Abstract
Background:Vitamin D is one of the considerable environmental factors exhibiting immunomodulatory and anti-inflammatory effects.
Objective: To conduct a systematic review and meta-analysis to estimate the effect of vitamin D supplements on IL-10 and INFγ levels in patients with multiple sclerosis.
Methods: We searched PubMed, Scopus, EMBASE, CINAHL, Web of Science, Ovid, The Cochrane Library and gray literature, including references of selected studies, conference abstracts which were published up to May 2019. We included single- or double-blinded RCTs or open-label trials in which one of the main outcomes was INFγ and/ or IL-10 levels after vitamin D supplementation. Only articles that had been published in English were included.
Results: The literature search yielded 369 articles, that were monitored by us. After eliminating duplicates, 128 studies remained; from these, we excluded observational studies, reviews, case reports and non-randomized trials, and 33 studies remained. Finally, only three articles were included. The mean difference for INFγ was 268.4 and 95 % CI 200.6-336.1. There was no significant heterogeneity (I ² = 0 %, Chi ² = 0.1, p = 0.7). The mean difference for IL-10 was 398.3 and 95% CI -528.05-1324.8). There was significant heterogeneity (I2 = 94 %, Chi2 = 31.1 p < 0.001).
Conclusion: The results of this systematic review were not satisfactory. More clinical trials are further needed to evaluate the effects of vitamin D supplements on IL-10 and INFγ levels in patients with multiple sclerosis.
Keywords:multiple sclerosis, systematic review, vitamin D, chemokines.
INTRODUCTION
Multiple sclerosis (MS) is an inflammatory demyelinating disease affecting the central nervous system and leading to a wide range of disabilities (1). Women are affected more than men and both genetics and environmental factors have roles in disease development (2). T helper 1 cells producing INFγ have been considered to be involved in the disease development (3). On the other hand, IL-10 is a regulatory cytokine controlling inflammatory progressions (4). The exact effects of IL-10 are not clear, while the literature shows that in the relapsing phase of MS, the number of IL-10-positive T cells decrease and promoter polymorphism is related to the disease severity (5, 6).
Vitamin D is one of the considerable environmental factors which has immunomodulatory and anti-inflammatory effects (7, 8). Lower serum levels of this vitamin have been considered as a risk factor of MS along with higher risk of disease activity (8, 9). It is considered that vitamin D modulate the immune system by prohibiting T cell proliferation and inhibiting pro-inflammatory cytokines production such as INFγ as well as regulating the transcription of IL-10 gene resulting in IL-10 level elevation (10-12).
The aim of this systematic review and meta-analysis is to evaluate the effect of vitamin D supplements on IL-10 and INFγ levels in MS, as there is no published systematic review on this specific topic so far.
METHODS
The protocol of this systematic review has been already published (13).
Literature search. We searched PubMed, Scopus, EMBASE, CINAHL, Web of Science, Ovid, The Cochrane Library and gray literature including reference of the selected studies, conference abstracts which were published up to May 2019.
Inclusion and exclusion criteria We included single- or double-blinded RCTs or open-label trials in which one of the main outcomes was INFγ and/ or IL-10 levels after vitamin D supplementation. Only articles that had been published in English were included. Studies comparing high and low dose vitamin D therapies as well as cohort studies, case-control studies, and any other types of studies were excluded.
Data extraction Two independent researchers independently assessed the articles. Data on the number of participants in each group, INFγ and/ or IL-10 levels in each treatment arm, study duration, first author, publication year and sample size were extracted from the included studies. In case of disagreement, the two researchers searched for a third reviewer’s opinion.
Statistical analysis All statistical analyses were performed using STATA Version 13.0 (Stata Corp LP, College Station, TX, USA).
We used the inverse variance with random effects model.
The mean difference was calculated for comparisons.
Inconsistency (I²) was calculated to determine heterogeneity.
Risk of bias assessment. We evaluated the risk of potential bias by using a specific tool of Cochrane Collaboration for assessing such risk (14).
A p value less than 0.05 was considered statistically significant.
RESULTS
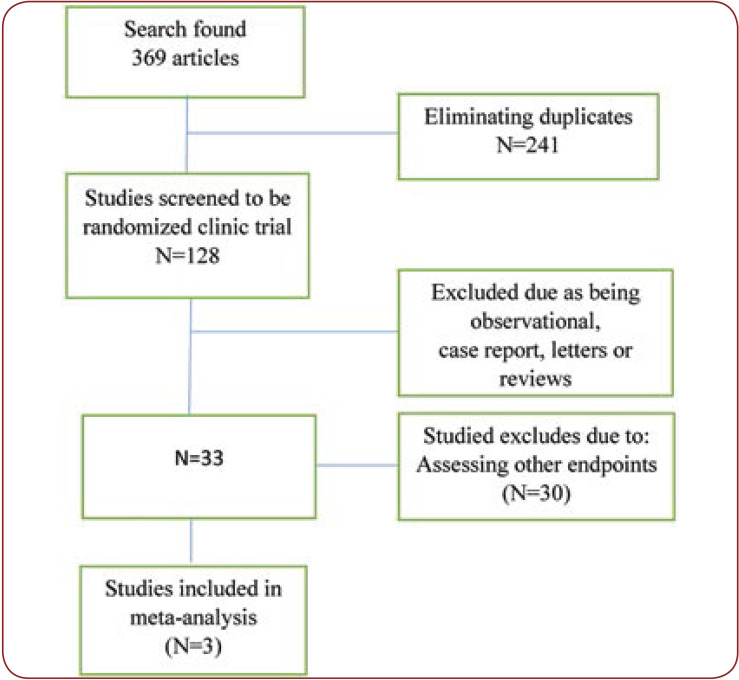
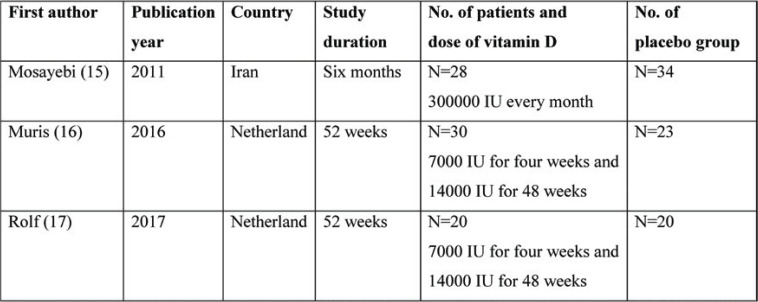
The literature search found 369 articles that were monitored. After eliminating duplicates, 128 studies remained; from these, we excluded observational studies, reviews, case reports and non-randomized trials, and 33 studies remained. Finally, only three articles were included for analysis (two for INFγ and three for IL-10) (Figure 1). Characteristics of included articles are summarized in Table 1.
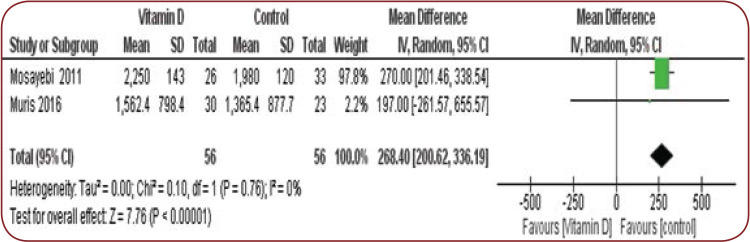
The mean difference for INFγ was 268.4 and 95% CI 200.6-336.1.
There was no significant heterogeneity (I² =0%, ChI² =0.1, p =0.7) (Figure 2).
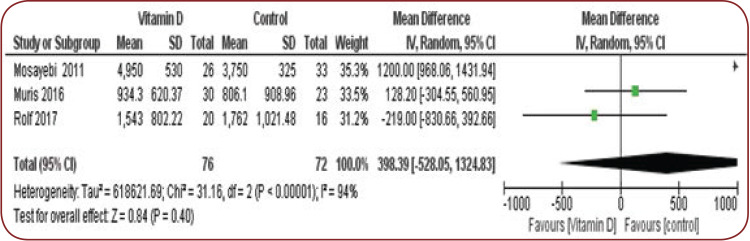
The mean difference for IL-10 was 398.3 and 95% CI -528.05-1324.8.
There was a significant heterogeneity (I² =94%, ChI² =31.1 p<0.001) (Figure 3).
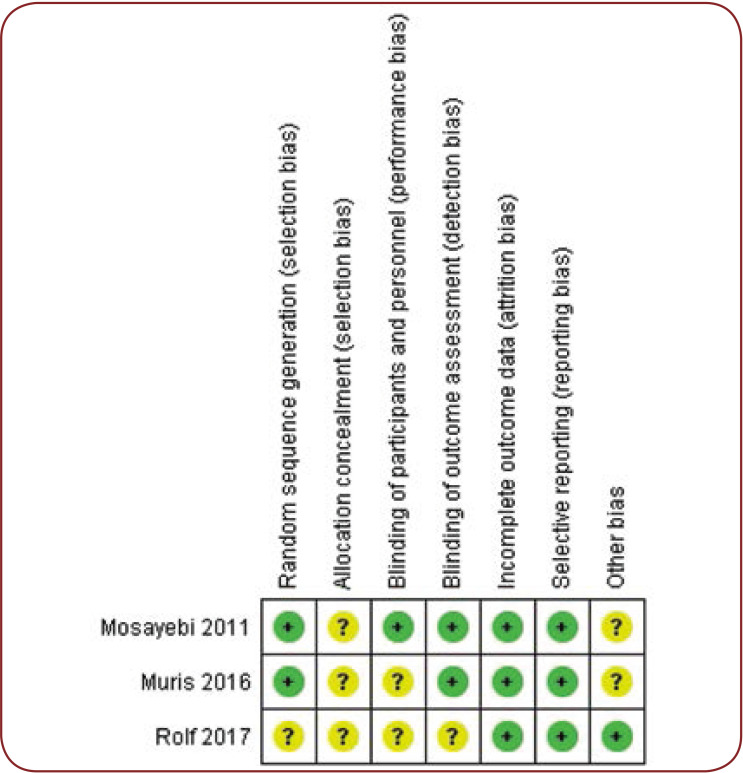
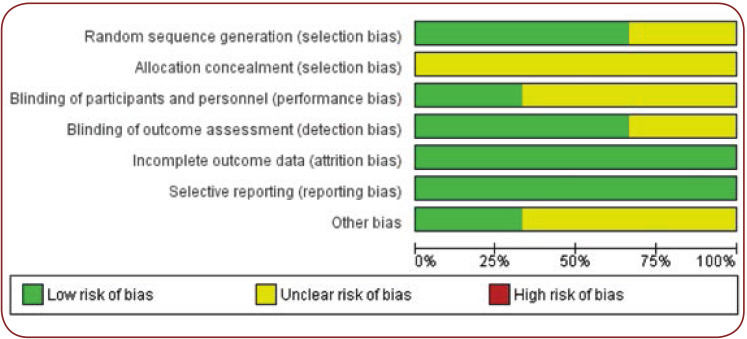
The risk of potential bias was evaluated by a specific tool of Cochrane Collaboration for assessing such risk (14) (Figures 4 and 5).
A p value less than 0.05 was considered statistically significant.
DISCUSSION
The result of this systematic review and metaanalysis showed that vitamin D supplements resulted in an significant increased level of INFγ (by including two studies) and had no significant effect on IL-10 level when comparing intervention and placebo groups.
In Rolf et al’s study, which was included in our systematic review, the vitamin D intervention group received 7000 IU for four weeks and 14000 IU for 48 weeks. According to their results, the mean IL-10 level was not significantly different at baseline and at the end in either the intervention or control group. The mean final levels were not statistically different between groups (final IL-10 was 1762 in the placebo group and 1543 in the vitamin D group) (17).
In Mosayebi et al’s study, the intervention group received 300000 IU vitamin D monthly for six months and the mean IL-10 level was reported to be 4950 at the final stage in the intervention group and 3750 in the control group, while the difference was not statistically significant between the two groups, although the level had increased in the intervention group (15).
In Muris et al’s study, the intervention group received 7000 IU of vitamin D for four weeks and 14000 IU for 48 weeks; the authors found a mean IL-10 level of 934 in the intervention group and 806 in the control group, while the difference was not statistically significant between the two groups, although the level had increased in the intervention group (16).
Given that the number of included studies for each item was limited, that of the included cases was limited too.
In Mosayebi et al’s study, the mean INFγ level decreased in both groups after six months, while the difference between the two groups was not significant (2250 in the intervention group and 1980 in the control group) (15).
In Muris et al’s study, the mean INFγ level decreased in both groups, while the difference between groups at the end of the study was not significant (16).
Experimental studies showed that 1,25-OH-vitamin D inhibited Th1 and Th17 cells and promoted Th2 and Treg cells (8, 18). On the other hand, prohibition of dendritic cell proliferation and maturation will result in secretion of IL-10 (19).
Previously, results of in vitro studies demonstrated that vitamin D prohibited T-cell proliferation and production of cytokines including IL-2, IL-12, and INFγ (20, 21).
An animal study revealed that 1,25-OH-vitamin D in an experimental autoimmune encephalomyelitis (EAE) model caused suppressed production of INFγ in spleen cells (22).
This systematic review has some limitations. Firstly, the number of included studies was limited. Secondly, we did not evaluate other chemokines.
CONCLUSION
The results of this systematic review was not satisfactory. There is a need for more clinical trials evaluating the effects of vitamin d supplements on IL-10 and INFγ levels in patients with multiple sclerosis.
Conflict of interests: none declared
Financial support: none declared
FIGURE 1.
Flow diagram summarizing the selection of eligible studies
FIGURE 2.
Mean difference of INFγ
FIGURE 3.
Mean difference of IL-10
TABLE 1.
Characteristics of included studies
FIGURE 4.
Methodologic quality assessment graph
FIGURE 5.
Risk-of-bias assessment for each study included in the meta-analysis
Contributor Information
Amirreza AZIMI, Multiple Sclerosis Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran.
Mahsa GHAJARZADEH, Universal Council of Epidemiology (UCE), Universal Scientific Education and Research Network (USERN), TUMS, Tehran, Iran.
Mohammad Ali SAHRAIAN, Multiple Sclerosis Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran.
Mehdi MOHAMMADIFAR, Department of Radiology, Zanjan University of Medical Sciences, Iran.
Bita ROOSTAEI, Department of Neurology, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Sara Mohammad Vali SAMANI, Department of Neurology, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Hamid Reza Farhadi SHABESTARI, TUMS, Tehran, Iran.
Sara HANAEI, Research Center for Immunodeficiencies (RCID), TUMS, Tehran, IranUSERN, Tehran, Iran.
References
- 1.Ghajarzadeh M, Jalilian R, Eskandari G, Sahraian AM, Azimi RA. Validity and reliability of Persian version of Modified Fatigue Impact Scale (MFIS) questionnaire in Iranian patients with multiple sclerosis. Disability and Rehabilitation. 2013;18:1509–1512. doi: 10.3109/09638288.2012.742575. [DOI] [PubMed] [Google Scholar]
- 2.Handel AE, Giovannoni G, Ebers GC, Ramagopalan SV. Environmental factors and their timing in adult-onset multiple sclerosis. Nature Reviews Neurology. 2010;3:156. doi: 10.1038/nrneurol.2010.1. [DOI] [PubMed] [Google Scholar]
- 3.Gutcher I, Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. The Journal of Clinical Investigation. 2007;5:1119–1127. doi: 10.1172/JCI31720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujio K, Okamura T, Yamamoto K. The Family of IL-10-secreting CD4+ T cells. Adv Immunol. 2010;105:99–130. doi: 10.1016/S0065-2776(10)05004-2. [DOI] [PubMed] [Google Scholar]
- 5.Spach KM, Nashold FE, Dittel BN, Hayes CE. IL-10 signaling is essential for 1, 25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. The Journal of Immunology. 2006;9:6030–6037. doi: 10.4049/jimmunol.177.9.6030. [DOI] [PubMed] [Google Scholar]
- 6.Luomala M, Lehtimäki T, Huhtala H, et al. Promoter polymorphism of IL-10 and severity of multiple sclerosis. Acta Neurologica Scandinavica. 2003;6:396–400. doi: 10.1034/j.1600-0404.2003.00165.x. [DOI] [PubMed] [Google Scholar]
- 7.Moore M, Piazza A, McCartney Y, Lynch M. Evidence that vitamin D3 reverses age-related inflammatory changes in the rat hippocampus. Portland Press Limited, 2005. [DOI] [PubMed]
- 8.Correale J, Ysrraelit MC, Gaitán MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain: a Journal of Neurology. 2009;5:1146–1160. doi: 10.1093/brain/awp033. [DOI] [PubMed] [Google Scholar]
- 9.Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;23:2832–2828. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 10.Muris A-H, Damoiseaux J, Smolders J. Monitoring in vivo immune modulation by vitamin D in multiple sclerosis. Handbook of vitamin D in human health. 2013. pp. 474–500.
- 11.Matilainen JM, Räsänen A, Gynther P, Väisänen S. The genes encoding cytokines IL-2, IL-10 and IL-12B are primary 1α, 25 (OH) 2D3 target genes. The Journal of Steroid Biochemistry and Molecular Biology. 2010;1-2:142–145. doi: 10.1016/j.jsbmb.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Heine G, Niesner U, Chang HD, et al. 1, 25-dihydroxyvitamin D3 promotes IL-10 production in human B cells. European Journal of Immunology. 2008;8:2210–2218. doi: 10.1002/eji.200838216. [DOI] [PubMed] [Google Scholar]
- 13.Ghajarzadeh M, Keshtkar AA, Azimi A, et al. The Effect of Vitamin D Supplements on Clinical and Para-Clinical Outcomes in Patients With Multiple Sclerosis: Protocol for a Systematic Review. JMIR Research Protocols. 2019;4:e12045. doi: 10.2196/12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosayebi G, Ghazavi A, Ghasami K, et al. Therapeutic effect of vitamin D3 in multiple sclerosis patients. Immunological Investigations. 2011;6:627–639. doi: 10.3109/08820139.2011.573041. [DOI] [PubMed] [Google Scholar]
- 16.Muris AH, Smolders J, Rolf L, et al. Immune regulatory effects of high dose vitamin D3 supplementation in a randomized controlled trial in relapsing remitting multiple sclerosis patients receiving IFNbeta; the SOLARIUM study. Journal of Neuroimmunology. 2016;300:47–56. doi: 10.1016/j.jneuroim.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Rolf L, Muris AH, Bol Y, et al. Vitamin D3 supplementation in multiple sclerosis: Symptoms and biomarkers of depression. J Neurol Sci. 2017;378:30–35. doi: 10.1016/j.jns.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Smolders J, Damoiseaux J, Menheere P, Hupperts R. Vitamin D as an immune modulator in multiple sclerosis, a review. Journal of Neuroimmunology. 2008;1-2:7–17. doi: 10.1016/j.jneuroim.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Raghuwanshi A, Joshi SS, Christakos S. Vitamin D and multiple sclerosis. Journal of Cellular Biochemistry. 2008;2:338–343. doi: 10.1002/jcb.21858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imitola J, Chitnis T, Khoury SJ. Cytokines in multiple sclerosis: from bench to bedside. Pharmacology & Therapeutics. 2005;2:163–177. doi: 10.1016/j.pharmthera.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Matheu V, Bäck O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D–induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. Journal of Allergy and Clinical Immunology. 2003;3:585–592. doi: 10.1016/s0091-6749(03)01855-4. [DOI] [PubMed] [Google Scholar]
- 22.Muthian G, Raikwar HP, Rajasingh J, Bright JJ. 1, 25 dihydroxyvitamin-D3 modulates JAK–STAT pathway in IL‐12/IFNγ axis leading to Th1 response in experimental allergic encephalomyelitis. Journal of Neuroscience Research. 2006;7:1299–1309. doi: 10.1002/jnr.20826. [DOI] [PubMed] [Google Scholar]