Abstract
Context:Phytoconstituents of medicinal plants have been playing a key role in treating various diseases all over the world since ancient times. The present study was focused on preliminary analysis of phytoconstituents and evaluation of anthelmintic property (in vitro) of different extracts of Cayratia auriculata (Family: Vitaceae) against earthworms (Pheretima posthuma).
Materials and methods:Four different hexane, chloroform, ethyl acetate and methanol extracts of Cayratia auriculata were screened for their phytoconstituents. Cayratia auriculata has been shown to have various phytoconstituents such as flavonoids, phenolic compounds, tannins, alkaloids, saponins, glycosides and steroids. Hexane, chloroform, ethyl acetate and methanol extracts of Cayratia auriculata were analyzed for their anthelmintic property on earthworms (Pheretima posthuma). Each extract at three different concentrations (20 mg/mL, 40 mg/mL and 80 mg/mL) was analysed to evaluate the time taken for paralysis (P) and death (D) of adult earthworms. Albendazole was used as standard and 2% Tween 80 in distilled water as control at a concentration of 10 mg/mL.
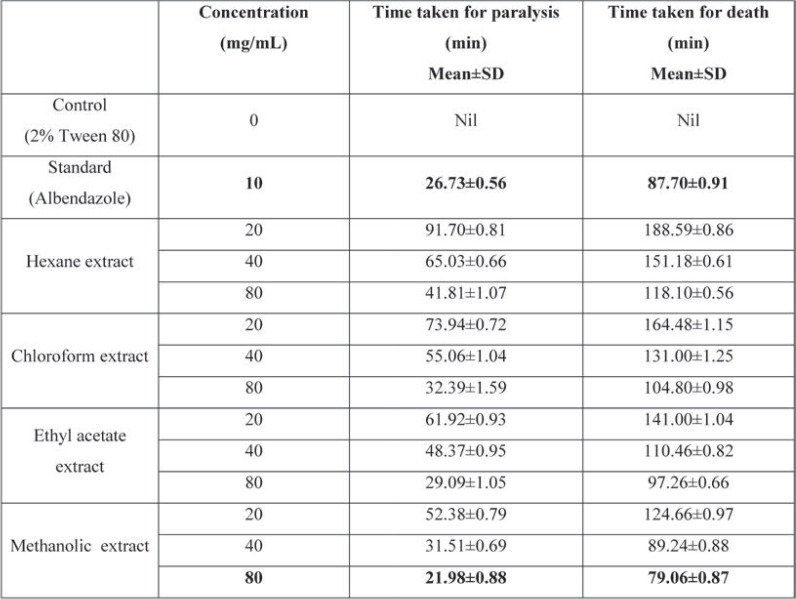
Results:Our study found that the methanolic extract of Cayratia auriculata at a concentration of 80 mg/mL [21.98±0.88 (P), 79.06±0.87(D)] expressed a stronger anthelmintic property than the standard albendazole (10 mg/mL) [26.73±0.56 (P), 87.70±0.91 (D)].
Conclusions:All extracts expressed a dose dependent anthelmintic activity, with an increasing pattern in the following order: hexane, chloroform, ethyl acetate and methanol.
Keywords:Cayratia auriculata, Pheretima posthuma, anthelmintic, albendazole.
INTRODUCTION
Natural products derived from medicinal plants with potent bioactive principles are the chief elements of phytomedicine. They have versatile applications in treating chronic as well as infectious diseases affecting mankind, which shows the immense potential of medicinal plants for use in various conventional systems (1). In modern medicine, highly marketed drugs such as artemisinin, quinine, and emetine are the best examples of pharmaceuticals derived from medicinal plants (2). Most of the drugs used in modern practice are very expensive for people living in developing countries, which gives a valuable tool for researchers to search for cheaper antibacterial substances from natural sources. Plants are the best natural sources for new safe, environment friendly and renewable drugs. Given the lack of scientific explanations for the therapeutic uses of medicinal plants by primitive peoples, a careful and well conducted investigation should explore plants as medicinal tools (3).
Helminths are known to be a big problem in livestock production along the tropics. The nature of helminthic diseases is persistent and debilitating, and it is likely to result in morbidity and significant economic and community distress in both humans and animals. Weakness, anorexia, decreased food capability, weight loss and decreased productivity are the characteristic features of parasitic gastroenteritis, which was caused by mixed infection of various species of intestinal worms. As no effective anthelminthic vaccines were developed yet, chemotherapy remains the only treatment and effective tool to control and cure helminthic infections. The use of synthetic anthelmintics unsystematically can results in parasitic resistance. Herbal medicine has been in use to prevent development of parasitic resistance since ancient times (4). The importance of phytochemicals such as tannins, terpenoids, flavonoids, alkaloids and glycosides with anthelmintic activity was reported by other investigators (5, 6).
Cayratia auriculata belongs to native species of Southern India. Cayratia auriculata (Roxb.) gamble is a synonym of Cyphostemma auriculatum (Roxb), a climber that is well grown in dry evergreen to dry deciduous forests and well distributed in Andhra Pradesh (7, 8), Tamilnadu (9), Maharashtra (10), Madhya Pradesh (11), Orissa (12), Gujarat, Goa, Karnataka, Kerala, Bihar, West Bengal, Bangladesh, Myanmar and Rajasthan (13). An ethnomedicinal survey on Cayaratia auriculata shows that this plant is used as a folklore medicine and blood purifier, in cardiac disorders (7), intestinal worm diseases (11), earache, wound abscess, dog bite, rheumatism, purulent wounds, wound healing, tumors, cough, colds and hydrocele, but also as a tonic (15) and an astringent. This plant has been also used in veterinary medicine to treat animal bloody dysentery and diarrhea (16). Phytochemical studies of Vitaceae family species suggest the presence of alkaloids, flavonoids, saponins, steroids, terpenoids, stilbenoids and tannins (17)..
Given that various phytoconstituents of medicinal plants are known for their anthelmintic properties and substantial literature review did not report anything about the anthelmintic activity of Cayratia auriculata, the present study has been carried out to perform a preliminary analysis on the phytoconstituents of this plant and to assess their anthelmintic properties (in vitro).
MATERIALS AND METHODS
Plant collection and authentication
The plant was collected in and around forest areas of Araku valley, Visakhapatnam district, Andhra Pradesh, and it was identified and authenticated by Associate Professor, Department of Botany, Andhra University, Visakhapatnam-530 003, Andhra Pradesh, India. The voucher herbarium specimen number 22228 was deposited in Andhra University Herbarium (AUH), Botany Department, Visakhapatnam.
Extraction of plant material
Fresh plant material of Cayratia auriculata was washed under running tap water to ensure the absence of any foreign organic matter, solid debris, organisms, fungi, etc, and then dried in the shade for seven days. About 1200 g of shade dried plant material was grinded with the help of grinder to make powder and preserved in an air-tight container. Successive solvent extractions were then performed by using a Soxhlet apparatus. About 120 g of Cayratia auriculata powdered material was packed into a thimble and extraction continued until the solvent in the extractor siphon tube became colourless by using various solvents in the order of increasing polarity: hexane, chloroform, 352 Maedica A Journal of Clinical Medicine, Volume 14, No. 4, 2019 ethyl acetate, and methanol, respectively. Whatman No. 1 filter paper (42) was used for extract filtration. The filtered extracts were concentrated by using a vacuum oven at 400 C and were preserved in a cool and dark place until further use (17, 18). Afterwards, extracts were subjected to a preliminary analysis of their phytoconstituents and in vitro evaluation of their anthelmintic properties.
Phytoconstituent analysis
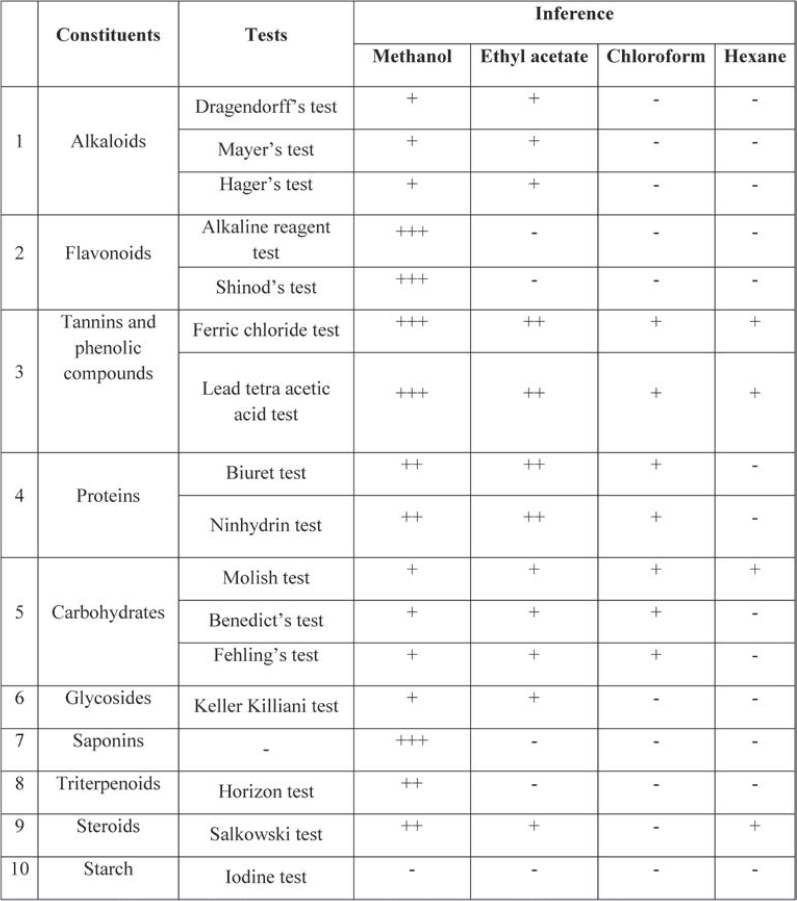
Preliminary analysis of extracts was carried out to identify the presence of various phytoconstituents by employing standard protocols (20, 21). The results were summarized in Table 1 after conducting the following chemical tests.
Tests for alkaloids
(a) Dragendorff ’s test. By adding 1 mL of Dragendorff”s reagent to 2 mL of extract, an orange red precipitate was formed, indicating the presence of alkaloids.
(b) Mayer’s test. Few drops of Mayer’s reagent were added to 1 mL of extract. A yellowish or white precipitate was formed, indicating the presence of alkaloids.
(c) Hager’s test. Two milliliters of extract were treated with few drops of Hager’s reagent. A yellow precipitate was formed, indicating the presence of alkaloids.
Tests for flavonoids
(a) Alkaline reagent test. Two to three drops of sodium hydroxide were added to 2 mL of extract. Initially, a deep yellow colour appeared but it gradually became colourless by adding few drops of dilute HCL, indicating that flavonoids were present.
(b) Shinod’s test. Ten drops of dilute HCL and a piece of magnesium were added to 1 mL of extract, the resulting deep pink colour indicating the presence of flavonoids.
Test for phenolic compounds and tannins
(a) Ferric chloride test. Two milliliters of 5% neutral ferric chloride solution were added to 1 mL of extract, the dark blue colouring indicating the presence of phenolic compounds and tannins.
(b) Lead tetra acetic acid test. One milliliter of lead tetra acetate solution was treated with 0.5 mL of extract, precipitate formation indicating the presence of phenolic compounds and tannins.
Tests for proteins
(a) Biuret test. Two drops of 3% copper sulphate and few drops of 10% sodium hydroxide were added to 1 mL of extract, violet or red colour formation indicating that proteins are present.
(b) Ninhydrin test. Two drops of 0.2% freshly prepared ninhydrin solution added to 1 mL of extract. Production of purple colour shows the presence of proteins.
Test for carbohydrates
(a) Molish test. Few drops of alcoholic a-naphthol solution were added to 2 mL of extract. Later, few drops of concentrated H2SO4 were added along the walls of test tube. At the junction of two liquids, a violet colour ring appeared, indicating that carbohydrates were present.
(b) Benedict’s test. To 5 mL of Benedict’s reagent, 8-10 drops extract were added, then heated for five minutes; the resulting dark red precipitate indicated the presence of carbohydrates.
(c) Fehling’s test. To 2 mL of extract, an equal volume of Fehling’s (A & B) solution was added and heated for five minutes, the resulting red/dark red precipitate indicating the presence of carbohydrates.
Tests for glycosides
Keller Killiani test. A solution of 0.5 mL, containing glacial acetic acid and 2-3 drops of ferric chloride, was mixed with 2 mL of extract. Later, 1 mL of concentrated H2SO4, was added along the walls of the test tube. The appearance of deep blue colour at the junction of two liquids indicated the presence of cardiac glycosides.
Tests for saponins
A drop of Na2CO3 solution was added to 5 mL of extract in a test tube. After vigorous shaking, it was left to rest for five minutes. Foam formation indicated the presence of saponins.
Test for triterpenoids
Horizon test. Two milliliters of trichloroacetic acid was added to 1 mL of extract. The presence of terpenoids was confirmed by the formation of a red precipitate.
Test for steroids
Salkowski test. The test extract was shaken with chloroform and concentrated H2SO4 was added along the walls of a test tube; a red colour appeared, indicating the presence of steroids.
Test for starch
Iodine test. Two milliliters of iodine solution with potassium iodine were added to 2 mL of test extract, and the appearance of a blue colour indicated that presence of starch.
Anthelmintic property
Adult Indian earth worm, Pheretima posthuma (Annelida) was used to evaluate the anthelmintic property of different extracts of Cayratia auriculata, due to its anatomical and physiological similarity with round worm parasite occurring in the alimentary tract of human beings. The method of Ajaiyeoba et al and Kaur et al with slight modifications was used to evaluate anthelmintic activity (22, 23). An average size of 4-6 cm of adult earth worms were collected from water logged soil areas of GSL Medical College and General Hospital campus, and all faecal matters were removed by proper washing in normal saline. Extracts at concentrations of 20 mg/mL, 40 mg/mL and 80 mg/mL were used to identify the time of paralysis and Preliminary Analysis of Phytoconstituents and Evaluation of Anthelminthic Property of Cayratia auriculata (In Vitro) 354 Maedica A Journal of Clinical Medicine, Volume 14, No. 4, 2019 death of earth worms. Albendazole at a concentration of 10 mg/mL was taken as standard and 2% Tween 80 in distilled water was used as control. Freshly prepared test solutions and standard drug solutions were used for the entire experimental protocol. Six earth worms in each group of approximately similar size were placed into 10 mL of different test solutions. The time taken for the paralysis and death of individual earth worms was noted. No movement of worms, except when they were vigorously shaken, was noted as time of paralysis. No motility and fading of worm body color was deemed as death of worms.
RESULTS
Preliminary phytoconstituent analysis
Successive extraction of Cayratia auriculata was done in the order of increasing polarity of the solvent, and hexane > chloroform > ethyl acetate > methanol extracts were prepared. Qualitative preliminary phytoconstituents screening of the various extracts of Cayratia auriculata showed the presence of various phytoconstituents. The methanolic extract was found to have a high content of flavonoids, saponins, tannins and phenolic compounds, moderate amounts of proteins, triterpenoids and steroids, and only traces of alkaloid carbohydrates and glycosides. The ethyl acetate extract was found to have moderate amounts of proteins, tannins and phenolic compounds, and traces of carbohydrates, glycosides, steroids, alkaloids. The chloroform extract had only traces of proteins, carbohydrates, tannins and phenolic compounds. The hexane extract had carbohydrate steroids, tannins and phenolic compounds. This shows that high polar phytoconstituents were extracted with methanol when compared to other solvents such as ethyl acetate, chloroform and hexane (Table 1).
Evaluation of anthelmintic property
Table 2 shows the mean paralysis and death time produced by the various extracts of Cayratia auriculata against adult earth worms. For the methanolic extract at a concentration of 80 mg/mL and albendazole at a concentration of 10 mg/mL, used as positive control, the mean paralysis time and death time were (P)21.98±0.88, (D)79.06±0.87 and (P)26.73±0.56, (D)87.70±0.91, respectively. The paralysis time and death time of all four extracts at 20 mg/mL was too long when compared to the standard drug and methanolic extract at a concentration of 40 mg/mL and 80 mg/mL. Chloroform and ethyl acetate extracts at 80 mg/mL showed a better paralysis time, but the death time was moderately long when compared to the standard drug. For the ethyl acetate extract at 80 mg/mL concentration, the paralysis time was closer to the standard drug but the death time was moderately high. All extracts expressed a dose dependent anthelmintic activity, with an increasing pattern in the following order: hexane, chloroform, ethyl acetate and methanol. The anthelmintic property of the Cayratia auriculata methanolic extract at a concentration of 80 mg/mL was found to be stronger than that of the standard albendazole at a concentration of 10 mg/mL.
DISCUSSION
Phytochemicals found in Cayratia auriculata during the preliminary phytoconstituent screening (Table 1) would be responsible for the evaluated anthelmintic activity (Table 2) and may optimistically deemed as a substitutive approach to control helminth infections (24, 25). All extracts of Cayratia auriculata exhibited concentration dependent anthelmintic properties on earthworms, probably due to the presence of alkaloids, glycosides, flavonoids, saponins, triterpenoids, tannins and phenolic compounds. Among all extracts, the methanolic one exhibited the most effective anthelmintic activity, most likely due to the involvement of glycosides, flavonoids, tannins and phenolic compounds; hence, it may be considered as a potent anthelmintic agent. The decreasing pattern of anthelmintic properties of the four different extracts was as follows: methanolic > ethyl acetate > chloroform> hexane extracts. At 40 mg/mL and 80 mg/mL, the methanolic extract of Cayratia auriculata exhibited a good anthelmintic property when compared to the standard at a concentration of 10 mg/mL. The methanolic extract of Cayratia auriculata expressed anthelmintic property in concentration dependent order with shortened time of paralysis (P) and death (D) at 40 mg/mL and 80 mg/mL when compared to the ethyl acetate, chloroform, and hexane extracts at the same concentrations. The methanolic extract of Cayratia auriculata at 80 mg/mL caused paralysis and death of Pheretima posthuma at 21.98±0.88 and 79.06±0.87 minutes, respectively, while when the standard drug, albendazole, was used at concentration of 10 mg/mL, paralysis occurred at 26.73±0.56 minutes and death at 87.70±0.91 minutes. Uptake of glucose by the larval and adult stages of the susceptible parasites is impaired by albendazole, resulting in depletion of their glycogen stores. Helminths’ metabolic pathways are disrupted by high concentrations of albendazole by inhibiting metabolic enzymes such as malate dehydrogenase and fumarate reductase, resulting in decreased energy produced by the Krebs cycle. Due to inadequate ATP production, the parasite is immobilized and ultimately dies (26).
Velebny GHS and Sirama et al reported that alkaloids exhibit neurotoxic properties by inhibiting acetylcholine esterase, resulting in acetylcholine induced body wall muscle spasms, flaccid paralysis and death of gastrointestinal parasites. On the other hand, plant glycosides at low concentrations, when ingested by human beings, kill intestinal parasites due to their neurotoxic properties (27, 28). Tannins are polyphenolic compounds with a highly effective action against internal nematodes in ruminants. Their great effectiveness as anthelmintic substances is thought to be related to their direct vermicidal activity. Tannins also play a role in increasing host resistance against intestinal parasites (29). Drugs such as oxyclosamide, bithinol and niclosamide are chemically belonging to synthetic phenolic compounds that exhibit their anthelmintic activity by uncoupling oxidative phosphorylation, which leads to a decreased ATP production, and as a result, the parasite is paralyzed and eventually dies. It was assumed that tannins present in the extracts produced similar results. Moreover, tannins bind to free proteins in the alimentary tract of host animals or glycoprotein in cuticle of the parasite produce anthelmintic activity (30). The current study showed that all extracts of Cayratia auriculata had an anthelmintic activity and the methanolic one could be a promising tool for an alternative approach to treat helminthic infections.
CONCLUSION
The present study aimed to perform a preliminary phytochemical analysis and evaluation of the anthelmintic activity (in-vitro) of various extracts of Cayratia auriculata (Family: Vitaceae) against earthworms (Pheretima posthuma). The phytochemical tests for various constituents revealed that the plant extracts contained alkaloids, flavonoids phenolic compounds, tannins, glycosides, saponins, and steroids. Our findings highlighted that the methanolic extract of Cayratia auriculata had a significant anthelmintic activity. Further studies are needed to determine the actual phytoconstituents and underlying mechanisms responsible for the anthelminthic property.
Conflict of interests: none declared
Financial support: none declared.
TABLE 1.
Preliminary phyto analysis of the methanol, ethyl acetate, chloroform and hexane extracts of Cayratia auriculata
TABLE 2.
Anthelminthic property of hexane, chloroform, ethyl acetate and methanolic extracts of Cayratia auriculata
Contributor Information
Nagaraju KANCHERLA, Department of Pharmacology, GSL Medical College and General Hospital, Rajahmundry, Andhra Pradesh-533296, India.
Anusha DHAKSHINAMOOTHI, Department of Pharmacology, Sri Ramachandra Institute of Higher Education &Research, Porur, Chennai-600116, India.
K CHITRA, Department of Pharmaceutical Chemistry, Sri Ramachandra Institute of Higher Education & Research, Porur, Chennai-600116, India.
Ravi Babu KOMARAM, Department of Pharmacology, GSL Medical College and General Hospital, Rajahmundry, Andhra Pradesh-533296, India.
References
- 1.Duraipandiyan V, Ayyanar M, Ignacimuthu S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. Complement Altern Med. 2006;6:35–41. doi: 10.1186/1472-6882-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hidayathula S, Chandra KK, Chandrashaker KR . Phytochemical evaluation and antibacterial activity of Pterospermum diversifolium blume. Int J Pharma Sci. 2011;3:165–167. [Google Scholar]
- 3.Perumal SR, Ignacimuthu S. Antibacterial activity of some folkloremedicinal plants used by tribes in Westernchats of India. J Ethanopharmacol. 2000;69:63–71. [Google Scholar]
- 4.Balamuguran G, Selvarajan S. Preliminary phytochemical Investigation and Anthelmintic activity of Indigofera tinctoria linn. Int J Drug Dev Res. 2009;1:157–160. [Google Scholar]
- 5.Acharya S, Dash GK, BrahmaDK, Chhetree RR. Preliminary phytochemical investigation and anthelmintic activity of acacia suma (Roxb) barks. IRJP. 2011;2:136–141. [Google Scholar]
- 6.Dash S, Das C, Sahoo DC. Phytochemical and anthelmintic screening of crude bark extract of Adenanthera pavonina Linn. IJCP. 2010;2:1–4. [Google Scholar]
- 7.Saheb TS. A Study on Medicinal Climbers of Nallamalais, Andhra Pradesh. International Journal of Multidisciplinary Research and Development. 2014;1:172–176. [Google Scholar]
- 8.Naidu MT, Kumar OA, Venkaiah M. Taxonomic diversity of lianas in tropical forests of northern eastern ghats of Andhra Pradesh, India. Not Sci Biol. 2014;6:59–65. [Google Scholar]
- 9.Nair NC, Henry AN. Flora of Tamil Nadu, India. 1983;1 [Google Scholar]
- 10.Ahirrao YA, Patil DA. Ethnomedicinal investigation in Nandurbar district of Maharasthtra. J Ancient Science of Life. 2007;17:50–56. [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava A, Patil SP, Mishra RK, et al. Ethnomedicinal importance of the plants of Amarkantak region, Madhya Pradesh, India. Int J Med Arom Plants. 2012;2:53–59. [Google Scholar]
- 12.Sadangi N, Sahu RK. Traditional veterinary herbal practice of Kalahandi district, Orissa, India. Journal of Natural Remedies. 2004;4:131–136. [Google Scholar]
- 13.Anita Jain A. Katewa SS, Galac P,Nag A. Some therapeutic uses of biodiversity among the tribals of Rajasthan. Indian Journal of Traditional Knowledge. 2008;2:256–262. [Google Scholar]
- 14.Reddy KN, Trimurthulu G, Reddy CS. Plants used by ethnic people of Krishna district, Andhra Pradesh. Indian Journal of Traditional Knowledge. 2009;9:313–317. [Google Scholar]
- 15.Wath M, Sangeeta JS. Ethnoveterinary survey of herbal therapy treating livestocks of Melghat region (Maharashtra). IJPAES. 2014;3:42–48. [Google Scholar]
- 16.Patil US, Deshmukh OS. Traditional ethno-veterinary practices in Betul district Madhya Pradesh India. International Journal of Current Research in Life Sciences. 2015;4:423–428. [Google Scholar]
- 17.Anuj SK, Srivastava P, Tiwari BN, et al. Plant (Cissus quadrangularis) with various ethnopharmacological action: A review. Journal of Pharmacy Research. 2011;4:1887–1890. [Google Scholar]
- 18.Igbinosa OO, Igbinosa EO, Aiyegoro OA. Antimicrobial activity and phytochemical screening of stem bark extracts from Jatropa curcas L. African Journal of Pharmacy and Pharmacology. 2009;3:58–62. [Google Scholar]
- 19.Obey JK, von Wright A, Orjala J, et al. Antibacterial activity of Croton macrostachyus stem bark extracts against several human pathogenic bacteria. Journal of Pathogens. 2016. [DOI] [PMC free article] [PubMed]
- 20.Chaudhary S, Negi A, Dahiya V. The study of in vitro antimicrobial activity and phytochemical analysis of some medicinal plants in chamoli garhwal region. Phcog J. 2010;2:481–485. [Google Scholar]
- 21.Evans WC. Trease & Evans. Pharmacognosy. 2009;16th edition [Google Scholar]
- 22.Ajaiyeoba EO, Onocha PA, Olarenwaju OT. In-vitro anthelmintic properties of Buchholzia coiaceae and Gynandropsis gynandra extract. Pharm Biol. 2001;39:217–220. [Google Scholar]
- 23.Kaur R, Kaur G, Kapoor A. Preliminary phytochemical screening and in-vitro anthelmintic activity of whole plant extracts of Barlera prionitis Linn against earth worms: Pheretima posthuma. World Journal of Pharmacy and Pharmaceutical Sciences. 2015;4:1340–347. [Google Scholar]
- 24.Bauri R, Tigga M, Kullu S. A review on use of medicinal plants to control parasites. Indian J Nat Prod Resour. 2015;6:268–277. [Google Scholar]
- 25.Poolperm S, Jiraungkoorskul W. An Update Review on the Anthelmintic Activity of Bitter Gourd, Momordica charantia. Pharmacogn Rev. 2017;11:31–34. doi: 10.4103/phrev.phrev_52_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waller DG, Sampson A. Medical Pharmacology and Therapeutics. E-Book. Elsevier Health Sciences. 2017;11:616. [Google Scholar]
- 27.Velebny GHS. Pharmacological Potential of Selected Natural Compounds in the Control of Selected Protozoan diseases. Springer Wien Heidelberg New York Dordrecht London. 2013. pp. 1325–1327.
- 28.Sirama V, Kokwaro J, Owuor B, et al. In-vitro anthelmintic activity of Vernonia amygdalina Del. (asteraceae) roots using adult Haemonchus contortus worms. International Journal of Pharmacological Research. 2015;5:1–7. [Google Scholar]
- 29.Mukherjee NMS, Saini P, Roy P, Babu SPS. Phenolics and Terpenoids; the Promising New Search for Anthelmintics. Mini Reviews in Medicinal Chemistry. 2016;16:1415–1441. doi: 10.2174/1389557516666151120121036. [DOI] [PubMed] [Google Scholar]
- 30.Poolperm S, Jiraungkoorskul W. An Update Review on the Anthelmintic Activity of Bitter Gourd. Momordica charantia. Pharmacogn Rev. 2017;11:31–34. doi: 10.4103/phrev.phrev_52_16. [DOI] [PMC free article] [PubMed] [Google Scholar]