Abstract
Anthropic activity in Antarctica has been increasing considerably in recent years, which could have an important impact on the local microbiota affecting multiple features, including the bacterial resistome. As such, our study focused on determining the antibiotic-resistance patterns and antibiotic-resistance genes of bacteria recovered from freshwater samples collected in areas of Antarctica under different degrees of human influence. Aerobic heterotrophic bacteria were subjected to antibiotic susceptibility testing and PCR. The isolates collected from regions of high human intervention were resistant to several antibiotic groups, and were mainly associated with the presence of genes encoding aminoglycosides-modifying enzymes (AMEs) and extended-spectrum β-lactamases (ESBLs). Moreover, these isolates were resistant to synthetic and semi-synthetic drugs, in contrast with those recovered from zones with low human intervention, which resulted highly susceptible to antibiotics. On the other hand, we observed that zone A, under human influence, presented a higher richness and diversity of antibiotic-resistance genes (ARGs) in comparison with zones B and C, which have low human activity. Our results suggest that human activity has an impact on the local microbiota, in which strains recovered from zones under anthropic influence were considerably more resistant than those collected from remote regions.
Subject terms: Antimicrobial resistance, Environmental impact
Introduction
The rise of antibiotic-resistant bacteria occurred few years after the beginning of the antibiotic era1, and is mediated either by mutations or by the horizontal transfer of foreign resistance genes among environmental and/or nosocomial bacteria. In this sense, it is well known that the environment can act as a reservoir of antibiotic-resistance genes (ARGs)2–4. Importantly, bacteria harboring AGRs can be disseminated to isolated regions and transfer these genes to endemic microorganisms5,6. Several factors related to this phenomenon have been described, in which anthropic activity and birds migration can mediate the dissemination of ARGs7–11. As such, the “One Health” initiative emerged as a global initiative oriented to generate a multidisciplinary approach to attain optimal health for humans, animals and the environment12. Accordingly, antibiotic-resistance is considered as an important threat to tackle under this new perspective.
Antarctica is considered the last pristine continent, due to its extreme weather conditions and geographical isolation13, which has allowed several ecosystems to be preserved almost unaltered. However, the presence of migrating animals and the increase in anthropogenic activity14, have favored the introduction of ARGs-harbouring bacteria13,15. Antibiotic-resistant isolates have been detected in both the South and North Poles, thus studies on the impact of human activity in these regions are highly needed in order to understand the effects of antibiotic-resistance beyond the clinical settings4,16,17. Due to the above, the aim of this study was to evaluate the antibiotic-resistance features of bacterial isolates recovered from freshwater samples collected in regions under differential anthropic influence in Fildes Peninsula, King George Island, Antarctica.
Results
Bacterial counts
Total counts of cultivable heterotrophic bacteria (CHB) from freshwater samples were 102 to 103 CFU/ml in zones A and B; whereas in zone C there were 101 CFU/ml. There were no significant differences between the counts of CHB performed at 4°C and 12°C, which could be due to the psychrotolerant characteristic of the isolates. In the case of heterotrophic bacteria with decreased susceptibility to antibiotics, we observed significant differences (p < 0.05) between the counts from zones B and C in the plates supplemented with NAL, STR, KAN and CTX. Specifically, the highest counts of bacteria with decreased susceptibility to antibiotics were from zone B in agreement with the antibiotic susceptibility patterns, as a higher number of resistant isolates was also present in this region. On the other hand, it is important to remark that there were no significant differences between zones A and B regarding CHB with decreased susceptibility, which is congruent with the susceptibility profiles previously determined (p < 0.05).
Forty-eight isolates representing different colony morphotypes (with respect to mucous phenotype, colony morphology or size, and pigment production) were recovered from zone A (42 Gram-negative and 6 Gram-positive bacteria); twenty were recovered from zone B (all Gram-negative); and thirty-four from zone C (27 Gram-negative and 7 Gram-positive).
Antibiotic resistance and ARGs
Differences were observed between the bacteria recovered from zone A and zone B, where more resistant isolates were detected, in comparison with zone C, which was defined as a remote region with lower animal and human impact (Fig. 1). Therefore, a relationship can be established between the Antarctic zones sampled and the resistance to antibiotics (p < 0.05) (Fig. 2). Accordingly, zone B showed the highest percentages of antibiotic-resistant isolates. These isolates displayed resistance to β-lactams (mainly third-generation cephalosporins) and aminoglycosides. In addition, resistance to chloramphenicol, ciprofloxacin and trimethoprim was also observed.
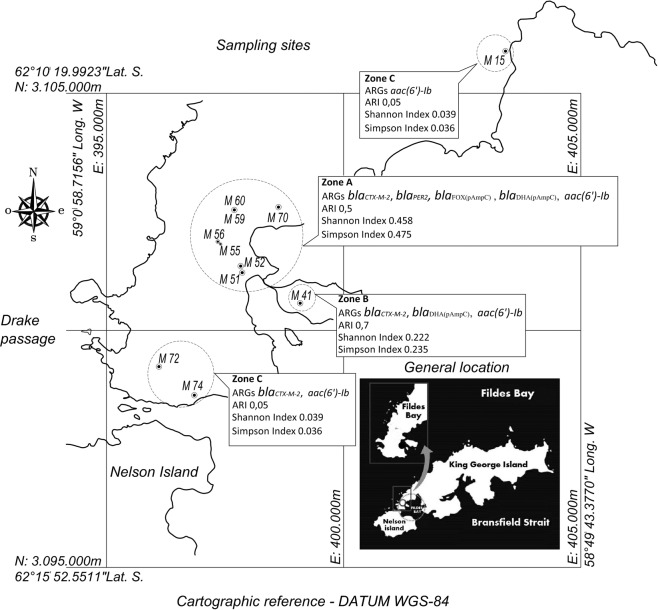
Figure 1.
Sampling sites in Peninsula Fildes showing ARGs detected, antibiotic resistance index (ARI) and richness (Simpson Index) and diversity (Shannon Index) of genes in each area. Zone A: places under human influence M51, M52, M55, M56, M59, M60, M70; Zone B: places without human influence but with possible animal influence M41; and Zone C: remote places without human or animal intervention M15, M72, M74.
Figure 2.
Percentage of antibiotic resistant strains in Antarctic areas. Antibiotics tested: ampicillin (AMP), cefalotin (CEF), cefuroxime (CXM), cefotaxime (CTX), ceftazidime (CAZ), cefepime (FEP), streptomycin (STR), kanamycin (KAN), amikacin (AMK), gentamicin (GEN), nalidixic acid (NAL), ciprofloxacin (CIP), tetracycline (TET), chloramphenicol (CHL). Antibiotics with p < 0.05 are indicated with (*).
In the case of zone A, the overall antibiotic-susceptibility patterns of the isolates were similar to zone B, but resistance to tetracycline and sulfamethoxazole was also observed. Resistance to β-lactams, aminoglycosides, ciprofloxacin and chloramphenicol was also observed in zone C.
Moreover, those isolates with inhibition zones ≤14 mm in diameter were screened for ARGs. Accordingly, in thirty-eight isolates from zone A, fifteen from zone B and seven from zone C the presence of 30 ARGs was investigated. The resistance to aminoglycosides was observed in the three zones studied, mediated by the presence of acetyltransferase-type AMEs, such as the aac(6′)-Ib gene, and resistance to beta-lactams in zones A and B was found to be due to the presence of extended-spectrum beta-lactamases (ESBL) and plasmid-mediated AmpC β-lactamases (Fig. 1). Zone A presented higher ARGs richness and diversity in comparison with zones B and C (Fig. 1). Interestingly, we determined that zone A was more dissimilar compared with zone C (Fig. 1), which could be due to the differences in anthropic activity. This could be indicating a distribution gradient of ARGs from zones under higher anthropic impact to less intervened regions.
Bacterial identification
Thirty-nine isolates were selected for identification according to ARG diversity and colony morphotypes. The Biolog System, despite having a limited database, allowed us to identify four isolates: one from zone A and three from zone B. Molecular identification (sequencing of 16S rRNA gene) was performed on isolates that could not be identified by phenotypic characterization. Thus, it was possible to establish the following strain identification: From zone A, Pseudomonas sp. (n = 2), P. veronii (n = 1), P. fluorescens (n = 2), Flavobacterium sp. (n = 2) and F. johnsoniae (n = 1). From zone B, Sphingobacterium thalpophilum (n = 1), Pseudomonas sp. (n = 1), P. fluorescens (n = 1) and P. tolaasii (n = 1). Finally, Janthinobacterium sp. (n = 1) and Hymenobacter sp. (n = 1) were identified in zone C.
Discussion
We quantified CHB recovered from freshwater samples in three zones of Antarctica, which are under different degrees of animal and human influence. The total counts of CHB were lower in zone C, which was defined as the less influenced area. These results are concordant with those published by Gonzalez-Rocha et al.18, in which they observed lower bacterial counts in remote zones in King George Island. The differences in bacterial counts could be attributed to the permanent presence of animals, such as migratory birds, in zone B. Settlements of migratory birds present in this zone could act as biological vectors of dissemination of antibiotic-resistant bacteria and ARGs from long distances19. Moreover, it is important to highlight that marine mammals also migrate long distances, increasing the probability of dissemination of these bacteria. Accordingly, resistant bacteria have been recovered from marine mammals and sharks in the west coast of the United States, of which 58% were resistant to at least one antibiotic, and 43% to more than one drug20. Despite these data, humans are more often associated to the dissemination of antibiotic-resistant bacteria. For instance, Salmonella enterica serovar Enteritidis related to human salmonellosis, has been detected in both Papua penguins (Pygoscelis papua) and Adelia penguins (Pygoscelis adeliae)21. Moreover, Pasteurella multocida, which is the etiological agent of avian cholera, has been detected in Rockhopper penguins (Eudyptes chrysocome)22, whereas other pathogenic bacteria such as Clostridium cadaveris, C. sporogenes and Staphylococcus sp. have been recovered from subcutaneous and muscular tissue of Adelia penguins23. Importantly, Antarctic migratory birds, such as skuas (Catharacta skuas) and seagulls (Larus dominicanus), whose habitats are under important anthropic influence, have been colonized by Campylobacter jejuni and Yersinia spp.24. On the other hand, we observed important differences in the antibiotic susceptibility patterns and in the bacterial richness and diversity of the ARGs detected among zones under human (zone A) and animal (Zone B) influence, in comparison with the more remote area (zone C). These differences could be due to the important influence of animals and humans that could be generating a selective pressure on the local microbiota12. It is also important to remark that the ARI indices, according to Krumperman24 showed differences between the zones, reflecting that the dissemination of the ARGs in the Antarctic environment could be influenced by the presence of both humans and animals. These results are in agreement with a previous report of ESBL-producing bacteria identified in freshwater samples collected in areas near the Bernardo O’Higgins (Antarctic Peninsula) and Arturo Prat (Greenwich Island) bases25. Even though the mechanisms of dissemination of ARGs in Antarctica are largely unknown, there is evidence that their spread is closely related to anthropogenic influence26 and to the presence of migratory animals11,27,28. Moreover, previous studies detected multidrug-resistant bacteria recovered from penguin feces in Torgensen Island and in the Palmer Station (Anvers Island)15. In addition we have previously published a study reporting E. coli resistant to STR and TET isolated from an area of Fildes Bay close to military and scientific bases14. In addition, Antelo and Batista (2013) detected bacterial isolates collected in Antarctica with high levels of antibiotic resistance, including aminoglycosides, β-lactams and trimethoprim, which is consistent with our findings29.
Interestingly, we detected isolates that were resistant to synthetic or semisynthetic antibiotics, such as SUL and TMP, in the zones with higher human activity, suggesting that both phenomena could be linked. While the data on antibiotic-resistance in Antarctic freshwater are scarce, a single report of Enterococcus sp. detected near Davis Station suggests that the discharge of insufficiently treated residual waters is introducing human pathogens that harbor ARGs into the Antarctic ecosystem30. The role of residual water is highly relevant since it is well known that resistant bacteria, ARGs and antibiotic debris can be disseminated through human feces. This was demonstrated by a study published by Karkman et al.31, in which the abundance of ARGs was correlated with fecal contamination and was not related to antibiotic selective pressure.
In the case of aminoglycosides resistance, we detected several AMEs, which could explain the resistant phenotypes observed among isolates. Our results revealed that aminoglycosides-acetylating enzymes were predominant among the resistant isolates. These enzymes have been previously identified in environmental isolates, in agreement with our results4. AMEs are normally plasmid-encoded, and also associated with transposons and integrons, which might contribute to their dissemination32. We screened for aac(6)-Ib and acc(3)-IIa genes, which account for resistance to KAN, TOB and AMK, and to GEN and TOB, respectively33,34. According to antibiotic-susceptibility patterns we detected resistance to GEN, STR, KAN and AMK in zones A, B and C. The presence of aac(6′)-Ib was detected in all areas and can explain the resistance to KAN and AMK. Interestingly, this gene has been commonly detected in Gram negative bacteria associated with humans, such as E. coli and P. aeruginosa35 and may represent a modification of the local resistome. A large number of genes can confer streptomycin resistance, including the phosphotransferase aph(6)-Ia gene (also named strA) and the aph(6)-Id gene (also named strB) which appear to be widely distributed in Gram-negative bacteria. strA-strB has been identified in bacteria circulating in humans, animals, and plants and these genes are frequently located on plasmids36.
Several β-lactamase genes were identified in our study; specifically, we detected the ESBL genes37–40 blaCTX-M2 and blaPER-2, and the plasmid-mediated AmpC β-lactamase genes pAmpCDHA, pAmpCFOX in zone A, while blaCTX-M2 and pAmpCDHA were identified in zone B. These enzymes mediate resistance to clinically relevant cephalosporins41–43, and were present in areas under human and wildlife influence. Interestingly, no β-lactamase genes were detected in zone C, where the collected isolates were considerably more susceptible to β-lactams. These findings suggest that these ARGs were introduced by either humans or animals into zones A and B. Our results are congruent with previous reports, in which ESBLs genes were detected in isolates collected in regions near scientific bases in Antarctica and native bacteria did not present any ARGs26.
According to the ARGs diversity analysis, we demonstrated that there is a gradient of richness and diversity from the less remote areas, where it is higher, to the more remote zones, reaffirming that ARGs are less prevalent in isolated regions. Similarly, Berglund9 demonstrated that ARGs and integrons were more prevalent in regions with anthropic activity, which includes the presence of residual water. Importantly, there is evidence that ARGs are present in the environment and are disseminated among bacteria44. Furthermore, it is important to remark that Antarctic bacteria are able to maintain and potentially disseminate ARGs, where it is possible that local microbiota could harbor naturally occurring ARGs, which could be potentially transmitted among bacteria45. It is difficult to measure the risk from the presence of antibiotic-resistant bacteria in this environment for both human and wildlife because there is a lack of data about the prevalence and persistence of ARGs in the environment46.
Even though more research is needed to achieve a better understanding of the dissemination routes of ARGs, our results suggest that human activity, together with migratory birds, could contribute to this phenomenon. These findings are illustrate the importance of the One Health approach, in which multi-disciplinary efforts are required to control the spread of ARGs and resistant bacteria among different environments12.
Conclusions
Our findings show that the presence of antibiotic-resistance bacteria, and therefore ARGs, are more predominant in the zones of Fildes Peninsula that are more influenced by both humans and wildlife in comparison with remote areas. Moreover, it is very interesting to remark the presence of resistance to synthetic and semisynthetic antibiotics, which was identified in zones associated to human activity, suggesting that these resistant isolates could be linked with the presence of humans.
Methods
Sampling sites
Eleven freshwater samples were collected during the 49th Antarctic Scientific Expedition (ECA49), January 2013. The samples were obtained from three areas: under human influence (zone A), animal influence (zone B) and areas with low animal and human influence (zone C), which are illustrated in Fig. 1. All the samples were transported on ice to the laboratory in Professor Julio Escudero Scientific Base (Chilean Antarctic Institute) and processed within 6 h from collection.
Bacterial counts
Total counts of cultivable heterotrophic bacteria (CHB) were performed by the surface dissemination method in R2A agar (Merck, Darmstadt, Germany) supplemented with cycloheximide (50 µg/ml)47,48. Additionally, total counts of CHB with decreased susceptibility to antibiotics were carried out with the same methodology, but using plates supplemented with: nalidixic acid (NAL) (0.5 µg/mL), ciprofloxacin (CIP) (0.5 µg/mL), tetracycline (TET) (4 µg/mL), ampicillin (AMP) (4 µg/mL), cefotaxime (CTX) (0.5 µg/mL), kanamycin (KAN) (8 µg/mL), streptomycin (STR) (0.5 µg/mL), erythromycin (ERY) (4 µg/mL), sulfamethoxazole (SUL) (128 µg/mL), and trimethoprim (TMP) (4 µg/mL). The plates were incubated at 4°C during 15 days and at 15°C for 7 days. Different bacteria morphotypes were selected, according to their macroscopic and microscopic characteristics, and were preserved in a R2A broth with glycerol (50% v/v) at −80°C.
Antibiotic susceptibility testing
Susceptibility tests were carried out by the disc diffusion method according to the CLSI guidelines48 using R2A as a replacement for Mueller-Hinton agar, except for TMP and SUL. The antibiotics tested were AMP (10 μg), CEF (30 μg), CXM (30 μg), CTX (30 µg), CAZ (30 μg), FEP (30 μg), STR (10 µg), KAN (30 µg), AMK (30 μg), GEN (10 μg), NAL (30 µg), CIP (5 µg), TET (30 µg) and chloramphenicol (CHL) (30 μg), and the plates were incubated at 15°C for 48 h. Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923 and Pseudomonas aeruginosa ATCC 27853 strains were used as susceptibility controls. Inhibition areas ≤14 mm in diameter were considered as breakpoints to define resistance. The antibiotic resistance index (ARI) was determined according to Krumperman et al.24.
Antibiotic resistance genes (ARGs)
Total bacterial DNA was extracted using the InstaGene matrix (Bio-Rad), according to the manufacturer’s instructions. ARGs were screened by conventional PCR using the primers listed in Table 1, covering diverse antibiotic groups.
Table 1.
Oligonucleotides used in the detection of antibiotic resistance genes.
| Gene | Primers | Nucleotide sequence (5′-3′) | Product size (bp) | Reference |
|---|---|---|---|---|
| 16S rRNA |
P0(16s) P6(16s) |
GAGAGTTTGATCCTGGCTCAG CTACGGCTACCTTGTTACG | 1400 | 49 |
| blaTEM |
TEMR TEMF |
TGGGTGCACGAGTGGGTTAC TTATCCGCCTCCATCCAGTC |
526 | 52 |
| blaSHV |
SHVR SHVF |
CTGGGGAAACGGAACTGAAATG GGGGTATCCCGCAGATAAAT |
389 | 53 |
| blaCTX-M-1 |
m-CTX-MG1R m-CTX-MG1F |
AAAAATCACTGCGCCAGTTC AGCTTATTCATCGCCACGTT | 551 | 54 |
| blaCTX-M-2 |
m-CTX-MG2R m-CTX-MG2F |
CGACGCTACCCCTGCTATT CCAGCGTCAGATTTTTCAGG | 742 | 54 |
| blaCTX-M-8 |
m-CTX-MG8R m-CTX-MG8F |
TCGCGTTAAGCGGATGATGC AACCCACGATGTGGGTAGC | 923 | 54 |
| blaCTX-M-9 |
m-CTX-MG9R m-CTX-MG9F |
CAAAGAGAGTGCAACGGATG ATTGGAAAGCGTTACTCACC | 803 | 54 |
| blaCTX-M-25 |
m-CTX-MG25R m-CTX-MG25F |
GCACGATGACATTCGGG AACCCACGATGTGGGTAGC | 876 | 54 |
| blaMOX-1, blaMOX-2, blaCMY-1, blaCMY-8 to blaCMY-11 |
MOXMR MOXMF |
CAC ATT GAC ATA GGT GTG GTG C GCT GCT CAA GGA GCA CAG GAT |
520 | 55 |
| blaLAT-1 to blaLAT-4, blaCMY-2 to blaCMY-7, blaBIL-1 |
CITMF CITR |
TGG CCA GAA CTG ACA GGC AAA TTT CTC CTG AAC GTG GCT GGC |
462 | 55 |
| blaDHA-1, blaDHA-2 |
DHAMF DHAMR |
AAC TTT CAC AGG TGT GCT GGG T CCG TAC GCA TAC TGG CTT TGC |
405 | 55 |
| blaACC |
ACCMF ACCMR |
AAC AGC CTC AGC AGC CGG TTA TTC GCC GCA ATC ATC CCT AGC |
346 | 55 |
| blaMIR-1T, blaACT-1 |
EBCMF EBCMR |
TCG GTA AAG CCG ATG TTG CGG CTT CCA CTG CGG CTG CCA GTT |
302 | 55 |
| blaFOX-1 to blaFOX-5b |
FOXMR FOXMF |
AAC ATG GGG TAT CAG GGA GAT G CAA AGC GCG TAA CCG GAT TGG |
190 | 55 |
| blaPER-2 |
PER-2 F PER-2REV |
GTAGTATCAGCCCAATCCCC CCAATAAAGGCCGTCCATCA | 738 | 56 |
| floR |
FloF FloR |
AATCACGGGCCACGCTGTATC CGCCGTCATTCTTCACCTTC |
215 | 57 |
| sul1 |
Sul1F Sul1R |
GTATTGCGCCGCTCTTAGAC CCGACTTCAGCTTTTGAAGG |
408 | 58 |
| sul2 |
Sul2F Sul2R |
GAATAAATCGETCATCATTTTCGG CGAATTCTTGCGGTTTCTTTCAGC |
810 | 59 |
| sul3 |
Sul3F Sul3R |
GAGCAAGATTTTTGGAATCG CATCTGCAGCTAACCTAGGGCTTTGGA | 790 | 60 |
| drfA6 | dfrIb |
GAGCAGCTICTITTIAAAGC TTAGCCCTTTIICCAATTTT |
393 | 61 |
| drfA1 |
D1 D2 |
ACGGATCCTGGCTGTTGGTTGGACGC CGGAATTCACCTTCCGGCTCGATGTC |
257 | 62 |
Species identification
Thirty-nine isolates harboring ARGs were selected for identification. They were initially run through the Biolog identification system (Biolog Inc.) using the MicroLog 1 software, following the manufacturer’s protocol. A probability >95% was set as threshold for species identification. Amplification and sequencing of 16S rRNA gene49 by conventional PCR using universal primers (Table 1) was performed on those isolates that could not be identified by the Biolog system. The sequences were compared against the National Center for Biotechnology Information (NCBI) nucleotide database using BLAST50.
Statistical analyses
All statistical analyses were performed using the IBM SPSS Statistics software (v23.0, SPSS Inc®, Chicago, IL, United States). The Student’s t-test for independent samples was used to compare the mean values of the tested parameters for all the different temperatures. In addition, one-way ANOVA and the Tukey’s multiple range tests were applied in order to compare the values of the tested parameters for all the different sampling sites. The p-value <0.05 was established for the statistical significance.
Pearson’s Chi-square test was applied to identify associations between the origin of strain and antibiotic resistance. The p-value <0.05 was established for the statistical significance.
In order to compare the sampled zones in terms of richness and diversity of ARGs, we built a binary matrix (multidimensional scaling, MDS) utilizing the Primer 6 software package51. Specifically, both richness and diversity were calculated by the Shannon-Wiener and Simpson’s indices. Genetic similarity among the strains was determined by parametric dimensional scaling based on the Bray-Curtis coefficient.
Acknowledgements
The contribution of Chilean Antarctic Institute (INACH project RT_06-12) to the funding of this study is greatly appreciated.
Author contributions
H.B.-T., L.V., M.D. and G.G.-R. managed the resources to carry out the research and made important contributions to the design of the work. G.G.-R. and L.V. obtained the samples in the field work in the Antarctica. D.J., C.C., M.Q.-A., A.O.-C., C.A.L. and P.F. contributed to the acquisition, analysis, and interpretation of the data. All authors provided approval for publication of the content and contributed drafting the work and critically revisiting the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tin T, et al. Impacts of local human activities on the Antarctic environment. Antarct. Sci. 2009;21:3–33. doi: 10.1017/S0954102009001722. [DOI] [Google Scholar]
- 2.Martinez JL. Antibiotics and Antibiotic Resistance Genes in Natural Environments. Science. 2008;321:365–367. doi: 10.1126/science.1159483. [DOI] [PubMed] [Google Scholar]
- 3.Walsh F, Duffy B. The Culturable Soil Antibiotic Resistome: A Community of Multi-Drug Resistant Bacteria. PLoS One. 2013;8:65567. doi: 10.1371/journal.pone.0065567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Goethem MW, et al. A reservoir of ‘historical’ antibiotic resistance genes in remote pristine Antarctic soils. Microbiome. 2018;6:40. doi: 10.1186/s40168-018-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lighthart B, Shaffer BT. Viable bacterial aerosol particle size distributions in the midsummer atmosphere at an isolated location in the high desert chaparral. Aerobiologia. 1995;11:19–25. doi: 10.1007/BF02136140. [DOI] [Google Scholar]
- 6.Levy SB, Bonnie M. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004;10:122. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 7.Bengtsson-Palme J, Kristiansson E, Larsson DGJ. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2018;42:68–80. doi: 10.1093/femsre/fux053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knapp CW, Dolfing J, Ehlert PAI, Graham DW. Evidence of increasing antibiotic resistance gene abundances in archived soils since 1940. Environ. Sci. Technol. 2010;44:580–587. doi: 10.1021/es901221x. [DOI] [PubMed] [Google Scholar]
- 9.Berglund B. Environmental dissemination of antibiotic resistance genes and correlation to anthropogenic contamination with antibiotics. Infect. Ecol. Epidemiol. 2015;5:28564. doi: 10.3402/iee.v5.28564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen HK, Moe LA, Rodbumrer J, Gaarder A, Handelsman J. Functional metagenomics reveals diverse β-lactamases in a remote Alaskan soil. ISME J. 2009;3:243. doi: 10.1038/ismej.2008.86. [DOI] [PubMed] [Google Scholar]
- 11.Wu J, Huang Y, Rao D, Zhang Y, Yang K. Evidence for environmental dissemination of antibiotic resistance mediated by wild birds. Front. Microbiol. 2018;9:745. doi: 10.3389/fmicb.2018.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rousham EK, Unicomb L, Islam MA. Human, animal and environmental contributors to antibiotic resistance in low-resource settings: Integrating behavioural, epidemiological and one health approaches. Proc. R. Soc. B Biol. Sci. 2018;285:20180332. doi: 10.1098/rspb.2018.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowan DA, et al. Non-indigenous microorganisms in the Antarctic: Assessing the risks. Trends Microbiol. 2011;19:540–548. doi: 10.1016/j.tim.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Rabbia V, et al. Antibiotic resistance in Escherichia coli strains isolated from Antarctic bird feces, water from inside a wastewater treatment plant, and seawater samples collected in the Antarctic Treaty area. Polar Sci. 2016;10:123–131. doi: 10.1016/j.polar.2016.04.002. [DOI] [Google Scholar]
- 15.Miller RV, Gammon K, Day MJ. Antibiotic resistance among bacteria isolated from seawater and penguin fecal samples collected near Palmer Station, Antarctica. Can. J. Microbiol. 2009;55:37–45. doi: 10.1139/W08-119. [DOI] [PubMed] [Google Scholar]
- 16.Sjölund M, et al. Dissemination of multidrug-resistant bacteria into the arctic. Emerg. Infect. Dis. 2008;14:70. doi: 10.3201/eid1401.070704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudha A, Augustine N, Thomas S. Emergence of multi-drug resistant bacteria in the Arctic,79N. J. Cell. Life Sci. 2013;1:1–5. [Google Scholar]
- 18.González-Rocha G, et al. Diversity structure of culturable bacteria isolated from the Fildes Peninsula (King George Island, Antarctica): A phylogenetic analysis perspective. PLoS One. 2017;12:e0179390. doi: 10.1371/journal.pone.0179390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen HK, et al. Call of the wild: Antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 2010;8:251. doi: 10.1038/nrmicro2312. [DOI] [PubMed] [Google Scholar]
- 20.Rosenblatt-Farrell, N. The landscape of antibiotic resistance. Environ. Health Perspect. A244–A250, 10.1289/ehp.117-a244 (2009). [DOI] [PMC free article] [PubMed]
- 21.Aronson RB, Thatje S, Mcclintock JB, Hughes KA. Anthropogenic impacts on marine ecosystems in Antarctica. Ann. N. Y. Acad. Sci. 2011;1223:82–107. doi: 10.1111/j.1749-6632.2010.05926.x. [DOI] [PubMed] [Google Scholar]
- 22.De Lisle GW, Stanislawek WL, Moors PJ. Pasteurella multocida infections in rockhopper penguins (Eudyptes chrysocome) from Campbell Island, New Zealand. J. Wildl. Dis. 1990;26:283–285. doi: 10.7589/0090-3558-26.2.283. [DOI] [PubMed] [Google Scholar]
- 23.Nievas VF, Leotta GA, Vigo GB. Subcutaneous clostridial infection in Adelie penguins in Hope Bay, Antarctica. Polar Biol. 2007;30:249–252. doi: 10.1007/s00300-006-0179-5. [DOI] [Google Scholar]
- 24.Krumperman PH. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 1983;46:165–70. doi: 10.1128/AEM.46.1.165-170.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernández J, et al. Human-associated extended-spectrum β-lactamase in the Antarctic. Appl. Environ. Microbiol. 2012;78:2056–2058. doi: 10.1128/AEM.07320-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernández J, González-Acuña D. Anthropogenic antibiotic resistance genes mobilization to the polar regions. Infect. Ecol. Epidemiol. 2016;6:32112. doi: 10.3402/iee.v6.32112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yogui GT, Sericano JL. Levels and pattern of polybrominated diphenyl ethers in eggs of Antarctic seabirds: Endemic versus migratory species. Environ. Pollut. 2009;157:957–980. doi: 10.1016/j.envpol.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Müller F, et al. Towards a conceptual framework for explaining variation in nocturnal departure time of songbird migrants. Mov. Ecol. 2016;4:24. doi: 10.1186/s40462-016-0089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antelo, V. & Batista, S. Presencia de integrones clase I en comunidades bacterianas terrestres de la Isla Rey Jorge. Avances en ciencia Antártica latinoamericana. Libro De Resúmenes Vii Congreso Latinoamericano De Ciencia Antártica VII, 59–62 (2013).
- 30.Spence, R. Distribution and taxonomy of Enterococci from the Davis Station wastewater discharge, Antarctica. (Doctoral dissertation, Queensland University of T (2014).
- 31.Karkman A, Pärnänen K, Larsson DGJ. Fecal pollution can explain antibiotic resistance gene abundances in anthropogenically impacted environments. Nat. Commun. 2019;10:80. doi: 10.1038/s41467-018-07992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guzmán M, et al. Identificación de genes que codifican enzimas modificadoras de aminoglucósidos en cepas intrahospitalarias de Klebsiella pneumoniae. Rev. la. Soc. Venez. Microbiol. 2016;36:10–15. [Google Scholar]
- 33.Vakulenko SB, Mobashery S. Versatility of aminoglycosides and prospects for their future. Clin. Microbiol. Rev. 2003;16:430–450. doi: 10.1128/CMR.16.3.430-450.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mella S, et al. Aminoglucósidos-aminociclitoles: Características estructurales y nuevos aspectos sobre su resistencia Aminoglycosides-aminocyclitols: Structural characteristics and new aspects on resistance. Rev. Chil. Infect. 2004;21:330–338. [Google Scholar]
- 35.Chow JW, et al. Aminoglycoside resistance genes aph (2″)-Ib and aac(6′)-Im detected together in strains of both Escherichia coli and Enterococcus faecium. Antimicrob. Agents Chemother. 2001;45:2691–2694. doi: 10.1128/AAC.45.10.2691-2694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pezzella C, Ricci A, Di Giannatale E, Luzzi I, Carattoli A. Tetracycline and streptomycin resistance genes, transposons, and plasmids in Salmonella enterica isolates from animals in Italy. Antimicrob. Agents Chemother. 2004;48:903–908. doi: 10.1128/AAC.48.3.903-908.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossolini GM, D’Andrea MM, Mugnaioli C. The spread of CTX-M-type extended-spectrum β-lactamases. Clin. Microbiol. Infect. 2008;14:33–41. doi: 10.1111/j.1469-0691.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- 38.Cantón R, Coque TM. The CTX-M β-lactamase pandemic. Curr. Opin. Microbiol. 2006;9:466–475. doi: 10.1016/j.mib.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: A clinical update. Clin. Microbiol. Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonnet R. Growing Group of Extended-Spectrum β-Lactamases: The CTX-M Enzymes. Antimicrob. Agents Chemother. 2004;48:1–14. doi: 10.1128/AAC.48.1.1-14.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rojas A. Revisión de la bibliografía sobre AmpC: Una importante β-lactamasa. Rev. Médica del. Hosp. Nac. Niños Dr. Carlos Sáenz Herrera. 2005;40:59–67. [Google Scholar]
- 42.Martínez Rojas, D. D. V. Betalactamasas tipo AmpC: generalidades y métodos para detección fenotípica. Rev. la Soc. Venez. Microbiol. 78–83 (2009).
- 43.Centrón, D., Ramírez, M. S., Merkier, A. K., Almuzara, M. & Vay, C. Analizan la diseminación de determinantes de resistencia a antibióticos del género Shewanella en el ambiente hospitalario. Salud Cienc.18, 651–652 (2011).
- 44.Davison J. Genetic exchange between bacteria in the environment. Plasmid. 1999;42:73–91. doi: 10.1006/plas.1999.1421. [DOI] [PubMed] [Google Scholar]
- 45.Vaz-Moreira I, Nunes OC, Manaia CM. Bacterial diversity and antibiotic resistance in water habitats: Searching the links with the human microbiome. FEMS Microbiol. Rev. 2014;38:761–778. doi: 10.1111/1574-6976.12062. [DOI] [PubMed] [Google Scholar]
- 46.Kümmerer K. Antibiotics in the aquatic environment - A review - Part I. Chemosphere. 2009;74:417–434. doi: 10.1016/j.chemosphere.2008.11.086. [DOI] [PubMed] [Google Scholar]
- 47.Clesceri, L., Greenberg, A. & Eaton, A. Standard Methods for the Examination of Water and Wastewater. American Public Health Association (1999).
- 48.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. Performance Standards for Antimicrobial susceptibility Testing; Twenty-Fourth Informational Supplement (2014).
- 49.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991;173:697–703. doi: 10.1128/JB.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 51.Mellado C, Campos V, Mondaca MA. Distribución de genes de resistencia a arsénico en bacterias aisladas de sedimentos con concentraciones variables del metaloide. Gayana. 2011;75:131–37. [Google Scholar]
- 52.Tenover, et al. D. H. Development of PCR assays to detect ampicillin resistance genes in cerebrospinal fluid samples containing Haemophilus influenzae. J. Clin. Microbiol. 1994;32:2729–2737. doi: 10.1128/JCM.32.11.2729-2737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bello-Toledo. Bases genéticas de la resistencia a cefalosporinas de tercera generación en cepas de Klebsiella pneumoniae subespecie pneumoniae aisladas de hospitales chilenos. Tesis para optar al grado de Doctor en Ciencias Biológicas, área Biología Celular y Molecular, Universidad de Chile. 175 (2005).
- 54.Woodford N. Rapid characterization of beta-lactamases by multiplex PCR. Methods Mol. Biol. 2010;642:181–192. doi: 10.1007/978-1-60327-279-7_14. [DOI] [PubMed] [Google Scholar]
- 55.Pérez-Pérez FJ, Hanson ND. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 2002;40:2153–2162. doi: 10.1128/JCM.40.6.2153-2162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Melano R. Multiple antibiotic-resistance mechanisms including a novel combination of extended-spectrum -lactamases in a Klebsiella pneumoniae clinical strain isolated in Argentina. J. Antimicrob. Chemother. 2003;52:36–42. doi: 10.1093/jac/dkg281. [DOI] [PubMed] [Google Scholar]
- 57.Bolton LF, Kelley LC, Lee MD, Fedorka-Cray PJ, Maurer JJ. Detection of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 based on a gene which confers cross-resistance to florfenicol and chloramphenicol. J. Clin. Microbiol. 1999;37:1348–1351. doi: 10.1128/JCM.37.5.1348-1351.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosser SJ, Young HK. Identification and characterization of class 1 integrons in bacteria from an aquatic environment. J. Antimicrob. Chemother. 1999;44:11–18. doi: 10.1093/jac/44.1.11. [DOI] [PubMed] [Google Scholar]
- 59.Grape M, Sundström L, Kronvall G. Sulphonamide resistance gene sul3 found in Escherichia coli isolates from human sources. J. Antimicrob. Chemother. 2003;52:1022–1024. doi: 10.1093/jac/dkg473. [DOI] [PubMed] [Google Scholar]
- 60.Perreten V, Boerlin P. A new sulfonamide resistance gene (sul3) in Escherichia coli is widespread in the pig population of Switzerland. Antimicrob. Agents Chemother. 2003;47:1169–1172. doi: 10.1128/AAC.47.3.1169-1172.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Navia MM, Ruiz J, Sanchez-Cespedes J, Vila J. Detection of dihydrofolate reductase genes by PCR and RFLP. Diagn. Microbiol. Infect. Dis. 2003;46:295–298. doi: 10.1016/S0732-8893(03)00062-2. [DOI] [PubMed] [Google Scholar]
- 62.Lee JC, et al. The prevalence of trimethoprim-resistance-conferring dihydrofolate reductase genes in urinary isolates of Escherichia coli in Korea. J. Antimicrob. Chemother. 2001;47:599–604. doi: 10.1093/jac/47.5.599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.