Abstract
PURPOSE:
Reports suggest that up to 50% of women with hormone receptor positive (HR+) breast cancer (BC) do not complete the recommended 5 years of adjuvant endocrine therapy (AET). We examined the impact of an outreach program at Kaiser Permanente Northern California (KPNC) on adherence and discontinuation of AET among patients who initiated AET.
METHODS:
We assembled a retrospective cohort of all KPNC patients diagnosed with HR+, stage I-III BC initiating AET before (n=4,287) and after (n=3,580) implementation of the outreach program. We compared adherence proportions and discontinuation rates before and after program implementation, both crude and adjusted for age, race/ethnicity, education, income and stage. We conducted a pooled analysis of data from six Cancer Research Network (CRN) sites that had not implemented programs for improving AET adherence, using identical methods and time periods, to assess possible secular trends.
RESULTS:
In the pre-outreach period, estimated adherence in years 1, 2 and 3 following AET initiation were 75.2%, 71.0% and 67.3%; following the outreach program, the estimates were 79.4%, 75.6% and 72.2% (p-values <.0001 for pairwise comparisons). Results were comparable after adjusting for clinical and demographic factors. The estimated cumulative incidence of discontinuation was 0.22 (0.21–0.24) and 0.18 (0.17–0.19) at 3-years for pre- and post-outreach groups (p-value<.0001). We found no evidence of an increase in adherence between the study periods at the CRN sites with no AET adherence program.
CONCLUSION:
Adherence and discontinuation after AET initiation improved modestly following implementation of the outreach program.
Introduction
Breast cancer is the most common malignancy among women.1 Given widespread mammographic screening, over 90% of breast cancers are diagnosed in the earlier, potentially curable stages of disease. Approximately 80–85% of women with early-stage breast cancer have hormone receptor-(HR) positive tumors2 and adjuvant treatment with endocrine therapy (AET) is one of their most effective treatment options. Adjuvant AET includes two drug classes, tamoxifen and aromatase inhibitors. AET is given orally and, to experience the significant benefit of the treatment, women are typically prescribed the drug for five years or longer.2 Numerous clinical trials using an intent-to-treat study design have demonstrated that AET reduces cancer recurrence risk by up to 50–70%.3
Despite the efficacy of AET for preventing recurrence, studies suggest that 7–10% of women prescribed these medications discontinue treatment annually, with only 40–60% completing the full course of treatment.4 Among women who do continue treatment, one study found that 64% are estimated to be non-adherent (i.e., have prolonged gaps in their treatment).5 Discontinuation of and non-adherence to AET may negatively impact womens’ outcomes. One study estimated that survival at 10 years was 80.7% for those who continued on AET for 5 years vs. 73.6% for those who discontinued. Furthermore, among those who continued AET, survival was better for those who were adherent than those who were non-adherent (81.7% vs. 77.8%).6
A recognized barrier to addressing gaps in optimal use of AET is the lack of data on pragmatic interventions that seek to improve adherence. We leveraged a unique opportunity to evaluate the impact of one such intervention. In 2011, Kaiser Permanente of Northern California (KPNC) implemented a surveillance and outreach program to improve medication adherence among breast cancer survivors who initiated AET, in part spurred by observations of low adherence.5 In the study presented here, we examined adherence to and continuation of AET during the period prior to and after the implementation of this outreach program. Understanding the program’s impact will be informative for community-based and other clinical settings seeking to improve AET adherence and long-term outcomes of breast cancer survivors.
Methods
Study Setting and Population
KPNC is an integrated healthcare system providing comprehensive primary and specialty care to over 4.3 million current members. The membership is diverse and largely representative of the demographics of Northern California’s general population, except it slightly under-represents the extremes of the socioeconomic spectrum.7 KPNC has more than 150 practicing medical oncologists as well as advanced practice cancer care providers, organized into 21 medical centers throughout the region.
In June 2011, KPNC implemented a region-wide surveillance and outreach program targeting nonmetastatic female breast cancer patients (stage I-III) who initiated (i.e., filled at least one prescription for) tamoxifen or aromatase inhibitor treatment and who, nine months after diagnosis, were identified from pharmacy records as being non-adherent, defined as failing to have received medication to cover 80% or more of days over the time period following initiation (medication possession ratio < 80%).8 In this program, non-adherent women identified by the KPNC Breast Cancer Tracking System (BCTS) received a mailed reminder letter, and if still non-adherent four weeks later, a message was sent to the treating oncologist or regional pharmacist to communicate with the patient regarding medication non-adherence. Those still remaining non-adherent received an additional mailed reminder letter from BCTS (Figure 1). This program was devised by KPNC Oncology leadership, including co-author Dr. Fehrenbacher, and shown to be feasible and beneficial based on a small pilot study.
Fig. 1.

Outreach and education program to improve adherence and continuation of adjuvant endocrine therapy (AET).
To evaluate the impact of this program on medication adherence and discontinuation, we assembled a retrospective cohort of women diagnosed with a first primary breast cancer, with no evidence of a previous cancer at any site. Eligible women were diagnosed with HR positive stage I, II, or III breast cancer in two calendar periods: five years prior to program implementation (2005–2009) and five years after (2012–2016). Women in our study were required to have at least 12 months of continuous enrollment (i.e., no health insurance coverage gap > 60 days) and pharmacy benefits prior to and after being diagnosed with breast cancer. Finally, our study was restricted to women who initiated adjuvant endocrine therapy within a year of diagnosis, as indicated by dispensing of first prescription.
Data Sources
Breast cancer cases were ascertained from the KPNC cancer registry, which also provided data on diagnosis date, patient age at diagnosis, disease and treatment characteristics, and patient demographic information. The KPNC cancer registry reports to the Greater Bay Area and Greater California cancer registries, both a part of the NCI Surveillance, Epidemiology and End Results (SEER) program, and has the same data elements and quality assurance procedures. Data on census tract-level measures of education and income, as well as comorbidities were drawn from KPNC’s electronic records. Finally, KPNC’s Pharmacy Information Management System provided information on AET prescriptions, including fill dates and number of pills dispensed, and pharmacy benefits. All data elements are extracted from these source databases and maintained in the Virtual Data Warehouse (VDW),9 a common data model that is implemented at all Cancer Research Network (CRM) institutions, a consortium of integrated health care systems.10,11
Outcome Measures
The main outcome of interest was the proportion of patients who were adherent to AET for each year following AET initiation, which we defined according to the anniversary date of the first prescription fill. To this end, we present definitions of discontinuation and adherence used to calculate these yearly estimates. For these purposes, prescription fills for either tamoxifen or AIs were considered jointly, such that switching from one to the other was not considered discontinuation of AET.
We considered a patient to have discontinued therapy if 180 days had elapsed since the exhaustion of her days’ supply of AET, including up to seven days of stockpiled medication accumulated from previous dispensings. The date of discontinuation was taken to be the last pill possession date before a 180-day gap.5,6,8,12
For each 12-month period following the index date of AET initiation, we considered a patient to be adherent to AET if her medication possession ratio (MPR), defined as the days’ supply divided by total days in a given period, met or exceeded 80%.
Statistical Analysis
We compared adherence proportions and continuation rates in each of three years before and after program implementation (June 2011). To ensure that follow-up on patients who were diagnosed before program implementation did not cross into the years post-implementation, we restricted our main pre-program implementation analyses to patients who were diagnosed in calendar years 2005, 2006 and 2007 and considered yearly adherence for three years following AET initiation. As a post-program implementation comparator group, we chose patients who were diagnosed in years 2012, 2013 and 2014.
We calculated the proportion of these patients who were adherent to AET in each year following their initiation,5 including in the denominator only patients who initiated AET and had health coverage for the entire year. Note that we did not exclude patients from the denominator if they had discontinued therapy, thereby providing a realistic estimate of the percentage of patients who were adherent to therapy in each year following AET initiation. We performed this calculation for patients who were diagnosed in: (i) the years before and after program implementation, 2005–2007 and 2012–2014, respectively, in aggregate; and (ii) each of calendar years 2005, 2006, 2007, 2012, 2013, 2014, separately. We used a test of equality of two proportions to test the difference in pre- and post-outreach implementation adherence for each of three years following AET initiation and logistic regression for covariate adjustment of age at diagnosis, stage, income, education, and race/ethnicity.
To examine trends in discontinuation, we treated discontinuation in a time-to-event framework, anchoring the analysis at time of AET initiation and following patients until time of discontinuation or censoring (loss to follow up or end of study at three years following initiation), accounting for death as a competing risk. We estimated the cumulative incidence of discontinuation, accounting for in-study mortality, for all diagnosis years separately and between the pre- and post-outreach program, and compared differences using Fine-Gray subdistribution hazard regression.13
Sensitivity Analyses
Methods for calculating adherence and discontinuation vary widely across the literature and we found few examples of yearly adherence calculations over consecutive years.5 When calculating adherence from one year to the next, we chose to include in the denominator all patients who had initiated hormone therapy and had health coverage for the entire year. Another choice, assumed by Hershman et al. (2010), further restricted the denominator for each 12-month time-period to patients who had not discontinued anytime during that period, so adherence was only considered among patients who were continuing therapy. For comparability, we also considered an additional method for calculating adherence where the denominator included patients who had health coverage for the entire year and were not classified as discontinued during the entire year under consideration.
As a sensitivity analysis we calculated trends in adherence and discontinuation using the date of discontinuation to be 90-days since last days’ supply. In addition, we performed stratified analyses based on race/ethnicity, age (<50 years, >50 years) and stage to assess possible differences in adherence and discontinuation across subgroup strata.
To explore whether changes in adherence observed within KPNC could have been due to factors other than the outreach program, we conducted a pooled analysis of data from six healthcare systems participating in the Cancer Research Network (CRN) using identical methods and time periods. The six sites were Henry Ford Health System, HealthPartners Institute, Kaiser Permanente Hawaii, Kaiser Permanente Washington, Marshfield Clinic, and the Meyers Primary Care Institute, affiliated with Reliant Medical Group. None of these sites had implemented system-wide programs for improving AET adherence.
The study was approved by the IRB at KPNC and by the IRBs at each of the CRN sites (some ceded to KPNC).
Results
The KPNC analyses included a total of 3,580 women who were diagnosed with hormone receptor positive stage I-III breast cancer in years 2005–2007 and initiated AET during the pre-outreach period and 4,287 during 2012–2014 in the post-outreach period. Characteristics of patients initiating AET were fairly similar in the two groups (pre- and post-outreach program). A typical patient was between the ages of 50 and 84 years old with stage I breast cancer. However, slightly more patients in the pre-outreach group were non-Hispanic White (Table 1).
Table 1.
Characteristics of the KPNC study population at time of breast cancer diagnosis of patient diagnosis in pre-outreach (2005–2007) and post-outreach (2012–2014) periods.
| 2005–2007 (n = 3,580) |
2012–2014 (n = 4,287) |
|||
|---|---|---|---|---|
| Characteristics | n | (%) | n | (%) |
| Stage | ||||
| Stage I | 1,951 | 54.5% | 2,442 | 57.0% |
| Stage II | 1,299 | 36.3% | 1,439 | 33.6% |
| Stage III | 330 | 9.2% | 406 | 9.5% |
| Chemotherapy | 1,306 | 36.5% | 1,555 | 36.3% |
| Radiation therapy | 1,015 | 28.4% | 2,071 | 48.3% |
| Age at diagnosis | ||||
| < 50 yrs | 641 | 17.9% | 764 | 17.8% |
| 50–59 yrs | 1,005 | 28.1% | 1,074 | 25.1% |
| 60–69 yrs | 989 | 27.6% | 1,391 | 32.4% |
| 70–84 yrs | 858 | 24.0% | 948 | 22.1% |
| >=85 yrs | 87 | 2.4% | 110 | 2.6% |
| Race/Ethnicity | ||||
| Asian/Pacific Islander | 461 | 12.9% | 707 | 16.5% |
| African American | 214 | 6.0% | 272 | 6.3% |
| Hispanic | 391 | 10.9% | 498 | 11.6% |
| Native American/Other | 4 | 0.1% | 15 | 0.3% |
| Unknown | 2 | 0.1% | 8 | 0.2% |
| Non-Hispanic White | 2,508 | 70.1% | 2,787 | 65.0% |
| Median household income | ||||
| < 50K | 652 | 18.2% | 737 | 17.2% |
| 50–<100K | 1,950 | 54.5% | 2,315 | 54.0% |
| 100K+ | 973 | 27.2% | 1,235 | 28.8% |
| Unknown | 5 | 0.1% | 0 | 0.0% |
| Education: less than high schoola | ||||
| < 10% | 2,910 | 81.3% | 3,463 | 80.8% |
| 10–<20% | 457 | 12.8% | 576 | 13.4% |
| 20+% | 208 | 5.8% | 248 | 5.8% |
| Unknown | 5 | 0.1% | 0 | 0.0% |
| Education: high schoolb | ||||
| < 10% | 444 | 12.4% | 503 | 11.7% |
| 10–<20% | 889 | 24.8% | 1,088 | 25.4% |
| 20+% | 2,242 | 62.6% | 2,696 | 62.9% |
| Unknown | 5 | 0.1% | 0 | 0.0% |
| Education: more than high schoolc | ||||
| < 25% | 43 | 1.2% | 63 | 1.5% |
| 25–<50% | 512 | 14.3% | 627 | 14.6% |
| 50+% | 3,020 | 84.4% | 3,597 | 83.9% |
| Unknown | 5 | 0.1% | 0 | 0.0% |
Income and education are based on U.S. Census block of residence. To interpret the “Education: less than high school” variable, 81.3% of patients in the pre-outreach period (diagnosis in 2005–2007) lived in a Census block in which <10% had less than high school education.
At KPNC, both adherence and discontinuation during each of the 3 years after AET initiation improved after the program was implemented. In the pre-outreach period, crude estimated adherence in years 1, 2 and 3 following AET initiation were 75.2%, 71% and 67.3%, respectively; following the outreach program, the estimates were 79.4%, 75.6% and 72.2% (p-values <.0001 for independent comparisons of yearly proportions between the two groups) (Figure 2 (a)). Thus, there was an absolute increase in adherence rates of 4.2%, 4.6%, and 4.9% for the first three years after diagnosis.
Fig. 2.
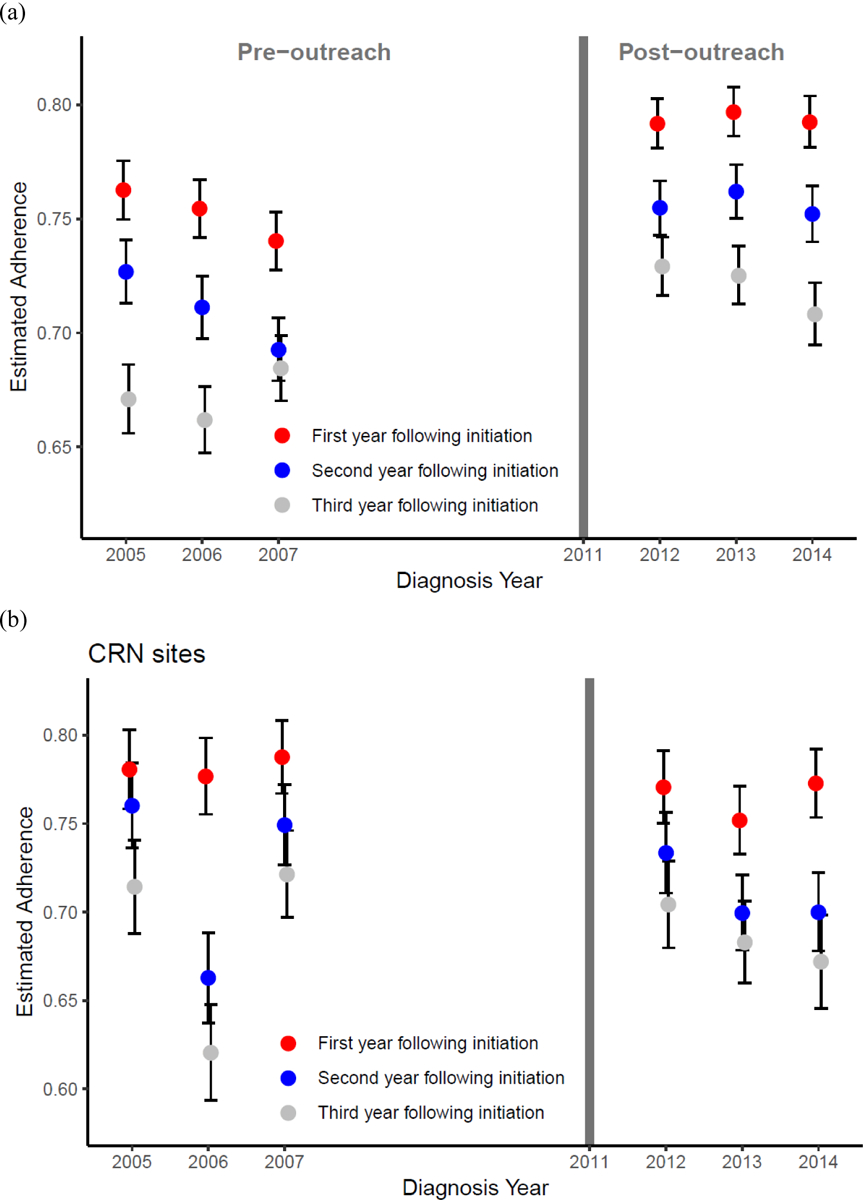
Adherence (%) for each diagnosis year during the periods (2005–2007) and (2012–2014) for (a) KPNC; and (b) using pooled data from six CRN sites. The six CRN sites were Henry Ford Health System, Health Partners Institute, and Kaiser Permanente Hawaii and Washington, Marshfield Clinic, and Meyers Primary Care Institute. Data from the Marshfield Clinic were not available for 2005–2007.
After adjusting for income, education, age and disease stage via logistic regression, the estimated model- based marginal adherence probabilities in years 1, 2 and 3 following AET were 69.3%, 57.7% and 55.2%; following the outreach program, the model-based marginal estimates were 74.0%, 63.2% and 60.8%. The absolute increase in adjusted adherence was 4.7%, 5.5% and 5.6%. In adjusted logistic regression models, the odds ratio comparing post- vs. pre-outreach was: 1.45 (1.22, 1.72) for adherence in the first year following initiation; 1.44 (1.22, 1.69) for adherence in the second year; and 1.28 (1.09,1.51) for adherence in the third year. Age at diagnosis and race/ethnicity were associated (p-value<0.05 from likelihood ratio test of nested models) with the outcome in each of the three models. In addition, income and stage were associated with adherence in both the first and second years, and less than high school education was associated with adherence in the second year.
The cumulative incidence curves for discontinuation (Figure 3), which account for the competing risk of death (5.2% of patients died before the end of follow-up), show a marked difference between the two groups. Estimates at three-years for pre- and post-outreach groups were 0.22 (0.21–0.24) and 0.18 (0.17–0.19), respectively (both unadjusted and adjusted subdistribution hazard regression p-values<.0001). In the adjusted model, race/ethnicity, less than high school education, income and stage were associated (p-value<0.05 from likelihood ratio test of nested models) with the cumulative incidence of discontinuation. Kaplan-Meier estimates of 10-year survival (not included) show no difference between the pre- and post-outreach groups, however, there is insufficient follow up on the post-outreach group.
Fig. 3.
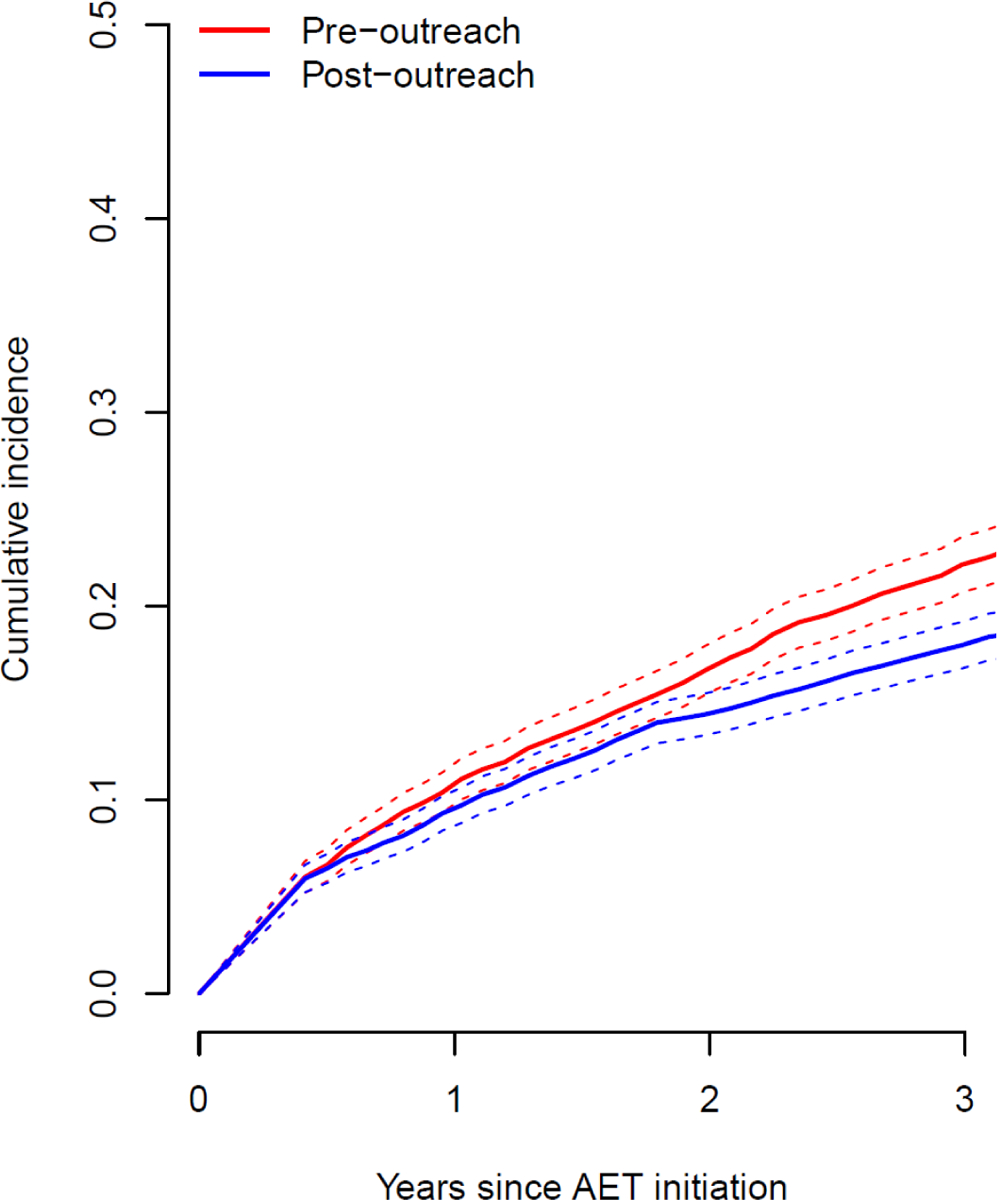
Estimated cumulative incidence curves for discontinuation, accounting for the competing risk of death, at KPNC pre (2005–2007) and post (2012–2014) outreach program. Discontinuation was defined by a 180-day gap since last pill possession, including up to seven days of stockpiled medication accumulated from previous dispensings.
In sensitivity analyses, adherence for patients diagnosed in all years in our analytic dataset (2005–2014) indicates an increase in adherence post-outreach intervention (2011). Defining discontinuation based on 90-days led to a rise in the cumulative incidence of discontinuation (Online Supplementary Figure SI) but did not negate post outreach improvements. Specifically, the estimated cumulative incidence for discontinuation, accounting for the competing risk of death, was 0.33 (0.31,0.35) at 3-years for the pre-outreach group vs. 0.29 (0.27,0.30) for the post outreach group. Note that adherence was not affected by altering the definition of discontinuation, as the definition of adherence that we employed did not exclude patients from the denominator if they had discontinued therapy. Excluding discontinued patients from the denominator in the alternate definition of adherence increased the adherence estimates in the second and third years following initiation, however, an increase between pre- and post-outreach periods remain (Online Supplementary Figure S2). In subgroup analyses based on age (<50, >50), race/ethnicity and stage of disease, we saw evidence of improvement among women age 50 and older, White women, and women with stage II cancer (Online Supplementary Figures S3–S5).
In a pooled analysis of six CRN sites (pre-KPNC outreach program N=1,200 breast cancer cases that initiated AET, post-KPNC outreach N=1,541, characteristics of women at each site in Supplementary Table SI), we found no evidence of an increase in adherence between years 2005–2007 and 2012–2014 (Figure 2 (b)). The estimated pooled proportion of patients who were adherent at one to three years after treatment initiation for the two time periods across all sites were 78.2%, 72.4%, and 68.5% for the earlier time period compared to 76.4%, 71.0% and 68.7% for the later time period. For discontinuation, the comparable proportions were 10.4%, 6.7%, and 4.1%, and 14.1 %, 6.7%, and 5.9%. Across all time periods and six CRN sites yearly adherence ranged from a low of 33.3% to a high of 100% with an interquartile range of (66.1 %, 80.0%), and discontinuation from 0% to 38.5% with an interquartile range of (4.5%, 11.9%). Note that 100% adherence and 0% discontinuation rates correspond to sites with a small number of women who initiated AET in a particular year (N=6 and 16, respectively).
Discussion
Our results indicate that an outreach program to remind patients to refill their AET prescriptions modestly improved women’s adherence to and continuation of therapy. At three years after treatment initiation, absolute rates of adherence increased by about 4.9%, while that for cumulative incidence of discontinuation decreased by 4.0%.
We understand that these improvements might be viewed as modest, however, there are several potential explanations for the magnitude of the changes observed in our study. First, adherence in our study population was already relatively high prior to the implementation of the outreach program and the potential for improvement was limited. Second, the program we evaluated was highly pragmatic, and relatively low-touch given that it was designed to fit into existing clinic infrastructure and workflows. Prior research indicates that AET non-adherence results from a complex set of factors, including side effects,14 interpersonal (e.g. social support) and intrapersonal factors (e.g. anxiety),15 and additional clinical and psychosocial factors as well as demographic characteristics.16 More comprehensive or personalized interventions may achieve larger improvements in adherence for some patients, but with potential trade-offs for implementation and sustainability in diverse practice settings.
Third, although the outreach program was rolled-out across all 21 KPNC medical centers in mid-2011, medical centers differed with respect to their implementation of and fidelity to the program’s components. Establishing a centralized system for outreach workflow and documentation, including a complete database of patients contacted, would enable evaluations of implementation alongside effectiveness.
Because the intervention occurred in 2011, there was insufficient follow up data to study 5-year adherence to AET at the time of program evaluation. However, if we assume that the cumulative incidence of discontinuation increases linearly (see Online Supplementary Figure S3), the estimated cumulative incidence of discontinuation at 5-years following diagnosis is 31.7% before outreach implementation and 24.1 % after, an absolute improvement of 7.6%. Although seemingly marginal, we provide a rough calculation of “number of years gained” by such a reduction in discontinuation that will illustrate the larger impact. Note that on average, 1,300 women at KPNC initiate AET each year. A reduction in discontinuation rates of 7.6% corresponds to 99 fewer women who discontinue AET at 5 years post-initiation. Based on estimates of 10-year survival comparing those who continued and discontinued AET at 5 years from Hershman et al.,6 the gain in restricted 10-year mean survival (or mean survival of those followed for 10 years) of those who continued vs. discontinued AET at 5 years is 4.3 months.17 Annually this translates to an expected cumulative gain in mean survival of 35 years for all the KPNC patients who initiate AET.
It is important to note that our rates of adherence and continuation are higher than prior published rates of adherence. Hershman et al.5 examined AET adherence and continuation in KPNC stage I-III breast cancer patients who were taking hormonal therapy during the period 1996–2006. During the first year following AET initiation, they reported that 19% of patients discontinued therapy. In comparison, taking the date of discontinuation to be the last pill possession date before a 90-day gap in drug coverage, which closely aligns with the definition used in Hershman et al., we found that 17.7% discontinued therapy in the pre-outreach period (2005–2007) and 15% in the postimplementation period (2012–2014). Among women who did not discontinue therapy during the first year following initiation, Hershman et al. reported that 78% were adherent, whereas adherence in the first year was 89.5% based on our data. The discrepancy in estimates between the two studies are likely due to several sources. In our study, the exact fill sizes were known, whereas in Hershman et al. fill sizes were estimated due to lack of prescription information. Second, subtle variations in the definitions of discontinuation and adherence in each year following AET initiation make it challenging to directly compare results across studies. Lastly, it is possible that adherence in the study population improved between the two study periods. During the review process, one reviewer observed that the adherence rates in the pooled analysis of six CRN sites was somewhat higher than in the KPNC cohort. There are a large number of factors that have been reported to be associated with adherence to AET that are generally not captured in the electronic health record (at least in a mineable way, i.e., structured variables) mentioned earlier in this section, which include symptoms, provision of care, SES. While we cannot be certain, we speculate that the CRN sites differ from KPNC with respect to some of these adherence-related factors. Unfortunately, data on these factors were not available.
This study has limitations that warrant discussion. The outreach program to improve AET adherence was implemented among insured members at KPNC, an integrated health care delivery system. The results of our study may not be immediately generalizable beyond Kaiser Permanente, particularly in health systems that do not have good coordination between pharmacies, pick up of medications and ordering clinicians. However, our patients tend to be representative of the population with respect to demographics, such as race/ethnicity.7 Although our study was retrospective in nature, we were able to identify and study all women who were HR+, stage I-III BC initiating AET during the study period through the KPNC cancer registry and breast cancer tracking system so that selection bias was minimized. However, variables that are not available in the electronic health record that may be relevant to the outcome and exposures were not collected. In addition, we used a pre/post quasi-experimental design to study the possible impact of the outreach program on AET adherence and discontinuation before and after program roll-out in 2011. It is possible that improvements in outcomes were due to secular trends. We were not able to test this directly in our population due to lack of a control arm. However, pooling data on adherence across six Cancer Research Network sites with no comparable outreach intervention showed no evidence of an increase in adherence between years 2005–2007 and 2012–2014. This suggests that there were no national factors that might have influenced changes in adherence or discontinuation rates in KPNC, and that the observed changes were more likely to be due to setting-specific factors, such as implementation of this program to improve adherence. We were only able to examine secondary adherence (adherence among patients who initiated) based on the data available, and not primary adherence (first fill). Finally, an analysis of prescription fills does not necessarily translate to actual patient adherence.
In conclusion, our results provide evidence that an outreach program adopted by KPNC in 2011 resulted in moderate improvements in adherence to AET by breast cancer patients. For a highly pragmatic, low-touch intervention consisting of mailed and follow-up telephone reminders, this is a substantial improvement, especially in the context of an overall adherence rate of about 67.3% and cumulative incidence of discontinuation of 22% before program implementation. The success of such a low-touch intervention was echoed in a recent randomized trial focused on breast cancer screening adherence where Luckman and colleagues found that the use of personal reminder letters and a reminder call was effective in increasing mammogram adherence even when baseline adherence is high.18 It is possible that we would see greater improvements in outcomes with more reliable program implementation across all KPNC facilities and more personalized interventions. Mougalian and colleagues showed in a recent pilot study that a daily bidirectional text-messaging system to monitor AET adherence and identify adverse events was well-received by patients.19 However, preliminary findings from another pilot randomized control trial using mobile text messaging to improve AET adherence only saw short-term benefits that diminished by 3-months post-initiation.20 Further research is needed on the evaluation of quality improvement programs, personalized interventions, and the use of different interventions based on technology on AET adherence. A longer period of follow-up would provide estimates of adherence rates at five years after treatment initiation. Overall, this modest program has the potential to be implemented in other community-based setting and result in improved outcomes for women with hormone-receptor-positive breast cancer which may translate to survival benefit. The results from this study will be used as evidence for continued improvements in regional practice consistency and enhancements in tracking.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Eva S. Thomas from KPNC Medical Oncology, and Karin Luikart, RN MSN, Jane Bethard-Tracy and Mary Callahan, RN MPH from the KPNC Breast Cancer Tracking System for their involvement in the project; Michelle Ross at the KPNC Division of Research (DOR) for her help with manuscript editing, formatting and the submission process; Lisa Moy at DOR for administrative support; Dr. Jessica Chubak at Kaiser Permanente Washington Health Research Institute for reviewing the manuscript, and the following CRN collaborators for contributing data to this study: Monica Fuji and Rebecca Ziebell at Kaiser Permanente Washington Health Research Institute; Dr. Stacey Honda (Hawaii Permanente Medical Group) and team at Kaiser Permanente Hawaii Center for Health Research; Dr. Robert Greenlee and team at Marshfield Clinic Research Institute; and the reviewers for critical and helpful feedback.
Funding:
The study was funded by Kaiser Permanente Northern California Community Benefit program and by the Cancer Research Network (U24 CA171524, L. Kushi, PI).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical Approval: This article does not contain any studies with human participants performed by any of the authors.
Disclaimers: Preliminary findings were presented at the 34th International Conference on Pharmacoepidemiology & Therapeutic Risk Management, August 2018, Prague, Czech Republic.
References:
- 1.Siegel RL, Miller KD, Jemal A: Cancer Statistics, 2017. CA Cancer J Clin 67:7–30, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Burstein HJ, Temin S, Anderson H, et al. : Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. J Clin Oncol 32:2255–69, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Group EBCTC: Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386:1341–1352, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Chlebowski RT, Kim J, Haque R: Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev Res (Phila) 7:378–87, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hershman DL, Kushi LH, Shao T, et al. : Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 28:4120–8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hershman DL, Shao T, Kushi LH, et al. : Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 126:529–37,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon N: Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2011 California Health Interview Survey, Kaiser Permanente Division of Research, 2015, pp 15 [Google Scholar]
- 8.Raebel MA, Schmittdiel J, Karter AJ, et al. : Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care 51: SI1–21, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross TR, Ng D, Brown JS, et al. : The HMO Research Network Virtual Data Warehouse: A Public Data Model to Support Collaboration. EGEMS (Wash DC) 2:1049, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner EH, Greene SM, Hart G, et al. : Building a research consortium of large health systems: the Cancer Research Network. J Natl Cancer Inst Monogr:3–l 1, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Chubak J, Ziebell R, Greenlee RT, et al. : The Cancer Research Network: a platform for epidemiologic and health services research on cancer prevention, care, and outcomes in large, stable populations. Cancer Causes Control 27:1315–1323, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goetz MP, Ingle J: Early discontinuation of tamoxifen: a lesson for oncologists. Cancer 110:2595–6; author reply 2596, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Fine JP, Gray RJ: A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 94:496–509,1999 [Google Scholar]
- 14.Smith SG, Sestak I, Howell A, et al. : Participant-Reported Symptoms and Their Effect on Long-Term Adherence in the International Breast Cancer Intervention Study I (IBIS I). J Clin Oncol 35:2666–2673, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Liew JR, Christensen AJ, de Moor JS: Psychosocial factors in adjuvant hormone therapy for breast cancer: an emerging context for adherence research. J Cancer Surviv 8:521–31, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Moon Z, Hunter MS, Moss-Morris R, et al. : Factors related to the experience of menopausal symptoms in women prescribed tamoxifen. J Psychosom Obstet Gynaecol 38:226–235, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DH, Uno H, Wei LJ: Restricted Mean Survival Time as a Measure to Interpret Clinical Trial Results. JAMA Cardiol 2:1179–1180, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luckmann R, Costanza ME, White MJ, et al. : A 4-year randomized trial comparing three outreach interventions to promote screening mammograms. Transl Behav Med 9:328–335, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mougalian SS, Epstein LN, Jhaveri AP, et al. : Bidirectional Text Messaging to Monitor Endocrine Therapy Adherence and Patient-Reported Outcomes in Breast Cancer. JCO Clin Cancer Inform 1:1–10, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Wen K-Y, Goldstein LJ, Smith R, et al. : Mobile text messaging to improve adjuvant hormone therapy and side effect management among breast cancer patients: A pilot RCT. Journal of Clinical Oncology 37: e23061–e23061, 2019 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


