Abstract
The zona incerta has recently become an important target for deep-brain stimulation (DBS) in Parkinson’s disease (PD). The present review summarizes clinical, animal and anatomical data which have indicated an important role of this structure in PD, and discusses potential mechanisms involved in therapeutic effects of DBS. Animal studies have suggested initially some role of neurons as well as GABAergic and glutamatergic receptors of the zona incerta in locomotion and generation of PD signs. Anatomical data have indicated that thanks to its multiple interconnections with the basal ganglia, thalamus, cerebral cortex, brainstem, spinal cord and cerebellum, the zona incerta is an important link in a neuronal chain transmitting impulses involved in PD pathology. Finally, clinical studies have shown that DBS of this structure alleviates parkinsonian bradykinesia, muscle rigidity and tremor. DBS of caudal zona incerta seemed to be the most effective therapeutic intervention, especially with regard to reduction of PD tremor as well as other forms of tremor.
Keywords: Deep-brain stimulation, Zona incerta, Parkinson’s disease, Animal studies, Anatomical studies, Clinical studies
Introduction
It is generally accepted that motor signs of Parkinson’s disease (PD) akinesia, bradykinesia, muscle rigidity and tremor are related to the loss of dopamine in the striatum (putamen and caudate nucleus) due to degeneration of the dopaminergic nigrostriatal pathway arising from the substantia nigra pars compacta (SNc) [1]. In line with this view, a dopamine precursor, levodopa, became a gold standard of antiparkinsonian therapy [2, 3]. However, after a few years of treatment with this drug extremely troublesome side effects, i.e. dyskinesias and on–off phenomenon appear. Introduction of deep-brain stimulation (DBS) to the therapy of PD patients with advanced disease demonstrating strong uncontrollable levodopa-induced motor complications allowed for alleviation of parkinsonian signs as well as reduction of levodopa doses and side effects [3]. DBS of a few brain regions, i.e. ventral intermediate nucleus (Vim) [a synonym of posterior portion of the ventrolateral nucleus (VLp)] of the thalamus, ventrolateral region of the internal segment of the globus pallidus (GPi), the subthalamic nucleus (STN) and tegmental pedunculopontine nucleus (PPN) have been found to be beneficial in PD [4–9]. Among these structures, the STN is currently the most frequently chosen target for this procedure [4, 6, 9]. However, a number of recent clinical data have indicated that the zona incerta (ZI) and white matter located in the vicinity of the STN may be an alternative or concomitant target for DBS [10].
Cortico-basal ganglia-thalamo-cortical network and DBS of the STN
It is generally accepted that voluntary movements are initiated in the motor cortex which sends projections to the brainstem, spinal cord and subcortical targets (the basal ganglia). According to the classic model, the role of the basal ganglia output pathways to the thalamus is to facilitate or suppress movements by controlling information transmitted via the thalamocortical projection to the cortex. The basal ganglia-induced inhibition of the thalamic neurons has been suggested to be related to reduction of movements, while their disinhibition—with movement activation [11].
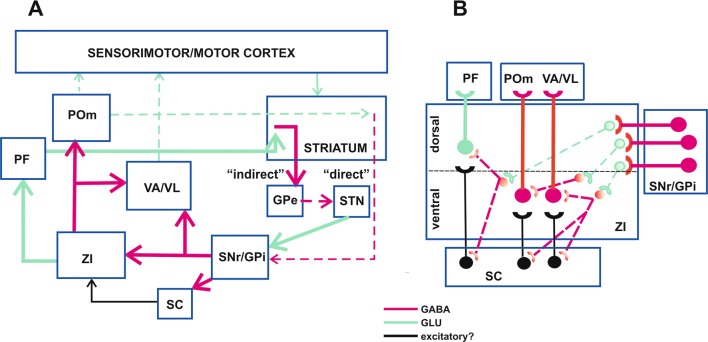
Degeneration of dopaminergic nigrostriatal pathway, arising from the SNc leads to a number of functional alterations in structures belonging to the above cortico-basal ganglia-thalamo-cortical neuronal loop which underlie motor signs of PD [6, 11–14] (Fig. 1). Its first link, i.e. the corticostriatal glutamatergic projection in humans starts from the precentral motor region (primary motor cortex, premotor cortex and supplementary cortex) and postcentral somatosensory cortex, and terminates largely in the putamen where it switches to medium-spiny GABAergic neurons which form two striatal efferents: the “direct” pathway leading to the substantia nigra pars reticulata (SNr) and ventrolateral region of the GPi, and the “indirect” pathway going to the external segment of the globus pallidus (GPe). The next link of the “indirect” pathway, i.e. the GABAergic pallidosubthalamic projection inhibits glutamatergic neurons of the STN which form subthalamonigral (leading to the SNr) and subthalamopallidal (leading to the GPi) pathways. Finally, glutamate released from terminals of these pathways activates GABAergic neurons of the SNr and GPi which send their axons to the motor thalamus, i.e. to the ventroanterior (VA) nucleus and anterior portion of the ventrolateral nucleus (VL), respectively. It has been suggested that the pallidothalamic pathway is involved in sequencing and execution of movements, while the nigrothalamic projection contributes to planning of movements. GABAergic basal ganglia-thalamic pathways, in turn, inhibit glutamatergic thalamocortical projections which close the cortico-basal ganglia-thalamo-cortical neuronal loop [6, 11–14].
Fig. 1.
A “classic” model of neuronal activity in the cortico-basal ganglia-thalamo-cortical circuit in Parkinson’s disease (PD) according to DeLong [13] (modified). Bold arrows indicate activated neuronal pathways, dashed arrows indicate inhibited neuronal pathways. Due to dopaminergic deficiency in PD, the “indirect” GABAergic pathway from the putamen (a part of the striatum) to GPe is disinhibited which results in inhibition of the GABAergic pathway from the GPe to the STN, and disinhibition of the glutamatergic route projecting from the STN to the SNr/GPi. On the other hand, loss of dopamine leads to decreased activation of the “direct” GABAergic pathway projecting from the putamen to the SNr/GPi. Finally, glutamatergic activation of the SNr/GPi prevails over GABAergic inhibition of these structures, which leads to activation of their output GABAergic pathways going to the VA/VL nuclei of the thalamus. As a result, glutamatergic efferents of these thalamic nuclei to the sensorimotor/motor cortex are inhibited. Brain structures: GPe the external segment of the globus pallidus, GPi the internal segment of the globus pallidus, SNr the substantia nigra pars reticulata, STN the subthalamic nucleus, VA/VL ventroanterior/ventrolateral nuclei of the thalamus. Neuronal pathways: GABA GABAergic pathway, GLU glutamatergic pathway
Several lines of evidence have indicated that the loss of dopamine in PD results in an imbalance between the “indirect” and “direct” pathways, i.e. the “indirect” pathway is released from inhibition and the activation of the “direct” pathway is reduced. That leads first to disinhibition of the STN neurons, then to activation of GABAergic basal ganglia output pathways which finally results in hyperinhibition of thalamocortical neurons [6, 11–14] (Fig. 1).
It must be stressed, however, that this model of PD-related neuronal network is, in fact, much more complicated and includes additionally some other connections, i.e. GABAergic projections from the SNr to the superior colliculus, PPN and intralaminar thalamic nuclei, and from the GPe to SNr and GPi, as well as glutamatergic projections from the cerebral cortex to the STN and thalamic nuclei, from the STN to the GPe, and others [6, 11, 13, 15]. Moreover, this model is not sufficient to explain pathomechanisms of PD tremor which seem to be distinct from those underlying bradykinesia and rigidity. According to recent studies, the tremor-related mechanisms include increased interaction between the cerebello-thalamo-cortical network and the basal ganglia [6, 16, 17]. In fact, the subcortical nuclei of the cerebellum communicate transsynaptically with the putamen and GPe via motor and intralaminar nuclei of the thalamus [18, 19] while the STN sends di-synaptic connections to the cerebellar cortex [20, 21]. Although a “pacemaker” of parkinsonian tremor has not been identified, yet, it has been suggested that “the basal ganglia network triggers the onset of tremor and the cerebellar network is responsible for maintaining the tremor rhythm and its amplification” [16, 17].
In line with the above model, pathological excitation of the STN neurons in PD patients or parkinsonian monkeys, characterized by irregular and bursty pattern or periodic oscillatory bursts has been suggested to be associated to akinesia/muscle rigidity and parkinsonian tremor, respectively [22–24]. This view was supported by animal studies which showed that lesions of this region in parkinsonian monkeys [25–27] and accidental hematoma in PD patient dramatically reduced akinesia, tremor and muscle rigidity [28]. These studies inspired researchers to introduce DBS of the STN, first in parkinsonian monkeys [29], then in PD patients [30]. Spectacular therapeutic results of these studies (reduction of PD signs and lack of dyskinesias) [29, 30] initiated a new age of surgical interventions in PD and DBS of the STN was approved by the United States Food & Drug Administration (FDA) in 2001 [4]. Since that time thousands of DBS of the STN carried out all over the world have supported a significant long-term therapeutic efficacy of this procedure.
The STN is functionally and anatomically divided into three subdivisions: motor (dorsolateral), associative (ventromedial) and limbic (medial) with separate input and output pathways, although, it remains unclear to what extent these regions overlap [31, 32]. According to this functional division, the dorsolateral part of this structure is usually chosen for DBS in PD which results in reduction of bradykinesia, muscle rigidity and tremor [10, 33].
However, according to some studies DBS of the anterior dorsolateral border of the STN [34, 35] or the interface between this region and ZI and thalamic fasciculus located dorsally to the STN [36] are equally [34, 36] or even more effective than DBS inside the STN [35].
Active target for DBS outside the STN: the ZI
The first evidence of the role of the ZI in PD came from the paper of Mundinger [37] who observed that high-frequency coagulation of a region which included the caudal ZI, H1/H2 Forel’s fields and partly prelemniscal radiation and nucleus ruber was highly effective in ameliorating muscle rigidity and tremor in PD patients. Similarly, Patel and coworkers [38] reported a strong antiparkinsonian, mainly tremorolytic, effect as a result of a lesion of the ZI/H2 region. Moreover, patients with a selective dorsolateral subthalamotomy demonstrated less clinical benefit than those with a combined lesion which included both the STN and the dorsally adjoining ZI/H2 region [38].
Research which compared clinical potency of DBS at different anatomical electrode locations in the STN and its surroundings has shown that stimulation of the region localized dorsally to this structure which included the ZI and/or H1/H2 Forel’s fields was weaker, equal or stronger than that of the STN.
While Herzog et al. [34] reported that DBS of the ZI and H1/H2 (located > 1.5 mm dorsally to the STN) was beneficial, its clinical effect in comparison to the STN appeared only suboptimal. Similarly, Welter et al. [39] claimed that “the best motor outcome was obtained when stimulating contacts were located within the STN as compared with the ZI”. Moreover, de Chazeron et al. [40] found that when both DBS contacts were located inside the STN or one inside the STN and the second one at its superior border, the motor improvement was better than when both contacts were localized outside, viz. superolaterally to this structure, presumably in the ZI. According to Yokoyama et al., [41] stimulation delivered dorsally to the STN to the rostral ZI/ pallidofugal fibres was as effective as stimulation inside the STN. Similarly, Henderson et al. [42] observed that when electrodes were positioned bilaterally: one at the border of the thalamus and ZI, and the second inside the STN, reduction of tremor, muscle rigidity and bradykinesia induced by DBS was similar on both sides of the body.
Voges and coworkers [43] and Lanotte et al. [33] were the first who claimed advantage of DBS of regions located dorsally to the STN. Voges et al. [43] observed that the relative amelioration of contralateral motor signs per energy unit was the highest after DBS of the region containing Forel’s fields and rostral part of the ZI. Lanotte et al. [33] found that 50% of the most effective electrode contacts (with respect to a decrease in the muscle rigidity of the wrist) were located 0.5 mm above the upper limit of the STN.
However, in the light of proximity of the above-mentioned stimulated regions of ZI and Forel’s fields to the STN and considering difficulties in determining the extent of current spread from electrodes, some doubts were raised as to their real contribution to DBS therapeutic effects. Since according to Pollak et al. [44], DBS is capable of affecting a tissue area of 2–3 mm in diameter, Yokoyama et al. [41] and Lanotte et al. [33] have suggested that the clinical effects described by them and induced by stimulation of areas located outside the STN might result from current spread to the latter structure.
Addressing this problem, Maks and coworkers [45] applied a method allowing for theoretical prediction and visualization of the volume of tissue activated (VTA) by clinically defined therapeutic parameters of STN stimulation with monopolar electrodes in 10 PD patients. In their work, the anatomical electrode localization was determined by neuroimaging and neurophysiological recordings. According to their calculations, which were based on neurostimulation parameters, capacitance and impedance of the electrode–tissue interface and electrical properties of tissue surrounding electrodes, the VTA was equal to 71 mm3 and was smaller than that of the whole STN (ca. 200 mm3) [45]. Using this method, the authors found that patients who had more than half of the VTA outside the STN (dorsally to this structure) demonstrated better therapeutic outcome than those who had more than a half of the VTA inside the STN [45].
While in the aforementioned studies therapeutic effects of DBS of more rostral regions of the ZI (and/or Forel’s fields) were examined, Kitagawa et al. [46] proposed that the region of caudal ZI and prelemniscal radiation located posteromedially to the STN can be considered another surgical target, especially for the tremor-dominant PD. They found that stimulation of this region significantly reduced contralateral muscle rigidity, tremor and akinesia, and improved handwriting, posture and gait. Plaha et al. [47] compared effects of stimulation of caudal ZI (located posteromedially and posterodorsally to the STN) to those of the dorsal half of the STN, and to the region located dorsomedially and medially to the STN, which comprised pallidofugal fibres and rostral ZI. Their caudal ZI targets were located more laterally than those investigated by Kitagawa et al. [46]. They found that although DBS of all regions strongly reduced contralateral PD signs (tremor, muscle rigidity, bradykinesia and timed hand movements), stimulation of the caudal ZI was the most effective. Moreover, stimulation of the rostral ZI induced side effects in some patients, i.e. speech deterioration and a sense of disequilibrium during walking [47], as well as irritability, psychomotor agitation and severe progressive insomnia [48]. The former disturbances were not present after stimulation of the caudal ZI [47]. Two years later Plaha and coworkers reported a dramatic mitigation of resting (94.8%) and postural (88.2%) tremor in PD patients as a result of bilateral stimulation of the caudal zone of the ZI [49]. Finally, Blomstedt and coworkers [50] chose this part of the ZI for DBS in 19 PD patients. The stimulated region was located between the red nucleus and STN, slightly posteromedially to the posterior tail of the latter structure. In an agreement with previous studies [47, 49], DBS of this region induced a profound effect on tremor, had less influence on akinesia and was free of negative influence on speech [50]. Special involvement of the caudal ZI and prelemniscal radiation (located posteromedially to the posterodorsal STN) in tremor, in general, was proven also by findings that DBS of this region had strong effect on PD voice tremor, proximal, distal and axial essential tremor, postural and intention component of multiple sclerosis tremor, Holmes tremor (resting, postural and intention) and dystonic tremor [49, 51–54]. When therapeutic effectiveness of DBS in essential tremor was compared in relation to the location of stimulated fields, the best region seemed to incorporate the superior part of the cerebellothalamic fasciculus and caudal ZI [52].
Besides motor signs, several non-motor disturbances, e.g. depression, apathy, decline in cognitive functions, hyposmia, sleep disorders as well as autonomic dysfunctions, are commonly observed in PD patients [55]. The influence of DBS of different brain structures on these disorders has been examined less frequently than that on motor signs. In general, DBS has been considered to have a positive impact on them either due to its direct action or because dopaminergic therapy could be reduced [55]. However, a number of non-motor adverse effects after DBS have also been reported [55]. With regard to the DBS of the ZI, only scarce and inconsistent data on non-motor signs are available.
Early case reports indicated that although DBS of the ZI/Forel’s fields in two PD patients (one with a history of previous depression) did not induce chronic depression, it evoked acute depressive/dysphoric mood states which were time-locked to the stimulation [56, 57].
De Chazeron and coworkers [40] did not find any significant differences between depression scores pre- and post-operation (3 and 6 months) in 18 patients treated with DBS of the ZI. However, Burrows and coworkers [58] reported, on the basis of observation of a small group of 11 PD patients who underwent initially DBS of the STN, that changing the stimulation contact to the ZI or to the area located near the latter structure decreased subjective anxiety, depression as well as mild and extreme fear, while worsened recognition of mild sadness. Moreover, Welter et al. [39] found that DBS of the STN region (which in some cases included the ZI/Forel’s field area) improved mood status in 26 of 45 patients who showed symptoms of depression before surgery, while worsened it in 27 initially non-depressive patients, within 1 year after the operation. Although it was not specified which area (STN vs. ZI/Forel’s fields) was stimulated in individual patients, postoperative depression was found to be related either to preexisting depressive signs or lower pre- and postoperative cognitive performance but not to contact locations [39].
Apathy, a common clinical feature of PD [59] seems to be worsened by DBS of the STN region [60, 61]. It has been suggested that both the surgery target and the reduction of dopaminergic medication are involved in aggravation of the apathetic condition after STN-DBS [60, 61]. However, apathy which was described to develop in one PD patient who had stimulating contact in the ZI (located dorsally to the STN) was insensitive to the treatment with the agonist of dopaminergic receptors ropinirole [60].
With regard to non-psychiatric signs, stimulation of the ZI was found to increase appetite [40], and stimulation of the region located ventrally to the motor thalamus (ZI and Forel’s field’s) was reported to induce weak olfactory deficits [62]. However, the latter deficit was only subclinical, because it could be found in specific tests but was not perceived by patients [62]. Moreover, heat sensation, sweating and oculomotor disturbances were sometimes seen during stimulation of the ZI [56, 57, 63, 64].
Anatomy of the ZI: chemoarchitecture and connections
Neuronal mechanisms underlying therapeutic efficiency of ZI DBS is unknown and may be complex because of differential neuroarchitecture of this structure and multiplicity of its connections.
The most knowledge about the ZI anatomy and physiology comes from studies in rodents, however, their results only partly can be compared with those obtained in some other species and humans [65, 66]. The ZI in rats extends from rostral regions of the thalamus to the rostral pole of the red nucleus [66, 67] and in humans from the rostral regions of the thalamus to the level of the rostral pole of the medial geniculate nucleus [66]. The ZI has been divided in rats into 4 main sectors: rostral, dorsal, ventral and caudal [66, 67], although borders between them are not clear [68]. Rostral ZI lies ventrally to the Vm thalamic nucleus [an equivalent of the medial portion of the VA [69] or VL [70] thalamic nucleus in primates], dorsomedially to the entopeduncular nucleus (an equivalent of the GPi) and dorsally to the lateral hypothalamus [67]. Dorsal and ventral sectors in rats are located in the central part of the ZI which lies dorsomedially to the STN and cerebral peduncle, dorsally to the lateral hypothalamus and ventrally to the Vm/ventroposterior thalamus [67] (Fig. 2). Caudal ZI in rats is located dorsally to the cerebral peduncle and the substantia nigra (pars lateralis) and ventrolaterally to parts of the posterior thalamic group of nuclei and medial lemniscus [66, 67]. The “rostral portion” of the ZI used by neurosurgeons for DBS of the region located dorsally/dorsomedially to the STN seems to correspond to the central region of ZI in rats [47, 66]. The caudal ZI stimulated by neurosurgeons lies between cerebral peduncle and substantia nigra, H1 Forel’s field and ventroposterior thalamic nucleus. From the rostral side, it is limited by the STN and from the caudal side by medial lemniscus [66]. The most posterior part of the caudal zona incerta in humans is not targeted by neurosurgeons [66].
Fig. 2.
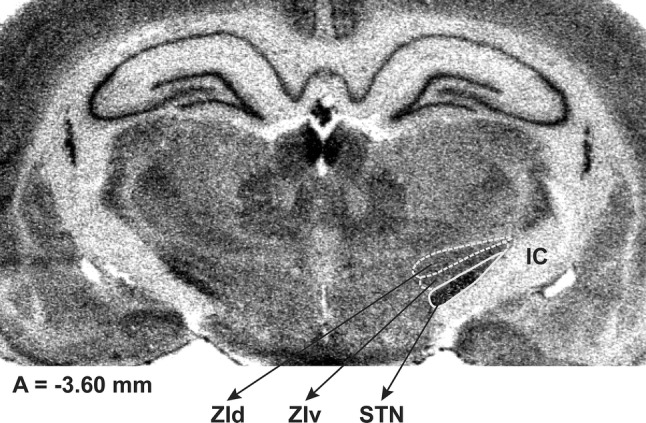
An autoradiogram of mRNA coding for COI (cytochrome oxidase subunit I) in a coronal section of the rat brain, at the level of A = − 3.60 mm from bregma, according to the Paxinos and Watson [67]. Borders of ZId, ZIv and STN are marked by white outlines. IC internal capsule, STN subthalamic nucleus, ZId dorsal subdivision of the zona incerta, ZIv ventral subdivision of the zona incerta. The autoradiogram has been generously delivered by prof. J. Wardas and Dr. B. Kosmowska (unpublished)
Rostral, dorsal, ventral and caudal sectors of the ZI differ significantly with regard to their chemoarchitecture. In rodents, the rostral sector of the ZI comprises the tyrosine hydroxylase-, somatostatin- and glutamate-immunoreactive neurons [71, 72]. Somatostatin-positive neurons are also present in the lateral edge of both dorsal and ventral ZI [68, 72] whereas tyrosine hydroxylase-positive ones are sparse in the medial parts of these sectors [68, 72]. GAD immunoreactive cells are present within all sectors of the ZI, however, most of them which are also parvalbumin-immunoreactive are found within the ventral sector of the ZI [66, 72-75]. The medial part of the dorsal ZI is also rich in parvalbumin-stained cells and neuropil, but the lateral part is not [66]. In the dorsal sector, NADPH-diaphorase, NOS, calretinin and glutamate-immunoreactive cells are the most abundant [66, 71–73]. In an agreement with the above immunoreactivity of neurons, the majority of fibres arising from the ventral tier of the ZI are GABAergic [68, 76, 77], whereas those extending from the dorsal ZI are mainly glutamatergic [71]. In contrast to the ventral ZI, the caudal ZI in rodents expresses little or no parvalbumin, GAD65/67 and GABA transporter immunoreactivity and includes a smaller number of NADPH-diaphorase-, NOS-, glutamate- and calretinin-positive cells than the dorsal tier of the ZI [66, 71]. Instead, the caudal ZI contains a number of calbindin-positive cells and its caudal pole is strongly positive for acetylcholinesterase both in rodents and primates. On the basis of chemoarchitecture of this structure, Watson et al. [66] have postulated that “the caudal pole of the ZI is sharply different in function to the remainder of the ZI”.
Besides the chemoarchitecture diversities, the above regions of the ZI differ with respect to their afferents and efferents (Fig. 3).
Fig. 3.
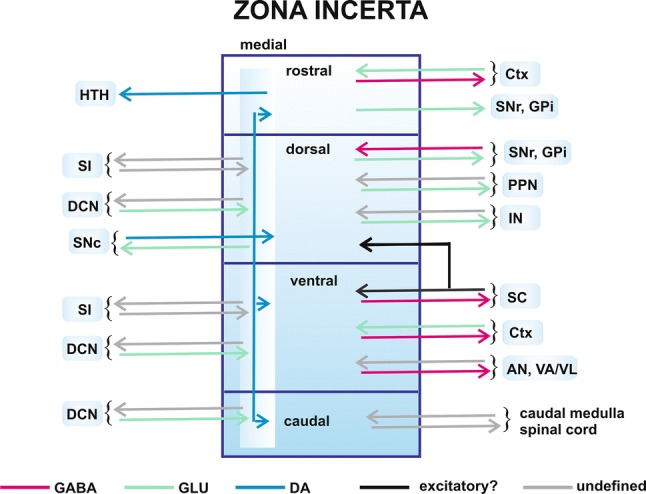
An overview of the main anatomical connections—outputs and input pathways (shown as arrows) of the zona incerta. Brain structures: AN association nuclei of the thalamus, Ctx cerebral cortex, DCN deep cerebellar nuclei, GPi the internal segment of the globus pallidus, HTH hypothalamus, IN intralaminar nuclei of the thalamus, PPN pedunculopontine nucleus, SC superior colliculus, SI substantia innominata, SNc substantia nigra pars compacta, SNr substantia nigra pars reticulata, VA/VL ventroanterior/ventrolateral nuclei of the thalamus. Neuronal pathways: DA dopaminergic pathway; excitatory?—putative excitatory pathways, GABA GABAergic pathways, GLU glutamatergic pathways; undefined—pathways of undefined neurotransmitter. Four subdivisions (rostral, dorsal, ventral and caudal) of the zona incerta are shown. The medial region extends through all the zona incerta subdivisions
Interconnections with the superior colliculus
The ventral sector of the ZI is the main area which gives rise to the GABAergic pathway to the superior colliculus in rats, cats and primates [78–84]. However, a reciprocal connection from the latter structure terminates in all subdivisions of the ZI, but mostly in its ventral tier in rats [77–79, 85] (Fig. 3) and in the main body, i.e. central ZI in cats and primates [80, 81].
Interconnections with the basal ganglia and brainstem
Although all sectors of the ZI send fibres to the basal ganglia structures [SNc and SNr, PPN (mainly pars dissipata), GPe and entopeduncular nucleus (GPi)], the incertal projection neurons (mainly glutamatergic) are the most abundant in the dorsal and rostral ZI in rats [71, 79, 82–84] (Fig. 3). Moreover, a few GABAergic neurons in the ventral subdivision of the ZI send their fibres to the SN, PPN and entopeduncular nucleus (GPi) [71, 78]. In contrast to more rostral regions, the caudal ZI has been reported to have only few incerto-basal ganglia neurons [71]. The ZI is traversed by fibres of topographically arranged projection from the SNr to motor higher order thalamic nuclei (VA/VL, intralaminar, and mediodorsal) which is GABAergic [86, 87]. Some of these fibres form terminals in the ZI [87]. Afferents from the SNr and other brainstem nuclei (midbrain reticular nucleus, pontine reticular nucleus, ventral tegmental area, PPN (pars dissipata), dorsal raphe, periaqueductal grey matter) terminate in all sectors of the ZI but mainly in its dorsal part [71, 79] (Fig. 3). On the other hand, dopaminergic projection from the SNc in rats terminates in the medial region of all sectors of the ZI, close to the border of Forel’s fields and lateral hypothalamus in rats [71] (Fig. 3). The ZI receives also the projection from the GPi and is traversed by pallidothalamic fibres (arising from the associative/limbic GPi), which lead to VA/VL and intralaminar thalamic nuclei, and some of them form terminals in the ZI in monkeys, humans and rats [71, 88–91] (Fig. 3).
Interconnections with the thalamus
All sectors of the ZI send projections to the dorsal thalamus which can be considered as a “gateway” to the neocortex for sensory information. The heaviest projections run to higher order thalamic nuclei: association nuclei (mainly from the ventral ZI) (lateral dorsal nucleus, lateral posterior nucleus and posterior thalamic nucleus) and intralaminar nuclei (mainly from the dorsal ZI) (central lateral nucleus, paracentral, central, medial, parafascicular nucleus (PF), posterior intralaminar nuclei) [68, 76, 77, 82–85, 92] (Fig. 3). Fewer fibres project to the first-order thalamic nuclei (lateral geniculate nucleus, medial geniculate nucleus, ventral geniculate nucleus). Scattered axons of the incerto-thalamic pathways arising from the central region of the ZI were also seen in the VA/VL nuclei in rats [76, 82] (Fig. 3). A vast majority of incerto-thalamic pathway neurons whose terminals innervate the proximal dendrites of relay thalamic neurons have been found to be GABAergic [76]. The ZI receives reciprocal connections from the dorsal thalamic nuclei. Most terminals belonging to fibres arising from intralaminar and association nuclei are present in all sectors of the ZI but predominantly in its dorsal and ventral sector, respectively [92] (Fig. 3). Afferents from primary relay nuclei are only scarce and were found scattered across all sectors of the ZI in rats [92].
Interconnections with the cerebral cortex
The rostral and central, but not caudal regions of the ZI send projections to the entire cerebral cortex in rats (especially to the frontal and parietal—somatosensory cortex) [75, 93–95] (Fig. 3). These projections are at least partly GABAergic [93]. Reciprocal excitatory projections from layer V of most cortical regions (frontal, cingulate, parietal, forelimb, and occipital) to the ZI have been shown [68, 96–98]. The heaviest projection originates in the cingulate cortex, whereas the weakest in the occipital cortex [97]. Among others, pathways originating in the parietal cortex, cortical representation of forelimbs, and vibrissae motor cortex terminate mainly in the ventral tier of the ZI (Fig. 3) with smaller representation in the dorsal ZI [68, 95, 99]. On the other hand, the cingulate cortex sends heavy projections to the dorsal and rostral sectors of the ZI [68, 97].
Interconnections with the cerebellum and red nucleus
The ZI (especially its more centrocaudal regions) is traversed by cerebellothalamic fibres arising from the dentate, interposed and fastigial subcortical cerebellar nuclei which terminate in VL nucleus (including its posterior portion), intralaminar and other associative thalamic nuclei in rats, monkeys and humans [88, 90, 100]. Some of these fibres form collaterals which terminate in the ZI [100]. Terminals of the projection from the interposed nucleus in rats are localized in all subregions of the ZI, but mainly medially in dorsal and ventral tiers of the ZI [101] (Fig. 3). Their density is especially prominent in the contralateral ZI thus ipsilateral caudal ZI is devoid of them. There is also a reciprocal small projection from the ZI (mainly from its medial region) to the interposed nucleus (Fig. 3). Although cells of the latter projection were found on both sides of the brain, they tended to be more numerous on the ipsilateral side [101].
There are also strong interconnections between the ZI and the red nucleus [102]. All sectors of the ZI (mainly their medial parts) send projections to the red nucleus, largely to its parvocellular lamina [102]. A reciprocal connection from the latter structure to medial region of each sector of the ZI was also noted [102].
Interconnections with caudal medulla and spinal cord
More caudal regions of the ZI send projections to the caudal medulla (to the inferior olivary complex, among others) and cervical and thoracic spinal cord in rodents [75, 82–84, 95, 103] (Fig. 3). The spinal cord (mainly its cervical levels) projects, in turn, especially to more caudal regions of the ZI [75, 95]. No interconnections with the spinal cord and the rostral ZI were observed [95].
Dopaminergic projections from the ZI
The medial region of the rostral ZI (A13 cells) sends dopaminergic projections to the hypothalamus (Fig. 3), horizontal limb of the diagonal band of Broca, central nucleus of the amygdala [104], as well as to the dorsolateral periaqueductal grey and mesencephalic locomotor region (MLR), i.e. PPN and cuneiform nucleus [105]. Dopaminergic component in the pathways extending from the medial ZI to PPN and cuneiform nucleus amounts 21.38 and 30.21%, respectively, in mice [105].
Other incertal interconnections
The ZI receives also somatosensory input from the trigeminal nuclei. This pathway terminates almost exclusively in the ventral part of the ZI [75, 95], however, a few trigeminal and medial lemniscus terminals can also be observed in the dorsal, rostral and caudal subdivisions of the ZI [75].
The region of the ZI located dorsally to the medial part of the STN contains terminals of the projection arising from the substantia innominata of the subpallidal forebrain which may be formed by collaterals of the subpallidal-PPN pathway [106] (Fig. 3). A reciprocal projection from the ZI to the subpallidal region was also described in the rat [82, 104].
Intrinsic incerto-incertal connections
A study carried out in monkeys has shown that besides projection (principal) neurons, the ZI contains also interneurons (presumably GABAergic) which make contacts with both principal neurons and other interneurons. That may lead to feedforward inhibition or disinhibition of principal neurons [107]. Moreover, widespread intranuclear recurrent GABAergic connections along the ZI neurons [76, 99], as well as incerto-incertal fibres connecting different sectors of the ZI ipsi- and contralaterally have been reported in rats [68, 82, 108]. For example, neurons of the caudal ZI (similarly to those of other sectors) heavily innervate dorsal, ventral and rostral ZI ipsilaterally and to a lesser extent contralaterally [68, 108]. In general, the strongest contralateral incerto-incertal projections are present between corresponding sectors of this structure [108].
The role of the ZI in motor behaviour and in models of parkinsonism in animals
In line with variety of its connections, the ZI has been suggested to be involved in different functions, i.e. in the control of arousal states and attention (orienting movements of the eyes and head, and whisking) [68, 78, 81, 95, 99, 109], visceral activities (ingestion, drinking, sexual cycles, cardiovascular activities and temperature control) [68, 110], posture, locomotion and other motor behaviours (see below), and neuropathic pain [96, 111–113]. It has been proposed that the global function of the ZI consists in translation of diverse sensory (exteroceptive and interoceptive) incoming signals to appropriate visceral, arousal, attention and posture-locomotion reactions [68].
The role of the region of ZI in locomotor behaviour was explored in animals already in the 1950s. Grossman [114] found that stimulation of the area which included H1 and H2 Forel’s fields and the medial portion of the ZI elicited walking movements in anaesthetized cats. However, the majority of studies of the ZI have been carried out in rats.
Almost 30 years after Grossman’s discovery [114], Mogenson and coworkers [106] proposed that a part of central region of the ZI located dorsally to the medial edge of the STN in rats belonged to the neuronal network involved in locomotor activity which includes pathways connecting nucleus accumbens with PPN (associated with the MLR) via the subpallidal region and ZI. According to their study, subpallidal neurons which are targets for impulses arising from the nucleus accumbens send projections to PPN through ZI where some fibres form collaterals and terminals. Moreover, procaine administered into the above region of ZI which blocked descending projections, reduced locomotor activity induced by a blockade of GABAA receptors by picrotoxin injected into the subpallidal region [106]. In line with the above suggestion and results of the classic paper by Grossman [114], it has been reported that electrical stimulation of ZI or a site within the field of Forel located dorsomedially to the STN triggered locomotor activity measured in an open field or on a treadmill in rats [115–118]. Moreover, such stimulation induced antidromical response in neurons of the subpallidal region, some of which responded also to stimulation of the PPN [116].
The above studies which used electrical stimulation or procaine-induced blockade of impulse flow were not able to differentiate between their influence on neurons located in the ZI and on fibres traversing this structure [106, 114–118]. Therefore, as a next step, Milner and Mogenson [115] found that intraincertal microinjections of bicuculline (an antagonist of GABAA receptors), picrotoxin (a blocker of GABAA receptor channel) or glutamate increased locomotor activity of rats. These results suggested real contribution of the activated incertal neurons to this behaviour. In the same year, we published a study [119] which showed hyperlocomotion induced by bicuculline injected bilaterally into the region which we called “zona incerta-lateral hypothalamus (ZI-LH)” in rats. The region explored by us included medial part of the central region of the ZI, dorsal part of the hypothalamus and, at the most caudal level, rostral extension of Forel’s fields [120]. It partly overlain the area described by Mogenson and coworkers [106, 115, 116]. Hypermotility in rats was observed also by others as a result of intraincertal (into the rostral, central and/or caudal regions of the ZI) injections of picrotoxin and bicuculline, as well as agonists of AMPA/kainate receptors [121–123].
The first report suggesting a role of the ZI in akinesia and muscle rigidity came from our laboratory. In 1987, Wardas and coworkers [124] published a paper showing that picrotoxin or bicuculline injections into the ZI–LH region in rats reduced or even blocked the morphine-induced catalepsy and muscle rigidity (measured as a tonic electromyographic activity in the gastrocnemius muscle). Moreover, we found that bicuculline administered into this region at doses of ≥ 0.5 ng/0.5 μl/side, which were much lower than those necessary to obtain similar results after injections into the Vm (≥ 25 ng/0.5 μl/side), reduced the catalepsy induced by haloperidol [119]. On the other hand, muscimol which is an agonist of GABAA receptors injected into this region evoked strong catalepsy which resembled that induced by haloperidol [119]. Since the haloperidol-induced catalepsy is a well-established rodent model of neuroleptic-induced parkinsonism in humans (mainly parkinsonian akinesia), the results of our study [119] were the first suggestion that ZI GABA synapses may be involved in parkinsonian signs independently of the Vm [119].
The neuroleptic-induced catalepsy is generally accepted to stem from blockade of dopamine D2 and D1 receptors localized in the striatum [125]. Therefore, in our following studies we tried to trace the route of impulse flow involved in this phenomenon from the striatum to the ZI. We found that catalepsy induced by intrastriatal injections of antagonists of D1 (SCH 23390) and D2 (sulpiride) receptors, or intrapallidal (into GPe) injections of muscimol was inhibited or even blocked by low doses of bicuculline administered into the ZI–LH region [126, 127]. Furthermore, the catalepsy induced by intrastriatal injections of sulpiride was inhibited by bicuculline administered into the GPe and muscimol into the SNr [127]. Finally, catalepsy induced by muscimol injected into the GPe was inhibited by muscimol injected to the SNr [127]. According to the above results, we concluded that ZI–LH was a link in a neuronal chain transmitting impulses pertinent to the neuroleptic-induced parkinsonism which were conveyed from the striatum to this region successively via GPe and SNr.
A role of the ZI region in transmission of neuronal impulses arising from the striatum was suggested also by Supko and coworkers [123] who found that the stereotypy induced by apomorphine or amphetamine was inhibited by the ibotenic acid-induced lesion or by a blockade of AMPA/kainate receptors of the central region of the ZI. On the other hand, the apomorphine- and amphetamine-induced stereotypy was also ameliorated by activation of AMPA receptors in the caudal portion of this structure [128].
Further support for a potential role of the ZI neurons in Parkinson’s disease came from the study of Périer and coworkers [129]. These authors found that a unilateral lesion of the nigrostriatal pathway increased both the level of mRNA coding for cytochrome oxidase subunit I [(COI), a metabolic marker of neuronal activity], and firing rate of neurons in the dorsal and lateral regions of the central ZI. In general, this region was located more caudally and more laterally to the region explored by us [119, 124, 126, 127]. In contrast, such lesion did not increase the number of Fos-immunoreactive cells in this region [130]. However, Heise and Mitrofanis [131] found that a unilateral lesion of the nigrostriatal tract resulted in a dramatic loss of parvalbumin expression in the neurons of central ZI (mainly in the ventral sector of this structure). These neurons are GABAergic [72, 75], and Heise and Mitrofanis [131] suggested that the decrease in parvalbumin (which is a calcium buffer protein) led to an increase in their excitability.
Potential mechanisms involved in therapeutic effects of DBS of the ZI
The mechanisms underlying the therapeutic effect of DBS delivered to different structures are unclear at present and may depend on the stimulated region. DBS is an unspecific procedure which may stimulate orthodromically and antidromically myelinated axons passing through a given region, may influence terminals of inhibitory and excitatory efferents and antidromically their sources, may affect neuronal cell bodies or even non-neuronal cells [6, 132]. Contribution of each of these elements to the therapeutic efficiency of DBS of the ZI has not been analyzed, yet. However, a huge effort has been made to clarify the mechanisms involved in DBS of other structures, e.g. the GPi, STN or Vim in humans or parkinsonian primates and rodents [7, 132, 133]. Since therapeutic effects of DBS of these structures in PD patients appeared to be similar to those induced by their lesions, it has been initially suggested that DBS induces “a reversible (functional) lesion”. However, because of conflicting results with regard to inhibitory vs. stimulatory influences of DBS on output axons of the stimulated nuclei [7, 132, 133], and DBS-induced de-coupling of somatic and axonal firing [7], therapeutic effectiveness of this procedure has recently been suggested to depend on interrupting a pathological pattern of neuronal firing in the stimulated nucleus and disrupting abnormal information flow through the cortico-basal ganglia-cortical loop [7, 9].
As mentioned above, the ZI is traversed by fibres of pallidothalamic, nigrothalamic and cerebellothalamic projections [87, 88, 90, 100] which convey pathological impulses involved in generation of bradykinesia, muscle rigidity and various forms of tremor [6, 11–14, 16]. Therefore, it cannot be excluded that stimulation of these fibres by DBS is the main cause of amelioration of these signs. However, animal (rodent) studies have shown that pharmacological manipulations within this structure influenced motor behaviour and induced or counteracted parkinsonian-like disturbances [115, 119, 121–124, 126–128]. Therefore, these animal studies clearly suggested that, besides fibres passing through, neurons of the ZI may also be important for the generation of parkinsonian signs.
Is firing pattern of the ZI neurons pathological in PD? It has been shown that background neuronal activity of the ZI recorded in PD patients during stereotaxic operation differs significantly from that of neighbouring STN with respect to its low amplitude and a lack of proprioceptive responses [22, 134]. Signal analysis showed heterogeneous firing patterns, ranging from tonic to burst and paused units [134]. Since there are no data available regarding the ZI neuronal activity in normal, healthy humans, it is difficult to conclude to what extent that observed in PD patients is pathological. However, bursting of some of ZI neurons (3.4% of total) was synchronized with tremor at rest which could indicate a certain role of neurons of this structure at least in this symptom [134]. Moreover, the aforementioned studies [129, 131] suggested an increase in both firing of the ZI GABAergic neurons and their metabolic activity in 6-hydroxydopamine-induced rodent model of PD.
However, behavioural experiments in rodents seem to be in contradiction to the concept that activation of the ZI neurons underlies parkinsonian signs. As mentioned above, these studies showed that intraincertal injections of antagonists of GABAA receptors or agonists of glutamate receptors, carried out along the whole extension (rostral–caudal) of the ZI, increased locomotor activity of rats [115, 119, 121, 122, 128]. Moreover, catalepsy induced by neuroleptics was antagonized by the blockade of GABAA receptors in the ZI, while stimulation of these receptors by their agonist, muscimol, induced catalepsy [119, 126, 127]. Since the stimulation of GABAA receptors can be expected to inhibit neurons, while their blockade or stimulation of glutamate receptors—to disinhibit or activate them, the above results may suggest that decreased, rather than increased, firing of some (undefined) ZI neurons is responsible for parkinsonian-like behaviour, at least in these models.
Anatomical data presented above indicate that the ZI is a big communication node in neuronal network involved in the generation of parkinsonian signs (Figs. 3, 4). This region is reciprocally connected with the basal ganglia, cerebellum, several thalamic nuclei, different cortical regions, brainstem nuclei, and spinal cord (see above) (Fig. 3). Therefore, the ZI not only receives impulses from these structures but may retrogradely modulate their function, as well. This creates the basis for therapeutic action of DBS of the ZI which may disrupt pathological impulses transmitted through this structure. Although all the above connections may be involved both in PD and DBS effects, available functional data allow for discussion of the role of at least some of them (Fig. 4). First, the ZI receives projections from output structures of the basal ganglia—the SNr and GPi (Figs. 3, 4) [71, 79, 87, 91]. These pathways are formed at least partly by fibres of GABAergic pallidothalamic and nigrothalamic pathways which on their way to ventral and intralaminar thalamic nuclei make synaptic contacts in the ZI [87, 91]. Since according to the present knowledge these pathways are activated in PD and strongly inhibit their targets in the thalamus [6, 11–14], they possibly influence neurons of the ZI in the same way (Fig. 4b). Our results showing cataleptic effect of intraincertal muscimol seem to support this view and may suggest that this compound mimicked hyperactive GABAergic input to this structure [119].
Fig. 4.
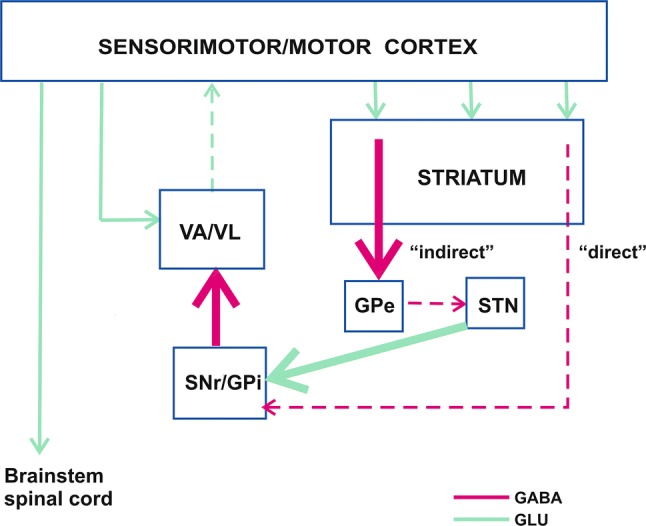
a The zona incerta (ZI) as a link in a neuronal chain responsible for conveying impulses potentially involved in motor signs of Parkinson’s disease. Due to dopaminergic deficiency in PD and imbalance between the “indirect” and “direct” GABAergic pathways arising from the striatum, the basal ganglia GABAergic output projections from the SNr/GPi to the VA/VL thalamic nuclei are overactive which results in inhibition of thalamic neurons and their projections to the sensorimotor/motor cortex [13]. The ZI receives GABAergic input from the SNr/GPi [71, 79, 87, 91] which is probably overactive, as well. The ZI, in turn, sends glutamatergic efferents to the PF [68, 77, 92] and GABAergic ones to the POm and VA/VL [68, 76, 77, 82] which are under excitatory influence of pathways originating from the SC [77], which also receives the GABAergic input from the SNr/GPi [15, 86]. Since glutamatergic pathways from the PF and POm to the striatum activate the “indirect” and “direct” pathways, respectively [77, 135, 136, 139], it has been suggested that the ZI, via inhibition of the POm, may suppress the “direct” pathway, while, via activation of the PF, may sensitize the “indirect” pathway to excitatory input from the sensorimotor/motor cortex [77, 136]. It may be speculated, therefore, that if both these incerto-thalamic pathways are overactive in PD they may contribute to the imbalance between the “direct” and “indirect” pathways and in this way, may be involved in appearance of motor signs of this disease. Moreover, putative activation of the GABAergic incerto-thalamic projection, which terminates in the VA/VL may constitute an additional, to the basal ganglia, source of inhibition of these nuclei in PD. b A hypothesis of potential, internal mechanisms of the ZI involved in modulation of incoming signals from the basal ganglia (SNr/GPi) and their transmission to thalamic nuclei (PF, POm, VA/VL) in PD. The ZI possesses extensive internal incerto-incertal network: GABAergic interneurons [107], collaterals of GABAergic incertal projection neurons [76, 99], and incerto-incertal connections linking different ZI regions utilizing undefined neurotransmitters [68, 108]. GABAergic incertal neurons are localized mainly in the ventral sector of this structure [66, 72, 77, 99], whereas glutamatergic ones concentrate mainly in its dorsal subdivision [66, 71, 72]. The GABAergic nigro(pallido)-incertal pathway (arising from the SNr/GPi) terminates mainly in the dorsal sector of the ZI [71, 79, 91], where it may switch to and inhibit glutamatergic neurons sending their axons to the ventral sector. A decrease in glutamatergic transmission in the ventral ZI may lead to inhibition of some GABAergic neurons terminating on incerto-thalamic neurons in the dorsal and ventral ZI, projecting to the PF, and POm/VA/VL, respectively [68, 76, 77, 92]. Inhibition of the internal GABAergic network may, in turn, lead to disinhibition of incerto-thalamic pathways, which are additionally under excitatory influence of signals coming from the SC [77]. Therefore, due to the above complex incerto-incertal interactions, overactivation of inhibitory basal ganglia input to the ZI, may increase output of this structure to the thalamus. Additionally, GABAergic incerto-collicular pathway starting in the ventral ZI [78–84] may be inhibited indirectly by the nigro(pallido)-incertal pathway that may result in disinhibition of the colliculo-incertal pathway and potentiation of its excitatory influence on incertal outputs to the thalamus. Brain structures: GPe external segment of the globus pallidus, GPi internal segment of the globus pallidus, PF parafascicular nucleus, POm posteromedial thalamic nucleus, SC superior colliculus, SNr substantia nigra, pars reticulata, STN subthalamic nucleus, VA/VL ventroanterior/ventrolateral nuclei of the thalamus, ZI zona incerta. Neuronal pathways: GABA GABAergic pathways, GLU glutamatergic pathways, excitatory?—putative excitatory pathways. Bold neurons and arrows—indicate activated neurons, pale neurons and dashed arrows—indicate inhibited neurons
What processes inside the ZI may be involved in reversing neuronal inhibition induced by the basal ganglia inputs [119] to neuronal excitation described in models of parkinsonism [129, 131]? In fact, intrinsic neuronal arrangement of the ZI is very complex. The ZI projection (principal) neurons seem to be under influence not only of afferents entering this structure but also of a network of inhibitory interneurons [107], as well as recurrent collaterals of inhibitory efferents [76, 99], and/or intranuclear incerto-incertal fibres, which connect different regions of this structure ipsi- and contralaterally [68, 108] (Fig. 4b). In this way, inhibition of neurons located at the site of input from the basal ganglia may lead to their own feedback disinhibition and inhibition or disinhibition of other neurons. Finally, flow of impulses in efferent pathways of the ZI depends on interactions between afferents of this structure and its entangled intrinsic network (Fig. 4b). In an agreement with complexity of ZI GABAergic neuronal network, Moon et al. [112] and Moon and Park [113] reported that both muscimol and bicuculline injected into the central region of this structure increased firing rate of some recorded neurons.
As mentioned above, the ZI sends intensive projections to intralaminar, associative and to a lesser degree also to ventral thalamic nuclei [68, 76, 77, 82–84, 92] (Fig. 3). At least some of these connections may be involved in parkinsonian signs.
First, incerto-thalamic pathway which terminates in VA/VL nuclei is, the most probably, GABAergic [76] and forms an additional, to the basal ganglia, source of their inhibition. Therefore, activation of this pathway (if present) may work concomitantly with basal ganglia-thalamic fibres to shut down neuronal activity in these thalamic regions in PD (Fig. 4).
Recently, a new concept has arisen with respect to the role of the ZI in regulation of thalamic gating of information flow from the cortex to the dorsolateral striatum [77, 135, 136]. The dorsolateral striatum, which is involved in the execution of habitual behaviours in a familiar sensory context, receives, besides the major corticostriatal input, also excitatory projections: from intralaminar—PF nucleus and from the higher order—posteromedial thalamic nucleus (a rodent equivalent of primate anterior pulvinar nucleus; POm [85, 135, 137–139]). These two thalamic projections influence responses of the striatal medium-spiny neurons to cortical signalling in an opposite way [135, 139]. Fibres arising from the PF nucleus activate burst-pause pattern of spiking of striatal cholinergic interneurons which initially, transiently interrupts, and then enhances responsiveness of striopallidal GABAergic neurons to corticostriatal input [135, 139]. The latter mechanism is probably important for redirection of attention and interruption of ongoing motor activity with the presentation of salient stimulus [135, 139]. On the other hand, the thalamostriatal projection from the POm activates the strionigral GABAergic pathway, and secondarily inhibits neurons in the SN [136], which leads to facilitation of movements [136]. The PF nucleus receives a projection from the dorsal sector of the ZI which is probably glutamatergic [68, 77, 92, 135], while POm is supplied by GABAergic fibres from the ventral subdivision of the ZI [68, 76, 77, 135] (Fig. 4). These anatomical data indicate that the ZI may suppress strionigral neurons indirectly by inhibition of the POm, and sensitize striopallidal neurons by activation of the PF. It may be speculated, therefore, that if both these incerto-thalamic pathways are overactive in PD they may enhance the imbalance between the “direct” and “indirect” pathways in PD and in this way, may contribute to appearance of motor signs of PD (Fig. 4).
On the other hand, inhibition of the ZI by DBS may restore normal functioning of striatal output neurons which may result in therapeutic effect of this procedure. In an agreement with this concept, Benazzouz et al. [140] found that DBS of the ventral sheet of the ZI reversed metabolic effects of the lesion of dopaminergic neurons in rats. While the lesion increased COI mRNA expression in the substantia nigra pars reticulata, and decreased it in the GPe, the DBS of the ZI had an opposite effect. Interestingly, this effect was similar to that of DBS of the STN [140].
Our hypothesis concerning contribution of the ZI and its internal network to a neuronal circuit responsible for motor signs of PD is shown in the Fig. 4a, b. This hypothesis is based on the assumption that an increased GABAergic inhibitory input to the ZI may diminish activity of incerto-incertal connections (glutamatergic and GABAergic), and in this way, may disinhibit the above incerto-thalamic pathways, which secondarily, via thalamostriatal projections, may disturb activity of striatal outputs. Future research in animals is warranted to verify this hypothesis. The first step may possibly consist in establishing how stimulation (optical?) of the nigral and pallidal GABAergic output pathways will influence activity of different, electrophysiologically and neurochemically identified populations of incertal neurons, and neurons of their thalamic targets.
As mentioned above, the ZI conveys also information arising from the nucleus accumbens and subpallidal region (substantia innominata) to the MLR (Fig. 3). This neuronal system has been suggested to be involved in initiation of exploratory locomotor activity [106, 116, 141, 142]. The MLR, which contains nuclei interconnected with the ZI (PPN, cuneiform nucleus), is a coordination centre for activation and control of spinal locomotor generator neurons [118, 141, 143]. The MLR is also connected with the basal ganglia, thalamus and other structures suggested to be involved in PD pathology [11, 144], and neurons of the PPN has been found to be inhibited in animal models of PD [145, 146]. What is interesting, the ZI is interconnected mainly with pars dissipata of the PPN, a subregion, which receives projections from the GPi and SNr, and contains bursting neurons (probably glutamatergic) involved in initiation of programmed movements [5]. In an agreement with the physiological function of the MLR, the DBS of PPN improves mainly postural instability and refractory gait freezing in PD [5, 8], and simultaneous stimulation of the PPN and caudal ZI [147, 148], PPN and STN [149] or PPN and GPi [8] has been used in PD patients to achieve an optimal therapeutic effect.
The last issue which should be mentioned here is that despite differential neuroarchitecture of the ZI and multiplicity of its connections DBS induces antiparkinsonian effects when applied either into the rostral/central or caudal region of this structure. Although, the reason of this phenomenon is not clear at present, the existence of extensive intrinsic network of the ZI [68, 107, 108] may explain it, at least partly. Thanks to incerto-incertal connections, the whole ZI is in a position to integrate signals coming from functionally diverse brain centres to its different sectors. Furthermore, neurons of one sector of the ZI may influence impulses leaving other sectors both ipsi- and contralaterally [108].
However, according to general opinion of neurosurgeons, DBS of the caudal sector of the ZI was the most therapeutically effective, especially with respect to reduction of different forms of tremor, including parkinsonian tremor [46, 47, 49–53]. The most probable explanation of this effect is that DBS affects the cerebellothalamic glutamatergic fibres arising from subcortical deep cerebellar nuclei [150] which traverse this region and form there collaterals and terminals [88, 90, 100].
Concluding remarks
The analysis of the available clinical and anatomical data, as well as results obtained in animal models of parkinsonism allows for drawing the following conclusions.
The ZI, which has interconnections with most of structures involved in PD pathology, is an important target for DBS, which alleviates parkinsonian bradykinesia, muscle rigidity and tremor. The therapeutic effect of DBS of the ZI may be related to disruption of abnormal information flow through this structure due to its influence on intrinsic incertal network and projection neurons, as well as fibres en passage (e.g. pallidothalamic and cerebellothalamic). The cerebellothalamic fibres traversing the caudal sector of the ZI, as well as their collaterals terminating in this region may contribute significantly to the tremorolytic efficiency of the DBS of this part of the ZI.
However, since the ZI is located close to the STN, the current spread to the STN during each session of ZI DBS, cannot be excluded. Therefore, the challenge for future research lies in discrimination of contribution of each structure to therapeutic effects of DBS. Studies on the effects of DBS of the ZI vs. STN in animal models of PD on activity of different neuronal populations in structures belonging to the PD-related circuits would be of help to solve this problem.
Acknowledgements
The study was supported by Statutory Funds of the Department of Neuropsychopharmacology, Maj Institute of Pharmacology, Polish Academy of Sciences, Kraków, Poland. The author wishes to express her gratitude to Prof. Jadwiga Wardas and Dr. Barbara Kosmowska (Department of Neuropsychopharmacology, Maj Institute of Pharmacology, Polish Academy of Sciences) for generous delivery of the coronal section of the rat brain with the zona incerta and subthalamic nucleus and preparation of the respective figure.
Compliance with ethical standards
Conflicts of interest
The authors state that any conflict of interest which might bias the present study does not exist.
References
- 1.Ehringer H, Hornykiewicz O. Verteilung von Noradrenalin und Dopamin (3-Hydroxytyramin) im Gehirn des Menschen und ihr Verhalten bei Erkrankungen des extrapyramidalen Systems. Klin Wochenschrift. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- 2.Ellis JM, Fell MJ. Current approaches to the treatment of Parkinson’s disease. Bioorg Med Chem Lett. 2017;27:4247–4255. doi: 10.1016/j.bmcl.2017.07.075. [DOI] [PubMed] [Google Scholar]
- 3.Fox SH, Katzenschlager R, Lim S-Y, Barton B, de Bie RMA, Seppi K, Coelho M, Sampaio C. International Parkinson and Movement Disorder Society Evidence-based Medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33:1248–1266. doi: 10.1002/mds.27372. [DOI] [PubMed] [Google Scholar]
- 4.Deep Brain Stimulation for Parkinson’s Disease Study Group Deep brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345:956–963. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 5.Hamani C, Moro E, Lozano AM. The pedunculopotine nucleus as a target for deep brain stimulation. J. Neural Transm. 2011;118:1461–1468. doi: 10.1007/s00702-010-0547-8. [DOI] [PubMed] [Google Scholar]
- 6.Kopell BH, Rezai AR, Chang JW, Vitek JL. Anatomy and physiology of the basal ganglia: implications for deep brain stimulation for Parkinson’s disease. Mov Disord. 2006;21(Suppl 14):S238–246. doi: 10.1002/mds.20958. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Postupna N, Falkenberg J, Anderson ME. High frequency deep brain stimulation: what are the therapeutic mechanisms? Neurosci Biobehav Rev. 2008;32:343–351. doi: 10.1016/j.neubiorev.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Thevathasan W, Debu B, Aziz T, Bloem BR, Blahak C, Butson C, Czernecki V, Foltynie T, Fraix V, Grabli D, Joint C, Lozano AM, Okun MS, Ostrem J, Pavese N, Schrader C, Tai CH, Krauss JK, Moro E, Movement Disorders Society PPN DBS Working Group in collaboration with the World Society for Stereotactic and Functional Neurosurgery Pedunculopontine nucleus deep brain stimulation in Parkinson's disease: a clinical review. Mov Disord. 2018;33:10–20. doi: 10.1002/mds.27098. [DOI] [PubMed] [Google Scholar]
- 9.Wichmann T, DeLong MR. Deep brain stimulation for movement disorders of basal ganglia origin: restoring function or functionality? Neurotherapeutics. 2016;13:264–283. doi: 10.1007/s13311-016-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caire F, Ranoux D, Guel D, Burbaud P, Cuny E. A systematic review of studies on anatomical position of electrode contacts used for chronic subthalamic stimulation in Parkinson’s disease. Acta Neurochir. 2013;155:1647–1654. doi: 10.1007/s00701-013-1782-1. [DOI] [PubMed] [Google Scholar]
- 11.Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol. 1996;6:751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- 12.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 13.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 14.Ossowska K. The role of excitatory amino acids in experimental models of Parkinson’s disease. J Neural Transm [P-D Sect] 1994;8:39–71. doi: 10.1007/BF02250917. [DOI] [PubMed] [Google Scholar]
- 15.Vila M, Herrero MT, Levy R, Faucheux B, Ruberg M, Guillen J, Luquin MR, Guridi J, Javoy-Agid F, Agid Y, Obeso JA, Hirsch EC. Consequences of nigrostriatal denervation on the gamma-aminobutyric acidic neurons of substantia nigra pars reticulata and superior colliculus in parkinsonian syndromes. Neurology. 1996;46:802–809. doi: 10.1212/wnl.46.3.802. [DOI] [PubMed] [Google Scholar]
- 16.Hallet M. Tremor: pathophysiology. Parkinsonism Relat Disord. 2014;20:S118–S122. doi: 10.1016/S1353-8020(13)70029-4. [DOI] [PubMed] [Google Scholar]
- 17.Helmich RC. The cerebellar basis of Parkinsonian tremor; a network perspective. Mov Disord. 2018;33:219–231. doi: 10.1002/mds.27224. [DOI] [PubMed] [Google Scholar]
- 18.Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 19.Ichinohe N, More F, Shoumura K. A di-synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via central lateral nucleus of the thalamus in the rat. Brain Res. 2000;880:191–197. doi: 10.1016/s0006-8993(00)02744-x. [DOI] [PubMed] [Google Scholar]
- 20.Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. PNAS. 2010;107:8452–8456. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jwair S, Coulon P, Ruigrok TJ. Disynaptic subthalamic input to the posterior cerebellum in rat. Front Neuroanat. 2017;11:13. doi: 10.3389/fnana.2017.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benazzouz A, Breit S, Koudsie A, Pollak P, Krack P, Benabid A-L. Intraoperative microrecordings of the subthalamic nucleus in Parkinson’s disease. Mov Disord. 2002;17(Suppl. 3):S145–S149. doi: 10.1002/mds.10156. [DOI] [PubMed] [Google Scholar]
- 23.Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- 24.Du G, Zhuang P, Hallet M, Zhang YQ, Li JY, Li YJ. Properties of oscillatory neuronal activity in the basal ganglia and thalamus in patients with Parkinson’s disease. Transl Neurodegener. 2018;7:17. doi: 10.1186/s40035-018-0123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aziz TZ, Peggs D, Sambrook MA, Crossman AR. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov Disord. 1991;6:288–292. doi: 10.1002/mds.870060404. [DOI] [PubMed] [Google Scholar]
- 26.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 27.Guridi J, Herrero MT, Luquin MR, Guillen J, Obeso JA. Subthalamotomy improves MPTP-induced parkinsonism in monkeys. Stereotact Funct Neurosurg. 1994;62:98–102. doi: 10.1159/000098603. [DOI] [PubMed] [Google Scholar]
- 28.Sellal F, Hirsch E, Lisovoski F, Mutschler V, Collard M, Marescaux C. Contralateral disappearance of parkinsonian signs after subthalamic hematoma. Neurology. 1992;42:255–256. doi: 10.1212/wnl.42.1.255. [DOI] [PubMed] [Google Scholar]
- 29.Benazzouz A, Gross C, Féger J, Boraud T, Biolac B. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci. 1993;5:382–389. doi: 10.1111/j.1460-9568.1993.tb00505.x. [DOI] [PubMed] [Google Scholar]
- 30.Pollak P, Benabid AL, Gross C, Gao DM, Laurent A, Benazzouz A, Hoffmann D, Gentil M, Perret J. Effects of the stimulation of the subthalamic nucleus in Parkinson disease. Rev Neurol (Paris) 1993;149:175–176. [PubMed] [Google Scholar]
- 31.Alkemade A, Schnitzler A, Forstmann BU. Topographic organization of the human and non-human primate subthalamic nucleus. Brain Struct Funct. 2015;220:3075–3086. doi: 10.1007/s00429-015-1047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Temel Y, Blokland A, Steinbusch HWM, Visser-Vandewalle V. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiol. 2005;76:393–413. doi: 10.1016/j.pneurobio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Lanotte M, Rizzone M, Bergamasco B, Faccani G, Melcarve A, Lopiano L. Deep brain stimulation of the subthalamic nucleus: anatomical, neurophysiological, and outcome correlations with the effects of stimulation. J Neurol Neurosurg Psychiatry. 2002;72:53–58. doi: 10.1136/jnnp.72.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herzog J, Fietzek U, Hamel W, Morsnowski A, Steigerwald F, Schrader B, Weinert D, Pfister G, Müller D, Mehdorn HM, Deuschl G, Volkmann J. Most effective stimulation site in subthalamic deep brain stimulation for Parkinson's disease. Mov Disord. 2004;19:1050–1054. doi: 10.1002/mds.20056. [DOI] [PubMed] [Google Scholar]
- 35.Zonenshayn M, Sterio D, Kelly PJ, Rezai AR, Beric A. Location of the active contact within the subthalamic nucleus (STN) in the treatment of idiopathic Parkinson’s disease. Surg Neurol. 2004;62:216–226. doi: 10.1016/j.surneu.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Garcia D, Guridi J, Toledo JB, Alegre M, Obeso JA, Rodriguez-Oroz MC. Stimulation sites in the subthalamic nucleus and clinical improvement in Parkinson’s disease: a new approach for active contact localization. J Neurosurg. 2016;125:1068–1079. doi: 10.3171/2015.9.JNS15868. [DOI] [PubMed] [Google Scholar]
- 37.Mundinger F. Stereotaxic interventions on the zona incerta area for treatment of extrapyramidal motor disturbances and their results. Conf Neurol. 1965;26:222–230. doi: 10.1159/000104030. [DOI] [PubMed] [Google Scholar]
- 38.Patel NK, Heywood P, O’Sullivan K, McCarter R, Love S, Gill SS. Unilateral subthalamotomy in the treatment of Parkinson’s disease. Brain. 2003;126:1136–1145. doi: 10.1093/brain/awg111. [DOI] [PubMed] [Google Scholar]
- 39.Welter M-L, Schüpbach M, Czernecki V, Karachi C, Fernandez-Vidal S, Golmard J-L, Serra G, Navarro S, Welaratne A, Hartmann A, Mesnage V, Pineau F, Cornu P, Pidoux B, Worbe Y, Zikos P, Grabli D, Galanaud D, Bonnet A-M, Belaid H, Dormont D, Vidailhet M, Mallet L, Houeto J-L, Bardinet E, Yelnik J, Agid Y. Optimal target localization for subthalamic stimulation in patients with Parkinson disease. Neurology. 2014;82:1352–1361. doi: 10.1212/WNL.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Chazeron I, Pereira B, Chereau-Boudet I, Durif F, Lemaire JJ, Brousse G, Ulla M, Derost P, Debilly B, Llorca PM. Impact of localization of deep brain stimulation electrodes on motor and neurobehavioural outcomes in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2016;87:758–766. doi: 10.1136/jnnp-2015-310953. [DOI] [PubMed] [Google Scholar]
- 41.Yokoyama T, Sugiyama K, Nishizawa S, Yokota N, Ohta S, Akamine S, Namba H. The optimal stimulation site for chronic stimulation of the subthalamic nucleus in Parkinson’s disease. Stereotact Funct Neurosurg. 2001;77:61–67. doi: 10.1159/000064598. [DOI] [PubMed] [Google Scholar]
- 42.Henderson JM, Pell M, O'Sullivan DJ, McCusker EA, Fung VS, Hedges P, Halliday GM. Postmortem analysis of bilateral subthalamic electrode implants in Parkinson's disease. Mov Disord. 2002;17:133–137. doi: 10.1002/mds.1261. [DOI] [PubMed] [Google Scholar]
- 43.Voges J, Volkmann J, Allert N, Lehrke R, Koulousakis A, Freund H-J, Sturm V. Bilateral high-frequency stimulation in the subthalamic nucleus for the treatment of Parkinson’s disease: correlation of therapeutic effect with anatomical electrode position. J Neurosurg. 2002;96:269–279. doi: 10.3171/jns.2002.96.2.0269. [DOI] [PubMed] [Google Scholar]
- 44.Pollak P, Benabid AL, Krack P, et al. Deep brain stimulation. In: Jankovic J, Tolosa E, et al., editors. Parkinson’s disease and movement disorders. Baltimore: Williams and Wilkins; 1998. pp. 1085–1101. [Google Scholar]
- 45.Maks CB, Butson CR, Walter BL, Vitek JL, McIntyre CC. Deep brain stimulation activation volumes and their association with neurophysiological mapping and therapeutic outcomes. J Neurol Neurosurg. 2009;80:659–666. doi: 10.1136/jnnp.2007.126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kitagawa M, Murata J, Uesugi H, Kikuchi S, Saito H, Tashiro K, Sawamura Y. Two-year follow-up of chronic stimulation of the posterior subthalamic white matter for tremor-dominant Parkinson’s disease. Neurosurgery. 2005;56:281–289. doi: 10.1227/01.neu.0000148167.49105.a3. [DOI] [PubMed] [Google Scholar]
- 47.Plaha P, Ben-Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain. 2006;129:1732–1747. doi: 10.1093/brain/awl127. [DOI] [PubMed] [Google Scholar]
- 48.Merello M, Cavanagh S, Perez-Lloret S, Roldan E, Bruno V, Tenca E, Leiguarda R. Irritability, psychomotor agitation and progressive insomnia induced by bilateral stimulation of the area surrounding the dorsal subthalamic nucleus (zona incerta) in Parkinson’s disease patients. J Neurol. 2009;256:2091–2093. doi: 10.1007/s00415-009-5285-1. [DOI] [PubMed] [Google Scholar]
- 49.Plaha P, Khan S, Gill SS. Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J Neurol Neurosurg Psychiatry. 2008;79:504–513. doi: 10.1136/jnnp.2006.112334. [DOI] [PubMed] [Google Scholar]
- 50.Blomstedt P, Persson RS, Hariz G-M, Linder J, Fredricks A, Häggström B, Philipsson J, Forsgren L, Hariz M. Deep brain stimulation in the caudal zona incerta versus best medical treatment in patients with Parkinson’s disease; a randomized blinded evaluation. J Neurol Neurosurg Psychiatry. 2018;89:710–716. doi: 10.1136/jnnp-2017-317219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eisinger RS, Wong J, Almeida L, Ramirez-Zamora A, Cagle JN, Giugni JC, Ahmed B, Bona AR, Monari E, Wagle Shukla A, Hess CW, Hilliard JD, Foote KD, Gunduz A, Okun MS, Martinez-Ramirez D. Ventral intermediate nucleus versus zona incerta region deep brain stimulation in essential tremor. Mov Disord Clin Pract. 2017;5:75–82. doi: 10.1002/mdc3.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fytagoridis A, Aström M, Samuelsson J, Blomsteadt P. Deep brain stimulation of the caudal zona incerta: tremor control in relation to the location of stimulation fields. Stereotact Funct Neurosurg. 2016;94:363–370. doi: 10.1159/000448926. [DOI] [PubMed] [Google Scholar]
- 53.Karlsson F, Malinova E, Olofsson K, Blomstedt P, Linder J, Nordh E. Voice tremor outcomes of subthalamic nucleus and zona incerta deep brain stimulation in patients with Parkinson disease. J Voice. 2018;33:545–549. doi: 10.1016/j.jvoice.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 54.Plaha P, Patel NK, Gill SS. Stimulation of the subthalamic region for essential tremor. J Neurosurg. 2004;101:48–54. doi: 10.3171/jns.2004.101.1.0048. [DOI] [PubMed] [Google Scholar]
- 55.Hogg E, Wertheimer J, Graner S, Tagliati M. Deep brain stimulation and nonmotor symptoms. Int Rev Neurobiol. 2017;134:1045–1089. doi: 10.1016/bs.irn.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 56.Stefurak T, Mikulis D, Mayberg H, Lang AE, Hevenor S, Pahapill P, Saint-Cyr J, Lozano A. Deep brain stimulation for Parkinson’s disease dissociates mood and motor circuits: a functional MRI case study. Mov Disord. 2003;18:1508–1541. doi: 10.1002/mds.10593. [DOI] [PubMed] [Google Scholar]
- 57.Tommasi G, Lanotte M, Albert U, Zibetti M, Castelli L, Maina G, Lopiano L. Transient acute depressive state induced by subthalamic region stimulation. J Neurol Sci. 2008;273:135–138. doi: 10.1016/j.jns.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 58.Burrows AM, Ravin PD, Novak P, Peters MLB, Dessureau B, Swearer J, Pilitsis JG. Limbic and motor function comparison of deep brain stimulation of the zona incerta and subthalamic nucleus. Oper Neurosurg. 2012;70(Suppl 1):125–131. doi: 10.1227/NEU.0b013e318232fdac. [DOI] [PubMed] [Google Scholar]
- 59.Pagonabarraga J, Kulisewsky J, Strafella AP, Krack P. Apathy in Parkinson’s disease: diagnosis, and treatment. Lancet Neurol. 2015;14:518–531. doi: 10.1016/S1474-4422(15)00019-8. [DOI] [PubMed] [Google Scholar]
- 60.Czernecki V, Schüpbach M, Yaici S, Lévy R, Bardinet E, Yelnik J, Dubois B, Agid Y. Apathy following subthalamic stimulation in Parkinson disease a dopamine responsive symptom. Mov Disord. 2008;23:964–969. doi: 10.1002/mds.21949. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Li Y, Zhang X, Xie A. Apathy following bilateral deep brain stimulation of subthalamic nucleus in Parkinson’s disease: a meta-analysis. Parkinson’s Dis. 2018 doi: 10.1155/2018/9756468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kronenbuerger M, Zobel S, Ilgner J, Finkelmeyer A, Reinacher P, Coenen VA, Wilms H, Kloss M, Kiening K, Daniels C, Falk D, Schulz JB, Deuschl G, Hummel T. Effect of deep brain stimulation of the cerebellothalamic pathways on the sense of smell. Exp Neurol. 2010;222:144–152. doi: 10.1016/j.expneurol.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 63.Ramirez-Zamora A, Smith H, Youn Y, Durphy J, Shin DS, Pilitsis JG. Hyperhidrosis associated with subthalamic deep brain stimulation in Parkinson’s disease: insights into central autonomic functional anatomy. J Neurol Sci. 2016;366:59–64. doi: 10.1016/j.jns.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 64.Blomstedt P. Hyperhidrosis caused by deep brain stimulation in the posterior subthalamic area. J Neurol Sci. 2017;380:277–279. doi: 10.1016/j.jns.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 65.Mitrofanis J, Ashkan K, Wallace BA, Benabid AL. Chemoarchitectonic heterogeneities in the primate zona incerta: clinical and functional implications. J Neurocytol. 2004;33:429–440. doi: 10.1023/B:NEUR.0000046573.28081.dd. [DOI] [PubMed] [Google Scholar]
- 66.Watson C, Lind CRP, Thomas MG. The anatomy of the caudal zona incerta in rodents and primates. J Anat. 2014;224:95–107. doi: 10.1111/joa.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 68.Mitrofanis J. Some certainty for the “zone of uncertainty”? Exploring the function of the zona incerta. Neuroscience. 2005;130:1–15. doi: 10.1016/j.neuroscience.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 69.Paxinos G, Huang X-F, Toga AW. The rhesus monkey brain in stereotaxic coordinates. San Diego: Academic; 1999. [Google Scholar]
- 70.Hintzen A, Pelzer EA, Tittgemeyer M. Thalamic interactions of cerebellum and basal ganglia. Brain Struct Funct. 2018;223:569–587. doi: 10.1007/s00429-017-1584-y. [DOI] [PubMed] [Google Scholar]
- 71.Heise CE, Mitrofanis J. Evidence for a glutamatergic projection from the zona incerta to the basal ganglia of rats. J Comp Neurol. 2004;468:482–495. doi: 10.1002/cne.10971. [DOI] [PubMed] [Google Scholar]
- 72.Kolmac C, Mitrofanis J. Distribution of various neurochemicals within the zona incerta: an immunocytochemical and histochemical study. Anat Embryol (Berl) 1999;199:265–280. doi: 10.1007/s004290050227. [DOI] [PubMed] [Google Scholar]
- 73.Benson DL, Isackson PJ, Gall CM, Lones EG. Contrasting pattens in the localization of glutamic acid decarboxylase and Ca2+/calmodulin protein kinase gene expression in the rat central nervous system. Neuroscience. 1992;46:825–849. doi: 10.1016/0306-4522(92)90188-8. [DOI] [PubMed] [Google Scholar]
- 74.Feldblum S, Erlender MG, Tobin AJ. Different distribution of GAD65 and GAD67 mRNAs suggest that the two glutamate decarboxylases play distinctive functional roles. J Neurosci Res. 1993;34:689–706. doi: 10.1002/jnr.490340612. [DOI] [PubMed] [Google Scholar]
- 75.Nicolelis MA, Chapin JK, Lin RC. Somatotopic maps within the zona incerta relay parallel GABAergic somatosensory pathways to the neocortex, superior colliculus, and brainstem. Brain Res. 1992;577:134–141. doi: 10.1016/0006-8993(92)90546-l. [DOI] [PubMed] [Google Scholar]
- 76.Barthó P, Freund TF, Acsády L. Selective GABAergic innervation of thalamic nuclei from the zona incerta. Neuroscience. 2002;16:999–1014. doi: 10.1046/j.1460-9568.2002.02157.x. [DOI] [PubMed] [Google Scholar]
- 77.Watson GDR, Smith JB, Alloway KD. The zona incerta regulates communication between the superior colliculus and the posteromedial thalamus: implications for thalamic interactions with the dorsolateral striatum. J Neurosci. 2015;35:9463–9476. doi: 10.1523/JNEUROSCI.1606-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim U, Gregory E, Hall WC. Connections between a ventral GABAergic subdivision of zona incerta and the superior colliculus. J Comp Neurol. 1992;321:555–575. doi: 10.1002/cne.903210405. [DOI] [PubMed] [Google Scholar]
- 79.Kolmac CI, Power BD, Mitrofanis J. Patterns of connections between zona incerta and brainstem in rats. J Comp Neurol. 1998;396:544–555. doi: 10.1002/(sici)1096-9861(19980713)396:4<544::aid-cne10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 80.May PJ, Basso MA. Connections between the zona incerta and superior colliculus in the monkey and squirrel. Brain Struct Funct. 2018;223:371–390. doi: 10.1007/s00429-017-1503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.May PJ, Sun W, Hall WC. Reciprocal connections between the zona incerta and the pretectum and superior colliculus of the cat. Neuroscience. 1997;77:1091–1114. doi: 10.1016/s0306-4522(96)00535-0. [DOI] [PubMed] [Google Scholar]
- 82.Ricardo JA. Efferent connections of the subthalamic region in the rat. II. The zona incerta. Brain Res. 1981;214:43–60. doi: 10.1016/0006-8993(81)90437-6. [DOI] [PubMed] [Google Scholar]
- 83.Romanowski CAJ, Mitchell IJ, Crossman AR. The organisation of the efferent projections of the zona incerta. J Anat. 1985;143:75–95. [PMC free article] [PubMed] [Google Scholar]
- 84.Watanabe K, Kawana E. The cells of origin of the incertofugal projections to the tectum, thalamus, tegmentum and spinal cord in the rat: a study using the autoradiographic and horseradish peroxidase methods. Neuroscience. 1982;7:2389–2406. doi: 10.1016/0306-4522(82)90203-2. [DOI] [PubMed] [Google Scholar]
- 85.Alloway KD, Smith JB, Watson GD. Thalamostriatal projections from the medial posterior and parafascicular nuclei have distinct topographic and physiologic properties. J Neurophysiol. 2014;111:36–68. doi: 10.1152/jn.00399.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Di Chiara G, Porceddu ML, Morelli M, Mulas ML, Gessa GL. Evidence for a GABAergic projection from the substantia nigra to the ventromedial thalamus and to the superior colliculus of the rat. Brain Res. 1979;176:273–284. doi: 10.1016/0006-8993(79)90983-1. [DOI] [PubMed] [Google Scholar]
- 87.Gulcebi MI, Ketenci S, Linke R, Hacioglu H, Yanali H, Veliskova J, Moshé SL, Onat F, Cavdar S. Topographical connections of the substantia nigra pars reticulata to higher-order thalamic nuclei in the rat. Brain Res Bull. 2012;87:312–318. doi: 10.1016/j.brainresbull.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 88.Gallay MN, Jeanmonod D, Liu J, Morel A. Human pallidothalamic and cerebellothalamic tracts: anatomical basis for functional stereotactic neurosurgery. Brain Struct Funct. 2008;212:443–463. doi: 10.1007/s00429-007-0170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kha HT, Finkelstein ID, Pow DV, Lawrence AJ, Horne MK. Study of projections from the entopeduncular nucleus to the thalamus of the rat. J Comp Neurol. 2000;426:366–377. [PubMed] [Google Scholar]
- 90.Sakai ST, Inase M, Tanji J. Comparison of cerebellothalamic and pallidothalamic projections in the monkey (Macaca fuscata): a double anterograde labeling study. J Comp Neurol. 1996;368:215–228. doi: 10.1002/(SICI)1096-9861(19960429)368:2<215::AID-CNE4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 91.Sidibé M, Bevan MD, Bolam P, Smith Y. Efferent connections of the internal globus pallidus in the squirrel monkeys; I. Topography and synaptic organization of the pallidothalamic projections. J Comp Neurol. 1997;382:323–347. [PubMed] [Google Scholar]
- 92.Power BD, Kolmac CI, Mitrofanis J. Evidence for a large projection from the zona incerta to the dorsal thalamus. J Comp Neurol. 1999;404:554–565. [PubMed] [Google Scholar]
- 93.Lin CS, Nicolelis MA, Schneider JS, Chapin JK. A major direct GABAergic pathway from zona incerta to neocortex. Science. 1990;248:1553–1556. doi: 10.1126/science.2360049. [DOI] [PubMed] [Google Scholar]
- 94.Lin RC, Nicolelis MAL, Chapin JK. Topographical and laminar organizations of the incertocortical pathway in rats. Neuroscience. 1997;81:641–651. doi: 10.1016/s0306-4522(97)00094-8. [DOI] [PubMed] [Google Scholar]
- 95.Shaw V, Mitrofanis J. Anatomical evidence for somatotopic maps in the zona incerta of rats. Anat Embryol. 2002;206:119–130. doi: 10.1007/s00429-002-0280-7. [DOI] [PubMed] [Google Scholar]
- 96.Cha M, Ji Y, Masri R. Motor cortex stimulation activates the incertothalamic pathway in an animal model of spinal cord injury. J Pain. 2013;14:260–269. doi: 10.1016/j.jpain.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mitrofanis J, Mikuletic L. Organisation of the cortical projection to the zona incerta of the thalamus. J Comp Neurol. 1999;412:173–185. [PubMed] [Google Scholar]
- 98.Veinante P, LaVallée P, Deschenes M. Corticothalamic projections from layer 5 of the vibrissal barrel cortex in the rat. J Comp Neurol. 2000;424:197–204. doi: 10.1002/1096-9861(20000821)424:2<197::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 99.Urbain N, Deschênes M. Motor cortex gates vibrissal responses in a thalamocortical projection pathway. Neuron. 2007;56:714–725. doi: 10.1016/j.neuron.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 100.Aumann TD, Horne MK. Ramification and termination of single axons in the cerebellothalamic pathway of the rat. J Comp Neurol. 1996;376:420–430. doi: 10.1002/(SICI)1096-9861(19961216)376:3<420::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 101.Mitrofanis J, de Fonseka R. Organisation of connections between the zona incerta and the interposed nucleus. Anat Embryol (Berl) 2001;204:153–159. doi: 10.1007/s004290100187. [DOI] [PubMed] [Google Scholar]
- 102.Mitrofanis J. Distinctive patterns of connectivity between the zona incerta and the red nucleus of rats. Anat Embryol (Berl) 2002;205:283–289. doi: 10.1007/s00429-002-0259-4. [DOI] [PubMed] [Google Scholar]
- 103.Peltier AC, Bishop GA. The site of origin of calcitonin gene-related peptide-like immunoreactive afferetns to the inferior olivary complex of the mouse. Neurosci Res. 1999;34:177–186. doi: 10.1016/s0168-0102(99)00045-0. [DOI] [PubMed] [Google Scholar]
- 104.Wagner CK, Eaton MJ, Moore KE, Lookingland KJ. Efferent projections from the region of the medial zona incerta containing A13 dopaminergic neurons: a PHA-L anterograde tract-tracing study in the rat. Brain Res. 1995;677:229–237. doi: 10.1016/0006-8993(95)00128-d. [DOI] [PubMed] [Google Scholar]
- 105.Sharma S, Kim LH, Mayr KA, Elliott DA, Whelan PJ. Parallel descending dopaminergic connectivity of A13 cells to the brainstem locomotor centers. Sci Rep. 2018;8:7972. doi: 10.1038/s41598-018-25908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mogenson GJ, Swanson LW, Wu M. Evidence that projections from substantia innominata to zona incerta and mesencephalic locomotor region contribute to locomotor activity. Brain Res. 1985;334:65–76. doi: 10.1016/0006-8993(85)90568-2. [DOI] [PubMed] [Google Scholar]
- 107.Ma TP, Hu XJ, Anavi Y, Rafols JA. Organization of the zona incerta in the macaque: a Nissl and Golgi study. J Comp Neurol. 1992;320:273–290. doi: 10.1002/cne.903200302. [DOI] [PubMed] [Google Scholar]
- 108.Power BD, Mitrofanis J. Evidence for extensive inter-connections within the zona incerta in rats. Neurosci Lett. 1999;267:9–12. doi: 10.1016/s0304-3940(99)00313-4. [DOI] [PubMed] [Google Scholar]
- 109.Shaw F-Z, Liao Y-F, Chen R-F, Huang Y-H, Lin RCS. The zona incerta modulates spontaneous spike-wave discharges in the rat. J Neurophysiol. 2013;109:2505–2516. doi: 10.1152/jn.00750.2011. [DOI] [PubMed] [Google Scholar]
- 110.Mok D, Mogenson GJ. Contribution of zona incerta to osmotically induced drinking in rats. Am J Physiol. 1986;251:R823–R832. doi: 10.1152/ajpregu.1986.251.5.R823. [DOI] [PubMed] [Google Scholar]
- 111.Lucas JM, Ji Y, Masri R. Motor cortex stimulation reduces hyperalgesia in an animal model of central pain. Pain. 2011;152:1398–1407. doi: 10.1016/j.pain.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moon HC, Lee YJ, Cho CB, Park YS. Supressed GABAergic signaling in the zona incerta causes neuropathic pain in a thoracic hemisection spinal cord injury rat model. Neurosci Lett. 2016;632:55–61. doi: 10.1016/j.neulet.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 113.Moon HC, Park YS. Reduced GABAergic neuronal activity in zona incerta causes neuropathic pain in a rat sciatic nerve chronic constriction injury model. J Pain Res. 2017;10:1125–1134. doi: 10.2147/JPR.S131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grossman RG. Effect of stimulation of non-specific thalamic system on locomotor movements in cat. J Neurophysiol. 1958;21:85–93. doi: 10.1152/jn.1958.21.1.85. [DOI] [PubMed] [Google Scholar]
- 115.Milner KL, Mogenson GJ. Electrical and chemical activation of the mesencephalic and subthalamic locomotor regions in freely moving rats. Brain Res. 1988;452:273–285. doi: 10.1016/0006-8993(88)90031-5. [DOI] [PubMed] [Google Scholar]
- 116.Mogenson GJ, Wu M. Subpallidal projections to the mesencephalic locomotor region investigated with a combination of behavioral and electrophysiological recording techniques. Brain Res Bull. 1986;18:383–390. doi: 10.1016/0361-9230(86)90060-2. [DOI] [PubMed] [Google Scholar]
- 117.Parker SM, Sinnamon HM. Forward locomotion elicited by electrical stimulation in the diencephalon and mesencephalon of the awake rat. Physiol Behav. 1983;31:581–587. [PubMed] [Google Scholar]
- 118.Skinner RD, Garcia-Rill E. The mesencephalic locomotor region (MLR) in the rat. Brain Res. 1984;323:385–389. doi: 10.1016/0006-8993(84)90319-6. [DOI] [PubMed] [Google Scholar]
- 119.Wardas J, Ossowska K, Wolfarth S. Evidence for the independent role of GABA synapses of the zone incerta-lateral hypothalamic region in the haloperidol-induced catalepsy. Brain Res. 1988;462:378–382. doi: 10.1016/0006-8993(88)90569-0. [DOI] [PubMed] [Google Scholar]
- 120.König JFR, Klippel RA. The rat brain. A stereotaxic atlas of the forebrain and lower parts of the brain stem. Baltimore: Wiliams and Wilkins; 1963. [Google Scholar]
- 121.Périer C, Tremblay L, Féger J, Hirsch EC. Behavioral consequences of bicuculline injection in the subthalamic nucleus and the zona incerta in rat. J Neurosci. 2002;22:8711–8719. doi: 10.1523/JNEUROSCI.22-19-08711.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Supko DE, Uretsky NJ, Wallace LJ. Activation of AMPA/kainic acid glutamate receptors in the zona incerta stimulates locomotor activity. Brain Res. 1991;564:159–163. doi: 10.1016/0006-8993(91)91367-a. [DOI] [PubMed] [Google Scholar]
- 123.Supko DE, Uretsky NJ, Wallace LJ. AMPA/kainic acid glutamate receptor antagonism in the zona incerta dorsal to the subthalamic nucleus inhibits amphetamine-induced stereotypy but not locomotor activity. Brain Res. 1992;576:89–96. doi: 10.1016/0006-8993(92)90612-d. [DOI] [PubMed] [Google Scholar]
- 124.Wardas J, Ossowska K, Wolfarth S. The role of γ-aminobutyric acid mechanisms of the zone incerta-lateral hypothalmaus in the catalepsy and muscle rigidity evoked by morphine. Brain Res. 1987;408:363–366. doi: 10.1016/0006-8993(87)90406-9. [DOI] [PubMed] [Google Scholar]
- 125.Ossowska K, Karcz M, Wardas J, Wolfarth S. Striatal and nucleus accumbens D1/D2 dopamine receptors in neuroleptic catalepsy. Eur J Pharmacol. 1990;182:327–334. doi: 10.1016/0014-2999(90)90291-d. [DOI] [PubMed] [Google Scholar]
- 126.Ossowska K, Wardas J, Gołembiowska K, Wolfarth S. Lateral hypothalamus-zona incerta region as an output station for the catalepsy induced by the blockade of striatal D1 and D2 dopamine receptors. Brain Res. 1990;505:311–315. doi: 10.1016/0006-8993(90)91269-m. [DOI] [PubMed] [Google Scholar]
- 127.Ossowska K, Karcz-Kubicha M, Wardas J, Krężołek A, Wolfarth S. Zona incerta-lateral hypothalamus as an output structure for impulses involved in neuroleptic drug-induced catalepsy. Naunyn-Schmiedeberg’s Arch Pharmacol. 1993;347:415–420. doi: 10.1007/BF00165392. [DOI] [PubMed] [Google Scholar]
- 128.Supko DE, Uretsky NJ, Wallace LJ. AMPA glutamate receptor activation in the posterior zona incerta inhibits amphetamine- and apomorphine-induced stereotypy. Brain Res. 1992;584:213–218. doi: 10.1016/0006-8993(92)90897-i. [DOI] [PubMed] [Google Scholar]
- 129.Périer C, Vila M, Féger J, Agid Y, Hirsch EC. Functional activity of zona incerta neurons is altered after nigrostriatal denervation in hemiparkinsonian rats. Exp Neurol. 2000;162:215–224. doi: 10.1006/exnr.1999.7331. [DOI] [PubMed] [Google Scholar]
- 130.Heise CE, Mitrofanis J. Fos immunoreactivity in some locomotor neural centres of 6-OHDA-lesioned rats. Anat Embryol. 2006;211:659–671. doi: 10.1007/s00429-006-0130-0. [DOI] [PubMed] [Google Scholar]
- 131.Heise CE, Mitrofanis J. Reduction in parvalbumin expression in the zona incerta after 6OHDA lesion in rats. J Neurocytol. 2005;34:421–434. doi: 10.1007/s11068-006-8728-y. [DOI] [PubMed] [Google Scholar]
- 132.Lozano AM, Dostrovsky J, Chen R, Ashby P. Deep brain stimulation for Parkinson’s disease: disrupting the disruption. Lancet Neurol. 2002;1:225–231. doi: 10.1016/s1474-4422(02)00101-1. [DOI] [PubMed] [Google Scholar]
- 133.Chiken S, Nambu A. Mechanism of deep brain stimulation: inhibition, excitation, or disruption. Neuroscientist. 2016;22:313–322. doi: 10.1177/1073858415581986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Merello M, Tenca E, Cerquetti D. Neuronal activity of the zona incerta in Parkinson’s disease patients. Mov Disord. 2006;21:937–943. doi: 10.1002/mds.20834. [DOI] [PubMed] [Google Scholar]
- 135.Alloway KD, Smith JB, Mowery TM, Watson GDR. Sensory processing in the dorsolateral striatum: the contribution of thalamostriatal pathways. Front Syst Neurosci. 2017;11:53. doi: 10.3389/fnsys.2017.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Watson GDR, Alloway KD. Opposing collicular influences on the parafascicular (Pf) and posteromedial (POm) thalamic nuclei: relationship to POm-induced inhibition in the substantia nigra pars reticulata (SNR) Brain Struct Funct. 2018;223:535–543. doi: 10.1007/s00429-017-1534-8. [DOI] [PubMed] [Google Scholar]
- 137.Butler AB, Hodos W. Comparative vertebrate neuroanatomy: evolution and adaptation. 2. Hoboken: Wiley; 2005. [Google Scholar]
- 138.Smith JB, Mowery TM, Alloway KD. Thalamic POm projection to the dorsolateral striatum of rats: potential pathway for mediating stimulus-response association for sensorimotor habits. J Neurophysiol. 2012;108:160–174. doi: 10.1152/jn.00142.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of cortical signaling by cholinergic interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Benazzouz A, Tai CH, Meissner W, Bioulac B, Bezard E, Gross C. High-frequency stimulation of both zona incerta and subthalamic nucleus induces a normalization of basal ganglia metabolic activity in experimental parkinsonism. FASEB J. 2004;18:528–530. doi: 10.1096/fj.03-0576fje. [DOI] [PubMed] [Google Scholar]
- 141.Jordan LM. Initiation of locomotion in mammals. Ann NY Acad Sci. 1998;860:83–93. doi: 10.1111/j.1749-6632.1998.tb09040.x. [DOI] [PubMed] [Google Scholar]
- 142.Sinnamon HM. Preoptic and hypothalamic neurons and the initiation of locomotor in the anesthetized rat. Prog Neurobiol. 1993;41:323–344. doi: 10.1016/0301-0082(93)90003-b. [DOI] [PubMed] [Google Scholar]
- 143.Noga BR, Sanchez FJ, Villamil LM, O’Toole C, Kasicki S, Olszewski M, Cabaj AM, Majczyński H, Sławińska U, Jordan LM. LFP oscillations in the mesencephalic locomotor region during voluntary locomotion. Front Neural Circ. 2017;11:34. doi: 10.3389/fncir.2017.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.French IT, Muthusamy KA. A review of the pedunculopontine nucleus in Parkinson’s disease. Front Aging Neurosci. 2018;10:99. doi: 10.3389/fnagi.2018.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mitchell IJ, Clarke CE, Boyce S, Robertson RG, Peggs D, Sambrook MA, Crossman AR. Neural mechanisms underlying parkinsonian symptoms based upon regional uptake of 2-deoxyglucose in monkeys exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neuroscience. 1989;32:213–226. doi: 10.1016/0306-4522(89)90120-6. [DOI] [PubMed] [Google Scholar]
- 146.Palombo E, Porrino LJ, Bankiewicz KS, Crane AM, Sokoloff L, Kopin IJ. Local cerebral glucose utilization in monkeys with hemiparkinsonism induced by intracarotid infusion of the neurotoxin MPTP. J Neurosci. 1990;10:860–869. doi: 10.1523/JNEUROSCI.10-03-00860.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Khan S, Mooney L, Plaha P, Javed S, White P, Whone AL, Gill SS. Outcomes from stimulation of the caudal zona incerta and pedunculopontine nucleus in patients with Parkinson's disease. Br J Neurosurg. 2011;25:273–280. doi: 10.3109/02688697.2010.544790. [DOI] [PubMed] [Google Scholar]
- 148.Khan S, Javed S, Mooney L, White P, Plaha P, Whone A, Gill SS. Clinical outcome from bilateral versus unilateral stimulation of the pedunculopontine nucleus with and without concomitant caudal zona incerta region stimulation in Parkinson’s disease. Br J Neurosurg. 2012;26:722–775. doi: 10.3109/02688697.2012.659297. [DOI] [PubMed] [Google Scholar]
- 149.Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130:1596–1607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- 150.Kuramoto E, Fujiyama F, Nakamura KC, Tanaka Y, Hioki H, Kaneko T. Complementary distribution of glutamatergic cerebellar and GABAergic basal ganglia afferents to the rat motor thalamic nuclei. Eur J Neurosci. 2011;33:95–109. doi: 10.1111/j.1460-9568.2010.07481.x. [DOI] [PubMed] [Google Scholar]