Abstract
Background:
This pilot study was performed to test our ability to administer neratinib monotherapy prior to clinically-recommended craniotomy in patients with human epidermal growth factor receptor 2 (HER2)-positive, metastatic breast cancer to the central nervous system (CNS), to examine neratinib’s CNS penetration at craniotomy, and to examine post-operative neratinib maintenance.
Patients and Methods:
Patients with HER2-positive brain metastases undergoing clinically-indicated cranial resection of a parenchymal tumor received neratinib 240 mg orally once daily for 7–21 days pre-operatively and resumed therapy post-operatively in 28-day cycles. Exploratory evaluations of time-to-progression, survival, as well as correlative tissue, cerebrospinal fluid (CSF), and blood-based analyses examining neratinib concentrations were planned.
Results:
We enrolled five patients between 5/22/2013–10/18/2016. As of March 1, 2019, patients had remained on study for 1–75+ postoperative cycles. Two patients had grade 3 diarrhea. Evaluation of the CSF showed low concentrations of neratinib, nonetheless two patients remained on therapy without progression for at least 13 cycles, with one on study treatment for nearly 6 years. Neratinib distribution in surgical tissue was variable for one patient, while specimens from two others did not produce conclusive results due to limited available samples.
Conclusions:
Neratinib resulted in expected rates of diarrhea in this small cohort, with 2 of 5 patients on study treatment for durable periods. Although logistically challenging, we were able to test a limited number of CSF- and parenchymal-based neratinib concentrations. Our findings from resected tumor tissue in one patient revealed heterogeneity in drug distribution and tumor histopathology.
ClinicalTrials.gov number:
Keywords: brain metastases, breast cancer, neratinib, HER2
MICROABSTRACT
We examined neratinib administration pre- and post-operatively in 5 patients with HER2-positive brain metastases. Two patients remained on therapy for 13 cycles and nearly 6 years postoperatively. We observed low CSF drug levels and variable craniotomy specimen levels among evaluable specimens. Inclusion of novel correlative analyses will continue to be crucial in advancing our understanding of CNS drug penetration.
INTRODUCTION
Patients with metastatic, human epidermal growth factor receptor 2 (HER2)-positive breast cancer have a high propensity to develop brain metastases,1–3 and treatment options remain limited. We have previously reported results from cohorts 1 and 3 of our multicenter phase II study4,5 (Translational Breast Cancer Research Consortium [TBCRC] 022) which evaluated the efficacy of neratinib monotherapy, an irreversible, pan HER tyrosine kinase inhibitor (cohort 1), and neratinib plus capecitabine (cohort 3) in patients with progressive HER2-positive parenchymal brain metastases ( NCT01494662).
Data on the CNS penetration of systemic therapies used to treat brain metastases are limited and were not evaluated in Cohorts 1 or 3. Although not previously explored in humans, pre-clinical data suggest CNS penetration of neratinib.6 As part of the initial design of this multi-cohort study, we included an exploratory cohort of 5 patients with HER2-positive breast cancer and brain metastases in whom craniotomy was clinically indicated (Cohort 2) to test our ability to administer neratinib in the unique clinical setting of a brief window prior to surgery. Postoperatively, patients received neratinib maintenance until progression. We aimed to utilize this window study’s opportunity to examine neratinib’s CNS penetration in patients undergoing craniotomy.
PATIENTS AND METHODS
Eligibility
Cohorts 1 and 3 of TBCRC 022 were designed for patients with progressive HER2-positive breast cancer brain metastases after receipt of prior therapy for parenchymal CNS disease. Key eligibility criteria for the cohort presented here (cohort 2) were the following: HER2-positive7 breast cancer with parenchymal CNS involvement without prior neratinib exposure and with candidacy for craniotomy. Measurable CNS disease was not required postoperatively and there were no minimums or limits to prior local or systemic treatments or enrollment. Key exclusions included escalating steroids during the week before baseline imaging, >2 seizures over the four weeks prior to study entry, significant malabsorption syndrome limiting oral drug administration, or any pre-existing grade ≥2 diarrhea. The study was conducted through the TBCRC and all women signed an informed consent, approved by the Institutional Review Boards of each institution.
Treatment Plan
Patients received neratinib 240 mg once daily for 7–21 days prior to a clinically-indicated craniotomy and held therapy on the morning of surgery. Diarrhea prophylaxis with loperamide 2 mg once daily for the first cycle was mandated. Postoperative stereotactic radiosurgery (SRS) but not whole brain radiotherapy (WBRT) was allowed and patients then re-initiated neratinib at the same dose, within 10 (+/− 3) days post-operatively or post-SRS, with a cycle length of 28 days. All patients were evaluated with a neurological exam on day 1 of each cycle (neurological symptoms, cranial nerve/motor strength assessments, presence of aphasia/dysphasia, ataxia, somnolence, sensation, and global assessment of worsening/stable/improved). Brain magnetic resonance imaging (MRI) and computed tomography (CT) of the chest-abdomen-pelvis occurred every 8 weeks. Upon CNS progression, patients had the option to add trastuzumab, though no patients enrolled on this study extension. Patients were to remain on study treatment until tumor progression, unacceptable toxicity, request to come off treatment, or any changes in the participant’s condition that rendered the participant unacceptable for further neratinib.
Correlative Studies
Patients had intra-operative cerebrospinal fluid (CSF), plasma (3 mL of blood was collected into a 5mL K3EDTA vacutainer tube), and intracranial tumor tissue collected for concentrations of neratinib. Samples of plasma and CSF were frozen immediately at −70 °C and shipped on dry ice to Covance Bioanalytical Services LLC (Indianapolis, IN). Neratinib concentrations were analyzed at Covance by a proprietary validated liquid chromatography-tandem mass spectrometry assay (Covance Bioanalytical Services Study #8266086).
Neratinib distribution in parenchymal brain tissue specimens was evaluated using liquid extraction surface analysis (LESA) ion trap mass spectrometry.8 Heme was imaged as a marker of vasculature using matrix assisted laser desorption ionization (MALDI) mass spectrometry imaging (MSI) using a 9.4 Tesla Fourier transform ion cyclotron resonance (FT ICR) mass spectrometer.9–11 More specifically, tissue sections were analyzed by LESA (Advion, Ithaca, NY) coupled to an amaZon Speed ion trap mass spectrometer (Bruker Daltonics, Billerica, MA). Sampling locations were selected with spacing of 1 mm in x and y dimensions to ensure there was no overlap between points. In this work we used an extraction/ESI solvent system (60/40/0.1 ACN/water/formic acid). Solvent (1.5 μL) was aspirated from a reservoir into a conductive pipette tip. The tip relocated to a pre-defined position above the sample and dispensed 0.2 μL solvent onto the surface. The liquid microjunction was maintained for 5 seconds to allow soluble analytes to dissolve before 1 μL was re-aspirated and injected into the mass spectrometer via a nano-electrospray ionization source, with a gas pressure of 0.3 psi and a tip voltage of 1.4 kV. All spectra were recorded in full scan and MS/MS mode with an isolation window recorded for 1 minute, and fragmentation for 2 minutes monitoring the m/z 512.1 fragment of neratinib. Concentration in tissue was calculated assuming standard spread over area of diameter 1 mm and tissue thickness 12 μm and tissue density approximated to water. A calibration curve plotted for standards under and on top of tissue, and the average of the two was used to measure concentrations in surgical specimens. MS data were analyzed in DataAnalysis (4.0) (Bruker Daltonics, Billerica, MA).
MALDI FT ICR MSI was performed on 12 μm sections of fresh frozen tissue mounted on microscopy glass slides. Sections were coated with 2,5-dihydroxybenzoic acid (DHB) (160 mg/mL in 70/30 methanol/water with 0.1% trifluoroacetic acid and 1%DMSO) matrix using a TM-Sprayer (HTX Imaging, Carrboro, NC). MSI was performed on a 9.4 Tesla SolariX XR FT ICR MS (Bruker Daltonics, Billerica, MA), and images were acquired in positive ion mode (m/z 155 – 1000) at a lateral spatial resolution (i.e. pixel size) of 100 μm. The transient length was 1.1185 seconds and the resulting mass resolving power was ~128,000 at m/z 557.2091. Online calibration was used to internally calibrate mass spectra from every location using the signal from heme (m/z 616.1776). MSI data were analyzed using SCiLS Lab software (Buker Daltonics, Bremen, Germany) and m/z values were searched against publicly available databases (Lipid Maps and the Human Metabolome Database (HMDB).12,13 Ions were preliminarily identified if assignments were associated with Δppm <2.
Serial tissue sections from the ones used for neratinib evaluation were stained with hematoxylin and eosin (H&E), digitally imaged, and annotated by a neuropathologist as previously described.14
Statistical Analyses
In descriptive analyses, we examined time-on-study treatment, toxicity, reasons for treatment cessation, and survival. We did not assess for response as postoperative measurable disease was not required. We also explored receptor status (estrogen receptor [ER], progesterone receptor [PR], HER2) from resected intracranial tumor specimens and described the agreement of primary and CNS tumor subtypes (all patients had CNS disease only). Results are based on data available as of March 1, 2019.
RESULTS
Patient characteristics
This cohort fully accrued, with five women enrolled on cohort 2 during 5/22/2013–10/18/2016. Table 1 shows the baseline characteristics of these patients. All patients had CNS disease only, with 1 also having leptomeningeal disease. Two patients (40%) had no prior CNS-directed treatments.
Table 1.
Patient Characteristics (N,% unless otherwise specified)a
| Characteristic (N=5) | n (%) |
|---|---|
| Age (years) (median, range) | 43.0 (27–45) |
| Female gender | 5 (100) |
| 2 | 2 (40) |
| White | 5 (100) |
| Other (non-CNS) sites | 0 (0) |
| Negative | 5 (100) |
| Positive | 1 (20) |
| No prior | 2 (40) |
| Other HER2/investigational agents | 1 (20) |
| ≥2 | 3 (60) |
Abbreviations: WBRT=whole brain radiotherapy; SRS=stereotactic brain surgery, CNS=central nervous system
Percentages may not always equal 100% due to rounding
All craniotomy specimens were ER-negative, PR-negative, and HER2-positive
Not including antibodies alone, hormonal therapy, or lapatinib unless coupled with chemotherapy
Efficacy and Survival
As of March 1, 2019, 1 patient remained on cohort 2 (cycle 75+). The number of cycles initiated by the five patients was 1 (patient with leptomeningeal disease), 2, 3, 13 and 75. The reasons for therapy cessation were CNS progression (n=2) and patient withdrawal (n=2), with one patient still on study treatment. At last follow-up, two patients had died (3 months after registration and receiving 1 cycle [n=1] and 6 months after study registration and receiving 3 cycles [n=1]); 1 was lost to follow-up (after 2 cycles and a total of 6 months of follow-up from study registration); and 2 remained alive (1 alive nearly 6 years since registration [and still on study treatment] and 1 patient alive nearly three years from registration who received a total of 13 cycles of study therapy).
Toxicity
Adverse events deemed possibly, probably, or definitely related to study therapy are shown in Table 2. One patient required a neratinib dose reduction and no patients came off study for toxicity.
Table 2.
Grade 2 and 3 toxicities reported as possibly, probably, or definitely attributed to neratiniba
| Cohort 2 (n=5) | Grade 2 (n, %) | Grade 3 (n, %) |
|---|---|---|
| Toxicity | ||
| Diarrhea | 1 (20) | 2 (40) |
| Hypokalemia | -- | 1 (20) |
| Syncope | -- | 1 (20) |
| Anemia | 2 (40) | -- |
| Folliculitis | 1 (20) | -- |
| Rash | 1 (20) | -- |
No grade 4–5 treatment toxicities reported. Each patient is counted once for the highest grade of each toxicity experienced
Neratinib concentrations
One patient’s craniotomy became urgent and no samples were collected. A second surgical patient’s CSF samples had questionable specimen integrity upon arrival to Covance. In total, three patients had CSF samples analyzed, with two of them also having corresponding plasma. In the three patients who had CSF collected at the time of craniotomy, all had neratinib concentrations below the limit of detection (<1.50 ng/mL) in their CSF, while the corresponding plasma concentrations for two of these patients were 34.3 (who went on to receive postoperative treatment for 1 cycle) and 53.8 ng/mL (who received postoperative treatment for 13 cycles). An additional patient without available plasma levels and with undetectable CSF neratinib concentrations was on study for 75+ cycles.
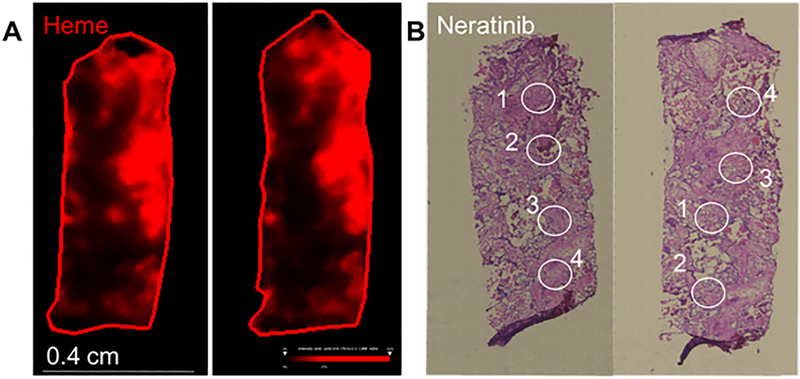
Drug distribution in the brain parenchyma was further evaluated in surgical tissue from one patient (who unfortunately did not have paired CSF or plasma with available results) using a spatially resolved surface sampling mass spectrometry approach (Figure 1). This patient came off study treatment after 2 cycles for CNS progression. Her intraoperative drug concentration from a total of 8 sampling locations of 1 mm diameter each over 2 tissue sections from the specimen ranged from 46 to 532 ng/ml, an approximate equivalent to 1–10 times plasma level concentrations measured for other patients in this cohort who had samples evaluated, revealing heterogenous drug distribution. Detailed histopathology evaluation immediately adjacent serial sections from the ones used for neratinib assessment (Supplemental figures 1 and 2) revealed complex tissue architecture with extensive heterogeneity comprising clusters of tumor cells, patches of necrosis, prominent vasculature with thickened walls, and extensive tissue reaction in the form of fibrosis. The distribution of selected phospholipids and phosphosphingolipids was mapped by MALDI FT ICR MSI and compared to the annotated histopathology image (Supplemental figure 1).
Figure 1.
Mass spectrometry analysis of neratinib distribution in a surgical specimen. A. MALDI 9.4 Tesla FT ICR MSI of heme distribution in red. B. Digital microscopy imaging of H&E staining of sister sections with drug distribution sampling location delineated as white circles. Each sampling location was analyzed directly by LESA MS/MS mass spectrometry for the drug neratinib fragment m/z 512.21 and drug concentrations were calculated from a standard curve. Sampling locations of approximately 1 mm diameter revealed heterogenous drug distribution associated with heterogenous histopathology features. More specifically, on the left panel, sampling location (1) revealed 533 ng/ml neratinib associated with mostly fibrous tissue and tumor surrounding the region, (2) showed 46 ng/ml in a fibrous and necrotic region, (3) 130 ng/ml with mostly fibrous tissue and scattered tumor cells, and (4) had 89 ng/ml with a cluster of tumor cells with surrounding fibrous tissue. A second section from the same specimen shown on the right panel showed a similar pattern with (4) 233 ng/ml neratinib associated with a mix of blood vessel, necrosis and some clusters of tumor cells, (3) 205 ng/ml with mostly a blood vessel and surrounding fibrous tissue with some neighboring tumor cells at the edge, (1) 48 ng/ml with a cluster of tumor cells with fibrous tissue and the edge of a blood vessel, and (2) 130 ng/ml with the highest percentage of tumor cells with some associated fibrosis.
Receptor status
All 5 patients had ER-negative and HER2-positive disease in primary tumors and in craniotomy specimens. One patient had PR-positive disease in her primary tumor but had PR-negative disease in her intracranial tumor.
DISCUSSION
We have previously reported efficacy results of neratinib monotherapy4 and neratinib plus capecitabine5 in the setting of progressive CNS disease. We demonstrated modest activity (CNS ORR 8%) in patients with progressive brain metastases treated with neratinib monotherapy, but a more robust response rate (CNS ORR 49%) in patients treated with neratinib plus capecitabine. Here, we report the results from an exploratory cohort of patients who were treated with neratinib prior to resection of brain metastasis(es) to better understand the degree to which neratinib penetrates the CNS. We found negligible neratinib concentrations in the CSF compared with plasma in a limited number of specimens and could not measure levels in parenchymal CNS samples using MALDI MSI despite adequate limit of detection, though were able to measure levels in one specimen using LESA MS/MS. However, despite the finding for low CSF concentrations of neratinib and heterogenous levels in a single parenchymal sample, patients may have benefitted from therapy, with 2 patients having CSF neratinib concentrations of <1.50 ng/mL but remaining on study treatment for 13 and over 75 cycles. The analysis of surgical tissue from one patient revealed neratinib concentrations ranging from 0.1 – 1 μM, providing levels well above the IC50 for neratinib of 17 nM (personal communication with Puma). Of note, diarrhea events for patients on this study cohort were similar to those reported previously and the prophylaxis for these events has since become more aggressive in subsequent cohorts to mitigate this well described toxicity.
A possible reason for our findings for negligible CSF levels of neratinib (and heterogenous levels in the one patient with an evaluable parenchymal sample) despite potential efficacy in some patients is that neratinib may only penetrate a disrupted blood-brain-barrier15 (or blood-CSF barrier) and that CSF levels do not necessarily reflect CNS penetration, resulting in a possible discordance between CNS levels and CNS activity. Indeed, there are multiple examples of agents with minimal penetration across an intact BBB but with demonstrated CNS activity, and support the recent American Society of Clinical Oncology (ASCO)-Friends of Cancer Research (FOCR) recommendations to encourage broader inclusion of patients with brain metastases into clinical trials.16 As potential preliminary evidence to support penetration into established brain metastases despite low CNS concentrations, there is a suggestion of a pharmacodynamic effect of neratinib in preclinical PDX models of HER2-positive brain metastases, as well as evidence of tumor regression, both as monotherapy, and in combination with ado-trastuzumab emtansine (TDM1).17 We have since initiated an additional cohort of the TBCRC 022 study which will test the combination of neratinib and TDM1 in patients with progressive, HER2+ breast cancer brain metastases.
Similar to our finding of variable drug levels within an individual brain metastasis sample, other groups have also shown variable concentrations of lapatinib, a different HER2 tyrosine kinase inhibitor, in breast cancer brain metastases, when tested after a brief preoperative exposure prior to craniotomy.18 Of interest, in preclinical models of other chemotherapy or targeted agents, the degree of brain metastasis penetration did not vary directly according to tumor size; instead factors other than tumor size appear also contribute to blood-tumor-barrier disruption.15,18,19
It is also possible that our assays used to detect concentrations of neratinib in the CSF were not sufficiently sensitive, or were not processed properly, though the higher levels detected in corresponding plasma samples makes this less likely. Our study design did not allow us to determine whether neratinib’s capability for CNS penetration evolved after craniotomy, with improved/higher levels achieved during the longer, ‘maintenance’ portion of postoperative treatment. It is also important to note that our exploratory and descriptive analyses cannot confirm who may have benefitted from pre- and post-operative neratinib and who would have benefitted from craniotomy alone.
Our findings in Cohort 2 highlight the challenges of incorporating and interpreting tissue-based analyses from CNS-based trials. In the future, continued inclusion of novel imaging studies and tissue-based analyses may further our understanding of drug penetration across both an intact and disrupted BBB. Further, the increasing availability of human brain metastasis-derived PDX and organoid preclinical models have tremendous potential to evaluate and prioritize novel therapeutic options to bring forward into the clinic.20 Future studies should continue to incorporate and explore these questions, though our small study highlights the challenges in execution and interpretation of this work, and the prerequisite for careful and thoughtful scientific collaborations.
Supplementary Material
Clinical Practice Points.
Neratinib has limited activity in the CNS when given alone but has increased activity when given with capecitabine. This small sub-study aimed to administer neratinib pre- and post-craniotomy to 5 patients with HER2-brain metastases and to examine neratinib concentrations in CSF, blood, and craniotomy specimens from enrolled patients. As of March 1, 2019, patients had remained on study for 1–75+ postoperative cycles. Evaluation of the CSF showed low neratinib concentrations, nonetheless two patients remained on therapy without progression for at least 13 cycles, with one on study treatment for nearly 6 years. The neratinib distribution in surgical tissue from one patient revealed heterogeneity in drug distribution and tumor histopathology. Inclusion of novel correlative analyses will continue to be crucial in advancing our understanding of CNS drug penetration.
Acknowledgments
We are grateful to all the patients who generously volunteered to participate in this study. We thank the TBCRC investigators, research nurses, and study coordinators for their efforts on behalf of the patients. We thank Kaitlyn Bifolck for her assistance with preparation of our submission. We thank Jean Zhao, PhD for her contributions to the pre-clinical work described.
REFERENCES
- 1.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13(6):1648–1655. [DOI] [PubMed] [Google Scholar]
- 2.Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–2977. [DOI] [PubMed] [Google Scholar]
- 3.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617. [DOI] [PubMed] [Google Scholar]
- 4.Freedman RA, Gelman RS, Wefel JS, et al. Translational Breast Cancer Research Consortium (TBCRC) 022: A Phase II Trial of Neratinib for Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases. J Clin Oncol. 2016;34(9):945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman RA, Gelman RS, Anders CK, et al. TBCRC 022: A Phase II Trial of Neratinib and Capecitabine for Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases. J Clin Oncol. 2019:JCO1801511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puma Biotechnology. Neratinib investigator brochure and personal communication with Puma Biotechnology. Puma Biotechnology: Los Angeles, CA; 2012. [Google Scholar]
- 7.Wolff AC, Hammond EH, Hicks DG, et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update http://www.asco.org/quality-guidelines/recommendations-human-epidermal-growth-factor-receptor-2-testing-breast-cancer; accessed May 15, 2015 J Clin Oncol. 2013;31(31):3997–4013. [DOI] [PubMed] [Google Scholar]
- 8.Kim AJ, Basu S, Glass C, et al. Unique Intradural Inflammatory Mass Containing Precipitated Morphine: Confirmatory Analysis by LESA-MS and MALDI-MS. Pain Pract. 2018;18(7):889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen PY, Touat M, Alexander BM, et al. Buparlisib in Patients With Recurrent Glioblastoma Harboring Phosphatidylinositol 3-Kinase Pathway Activation: An Open-Label, Multicenter, Multi-Arm, Phase II Trial. J Clin Oncol. 2019:JCO1801207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Randall EC, Emdal KB, Laramy JK, et al. Integrated mapping of pharmacokinetics and pharmacodynamics in a patient-derived xenograft model of glioblastoma. Nature communications. 2018;9(1):4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Ide JL, Norton I, et al. Molecular imaging of drug transit through the blood-brain barrier with MALDI mass spectrometry imaging. Sci Rep. 2013;3:2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wishart DS, Feunang YD, Marcu A, et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018;46(D1):D608–D617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fahy E, Sud M, Cotter D, Subramaniam S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007;35(Web Server issue):W606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberlin LS, Liu X, Ferreira CR, Santagata S, Agar NY, Cooks RG. Desorption electrospray ionization then MALDI mass spectrometry imaging of lipid and protein distributions in single tissue sections. Anal Chem. 2011;83(22):8366–8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin NU, Prowell T, Tan AR, et al. Modernizing Clinical Trial Eligibility Criteria: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Brain Metastases Working Group. J Clin Oncol. 2017;35(33):3760–3773. [DOI] [PubMed] [Google Scholar]
- 17.Ni J, Wang Y, Diala I, et al. Preclinical evaluation of neratinib plus T-DM1 in orthotopic PDX models of HER2-positive breast cancer brain metastases. Presented at the American Association for Cancer Research (AACR) Annual meeting, April 3, 2019, abstract #4832. [Google Scholar]
- 18.Morikawa A, Peereboom DM, Thorsheim HR, et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: a prospective study. Neuro Oncol. 2015;17(2):289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taskar KS, Rudraraju V, Mittapalli RK, et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm Res. 2012;29(3):770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Wang Y, Kabraji S, et al. Improving orthotopic mouse models of patient-derived breast cancer brain metastases by a modified intracarotid injection method. Sci Rep. 2019;9(1):622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.