Abstract
We investigated the beta rhythm in 10 children with autism disorders (six boys and four girls) aged 5-12 (mean± SD: 8.3± 2.1) before and after the application of pico Tesla transcranial magnetic stimulation (pT-TMS) using magnetoencephalography (MEG). The MEG was car-ried out in a magnetically shielded room with a whole-head 122-channel gradiometer. After applying pT-TMS, we observed a beta rhythm increase towards the frequency range of 18-26 Hz in seven out of 10 patients (70%). We created a score that rated the level of improvement for each patient: 1=some change; 2=minor change; and 3=major change. All patients had an improvement in their clinical symptoms after the application of pT-TMS. There was a correlation between the clinical score and the increase of channels in the frequency range 18-26 Hz after pT-TMS. We concluded that the application of pT-TMS affected the beta rhythm in children with autism disorder. Therefore, more studies need to be further conducted.
Keywords:MEG, Fourier transform, autism disorder, beta rhythm.
INTRODUCTION
Autism disorder is a persistent developmental disorder characterized by deficits in public communication, language, stereotyped behaviors, and limited range of interests. Low amplitude beta waves with multiple and varying frequencies are often associated with active, busy or anxious thinking and active concentration (1-3).
Leuchter et al (4) examined the tolerability of low field magnetic stimulation synchronized to an individual’s alpha frequency for treatment of Major Depressive Disorder and suggested that synchronized transcranial magnetic stimulation (TMS) may be an effective, safe, and well tolerated method. In a pilot study (5), Jin and Phillips have also suggested that synchronized TMS might be an effective treatment for Major Depressive Disorder.
In a previous study (6) we used a pT-TMS electronic device for the therapeutic treatment of children suffering from autism. In the present study, we used our pT-TMS electronic device for the therapeutic treatment of children with autism because the higher level of TMS might have side effects on pediatric population. This device is a modified helmet containing up to 122 coils arranged in five array groups, so as to cover the main seven brain regions (frontal, vertex, right and left temporal, right and left parietal and occipital regions) of the subject. It is designed to create pT-TMS range modulations of magnetic flux in the alpha frequency range of 8-13 Hz for each patient. We used the a-rhythm because it is the individual physiological rhythm. We have also tested other frequencies as well as other forms of oscillations before, but there were no effects. The pT-TMS device was configured for each individual patient to generate a square wave, so as to resemble the firing activity of neurons in the brain (7-14).
This research is a continuation of our previously published work investigating the delta and theta rhythm after the application of pT-TMS in children with autism disorder using a double blind experimental design (8). In the current study we investigate the beta rhythm in children with autism disorder after the application of pT-TMS.
METHOD
Biomagnetic measurements were performed using a whole-head 122-channel magnetoencephalograpy (MEG) system (Neuromag-122, Neuromag Ltd. Helsinki, Finland) (7-14) in an electromagnetically shielded room. Ten children with autism (six boys and four girls, aged 5-12 (mean± SD: 8.3± 2.1) were included in the study. The Investigational Review Board of our University approved the research. For all children, informed consent for the study methodology and aim was obtained by their parents prior to procedure.
All children were under similar conditions (at rest with eyes closed in order to avoid artifacts) during the MEG. All MEG data tracings were visually inspected off-line for movement artifacts and periods contaminated with artifacts were cut off. The time taken for each recording was three minutes in order to ensure alertness for each child.
Each child was comfortably seated on a non-magnetic chair in a magnetically shielded room covered by the helmet-shaped Dewar. The resting state MEG recordings were obtained with a sampling frequency of 256 Hz and filtered with cut-off frequencies between 0.3 and 40 Hz. Our laboratory developed a software program identifying the amplitude of the primary dominant frequency of MEG power spectra after the application of Fast Fourier Transform (FFT) (7-10). We used the FFT algorithm to obtain maps of power spectra. Different colors in the maps correspond to different dominant frequencies. The numbers in the map squares represent the 122 MEG channels.
RESULTS
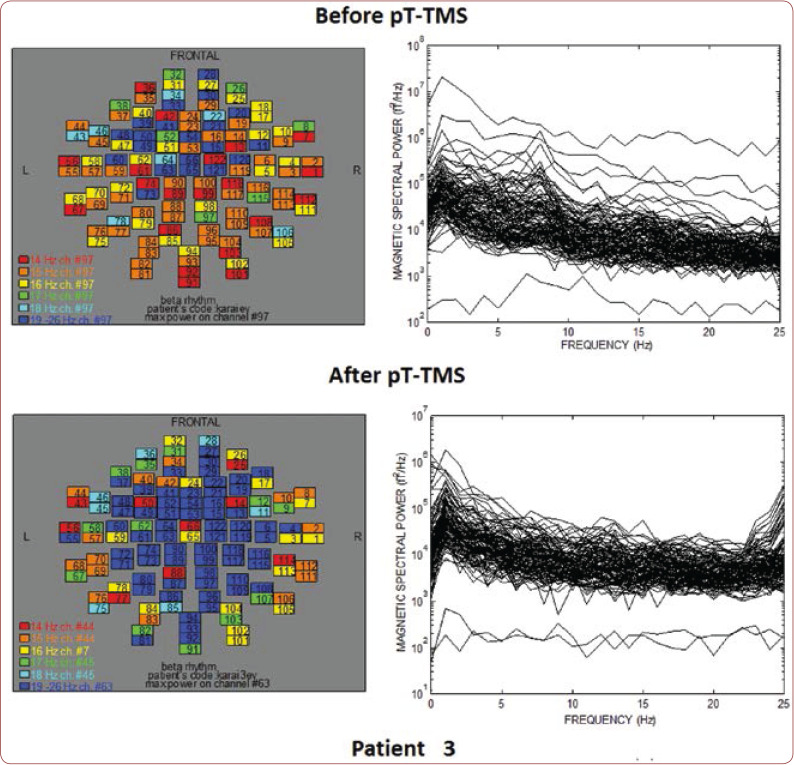
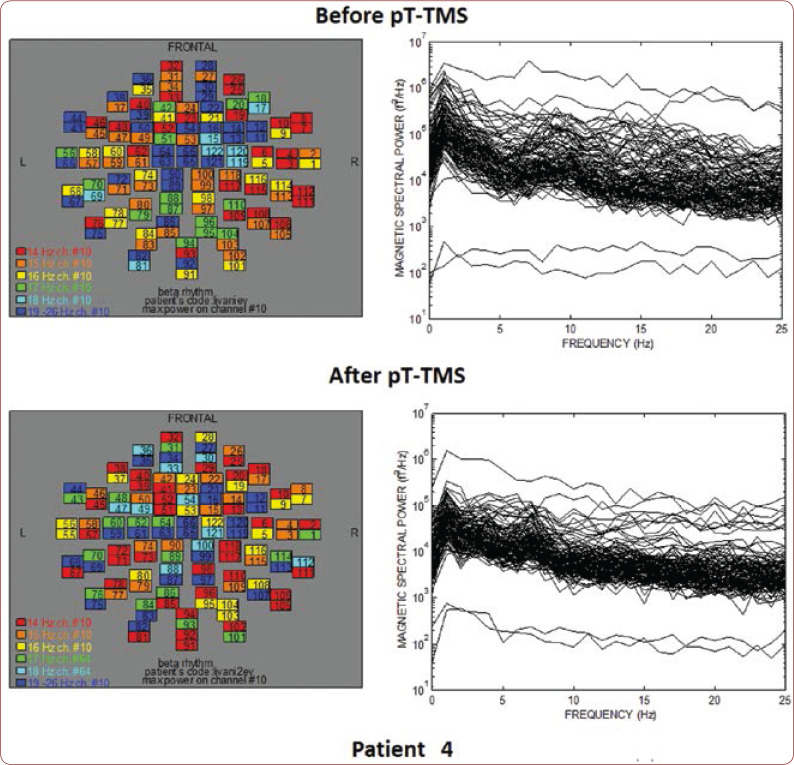
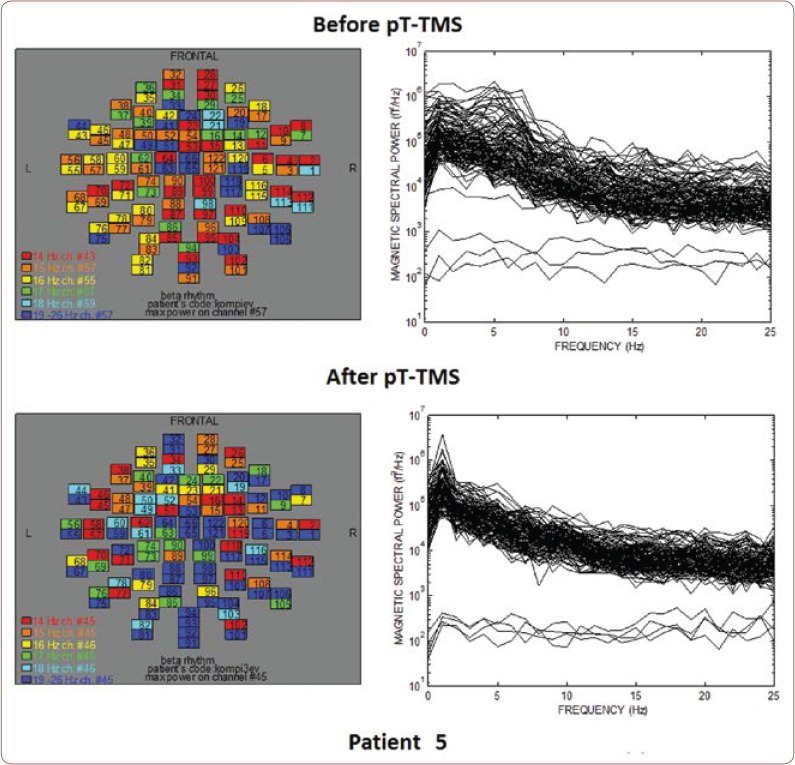
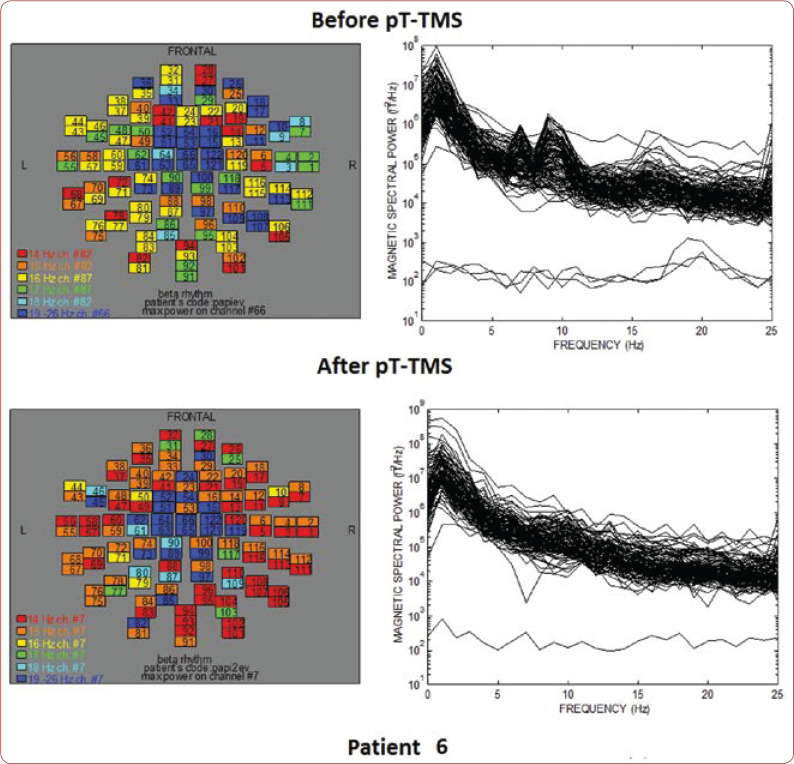
Table 1 shows the clinical characteristics of each child that have already been described previously (8). We created a score that rates the level of improvement for each patient: 1=some change; 2=minor change; and 3=major change. All patients had an improvement in their clinical symptoms after the application of pT-TMS, so they had clinical score 1-3. Figures 1-10 represent the maps and overlapping of the power spectra in the frequency range of 14-26 Hz (beta rhythm) before and after pT-TMS application for each child. Patients 4, 6 and 7 had no increase in channels in the frequency range 18-26 Hz after pT-TMS.
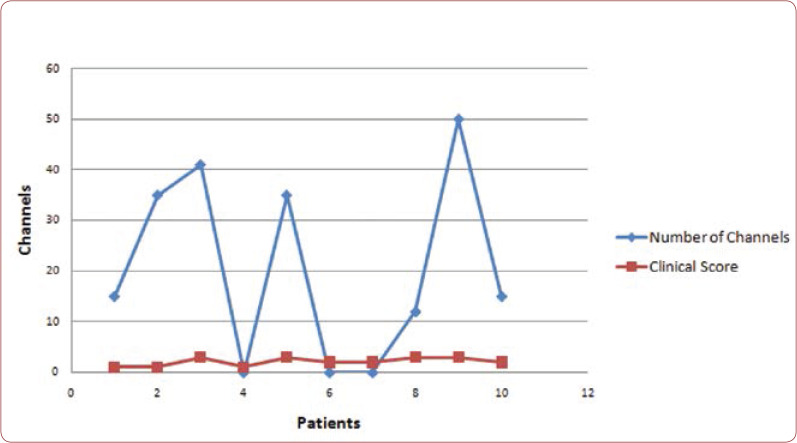
Figure 11 shows the correlation between the clinical score and increase of channels in the range of 18-26 Hz after pT-TMS. In the diagram, the number of channels for each patient is the difference before and after pT-TMS. If the difference was positive, we put the corresponding number, whereas if it was negative (in patients 4, 6, and 7) we put zero, meaning that we have not seen any increase of channels in the frequency range 18-26 Hz after the application of pT-TMS.
DISCUSSION
In our study we found a correlation between the number of channels that increased in the range of 18-26 Hz with the clinical score after pT-TMS. The mechanism by which the application of the pT-TMS has some beneficial effects in the autistic patients is unidentified. The mechanism of action is different from the traditional TMS because of the extremely small magnetic field. However, one possible cause is that these magnetic fields (pT-TMS) have been shown to influence the action of the pineal gland (PG), which regulates the endogenous opioid functions, dopaminergic modulation and GABA. Two patents established the role of PG after pT-TMS (7, 14). Anninou and Tsagas’s patent (15) revealed the strengthening of the immune system which is controlled by the PG. Anninos et al’s patent (6) demonstrated the decalcification of epiphysis by means of magnetic fields with characteristics determined by MEG and our pT-TMS device.
Another point which is related to PG is the alpha rhythm. Since the full expression of alpha rhythm has been known to occur with puberty, it is possible that the establishment of alpha rhythm is subject to neuroendocrine influences. Nocturnal plasma melatonin levels have been shown to decline gradually during childhood, reaching a lower point at puberty. This progressive decline in melatonin secretion throughout childhood facilitates the maturation of alpha rhythm. So, the change of alpha rhythm could be used as a neurophysiological indicator for the action of PG and for the disorders associated with missing or delayed maturation of the alpha rhythm such as autism, dyslexia, epilepsy, Parkinson disease, etc, which might be related to disturbances of PG melatonin functions in early life (16).
CONCLUSION
The pT-TMS has the potential to non-invasively improve symptoms in autism patients. Additional investigations with a larger number of patients and replication of results provided by the pilot study by other research centers are required.
Conflict of interests: none declared
Financial support: This study was funded by a partnership of GGET (General Secretariat of Research and Technology, GR) and ERGO AEBE, INC, GR [Grant Number: 80623].
Acknowledgements: We would like to express our deep appreciation and thanks to Professor Lothar Krinke for his valuable contribution in our paper.
TABLE 1.
The clinical characteristic of each patient
TABLE 1.
The clinical characteristic of each patient
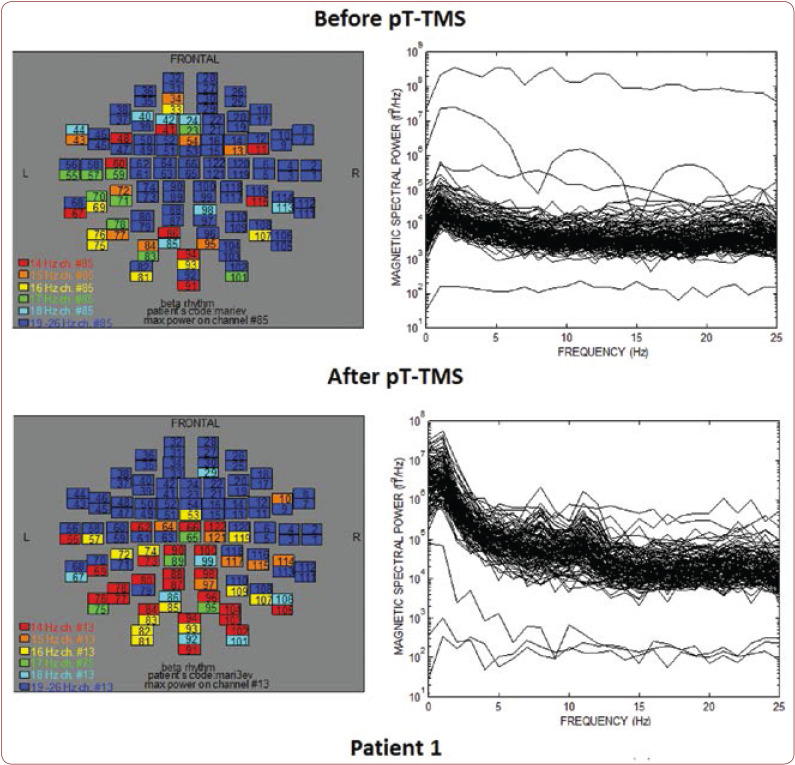
FIGURE 1.
The maps and overlapping of power spectra before and after pT-TMS for patient 1. The patient expressed a small improvement in the list of disorders.
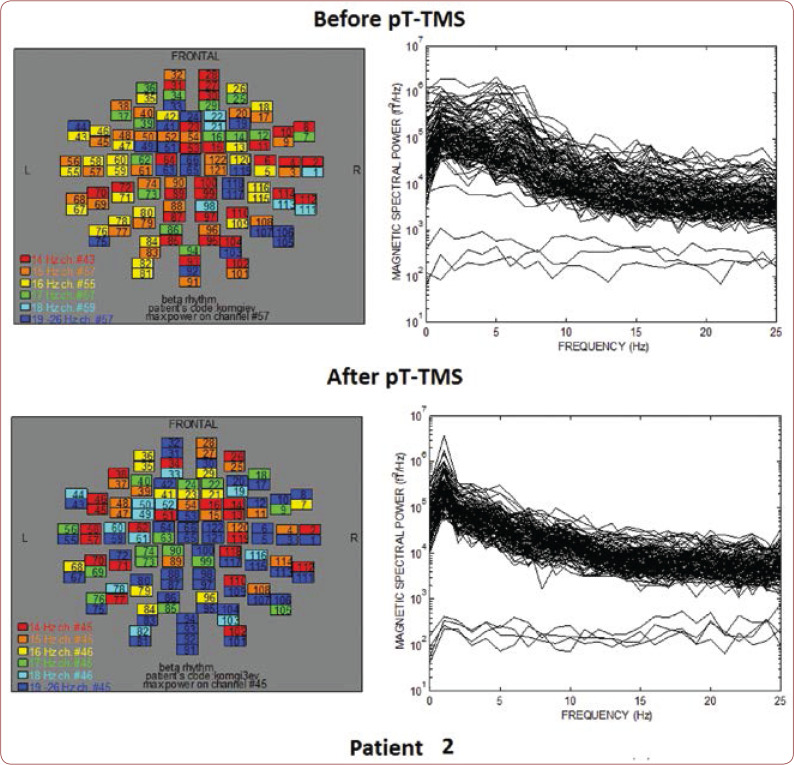
FIGURE 2.
The maps and overlapping of power spectra before and after pT-TMS for patient 2. The patient expressed a small improvement in the list of disorders.
FIGURE 3.
The maps and overlapping of power spectra before and after pT-TMS for patient 3. The patient expressed major changes.
FIGURE 4.
The maps and overlapping of power spectra before and after pT-TMS for patient 4. The patient expressed mixed changes.
FIGURE 5.
The maps and overlapping of power spectra before and after pT-TMS for patient 5. The patient expressed major changes.
FIGURE 6.
The maps and overlapping of power spectra before and after pT-TMS for patient 6. The patient expressed minor changes.
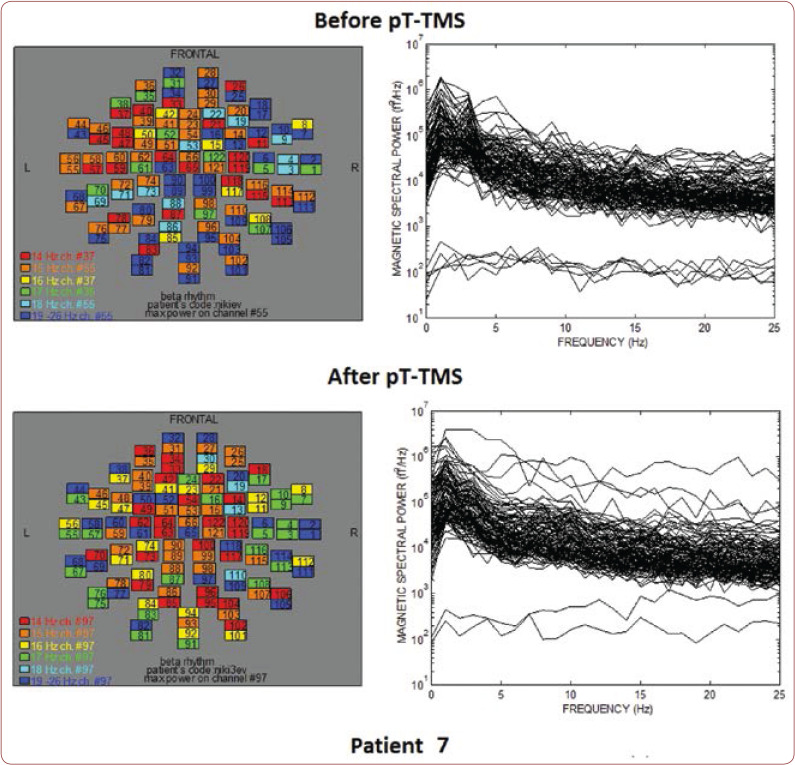
FIGURE 7.
The maps and overlapping of power spectra before and after pT-TMS for patient 7. The patient expressed minor changes.
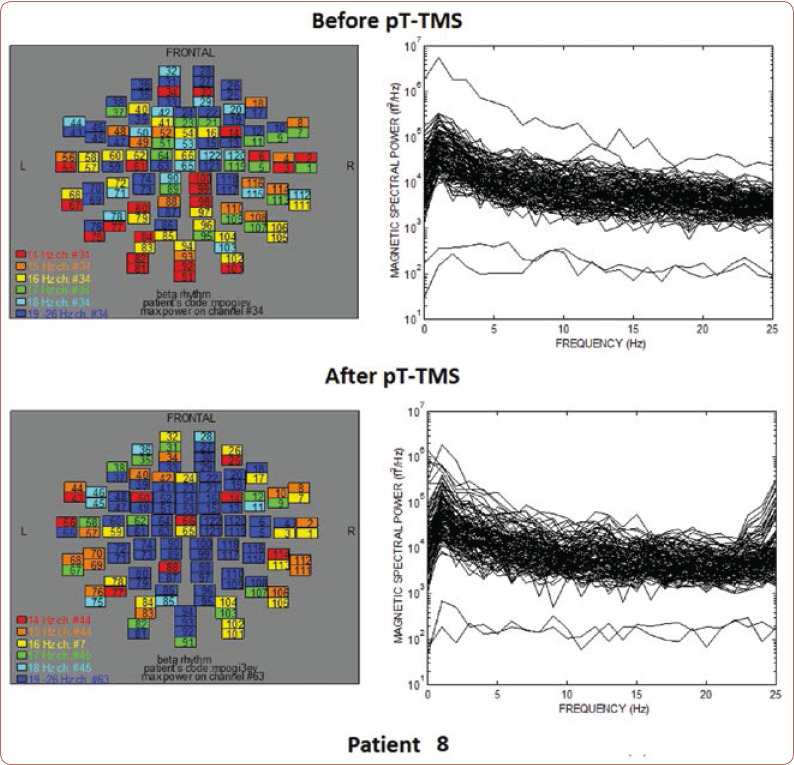
FIGURE 8.
The maps and overlapping of power spectra before and after pT-TMS for patient 8. The patient expressed major changes.
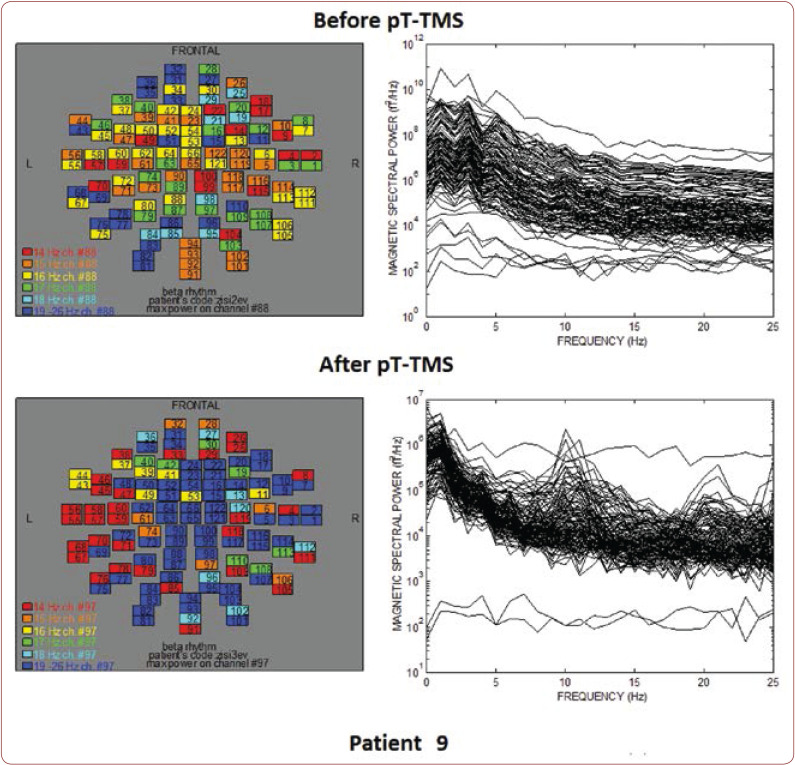
FIGURE 9.
The maps and overlapping of power spectra before and after pT-TMS for patient 9. The patient expressed major changes.
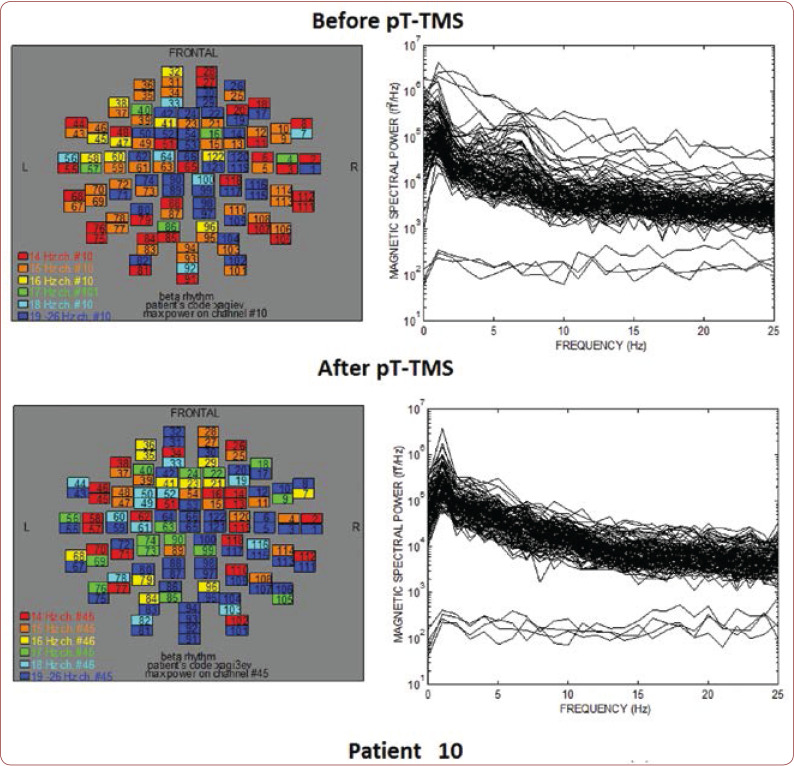
FIGURE 10.
The maps and overlapping of power spectra before and after pT-TMS for patient 10. The patient expressed minor changes.
FIGURE 11.
Correlation between the number of channels that increased in the range of 18-26 Hz and the clinical score after pT-TMS.
Contributor Information
Photios ANNINOS, Laboratory of Medical Physics, Department of Medicine, School of Health Sciences, Democritus University of Thrace, University Campus, Alexandroupoli, Greece.
Athanasios CHATZIMICHAEL, Department of Paediatrics, University Hospital of Alexandroupoli, Democritus University of Thrace, University Campus, Alexandroupoli, Greece.
Nicolia ANNINOU, Laboratory of Medical Physics, Department of Medicine, School of Health Sciences, Democritus University of Thrace, University Campus, Alexandroupoli, Greece.
Athanasia KOTINI, Laboratory of Medical Physics, Department of Medicine, School of Health Sciences, Democritus University of Thrace, University Campus, Alexandroupoli, Greece.
Adam ADAMOPOULOS, Laboratory of Medical Physics, Department of Medicine, School of Health Sciences, Democritus University of Thrace, University Campus, Alexandroupoli, Greece.
Triandafillos GEMOUSAKAKIS, Laboratory of Medical Physics, Department of Medicine, School of Health Sciences, Democritus University of Thrace, University Campus, Alexandroupoli, Greece.
Nicolaos TSAGAS, Department of Electrical Engineering, Engineering Faculty, Democritus University of Thrace, Greece.
References
- 1.Baumeister J, Barthel T, Geiss KR, Weiss M. Influence of phosphatidylserine on cog-nitive performance and cortical activity after induced stress. Nutr Neurosci. 2008;11:103–110. doi: 10.1179/147683008X301478. [DOI] [PubMed] [Google Scholar]
- 2.Sokhadze EM, El-Baz AS, Tasman A, et al. Neuromodulation integrating rTMS and neurofeedback for the treatment of autism spectrum disorder: An exploratory study. Appl Psychophysiol Biofeedback. 2014;39:237–257. doi: 10.1007/s10484-014-9264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masuda F, Nakajima S, Miyazaki T, et al. Motor cortex excitability and inhibitory im-balance in autism spectrum disorder assessed with transcranial magnetic stimulation: a sys-tematic review. Transl Psychiatry. 2019;9:110. doi: 10.1038/s41398-019-0444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leuchter AF, Cook IA, Feifel D, et al. Efficacy and Safety of Low-field Synchronized Transcranial Magnetic Stimulation (sTMS) for Treatment of Major De-pression. Brain Stimul. 2015;8:787–794. doi: 10.1016/j.brs.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Jin Y, Phillips B. A pilot study of the use of EEG based synchronized Transcranial Magnetic Stimulation (sTMS) for treatment of Major Depression. BMC Psychiatry. 2014;14:13. doi: 10.1186/1471-244X-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anninos PA, Tsagas N. Electronic apparatus for treating epileptic individuals. USA patent 5453072. 1995.
- 7.Anninos P, Chatzimichael A, Adamopoulos A, Kotini A, Tsagas N. A combined study of MEG and pico-Tesla TMS on children with autism disorder. J IntegrNeurosci. 2016;15:497–513. doi: 10.1142/S0219635216500278. [DOI] [PubMed] [Google Scholar]
- 8.Anninos P, Chatzimichael A, Adamopoulos A, Kotini A, Tsagas N. Brain Mapping in the Frequency Domain for Autism Children: A Magnetoencephalographic Study with pT-Transcranial Brain Stimulation. Clin Res Neurol. 2018;1:1–4. [Google Scholar]
- 9.Anninos P, Chatzimichael A, Adamopoulos A, Kotini A, Tsagas N. Autism disorder and pico-Tesla TMS. Arch Paediatr Dev Pathol. 2016;1:1008. doi: 10.1142/S0219635216500278. [DOI] [PubMed] [Google Scholar]
- 10.Kotini A, Anninos P, Gemousakakis T, Adamopoulos A. The Effects of Sweet, Bitter, Salty and Sour Stimuli on Alpha Rhythm. A Meg Study. Maedica J Clin Med. 2016;11:208–213. [PMC free article] [PubMed] [Google Scholar]
- 11.Anninos P, Adamopoulos A, Kotini A, Tsagas N. MEG evaluation of pico-Tesla ex-ternal TMS on multiple sclerosis patients. Mult Scler Relat Disord. 2016;8:45–53. doi: 10.1016/j.msard.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Anninos P, Adamopoulos A, Kotini A, Tsagas N, Tamiolakis D, Prassopoulos P. MEG evaluation of Parkinson’s diseased patients after external magnetic stimulation. Acta Neurol Belg. 2007;107:5–10. [PubMed] [Google Scholar]
- 13.Anninos P, Adamopoulos A, Kotini A, Tsagas N. Combined MEG and pT-TMS study in Parkinson’s disease. J IntegrNeurosci. 2016;15:145–162. doi: 10.1142/S0219635216500102. [DOI] [PubMed] [Google Scholar]
- 14.Anninos P, Kotini A, Anninou N, Adamopoulos A, Papastergiou A, Tsagas N. MEG recordings of patients with CNS disorders before and after external magnetic stimulation. J Integr Neurosci. 2008;7:17–27. doi: 10.1142/s0219635208001745. [DOI] [PubMed] [Google Scholar]
- 15.Anninou N, Tsagas I. Electronic device for strengthening the immune system. USA patent 20060058572. 2006.
- 16.Sandyk R. Alpha rhythm and the pineal gland. Int J Neurosci. 1992;63:221–227. doi: 10.3109/00207459208987198. [DOI] [PubMed] [Google Scholar]