Abstract
Background:
Insight into type 5 long QT syndrome (LQT5) has been limited to case reports and small family series. Improved understanding of the clinical phenotype and genetic features associated with rare KCNE1 variants implicated in LQT5 was sought through an international multi-center collaboration.
Methods:
Patients with either presumed autosomal dominant LQT5 (N = 229) or the recessive Type 2 Jervell and Lange-Nielsen syndrome (JLNS2, N = 19) were enrolled from 22 genetic arrhythmia clinics and 4 registries from 9 countries. KCNE1 variants were evaluated for ECG penetrance (defined as QTc > 460ms on presenting ECG) and genotype-phenotype segregation. Multivariable Cox regression was used to compare the associations between clinical and genetic variables with a composite primary outcome of definite arrhythmic events, including appropriate implantable cardioverter-defibrillator shocks, aborted cardiac arrest, and sudden cardiac death.
Results:
A total of 32 distinct KCNE1 rare variants were identified in 89 probands and 140 genotype positive family members with presumed LQT5 and an additional 19 JLNS2 patients. Among presumed LQT5 patients, the mean QTc on presenting ECG was significantly longer in probands (476.9 ± 38.6ms) compared to genotype positive family members (441.8 ± 30.9ms, p<0.001). ECG penetrance for heterozygous genotype positive family members was 20.7% (29/140). A definite arrhythmic event was experienced in 16.9% (15/89) of heterozygous probands in comparison with 1.4% (2/140) of family members (adjusted hazard ratio [HR]: 11.6, 95% confidence interval [CI]: 2.6-52.2; p=0.001). Event incidence did not differ significantly for JLNS2 patients relative to the overall heterozygous cohort (10.5% [2/19]; HR: 1.7, 95% CI: 0.3-10.8, p=0.590). The cumulative prevalence of the 32 KCNE1 variants in the Genome Aggregation Database (gnomAD), which is a human database of exome and genome sequencing data from now over 140,000 individuals, was 238-fold greater than the anticipated prevalence of all LQT5 combined (0.238% vs. 0.001%).
Conclusions:
The present study suggests that putative/confirmed loss-of-function KCNE1 variants predispose to QT-prolongation, however the low ECG penetrance observed suggests they do not manifest clinically in the majority of individuals, aligning with the mild phenotype observed for JLNS2 patients.
Keywords: long QT syndrome, genetics, penetrance, arrhythmia, sudden cardiac death
Introduction
Long QT syndrome (LQTS) is an inherited channelopathy characterized by impaired cardiac repolarization that confers an increased risk of syncope and sudden cardiac death (SCD) secondary to torsades de pointes.1 The prevalence of LQTS is approximately 1 in 2,000 and 17 genes have been implicated in its pathogenesis, though the majority of cases stem from mutations within KCNQ1 (LQT1), KCNH2 (LQT2), and SCN5A (LQT3), considered the major LQTS genetic subtypes.2-4 The KCNQ1 gene encodes the Kv7.1 α-subunit responsible for the slow component of the delayed rectifier potassium current (IKs), whereas the Kv11.1 α-subunit of the rapid component of the delayed rectifier potassium current (IKr) is encoded by KCNH2.5-7 Loss-of-function mutations within these voltage-gated potassium channels impair ventricular repolarization during Phase 3 of the cardiac action potential leading to LQT1 and LQT2.8,9
LQT5 is a minor LQTS genetic subtype accounting for approximately 1-2% of LQTS cases. LQT5 develops secondary to loss-of-function variants within KCNE1, which encodes minK, a voltage-gated potassium channel β-subunit felt to primarily interact with the Kv7.1 α-subunit responsible for IKs, though reports have also suggested a role for minK in IKr through an interaction with the Kv11.1 α-subunit.5,10-12 The most intensively investigated KCNE1 rare variant, p.Asp76Asn, has been implicated in both congenital and drug-induced forms of LQTS.10,13 The relative rarity of LQT5 has led to limited insight into its clinical and genetic attributes and management is often extrapolated from knowledge of the canonical LQT1-3 subtypes.
Recent work has revealed that loss-of-function variants in KCNE2, another voltage-gated potassium channel β-subunit, are more aptly characterized as arrhythmia predisposing variants or functional risk alleles, leading to recognition that LQT6 is not a monogenic form of LQTS and a corresponding alteration to the treatment approach for individuals possessing these variants.14,15 The KCNE2 and KCNE1 genes have many similarities, though only KCNE1 loss-of-function homozygotes and compound heterozygotes manifest with sensorineural deafness in association with QT-prolongation, referred to as Type 2 Jervell and Lange-Nielsen syndrome (JLNS2).16-18 Notably, in contrast to the severe and often complete loss-of-function observed for pathogenic KCNQ1 and KCNH2 mutations, the reductions in cardiac potassium currents observed on experimental in vitro patch clamp analysis for KCNE2 and KCNE1 variants have been modest.10,19,20
The growing recognition that each genetic LQTS subtype may require its own tailored approach to management led to the pursuit of an international multi-center collaboration to further define the clinical and genetic features of LQT5.21-25
Methods
Transparency and Openness Promotion
Data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
The study population consisted of 4 LQTS registries, including the Canadian LQTS registry, the Rochester (New York) LQTS registry, the Japanese LQTS registry, and the National Cardiac Inherited Disease Registry of New Zealand, along with 22 inherited arrhythmia clinics from 9 countries. Care was taken to ensure that no study participants were included twice through consultation with study investigators. Inclusion criteria for living probands required the presence of a rare KCNE1 variant, defined as an allele frequency < 0.1% in the Genome Aggregation Database (gnomAD; a database comprised of 141,456 individuals from multiple population-based and disease-specific genetic cohort studies),26 and presence of a resting QTc >460ms on a surface ECG. An allele frequency of < 0.1% was chosen, as this rate may be sufficiently rare to contribute to a low penetrant form of LQTS. Genotype positive family members identified on cascade screening, which refers to clinical and genetic evaluation with variant-specific genetic testing of blood relatives at risk of being affected, were also included.
Cases of SCD that remained unexplained following cardiac autopsy were eligible for inclusion when molecular autopsy identified a rare KCNE1 variant that had been observed in at least one living proband in our study that possessed a QTc > 460ms on ECG. Homozygotes and compound heterozygotes of rare KCNE1 variants that exhibited sensorineural deafness consistent with JLNS2 were also eligible for the study. All living probands presenting with an arrhythmic event were required to have undergone clinical testing with an ECG, exercise treadmill test, and echocardiogram, at minimum, and exhibit no evidence of another channelopathy or cardiomyopathy. Probands entered into the study were also required to have undergone screening of all exons and associated exon-intron boundaries within the KCNQ1, KCNH2, SCN5A, KCNE1, and KCNE2 genes.
Exclusion criteria for living probands and genotype positive family members consisted of a pathogenic or likely pathogenic mutation, as per American College of Medical Genetics and Genomics (ACMG) guidelines, in another LQTS gene and deceased probands were excluded when a pathogenic or likely pathogenic mutation was identified in a gene known to be causative for either a cardiac channelopathy or cardiomyopathy.27 Individuals possessing the known loss-of-function, pro-arrhythmic risk allele KCNE1-p.Asp85Asn in isolation were not included due to its presence in 0.1-2.5% of the general population (depending on ancestry; 1.6% in European ancestry subjects) and its being considered too common to function as a monogenic culprit for LQTS.15,28
The following variables were collected retrospectively for all living probands and genotype positive family members: date of birth, date of initial presentation, reason for presentation, sex, familial status (proband versus family member), Bazett corrected QT-intervals (QTc) recorded on ECGs at initial presentation and during follow-up, date at the time of cardiac events (including presumed cardiac syncope, appropriate implantable cardioverter defibrillator [ICD] shock, aborted cardiac arrest [ACA] requiring resuscitation, and SCD with normal cardiac autopsy), activity at the time of the cardiac event, secondary QT stressors present at the time of the cardiac event (including QT prolonging medication, electrolyte abnormality, and heart block), and details of β-blocker usage, including dates of initiation and discontinuation, if applicable. Genetic details of the KCNE1 variant, including the nucleotide and amino acid change, were obtained for each case.
The study was performed as part of a protocol approved by the research ethics boards of Western University, London, Ontario, Canada and the collaborating institutions. All study participants provided informed consent for their clinical and genetic data to be used for research.
Assessment of ECG Penetrance and Genotype-Phenotype Segregation
ECG penetrance was assessed in genotype positive family members. Consistent with prior work, an electrocardiographically manifest (penetrant) LQTS phenotype was defined as a QTc value on the presenting ECG > 460ms.22 Evaluation for genotype-phenotype segregation was performed in each family in an effort to clarify the role of rare KCNE1 variants in predisposing to QT-prolongation and was considered present if 2 or more individuals possessing the variant were phenotype positive.
Evaluation of KCNE1 Variants
All KCNE1 variants included in the study were subjected to computer-based analyses and their prevalence in the general population and among individuals of European ancestry in isolation was assessed using gnomAD.26 Computer model predicting effects of mutations on protein function was performed using Polymorphism Phenotyping v2 (PolyPhen-2), Sorting Intolerant From Tolerant (SIFT), and Combined Annotation Dependent Depletion (CADD).29-31 Prior in vitro functional analyses of KCNE1 variants reported in the literature were reviewed. Variants were presumed to be loss-of-function if they manifested with sensorineural deafness consistent with a JLNS2 phenotype when present in a homozygous or compound heterozygous state.
Although variant classification was performed according to ACMG guidelines, this was ultimately deemed inappropriate secondary to the low level of penetrance observed for KCNE1 variants; ACMG criteria have been designed for classification of highly penetrant variants.27
Statistical Analysis
Continuous variables are presented as means ± standard deviation and those exhibiting normal and non-normal distributions were compared using Student’s t-test and the Wilcoxon rank-sum test, respectively. Comparison of categorical values was performed using Fisher’s exact test. Cox proportional hazards models were used to estimate the associations between clinical and genetic variables and age at first presumed primary arrhythmic event (composite of presumed cardiac syncope, appropriate ICD shock, ACA, or SCD with normal autopsy; subsequently referred to as the composite arrhythmic outcome with syncope) and the first definite primary arrhythmic event (composite of appropriate ICD shock, ACA, or SCD with normal autopsy; subsequently referred to as the composite arrhythmic outcome without syncope) among heterozygotes possessing rare KCNE1 variants and JLNS2 patients.
Variables evaluated in both uni-/multivariable analyses included familial status (proband versus family member), sex, QTc on initial presenting ECG, β-blocker therapy, and missense variant location (extracellular, transmembrane, intracellular) in the KCNE1-encoded β-subunit. The QTc on the initial presenting ECG was treated as a categorical variable divided into tertiles (<470 ms, ≥ 470 ms but ≤ 500 ms, and > 500 ms). Cumulative years on β-blocker therapy was treated as a time-dependent covariable in order to account for patients starting and stopping treatment throughout their lifetime and enabled comparison of event rates during time on β-blocker therapy relative to time off β-blocker therapy. Risk of arrhythmic events was also evaluated based on KCNE1-p.Asp76Asn variant status (KCNE1-p.Asp76Asn carriers versus carriers of another KCNE1 variant). Robust standard errors were used to account for familial relatedness. Due to minimal missing data, which only consisted of ECG values and age at LQTS diagnosis among 2 SCD cases identified to possess KCNE1 variants on molecular autopsy, complete case analysis was used. Two-tailed p-values < 0.05 were considered statistically significant. Statistical analyses were performed using Stata version 16 (College Station, TX, USA).
Results
Study Population
Eighty-nine probands heterozygous for a rare KCNE1 variant in the setting of a phenotype compatible with LQTS and 140 genotype positive family members were enrolled into the study (Table 1). The mean age at the time of first ECG was 25.4 ± 19.7 years and 61.6% were female. The mean QTc on the presenting ECG among probands was significantly longer relative to genotype positive family members (476.9 ± 38.6ms vs. 441.8 ± 30.9ms, p< 0.001). β-blocker therapy was used at some point in 78.7% of probands and 55.0% of genotype positive family members. A total of 41.6% of probands experienced a presumed cardiac event during their lifetime, defined as presumed cardiac syncope, appropriate ICD shock, ACA, or SCD, compared to only 5.7% of KCNE1 variant-positive family members (p<0.001). The number of individuals that experienced each of these events is provided in Table 1. Within the overall heterozygous cohort, the median ages of onset of the composite arrhythmic outcomes with and without syncope were 23.5 (interquartile range [IQR]: 14.2-43.4) and 27.0 (15.2-45.4) years, respectively.
Table 1:
Clinical Features of Probands and Genotype Positive Family Members Possessing Rare KCNE1 Variants
| LQT5 | JLNS2 | ||||
|---|---|---|---|---|---|
| Clinical Variable | Overall n = 229 |
Probands n = 89 |
Genotype +ve FM n = 140 |
p value* | n = 19 |
| Age at First ECG (years) | 25.4 (19.7) | 26.8 (19.2) | 24.5 (19.9) | 0.174 | 14.6 (14.0) |
| Female (%) | 141 (61.6) | 59 (66.3) | 82 (58.6) | 0.211 | 9 (47.4) |
| European Ancestry (%) | 219 (95.6) | 83 (93.3) | 136 (97.1) | 0.016 | 16 (84.2) |
| QTc on Presenting ECG (ms) | 455.6 (38.2) | 476.9 (38.6) | 441.8 (30.9) | <0.001 | 471.1 (43.5) |
| Males | 448.5 (36.2) | 469.3 (38.2) | 437.7 (30.2) | <0.001 | 468.9 (53.5) |
| Females | 460.1 (38.8) | 480.8 (38.6) | 444.8 (31.3) | <0.001 | 473.6 (32.0) |
| Atrial Fibrillation | 7 (3.1) | 6 (6.7) | 1 (0.7) | 0.017 | 0 (0) |
| Treatment | |||||
| β-Blocker | 147 (64.2) | 70 (78.7) | 77 (55.0) | 0.001 | 8 (42.1) |
| LCSD | 5 (2.2) | 2 (2.2) | 3 (2.1) | 1.000 | 1 (5.3) |
| ICD | 28 (12.2) | 23 (25.8) | 5 (3.6) | <0.001 | 0 (0) |
| Cardiac Event | |||||
| Syncope | 31 (13.5) | 25 (28.1) | 6 (4.2) | <0.001 | 3 (15.8) |
| Appropriate ICD Shock | 4 (1.8) | 3 (3.4) | 1 (0.7) | 0.304 | 0 (0) |
| Aborted Cardiac Arrest | 12 (5.2) | 12 (13.5) | 0 (0) | <0.001 | 1 (5.3) |
| Sudden Cardiac Death | 4 (1.8) | 3 (3.4) | 1 (0.7) | 0.304 | 1 (5.3) |
| CAO with Syncope | 45 (19.7) | 37 (41.6) | 8 (5.7) | <0.001 | 4 (21.1) |
| CAO Without Syncope | 17 (7.4) | 15 (16.9) | 2 (1.4) | <0.001 | 2 (10.5) |
Data are n (%) or mean (SD).
p-value compares LQT5 probands and family members. LQT5 = Type 5 Long QT syndrome, JLNS2 = Type 2 Jervell and Lange-Nielsen Syndrome, Genotype +ve FM = genotype positive family members, ms = milliseconds, LCSD = left cardiac sympathetic denervation, ICD = implantable cardioverter defibrillator, CAO = composite arrhythmic outcome
The KCNE1-p.Asp76Asn variant was present in 98 of 229 heterozygous individuals (42.8%) and the mean QTc among carriers (455.1 ± 35.5ms) was similar to the mean QTc value observed among the remaining individuals in the heterozygous cohort (455.9 ± 40.2ms, p = 0.873). An additional 19 JLNS2 individuals, including 15 homozygotes and 4 compound heterozygotes, were enrolled into the study and their clinical features are reported in Table 1. The composite arrhythmic outcome with syncope was experienced in a total of 13.3% (2/15) of homozygotes and 50% (2/4) of compound heterozygotes.
Among KCNE1 heterozygotes, only 2 genotype positive family members had definite arrhythmic events; their details are provided in the Online Supplement. The median age at the time of last follow up for the overall heterozygous cohort was 27.3 years (IQR: 15.2-45.6).
Disease Penetrance and Genotype-Phenotype Segregation
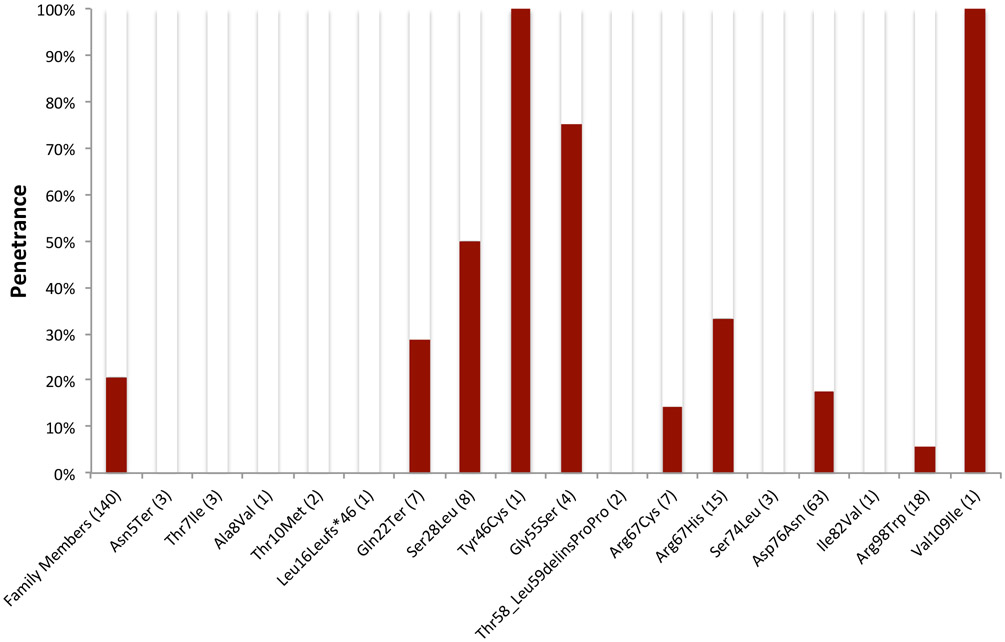
Disease penetrance was assessed in genotype positive family members based on the definition for an electrocardiographically manifest LQTS phenotype being a QTc value > 460ms on presenting ECG. The overall penetrance was 20.7% (29/140). Penetrance values for each individual KCNE1 variant possessed in a heterozygous state by a family member are illustrated in Figure 1. Among the 10 KCNE1 variants possessed by ≥ 3 individuals, penetrance values ranged from 0% (p.Asn5Ter and p.Thr7Ile) to 75% (p.Gly55Ser). The KCNE1-p.Asp76Asn variant, present in a heterozygous state in 63 family members, exhibited an overall penetrance of 17.5%. Among JLNS2 patients, the electrocardiographic penetrance was 66.7% (10/15) in homozygotes and 75% (3/4) in compound heterozygotes.
Figure 1:
ECG Penetrance of Rare KCNE1 Variants. Penetrance is defined as a QTc > 460ms on their presenting ECG.
(N) indicates the number of individuals with the KCNE1 variant
Genotype-phenotype segregation was assumed to be present if at least 2 individuals in a single family were phenotype positive. Thirteen of 52 (25%) families with at least 2 genotype positive individuals possessed evidence of genotype-phenotype segregation (Supplemental Table 1). Genotype-phenotype segregation was observed for 8 KCNE1 variants (KCNE1-p.Gln22Ter, -p.Ser28Leu, -p.Tyr46Cys, -p.Gly55Ser, -p.Arg67Cys, p.Arg67His, -p.Asp76Asn, and -p.Val109Ile; Supplemental Table 1).
Arrhythmic Risk Associations
Univariable Analyses
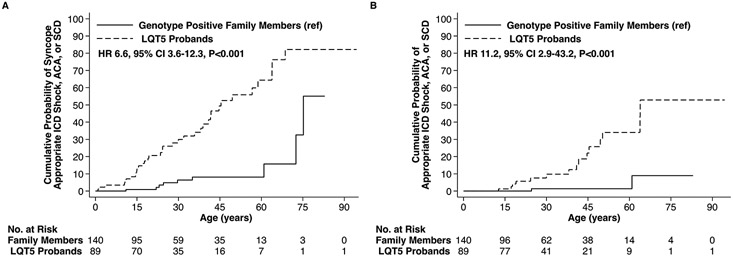
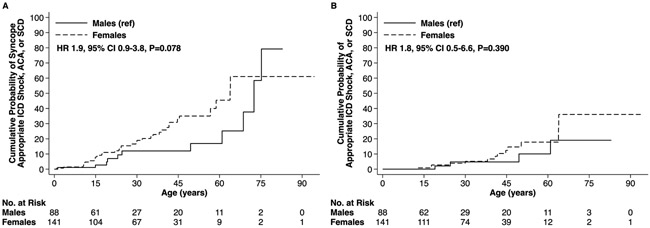
Probands possessing a rare KCNE1 variant had a 6.6-fold (95% confidence intervals [CI]: 3.6-12.3, p< 0.001) higher hazard of experiencing the composite arrhythmic outcome with syncope relative to genotype positive family members (Figure 2A and Table 2) and a 11.2-fold (95% CI: 2.9-43.2, p<0.001) higher hazard of the composite arrhythmic outcome without syncope (Figure 2B and Table 2). Evaluation of QTc values on presenting ECG revealed that the upper 2 tertiles were both associated with a higher risk of the composite arrhythmic outcome with syncope, whereas only the QTc > 500ms tertile exhibited a statistically significant association for the composite arrhythmic outcome without syncope, respectively (Table 2 and Supplemental Figure 1). Neither sex (Figure 3), nor β-blocker therapy, nor missense variant location within the KCNE1-encoded Kv7.1 β subunit (Supplemental Figure 2) were associated with an altered risk of the composite arrhythmic outcomes on univariable analysis (Table 2). The arrhythmic risk associated with the p.Asp76Asn variant, the most prevalent KCNE1 variant in the cohort carried by 42.8% of heterozygotes, did not differ statistically relative to the collective remainder of the KCNE1 variants evaluated (Supplemental Figure 3).
Figure 2:
Arrhythmic Events Among Probands and Genotype Positive Family Members Possessing a Rare KCNE1 Variant. Outcomes of (A) Syncope, Appropriate ICD Shock, ACA, or SCD and (B) Appropriate ICD Shock, ACA, or SCD.
ICD = implantable cardioverter-defibrillator, ACA = aborted cardiac arrest, SCD = sudden cardiac death, ref = reference, HR = hazard ratio, CI = confidence intervals.
Table 2:
Association of Clinical and Genetic Variables with Cardiac Events Among Individuals Heterozygous for Rare KCNE1 Variants
| Clinical and Genetic Variables | Composite of Syncope, Appropriate ICD Shock, ACA, SCD |
Composite of Appropriate ICD Shock, ACA, SCD |
||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) |
p-value | Adjusted HR (95% CI) |
p-value | Unadjusted HR (95% CI) |
p-value | Adjusted HR (95% CI) |
p-value | |
| Familial Status | 6.6 (3.5-12.3) | <0.001 | 4.7 (1.9-11.7) | <0.001 | 11.2 (2.9-43.2) | <0.001 | 11.6 (2.6-52.2) | 0.001 |
| Female Sex | 1.9 (0.9-3.8) | 0.08 | 0.9 (0.4-1.8) | 0.75 | 1.8 (0.5-6.6) | 0.39 | 0.4 (0.1-1.3) | 0.13 |
| QTc tertiles (ms) <470 | Reference | - | - | - | Reference | - | - | - |
| 470-500 | 3.6 (1.8-7.2) | <0.001 | 1.8 (0.8-4.4) | 0.17 | 2.1 (0.6-7.3) | 0.23 | 0.9 (0.2-3.9) | 0.90 |
| >500 | 3.4 (1.5-7.9) | 0.004 | 1.3 (0.4-4.6) | 0.65 | 7.9 (2.4-25.3) | <0.001 | 3.3 (0.7-15.7) | 0.13 |
| Time on β-Blocker* | 1.0 (0.9-1.2) | 0.53 | 1.0 (0.9-1.2) | 0.69 | 1.0 (0.9-1.1) | 0.75 | 1.0 (0.9-1.1) | 0.80 |
| Variant Location | ||||||||
| Extracellular | Reference | - | - | - | Reference | - | - | - |
| Transmembrane | 1.6 (0.4-6.9) | 0.51 | 1.4 (0.4-5.4) | 0.65 | 1.1 (0.1-10.0) | 0.93 | 0.5 (0.1-5.0) | 0.55 |
| Intracellular | 0.9 (0.3-2.9) | 0.88 | 0.7 (0.2-2.2) | 0.53 | 0.5 (0.1-2.7) | 0.44 | 0.3 (0.1-1.8) | 0.21 |
β-blocker treated as a time dependent covariable. ICD = implantable cardioverter defibrillator, ACA = aborted cardiac arrest, SCD = sudden cardiac death, HR = hazard ratio, CI = confidence interval, ms = milliseconds.
Figure 3:
Arrhythmic Events Among Males and Females Possessing a Rare KCNE1 Variant. Outcomes of (A) Syncope, Appropriate ICD Shock, ACA, or SCD and (B) Appropriate ICD Shock, ACA, or SCD.
ICD = implantable cardioverter-defibrillator, ACA = aborted cardiac arrest, SCD = sudden cardiac death, ref = reference, HR = hazard ratio, CI = confidence intervals.
Univariable analyses for probands in isolation revealed measures of association that were generally consistent with the overall heterozygous cohort with no point estimates that extended beyond the 95% CI boundaries (Supplemental Table 2).
Multivariable Analysis
A multivariable Cox regression model was constructed including the variables for familial status, sex, QTc tertile on presenting ECG, β-blocker therapy, and location of the missense variant within the KCNE1-encoded Kv7.1 β subunit. Following adjustment, familial status was the only predictor that continued to exhibit a statistically significant association for the arrhythmic outcomes (Table 2). Similar results were obtained for probands in isolation with no point estimates that extended beyond the 95% CI boundaries for the overall heterozygous cohort (Supplemental Table 2).
JLNS2 Arrhythmic Outcomes
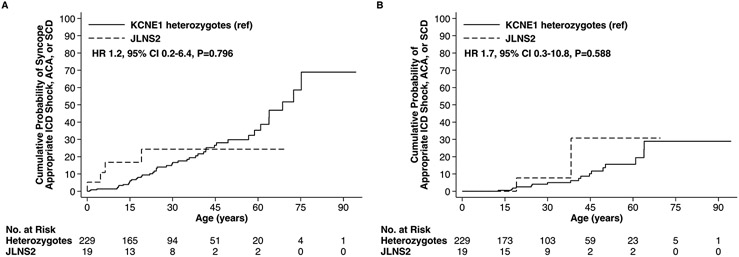
The mean QTc values on presenting ECG in JLNS2 patients trended towards being longer relative to individuals possessing a KCNE1 variant in a heterozygous state, but did not reach statistical significance (471.1 ± 43.5ms versus 455.6 ± 38.2ms, p = 0.050) (Table 1). JLNS2 patients had event rates that also did not exhibit statistically significant differences relative to KCNE1 heterozygotes for the composite arrhythmic outcomes including syncope (hazard ratio [HR] = 1.2, 95% CI 0.2-6.4, p= 0.800, Figure 4A) and excluding syncope (HR = 1.7, 95% CI 0.3-10.8, p= 0.590, Figure 4B). The median age at the time of last follow up for the JLNS2 cohort was 27.2 years (IQR: 15.8-38.0).
Figure 4:
Arrhythmic Events Among Type 2 Jervell and Lange-Nielsen Syndrome Patients and KCNE1 Heterozygotes. Outcomes of (A) Syncope, Appropriate ICD Shock, ACA, or SCD and (B) Appropriate ICD Shock, ACA, or SCD.
JLNS2 = Type 2 Jervell and Lange-NieIsen syndrome, ICD = implantable cardioverter-defibrillator, ACA = aborted cardiac arrest, SCD = sudden cardiac death, ref = reference, HR = hazard ratio, CI = confidence intervals.
Secondary QT Stressors and Triggers for Cardiac Events
A total of 62 cardiac events were experienced among the entire cohort during a collective 7,844 patient years beginning from birth. Three events were reported to have occurred in the setting of a QT-prolonging medication, 1 in the context of a severe electrolyte abnormality, and 1 was attributed to torsades de pointes in the setting of complete heart block. No secondary QT-prolonging stressors were identified in association with the remaining events. Activities reported at the time of events included awake at rest in 37 (60.0%), exertion in 17 (27.4%), auditory stimuli in 2 (3.2%), post-exertion in 1 (1.6%), sleep in 1 (1.6%), and the activity at the time of the event was unknown in 4 (6.5%).
Evaluation of KCNE1 Variants
Population Allele Frequencies
Among the 32 KCNE1 variants possessed by the study participants, 22 were observed in gnomAD, with individual allele frequencies ranging up to 0.02094% for the Thr10Met variant (0.02134% when restricted to European ancestry; Supplemental Table 3). The collective prevalence of these variants in the overall gnomAD cohort was 0.238% and 0.169% among the European ancestry subgroup. Based on the assumptions that the prevalence of LQTS is 0.05% and LQT5 accounts for 2% of LQTS, its prevalence is estimated at 0.001%. The collective prevalence of KCNE1 variants implicated in LQT5 is 238-fold the anticipated prevalence of LQT5 when the overall gnomAD cohort is considered and 169-fold when the analysis is restricted to individuals of European ancestry. Eight of the 32 KCNE1 variants were observed in JLNS2, confirming their status as loss-of-function given their being causative for sensorineural deafness (Supplemental Table 3). The collective prevalence of KCNE1 variants identified in the context of JLNS2 in the overall gnomAD cohort was 0.0162% and 0.0240% among Europeans.
Computer-Based and Previously Reported In Vitro Analyses
Computer-based analysis of KCNE1 variants possessed by study participants was performed using PolyPhen-2, SIFT, and CADD (Supplemental Table 3). PolyPhen-2 and SIFT both identified 14 of 24 missense variants as probably/possibly damaging or damaging, respectively. A total of 18 of 27 single nucleotide variants had a CADD score greater than 20, predicting their being among the top 1% of most damaging variants within the genome.31 Classification of the variants using the 2015 ACMG guidelines identified 3 as pathogenic, 5 as likely pathogenic, 17 as a variant of unknown significance, and 7 as likely benign (Supplemental Table 3). Assignment of likely benign status to 7 variants was primarily driven by their minor allele frequencies being greater than the anticipated prevalence of LQT5 (0.001%), which is not considered appropriate when variant penetrance is anticipated to be low. On review of the literature, in vitro patch-clamping analysis using heterologous expression of mutant KCNE1 in association with wild-type KCNQ1 had been performed for only 4 of 25 KCNE1 missense variants (Supplemental Table 3) and each was consistent with a loss-of-function.10,19,20
Discussion
This international multicenter study represents the first large-scale evaluation of rare KCNE1 variants implicated as monogenic culprits for LQTS. Their low ECG penetrance in family members, coupled with their excess prevalence in gnomAD, suggests that loss-of-function KCNE1 variants do not manifest clinically in a majority of individuals. The benign phenotype observed in the vast majority of genotype positive family members strongly suggests that loss-of-function KCNE1 variants require additional genetic and/or non-genetic factors to manifest with a positive LQTS phenotype. However in contrast to KCNE214, QT-prolongation and clinical events occurred in the overwhelming majority of individuals in the absence of an identifiable QT prolonging stressor, suggesting that LQT5 should be viewed as a low penetrant primary arrhythmic condition rather than an exclusively provoked syndrome. These findings, which align with the conclusions drawn for KCNE1 from the recent Clinical Genome Resource Consortium reappraisal of LQTS genes, have important clinical implications for probands and genotype positive family members.32
Evaluation of arrhythmic events among probands initially suggested that LQT5 may be a highly malignant disorder, however mirroring prior work in LQTS, the striking event rate observed among probands differed dramatically relative to the findings among genotype positive family members.33 The contrasting arrhythmic profiles of probands and genotype positive family members, coupled with clinical and genetic evidence suggesting KCNE1 variants do not manifest clinically in the majority of individuals, strongly suggests that the high event rate observed among LQT5 probands was secondary to selection bias. Although operative in all forms of LQTS, the impact of selection bias is expected to be more extreme for low penetrant variants when the contribution of genomic background and environmental influences to arrhythmic events and QT prolongation is anticipated to be much greater. This concept is effectively illustrated by a recent study that identified hazard ratios ranging from 2.48- to 3.21 for a composite outcome of syncope, ACA, or SCD among probands relative to family members in the major LQTS genetic subtypes (1-3), in comparison to the unadjusted 6.6-fold increased hazard ratio reported here for LQT5.34
Aside from familial status, no other intrinsic clinical or genetic factors, including QTc on presenting ECG, sex, β-blocker therapy, and missense variant location, were associated with an altered risk of events on multivariable analyses (Table 2). Notably, only 64.2% of individuals were treated with β-blocker during their lifetime and the mean QTc of those administered β-blockade was 464.4 ± 39.0 ms in comparison with a mean value of 439.4 ± 30.8 ms for those not treated (p<0.001). These findings suggest that patients with milder phenotypes were not treated, which is anticipated to lead to biased measures of association secondary to confounding by indication. It is possible that confounding by indication, coupled with the low event rate, may have led to the lack of an apparent protective effect with β-blocker.
Although the findings from the current study serve as strong evidence that many KCNE1 variants are insufficient in isolation to cause LQTS, it could be argued that only a minority of these variants have undergone functional work and hence the physiological relevance for the majority is unclear. Eight of the 32 variants were observed among cases of JLNS2 providing definitive evidence for their being loss-of-function. Penetrance of these variants was 15.7% among family members, which was consistent with findings from the overall sample (20.7%). In addition, QTc values and event rates among study participants possessing the most prevalent KCNE1 variant (p.Asp76Asn), known to be loss-of-function and present in 98 of the 229 heterozygous individuals, were consistent with those from the remainder of the cohort (Supplemental Figure 2).10,19
Attempted evaluation of the KCNE1 variants using ACMG criteria was ultimately deemed inappropriate due to their low penetrance given that ACMG criteria are tailored for highly penetrant variants.27 Notably, the KCNE1-p.Asp76Asn variant has a prevalence among individuals with European ancestry of 0.02212%, which exceeds the anticipated prevalence of LQT5 (0.001%) by >22-fold. A greater than expected allele frequency for the disorder being evaluated is considered a strong ACMG criterion for classifying a variant as benign. Although the p.Asp76Asn variant had sufficient additional supporting evidence to still receive a likely pathogenic designation, 7 KCNE1 variants were demoted to likely benign status primarily owing to their prevalence being greater than anticipated for LQT5 (Supplemental Table 3). In the collective view of the investigators, given that KCNE1-p.Asp76Asn is an established genetic culprit for LQT5, it is not felt that demotion of other variants with similar allele frequencies to likely benign status on the basis of their apparent excess prevalence is appropriate.15
The study also builds upon prior work and provides additional insight into the JLNS2 phenotype.18 In contrast to JLNS1, an autosomal recessive condition secondary to homozygous or compound heterozygous KCNQ1 loss-of-function mutations and characterized by marked QT prolongation and a highly malignant arrhythmic phenotype, the phenotype of JLNS2 appeared surprisingly mild, which aligns with earlier work.18 Although the apparent lack of an effect on phenotypic severity for increasing gene dosage may be secondary to inadequate power given that only 19 JLNS2 patients were included in the study, the finding that JLNS2 has a relatively mild phenotype lends further support to dysfunction of the KCNE1-encoded β-subunit often being clinically concealed..
Although a functional copy of KCNE1 is necessary for sensorineural hearing, the findings from this study suggest that the KCNE1-encoded β-subunit may either exert a modest role in cardiac repolarization or, alternatively, the heart, in contrast to the inner ear, may have established a redundancy for β-subunits that allows for effective compensation in response to the loss of one constituent. The notion that a single β-subunit may be able to interact interchangeably with multiple pore forming α-subunits is alluded to by evidence that minK not only contributes to IKs, but also IKr through an interaction with the Kv11.1 α-subunit.5,11,12
Whereas possessing a pathogenic mutation causative for the major genetic LQTS subtypes results in a diagnosis of LQTS and most often triggers initiation of a β-blocker regardless of phenotype35, evidence from the current study suggests that an alternative approach to management for individuals possessing a KCNE1 rare variant in the absence of an LQTS phenotype may be desired. While it is felt that all individuals possessing a loss-of-function KCNE1 variant should be advised to avoid QT-prolonging drugs13, in the presence of a normal phenotype intensive measures such as β-blockade and exercise restriction may not be merited. Although a protective effect of β-blockade was not observed in the study, given the potential limitations highlighted above that may have led to both biased and underpowered results, it is felt that β-blocker therapy should still be recommended in the presence of a positive LQTS phenotype. Due to the presence of study participants that experienced presumed arrhythmic events despite QTc values considered within normal limits on presenting ECG, highlighting the limitations of a single ECG to assess disease penetrance, it is advocated that all individuals possessing true loss-of-function variants be followed for serial monitoring of QTc values. Routine use of cascade screening for these variants is also advocated given their potential to manifest with a malignant LQTS phenotype, as highlighted by the natural history of the probands in the study.
Limitations
Although the largest dedicated evaluation for rare KCNE1 variants to date, the study may be underpowered to detect statistically significant associations between relevant clinical and genetic factors and arrhythmic risk. As an observational study, it is also vulnerable to various unavoidable forms of bias. The cohort consisted of probands referred to specialized inherited arrhythmia clinics due to worrisome clinical findings and likely led to selection of a malignant subset of KCNE1 heterozygotes and a correspondingly inflated arrhythmic event rate. In addition, evaluation for a potential protective effect of β-blocker therapy will unavoidably be biased secondary to confounding by indication.
Conclusions
The present study reveals that KCNE1 loss-of-function variants are weakly penetrant and individuals manifesting with an LQTS phenotype in the presence of a loss-of-function KCNE1 variant likely possess additional genetic or environmental factors that predispose to QT prolongation. In contrast to KCNE2, the overwhelming majority of probands and genotype positive family members manifesting with QT-prolongation and arrhythmic events did so in the absence of a QT-prolonging stressor suggesting that LQT5 should be viewed as a low penetrant primary arrhythmic condition rather than an exclusively provoked syndrome. Following identification of a rare KCNE1 loss-of-function variant, clinical management should consist of meticulous evaluation for an LQTS phenotype and counselling regarding the avoidance of QT prolonging drugs.
Supplementary Material
Clinical Perspective.
What is new?
Rare loss-of-function KCNE1 variants are weakly penetrant and do not manifest with an LQTS phenotype in a majority of individuals.
QT-prolongation and arrhythmic risk associated with Type 2 Jervell and Lange-Nielsen syndrome is mild in comparison with the more malignant phenotype observed for Type 1 Jervell and Lange-Nielsen syndrome.
What are the clinical implications ?
All individuals possessing a rare loss-of-function KCNE1 variant should be counseled to avoid QT-prolonging medication and undergo a meticulous clinical evaluation to screen for an LQTS phenotype
In the absence of an LQTS phenotype, more intensive measures such as β-blockade and exercise restriction may not be merited.
Acknowledgments
Funding Sources
J.D.R. is supported by the Marianne Barrie Philanthropic Fund, the Canadian Institutes of Health Research, and the Heart and Stroke Foundation of Canada, J.M.B., J.R.G., and M.J.A. are supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program, A.D.K. receives support from the Sauder Family and Heart and Stroke Foundation Chair in Cardiology (Vancouver, British Columbia, Canada), the Paul Brunes Chair in Heart Rhythm Disorders (Vancouver, British Columbia, Canada) and the Paul Albrechtson Foundation (Winnipeg, Manitoba, Canada), the Heart and Stroke Foundation of Canada (G-14-0005732), and the Canadian Institutes of Health Research (MOP-142218 and SRG-15-P09-001; Ottawa, Ontario, Canada), T.A. and W.S. acknowledge support from a Health Science Research Grant from the Ministry of Health, Labor and Welfare of Japan for Clinical Research on Measures for Intractable Diseases (H24-033, H26-040, and H27-032), A.P.L is supported by National Institutes of Health (K08-HL136839), Centers for Disease Control and Prevention (5NU50-DD004933), and Duke MEDx, A.A.M.W. acknowledges support from the Netherlands CardioVascular Research Initiative, the Dutch Heart Foundation, the Dutch Federation of University Medical Centres, the Netherlands Organisation for Health Research and Development, and the Royal Netherlands Academy of Sciences (Predict2 to A.A.M.W.), E.R.B receives research funds from the Robert Lancaster Memorial Fund, sponsored by McColl’s Retail Group, and P.J.S. is supported by the Leducq Foundation for Cardiovascular Research grant 18CVD05 “Towards Precision Medicine with Human iPSCs for Cardiac Channelopathies”.
Non-standard Abbreviations and Acronyms:
- LQTS
long QT syndrome
- SCD
sudden cardiac death
- JLNS2
Type 2 Jervell and Lange-Nielsen syndrome
- gnomAD
Genome Aggregation Database
- ACMG
American College of Medical Genetics and Genomics
- ICD
implantable cardioverter defibrillator
- ACA
aborted cardiac arrest
- PolyPhen-2
Polymorphism Phenotyping v2
- SIFT
Sorting Intolerant From Tolerant
- CADD
Combined Annotation Dependent Depletion
- IQR
interquartile range
- CI
confidence intervals
- HR
hazard ratio
Footnotes
Disclosures
None.
References
- 1.Schwartz PJ, Crotti L, Insolia R. Long-QT syndrome: from genetics to management. Circ Arrhythm Electrophysiol. 2012;5:868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzanti A, Maragna R, Priori S. Genetic causes of sudden cardiac death in the young. Current Opinion in Cardiology. 2017;32:253–261. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz PJ, Ackerman MJ, Wilde AAM. Channelopathies as causes of sudden cardiac death. Card Electrophysiol Clin. 2017;9:537–549. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, Gabbarini F, Goulene K, Insolia R, Mannarino S, Mosca F, Nespoli L, Rimini A, Rosati E, Salice P, Spazzolini C. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. [DOI] [PubMed] [Google Scholar]
- 6.Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. [DOI] [PubMed] [Google Scholar]
- 7.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. [DOI] [PubMed] [Google Scholar]
- 9.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. [DOI] [PubMed] [Google Scholar]
- 10.Splawski I, Tristani-Firouzi M, Lehmann MH, Sanguinetti MC, Keating MT. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat Genet. 1997;17:338–340. [DOI] [PubMed] [Google Scholar]
- 11.McDonald TV, Yu Z, Ming Z, Palma E, Meyers MB, Wang KW, Goldstein SA, Fishman GI. A minK-HERG complex regulates the cardiac potassium current I(Kr). Nature. 1997;388:289–292. [DOI] [PubMed] [Google Scholar]
- 12.Nishio Y, Makiyama T, Itoh H, Sakaguchi T, Ohno S, Gong Y-Z, Yamamoto S, Ozawa T, Ding W-G, Toyoda F, Kawamura M, Akao M, Matsuura H, Kimura T, Kita T, Horie M. D85N, a KCNE1 polymorphism, is a disease-causing gene variant in long QT syndrome. J Am Coll Cardiol. 2009;54:812–819. [DOI] [PubMed] [Google Scholar]
- 13.Weeke P, Mosley JD, Hanna D, Delaney JT, Shaffer C, Wells QS, Van Driest S, Karnes JH, Ingram C, Guo Y, Shyr Y, Norris K, Kannankeril PJ, Ramirez AH, Smith JD, Mardis ER, Nickerson D, George AL, Roden DM. Exome sequencing implicates an increased burden of rare potassium channel variants in the risk of drug-induced long QT interval syndrome. J Am Coll Cardiol. 2014;63:1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts JD, Krahn AD, Ackerman MJ, Rohatgi RK, Moss AJ, Nazer B, Tadros R, Gerull B, Sanatani S, Wijeyeratne YD, Baruteau A-E, Muir AR, Pang B, Cadrin-Tourigny J, Talajic M, Rivard L, Tester DJ, Liu T, Whitman IR, Wojciak J, Conacher S, Gula LJ, Leong-Sit P, Manlucu J, Green MS, Hamilton R, Healey JS, Lopes CM, Behr ER, Wilde AA, Gollob MH, Scheinman MM. Loss-of-function KCNE2 variants: True monogenic culprits of long-QT syndrome or proarrhythmic variants requiring secondary provocation? Circ Arrhythm Electrophysiol. 2017;10:e005282. [DOI] [PubMed] [Google Scholar]
- 15.Giudicessi JR, Roden DM, Wilde AAM, Ackerman MJ. Classification and reporting of potentially proarrhythmic common genetic variation in long QT syndrome genetic testing. Circulation. 2018;137:619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbott GW. The KCNE2 K+ channel regulatory subunit: Ubiquitous influence, complex pathobiology. Gene. 2015;569:162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulze-Bahr E, Wang Q, Wedekind H, Haverkamp W, Chen Q, Sun Y, Rubie C, Hördt M, Towbin JA, Borggrefe M, Assmann G, Qu X, Somberg JC, Breithardt G, Oberti C, Funke H. KCNE1 mutations cause Jervell and Lange-Nielsen syndrome. Nat Genet. 1997;17:267–268. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz PJ, Spazzolini C, Crotti L, Bathen J, Amlie JP, Timothy K, Shkolnikova M, Berul CI, Bitner-Glindzicz M, Toivonen L, Horie M, Schulze-Bahr E, Denjoy I. The Jervell and Lange-Nielsen syndrome: natural history, molecular basis, and clinical outcome. Circulation. 2006;113:783–790. [DOI] [PubMed] [Google Scholar]
- 19.Bianchi L, Shen Z, Dennis AT, Priori SG, Napolitano C, Ronchetti E, Bryskin R, Schwartz PJ, Brown AM. Cellular dysfunction of LQT5-minK mutants: abnormalities of IKs, IKr and trafficking in long QT syndrome. Hum Mol Genet. 1999;8:1499–1507. [DOI] [PubMed] [Google Scholar]
- 20.Schulze-Bahr E, Schwarz M, Hauenschild S, Wedekind H, Funke H, Haverkamp W, Breithardt G, Pongs O, Isbrandt D, Hoffman S. A novel long-QT 5 gene mutation in the C-terminus (V109I) is associated with a mild phenotype. J Mol Med. 2001;79:504–509. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz PJ, Priori SG, Locati EH, Napolitano C, Cantù F, Towbin JA, Keating MT, Hammoude H, Brown AM, Chen LS, Colatsky TJ. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation. 1995;92:3381–3386. [DOI] [PubMed] [Google Scholar]
- 22.Mazzanti A, Maragna R, Vacanti G, Monteforte N, Bloise R, Marino M, Braghieri L, Gambelli P, Memmi M, Pagan E, Morini M, Malovini A, Ortiz M, Sacilotto L, Bellazzi R, Monserrat L, Napolitano C, Bagnardi V, Priori SG. Interplay between genetic substrate, QTc duration, and arrhythmia risk in patients with long QT syndrome. J Am Coll Cardiol. 2018;71:1663–1671. [DOI] [PubMed] [Google Scholar]
- 23.Mazzanti A, Maragna R, Faragli A, Monteforte N, Bloise R, Memmi M, Novelli V, Baiardi P, Bagnardi V, Etheridge SP, Napolitano C, Priori SG. Gene-specific therapy with mexiletine reduces arrhythmic events in patients with long QT syndrome Type 3. J Am Coll Cardiol. 2016;67:1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bos JM, Crotti L, Rohatgi RK, Castelletti S, Dagradi F, Schwartz PJ, Ackerman MJ. Mexiletine shortens the QT Interval in patients with potassium channel-mediated Type 2 long QT syndrome. Circ Arrhythm Electrophysiol. 2019;12:e007280. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz PJ, Gnecchi M, Dagradi F, Castelletti S, Parati G, Spazzolini C, Sala L, Crotti L. From patient-specific induced pluripotent stem cells to clinical translation in long QT syndrome Type 2. Eur Heart J. 2019;40:1832–1836. [DOI] [PubMed] [Google Scholar]
- 26.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won H-H, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG, Exome Aggregation Consortium. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lane CM, Giudicessi JR, Ye D, Tester DJ, Rohatgi RK, Bos JM, Ackerman MJ. Long QT syndrome type 5-Lite: Defining the clinical phenotype associated with the potentially proarrhythmic p.Asp85Asn-KCNE1 common genetic variant. Heart Rhythm. 2018;15:1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–1081. [DOI] [PubMed] [Google Scholar]
- 31.Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adler A, Novelli V, Amin A, Abiusi E, Care M, Nannenberg E, Feillotter H, Amenta S, Mazza D, Bikker H, et al. An international, multicentered evidence-based reappraisal of genes reported to cause congenital long QT syndrome. Circulation In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locati EH, MacCluer J, Hall WJ, Weitkamp L, Vincent GM, Garson A. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991;84:1136–1144. [DOI] [PubMed] [Google Scholar]
- 34.Kutyifa V, Daimee UA, McNitt S, Polonsky B, Lowenstein C, Cutter K, Lopes C, Zareba W, Moss AJ. Clinical aspects of the three major genetic forms of long QT syndrome (LQT1, LQT2, LQT3). Ann Noninvasive Electrocardiol. 2018;23:e12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang C-E, Huikuri H, Kannankeril P, Krahn A, Leenhardt A, Moss A, Schwartz PJ, Shimizu W, Tomaselli G, Tracy C. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.