Abstract
We report on the case of an 8-year-old Mexican male, with a 3-year-old clinical diagnosis of familial hypercholesterolemia, and the difficulties encountered in his treatment while in our care. His treatment started with a regimen consisting of ezetimibe/simvastatin, cholestyramine, and a dietary plan of 1600 calories, with a limited intake of 200 mg of cholesterol per day. Problems arose when the patient’s low-density lipoprotein cholesterol (LDL) levels did not meet ideal targets, which prompted the use of LDL cholesterol apheresis (not available in Mexico) for 6 months. As a last resort, PCSK9 inhibitors were administered but the LDL levels remained in the 600 mg/dL range. AmbryGenetics conducted a genetic test employing the Sanger method. The results suggested that there were 2 different mutations for each allele of the same LDL receptor gene (c.249delTinsGG and p.(Cys109Arg)), located in exons 3 and 4, respectively. We identified compound heterozygous mutations in our index case, with him having both the p.C109R mutation (from the maternal lineage), as well as a c.249delTinsGG mutation (from the paternal lineage). The p.C109R mutation has been previously reported, not only in Mexico, but in European regions (Germany, Czech Republic, Ireland, Italy) as well. Functional studies indicated a residual enzymatic activity of 15% to 30% for heterozygotes. To date, the variant c.249delTinsGG has not been reported. This case study illustrates the fact that in Mexico there are limited options available for treatment in such a scenario. As medical professionals, we are limited by the tools at our disposal.
Keywords: familial hypercholesterolemia, premature cardiovascular disease, protein convertase subtilisin, kexin type 9 gene, LDL receptor gene, familial heterozygous hypercholesterolemia, familial homozygous hypercholesterolemia
1. Introduction
Familial hypercholesterolemia (FH) is the most frequent monogenic disease associated with premature cardiovascular disease, resulting from extremely elevated concentrations of low-density lipoprotein cholesterol (LDL-C) from birth (1,2). FH has a clinical distinction based on phenotype severity consisting of heterozygous (heFH), which presents with a mild form of the disease (LDL-C levels 200–400 mg/dL) and homozygous (hoFH), which presents with severe disease phenotype (LDL-C levels > 600 mg/dL) (2). FH is caused by three mutations in the LDLR (19p13.2), APOB (2p24.1), and/or PCSK9 (1p32.3) genes, respectively (2). Mutations in the LDLR gene (autosomal dominant hypercholesterolemia) are the major cause of FH (2), with more than 2000 mutation variants distributed throughout the gene, which can cause an alteration in the function or a decrease in the number of receptors that internalize LDL-C (3–6). The extremely rare recessive form of hypercholesterolemia (autosomal recessive hypercholesterolemia) (2) is due to a mutation in the LDLRAP1, which is located on chromosome 1p36-35 and a mutation in SLCO1B1, located on chromosome 12p12.1. SLCO1B1 forms part of a family of solute carrier organic anion transporters also known as organic transporting polypeptides. They constitute a major influx transporter family responsible for the uptake of a broad range of drugs (7).
With the advances made in identifying the molecular basis of FH, patients with clinical and biochemical characteristics of hoFH are classified into 3 groups: true homozygotes, compound heterozygotes, and heterozygous doubles (8, 9). The true homozygotes are those that present the same mutation in each of the 2 alleles of the same gene, either in the LDLR, APOB, or PCSK9 gene. The compound heterozygotes show different variations in each of the 2 alleles of the same gene. Finally, heterozygous doubles are those that present one of the two alleles mutated in one gene, and another of the 2 alleles mutated in another gene; for example, mutation “A” in the LDLR gene and mutation “B” in the APOB gene (9).
This work present a clinical case with a severe form of the disease, a male patient with clinical and biochemical manifestations, and confirmed mutations at a molecular level that correlate to compound heterozygous FH. The results obtained by studying the family are presented as well. This case study attempts to highlight the challenges in providing an appropriate treatment with the tools at our disposal in Mexico.
2. Case Report
The patient was initially evaluated at age 5, because of subcutaneous tumors of semisoft consistency, compatible with xanthomas, present in the extensor tendons of the fingers, both elbows, the right popliteal gap, and in the right Achilles tendon. He is a native and resident of Mexico City, the product of a second pregnancy with no complications. His father is 36 years old, born in Puebla, and his mother is 34 years old, originally from Estado de Mexico. Both parents were in good health and noconsanguineous. The index case has a brother, 4 years older than him, with physical stigmata of FH.
At the time of his initial evaluation at the age of 5 years and 11 months, the physical examination revealed a normal weight of 21.8 kg (73rd percentile), height of 118 cm (79th percentile, and a body mass index of 15.7 kg/m2 (60th percentile). His lipid profile was as follows: total cholesterol 842 mg/dL, LDL-C 799 mg/dL, high-density lipoprotein (HDL-C) 33 mg/dL, and triglycerides 96 mg/dL. He was started on a daily regimen of ezetimibe/simvastatin 20/40 mg, a twice daily regimen of 4 g of cholestyramine, and a dietary plan consisting of 1600 calories with a limited intake of 200 mg of cholesterol per day.
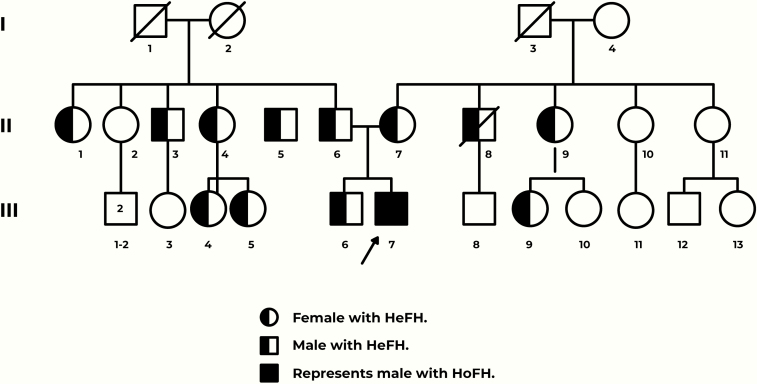
Secondary causes of hypercholesterolemia were ruled out indicating that the patient was most likely afflicted with HoFH. This prompted a cascade of tests for the parents and a comparison of their lipid profiles to other first and second relatives. (Fig. 1; Table 1).
Figure 1.
Graphical representation of the family tree. Arrow depicts the case study (III-7).
Table 1.
Lipid report of the family tree
| Patient | Age | Cholesterol | LDL | HDL | TGS | Glu | |
|---|---|---|---|---|---|---|---|
| II | 1 | 46 | 330 | 257 | 50 | 109 | 77 |
| 3 | 41 | 267 | 182 | 59 | 125 | 155 | |
| 4 | 37 | 353 | 248 | 35 | 342 | 85 | |
| 5 | 32 | 434 | 317 | 40 | 382 | 89 | |
| 6 (Father) | 39 | 456 | 362 | 44 | 245 | 89 | |
| 7 (Mother) | 37 | 323 | 266 | 39 | 84 | 90 | |
| 8 | 52 | 388 | 259.5 | 58.3 | 351 | 348 | |
| 9 | 50 | 200 | 125 | 34 | 202 | 88 | |
| III | 4 | 19 | 325 | 261.2 | 47 | 84 | 74 |
| 5 | 15 | 141 | 87.1 | 42.9 | 55 | 78 | |
| 6 (Brother) | 12 | 312 | 244 | 45 | 107 | 78 | |
| 7 (Case index) | 8 | 842 | 799 | 33 | 96 | 76 | |
| 9 | 10 | 346 | 285 | 39 | 106 | 84 |
Column 1 represents the generations of the family. Column 2 is correlated to the number assigned in the graphical representation of the family tree (see Fig. 1). Note the case study highlighted. All units are presented in mg/dL.
Abbreviations: Glu, glucose. HDL, high-density lipoprotein; LDL, low-density lipoprotein; TGS, triglycerides.
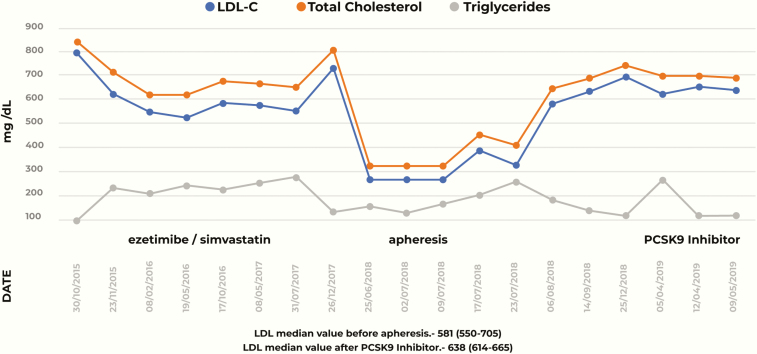
At follow-up 7 months later, the patient’s lipid profile demonstrated a 34% decrease in LDL-C from baseline levels (527 mg/dL) (P = .42) (Fig. 2). Due to recurrent episodes of gastrointestinal intolerance, administration of cholestyramine was suspended. Doppler ultrasonography to assess the condition of both arterial carotids demonstrated a right common carotid stenosis of 21% and a left common carotid stenosis of 20%. The remainder of the vascular structures were normal. An echocardiogram was also carried out, with no observed pathological alterations.
Figure 2.
Lipid profile of the patient (total cholesterol, low-density lipoprotein cholesterol, and triglycerides) through time.
Molecular analysis of the index case was carried out by AmbryGenetics using an FHNext test (https://www.ambrygen.com/clinician/genetic-testing/13/cardiology/fhnext), which consists of the study of 4 genes associated with familial hypercholesterolemia. The results demonstrated the presence of two mutations in the LDLR gene, specifically in exons 3 (c.249delTinsGG) and 4 (p. P109R), which encode the ligand-binding domain.
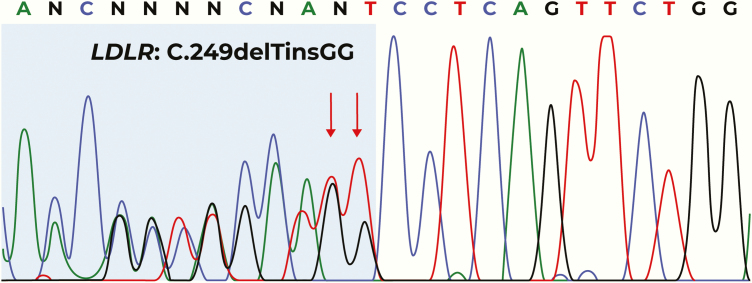
A cascade screening of both parents showed the presence of mutations. The mother was screened using a modified CTAB/DTAB method. Molecular analysis revealed a change in proline to arginine in exon 4; the position of the altered nucleotide was determined to be c.325 T > C, leading to the mutation p. P109R in heterozygous state. The father was screened using the Sanger method, revealing the c.249delTinsGG mutation in heterozygous state (Fig. 3). Both parents started statin treatment immediately, only to be suspended due to intolerance. With no other option available, both parents were administered PCSK9 inhibitors. The father was treated with the anti-PCKSK9 antibody, evolocumab, which reduced serum LDL-C by 60%. The mother was treated with the anti-PCKSK9 antibody, alirocumab, and achieved the desired treatment goals. The brother was treated with a combination of lipid-lowering drugs, ezetimibe and simvastatin (10 mg and 20 mg, respectively), achieving the treatment objectives.
Figure 3.
Partial electropherogram of the LDLR gene, of the father’s DNA sample. The red arrows indicate the position affected by the pathogenic variant NM_000527.4 (LDR_v001).—c249delinsGG in a heterozygous state.
After a year of treatment with ezetimibe/simvastatin 20/40 mg daily and lifestyle changes, the patient’s LDL-C levels did not reach the therapeutic goal of 50% from baseline. This prompted the use of apheresis. The patient received LDL apheresis in the United States but could not continue beyond 6 months because of the lack of this treatment in Mexico.
With no other option available, the medical team decided to allow the compassionate use of PCSK9 inhibitors in addition to previous therapy. The index case started evolocumab therapy with a dose of 140 mg administered subcutaneously every 15 days by a heath professional, starting in July 2018. The results were unsatisfactory (patient’s LDL-C levels were 640 mg/dL); there was a transient decrease in LDL-C levels for 1 month, only to return to similar values in a follow-up 2 months after this decision. Because of suboptimal response to LDL reduction, the patient was started on a weekly regimen of alirocumab (150 mg) along with a daily regimen of ezetimibe/rosuvastatin 10/20 mg, since LDL apheresis is currently unavailable in Mexico.
3. Discussion
Unlike heterozygous FH, which is a common disease, HoFH is considered rare, affecting 1 in 300 000 to 600 000 individuals of the general population (9–11). In Latin America, the prevalence of FH is unknown (12). Mehta et a performed a systematic review of all published data regarding FH in Latin American populations. They emphasized the significant gap in studies of FH in Latin America and the need for prospective randomized trials (12). In Mexico, it is estimated that at least 200 affected patients exist; however, most of them have not been diagnosed or treated. Martinez et al provided evidence for 16 cases with the same diagnosis; most of them belonging to communities that are geographically isolated and commonly practice endogamy (13).
The index case was categorized as compound heterozygous, having both the p.(Cys109Arg) mutation, as well as a c.249delTinsGG mutation. The p.(Cys109Arg) mutation has been previously reported, not only in Mexico, but in European regions as well (Germany, Czech Republic, Ireland, Italy). This mutation is a common class 2B mutation that affects transport between the endoplasmic reticulum and the Golgi apparatus (transport-defective alleles) (6). Functional studies report a residual enzymatic activity of 15% to 30% for heterozygotes (8,13). The variant c.249delTinsGG mutation has not yet been reported in any database (10–11).
The American Academy of Pediatrics recommends starting treatment as soon as possible, preferably after 2 years of age, because of the high risk of premature death (14). The National Lipid Association recommends a therapeutic upper limit for therapeutic atherogenic cholesterol goal ranges for managing children and adolescents of 144 mg/dL for non- high-density lipoprotein cholesterol and 129 mg/dL for LDL-C. However, in patients with FH for whom these targets are unachievable, an atherogenic cholesterol reduction of 50% should be the target. (15). In this case, the patient was diagnosed with compound heterozygous FH at the age of 6, which translates to cardiovascular disease risk in the teens. Monoclonal antibodies to PCSK9 are useful for homozygous patients of pediatric age (4, 16–17); however, since the regulatory agencies have not yet approved use of these drugs, their compassionate use is accepted. In this case, even with the use of these monoclonal antibodies, patient LDL-C did not meet the therapeutic target of 129 mg/dL or a 50% reduction from baseline values. Comparing our case report to other studies that use evolocumab, the treatment response did not achieve the expected goals, this may be due to lack of residual LDL receptor activity (18). The statistical analysis of the median of the LDL-C levels in the 3 different moments in the case showed that before the apheresis (treatment with ezetimibe/simvastatin), and during the PCSK9 inhibitor time, the difference between them is 0 (P = .345). However, during the time of apheresis, it had a significant LDL-C level reduction (*P = .042 and P = .43 when comparing apheresis and ezetimibe/simvastatin/PCSK9 inhibitors, respectively). Additional therapeutic measures, particularly LDL-C apheresis may prove beneficial; however, this technology is not currently available in Mexico (9, 19), making it difficult to provide a conclusive solution to the problem at hand.
Acknowledgments
Special thanks to Alfredo Hernández-Moreno on language and writing assistance.
Funding Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors Contributions: CMJJ, MPR, JAFR, JVS, and GOG collected the data and wrote the article, VCA and ACS contributed to reviewing the article. TLMT contributed to the reception of the index case sample and genetic analysis.
Additional Information
Disclosure statement: The authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Mata P, Alonso R, Ruiz A, et al. ; Otros Colaboradores [Diagnosis and treatment of familial hypercholesterolemia in Spain: consensus document]. Aten Primaria. 2015;47(1):56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hovingh GK, Davidson MH, Kastelein JJ, O’Connor AM. Diagnosis and treatment of familial hypercholesterolaemia. Eur Heart J. 2013;34(13):962–971. [DOI] [PubMed] [Google Scholar]
- 3. Fouchier SW, Kastelein JJ, Defesche JC. Update of the molecular basis of familial hypercholesterolemia in The Netherlands. Hum Mutat. 2005;26(6):550–556. [DOI] [PubMed] [Google Scholar]
- 4. Robles-Osorio L, Huerta-Zepeda A, Ordóñez ML, et al. . Genetic heterogeneity of autosomal dominant hypercholesterolemia in Mexico. Arch Med Res. 2006;37(1):102–108. [DOI] [PubMed] [Google Scholar]
- 5. Blesa S, Vernia S, Garcia-Garcia AB, et al. . A new PCSK9 gene promoter variant affects gene expression and causes autosomal dominant hypercholesterolemia. J Clin Endocrinol Metab. 2008;93(9):3577–3583. [DOI] [PubMed] [Google Scholar]
- 6. Hobbs HH, Brown MS, Goldstein JL. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat. 1992;1(6):445–466. [DOI] [PubMed] [Google Scholar]
- 7. Kim TE, Shin D, Gu N, et al. . The effect of genetic polymorphisms in SLCO2B1 on the lipid-lowering efficacy of rosuvastatin in healthy adults with elevated low-density lipoprotein. Basic Clin Pharmacol Toxicol. 2017;121(3):195–201. [DOI] [PubMed] [Google Scholar]
- 8. Ascaso JF, Mata P, Arbona C, Civeira F, Valdivielso P, Masana L. Hipercolesterolemia familiar homocigota: adaptación a España del documento de posición del grupo de consenso sobre hipercolesterolemia familiar de la Sociedad Europea de Arteriosclerosis: documento de consenso de la Sociedad Española de Arteriosclerosis (SEA) y la Fundación Hipercolesterolemia Familiar (FHF). Clin Invest Arterioscl. 2015; 27(2): 80–96. 10.1016/j.arteri.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 9. Ito MK, Watts GF. Challenges in the diagnosis and treatment of homozygous familial hypercholesterolemia. Drugs. 2015;75(15):1715–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varret M, Rabés JP, Thiart R, et al. . LDLR Database (second edition): new additions to the database and the software, and results of the first molecular analysis. Nucleic Acids Res. 1998;26(1):248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Villéger L, Abifadel M, Allard D, et al. . The UMD-LDLR database: additions to the software and 490 new entries to the database. Hum Mutat. 2002;20(2):81–87. [DOI] [PubMed] [Google Scholar]
- 12. Mehta R, Zubirán R, Martagón AJ, et al. . The panorama of familial hypercholesterolemia in Latin America: a systematic review. J Lipid Res. 2016;57(12):2115–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martínez L, Ordóñez Sánchez ML, Letona R, et al. . [Familial homozygous hypercholesterolemia due to the c2271delT mutation in the LDL receptor gene, detected exclusively in Mexicans]. Gac Med Mex. 2011;147(5):394–398. [PubMed] [Google Scholar]
- 14. Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, Grunberger G, Guerin CK, Bell DSH, Mechanick JI, Pessah-Pollack R, Wyne K, Smith D, Brinton EA, Fazio S, Davidson M. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocrine Practice. 2017;23(Suppl 2):1–87. 10.4158/EP171764.APPGL [DOI] [PubMed] [Google Scholar]
- 15. Jacobson TA, Ito MK, Maki KC, et al. . National lipid association recommendations for patient-centered management of dyslipidemia: Part 1—Full report. J Clin Lipidol. 2015; 9(2): 129–169. 10.1016/j.jacl.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 16. Watts GF, Gidding S, Wierzbicki AS, et al. . Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation. Int J Cardiol. 2014;171(3):309–325. [DOI] [PubMed] [Google Scholar]
- 17. Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res. 2009;50 Suppl:S172–S177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raal FJ, Honarpour N, Blom DJ, et al. ; TESLA Investigators Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):341–350. [DOI] [PubMed] [Google Scholar]
- 19. Kastelein JJ, Besseling J, Shah S, et al. . Anacetrapib as lipid-modifying therapy in patients with heterozygous familial hypercholesterolaemia (REALIZE): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet. 2015;385(9983):2153–2161. [DOI] [PubMed] [Google Scholar]