapse 18:343–353. [DOI] [PubMed] [Google Scholar]
- 59.Vargo JM, Marshall JF (1995) Time-dependent changes in dopamine agonist-induced striatal Fos immunoreactivity are related to sensory neglect and its recovery after unilateral prefrontal cortex injury. Synapse 20:305–315. [DOI] [PubMed] [Google Scholar]
- 60.Steiner H, Kitai ST (2000) Regulation of rat cortex function by D1 dopamine receptors in the striatum. J Neurosci 20:5449–5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alam M, Rumpel R, Jin X, von Wrangel C, Tschirner SK, Krauss JK, Grothe C, Ratzka A, Schwabe K (2017) Altered somatosensory cortex neuronal activity in a rat model of Parkinson’s disease and levodopa-induced dyskinesias. Exp Neurol 294:19–31. [DOI] [PubMed] [Google Scholar]
- 62.Murer MG, Moratalla R (2011) Striatal signaling in L-DOPA-induced dyskinesia: Common mechanisms with drug abuse and long term memory involving D1 dopamine receptor stimulation. Front Neuroanat 5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alcacer C, Andreoli L, Sebastianutto I, Jakobsson J, Fieblinger T, Cenci MA (2017) Chemogenetic stimulation of striatal projection neurons modulates responses to Parkinson’s disease therapy. J Clin Invest 127:720–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.F Hernández L, Castela I, Ruiz-DeDiego I, Obeso JA, Moratalla R (2017) Striatal activation by optogenetics induces dyskinesias in the 6-hydroxydopamine rat model of Parkinson disease. Mov Disord 32:530–537. [DOI] [PubMed] [Google Scholar]
- 65.Girasole AE, Lum MY, Nathaniel D, Bair-Marshall CJ, Guenthner CJ, Luo L, Kreitzer AC, Nelson AB (2018) A subpopulation of striatal neurons mediates Levodopa-induced dyskinesia. Neuron 97:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ryan MB, Bair-Marshall C, Nelson AB (2018) Aberrant striatal activity in Parkinsonism and levodopa-induced dyskinesia. Cell Rep 23:3438–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keifman E, Ruiz-DeDiego I, Pafundo DE, Paz RM, Solís O, Murer MG, Moratalla R (2019) Optostimulation of striatonigral terminals in substantia nigra induces dyskinesia that increases after L-DOPA in a mouse model of Parkinson’s disease. Br J Pharmacol 176:2146–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Groenewegen HJ, Wouterlood FG, Uylings HBM (2017) Organization of prefrontal-striatal connections In: Steiner H, Tseng KY (eds) Handbook of Basal Ganglia Structure and Function, Second Edition Academic Press/Elsevier, London, pp 423–438. [Google Scholar]
- 69.Haber SN (2017) Integrative networks across basal ganglia circuits In: Steiner H, Tseng KY (eds) Handbook of Basal Ganglia Structure and Function, Second Edition Academic Press/Elsevier, London, pp 535–552. [Google Scholar]
- 70.Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381. [DOI] [PubMed] [Google Scholar]
- 71.Joel D, Weiner I (1994) The organization of the basal ganglia-thalamocortical circuits: open interconnected rather than closed segregated. Neuroscience 63:363–379. [DOI] [PubMed] [Google Scholar]
- 72.Haber SN, Fudge JL, McFarland NR (2000) Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 20:2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carta M, Carlsson T, Kirik D, Björklund A (2007) Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain 130:1819–1833. [DOI] [PubMed] [Google Scholar]
- 74.Cenci MA (2014) Presynaptic mechanisms of l-DOPA-induced dyskinesia: the findings, the debate, and the therapeutic implications. Front Neurol 5:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bezard E, Tronci E, Pioli EY, Li Q, Porras G, Björklund A, Carta M (2013) Study of the antidyskinetic effect of eltoprazine in animal models of levodopa-induced dyskinesia. Mov Disord 28:1088–1096. [DOI] [PubMed] [Google Scholar]
- 76.Svenningsson P, Rosenblad C, Af Edholm Arvidsson K, Wictorin K, Keywood C, Shankar B, Lowe DA, Björklund A, Widner H (2015) Eltoprazine counteracts l-DOPA-induced dyskinesias in Parkinson’s disease: a dose-finding study. Brain 138:963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meadows SM, Chambers NE, Conti MM, Bossert SC, Tasber C, Sheena E, Varney M, Newman-Tancredi A, Bishop C (2017) Characterizing the differential roles of striatal 5-HT1A auto- and hetero-receptors in the reduction of l-DOPA-induced dyskinesia. Exp Neurol 292:168–178. [DOI] [PubMed] [Google Scholar]
- 78.Boschert U, Amara DA, Segu L, Hen R (1994) The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience 58:167–182. [DOI] [PubMed] [Google Scholar]
- 79.Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, Vergé D (1999) Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience 88:899–915. [DOI] [PubMed] [Google Scholar]
- 80.Stanford IM, Lacey MG (1996) Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra pars reticulata neurons in vitro. J Neurosci 16:7566–7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ding S, Li L, Zhou FM (2015) Robust presynaptic serotonin 5-HT(1B) receptor inhibition of the striatonigral output and its sensitization by chronic fluoxetine treatment. J Neurophysiol 113:3397–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]