Abstract
In recent decades, different methods have been introduced for the genotyping of Single Nucleotide Polymorphisms (SNPs) and mutations in nucleic acid sequences. These methods have several applications ranging from agriculture to medicine. The Loop-mediated isothermal amplification (LAMP) method was first introduced by Notomi et al. Since then, different methods derived from LAMP have been extensively applied in detecting pathogens. The LAMP method is an isothermal technique that amplifies the target DNA segment using four different primers that have been uniquely designed for recognizing six distinct zones on the objective gene; the process of reaction continues at a constant temperature via a strand displacement reaction. Amplifying and detecting the targeted zone can be accomplished in one stage. Although the LAMP method is mostly used for pathogen detection, several studies have used this method for genotyping. The present article reviewed various studies that used the LAMP method for SNP detection. The outcomes indicated that the LAMP technique could be a reliable and alternative technique for genotyping. Further studies are recommended to use this approach for genotyping.
Keywords: AS-LAMP, Genotyping, LAMP, Single nucleotide polymorphisms
Introduction
Finding the changes that occur in nucleic acid sequences is an important issue in various fields ranging from medicine to agriculture. Because the genomes of all organisms consist of a huge amount of DNA, it is very difficult to directly detect Single Nucleotide Polymorphisms (SNPs) or nucleotide changes in a genome. Currently, nucleic acid amplification methods are extensively utilized in many labs for identifying variations in previously well-characterized genomic regions 1. Since its innovation in 1993, the Polymerase Chain Reaction (PCR) has been the basis for most widespread techniques for applying nucleic acid amplification 2–6. Although PCR-based methods are cost-effective techniques that are very robust for nucleic acid amplification, they cannot be performed in every lab. In addition, the methods require a thermocycling system, which limits their application in the field 7,8.
In the past years, numerous nucleic amplification approaches have been introduced that do not need a thermocycling system. Isothermal techniques to amplify nucleic acids include Loop-mediated isothermal Amplification (LAMP), Nucleic Acid Sequence-Based Amplification (NASBA), Helicase-Dependent Amplification (HDA), Rolling Circle Amplification (RCA), Multiple Displacement Amplification (MDA), and Re-combinase Polymerase Amplification (RPA); these new approaches can easily be used at a constant temperature on a simple heater block 9–16.
LAMP Method
The LAMP method was first introduced by Notomi et al in 2000 17. Since then, different methods derived from LAMP have been widely used for pathogen detection 18–21. The LAMP method uses four sets of primers that are specially aimed to identify six distinct zones on the objective gene. These sets of primers are the outer primers (F3 and B3) and the inner primers. The forward inner primer (FIP) consisting of the F2 zone at the 3′ end with the sequence similar to the F1c zone at the 5′ end, is complementary to the F2c zone, while the Backward Inner Primer (BIP) consisting of the B2 zone at the 3′ end with the sequence similar to the B1c region at the 5′ end, is complementary to the B2c zone. The two primers are complementary to the downstream zone of the opposite strand in the objective (F1 and B1) 17. In addition to the specific primers, the LAMP reaction requires Bst polymerase, deoxynucleotide triphosphates (dNTPs), magnesium sulfate, betaine, and buffer for enzyme 18. The products of amplification, which have a stem–loop DNA structure, contain numerous inverted repeats of the objective region and cauliflower-like structures with multiple loops 22 (Figure 1). The LAMP method can quickly amplify a large number of DNA usually at 60°C, which leads to the production of pyrophosphate and the production of a white sediment, which is magnesium pyrophosphate 23. Although observing the white precipitate can indicate that the nucleic acid was amplified, LAMP products can also be identified using gel electrophoresis, real-time turbidimetry, fluorescent probes, and SYBR green dye 23–25. The LAMP method yields a considerable amount of amplicons in a short time (around 60 min), which is 103 times higher than the amount produced with simple PCR 26. Because the LAMP method uses four primer sets that are specific to the objective regions, it has rapid amplification, a higher amount of amplification products, and smaller detection limits compared with PCR-based methods 26.
Figure 1.
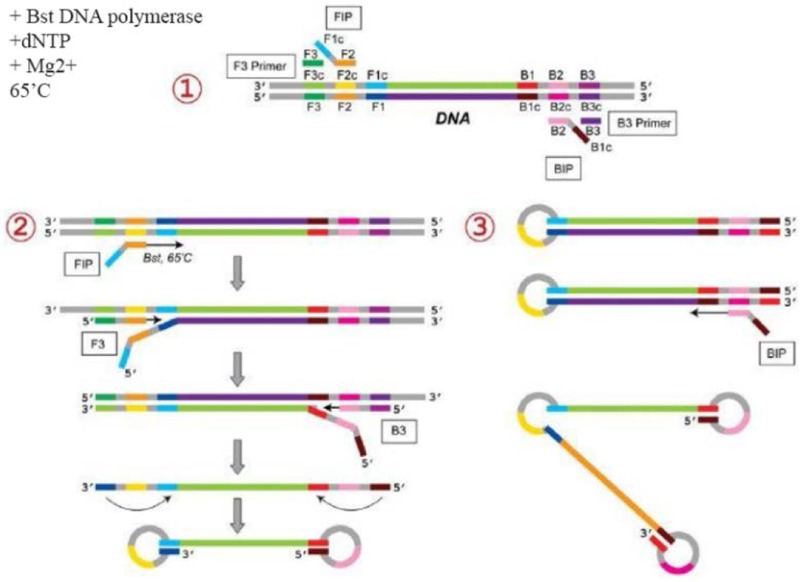
Schematic representation of the Loop-mediated isothermal Amplification for (LAMP). In the first step, the 435 designed primers bind to the complementary sequences. Then, the dumbbell-like DNA form is generated. Next, 436 in the cycling amplification step, DNAs of this form are generated continuously. The elongation reactions are 437 started from the products of the cycling amplification step, generating various sizes of the products.
Unlike PCR, which needs thermocycling system, the LAMP reaction can be performed at a fixed temperature and does not need a thermocycling system. Hence, LAMP is more beneficial for Point-Of-Care Testing (POCT) than PCR 21. It should be mentioned that the LAMP procedure also has some limitations, such as the complications involved in designing primers and the difficulties in using LAMP in multiplex reactions.
SNP detection using the LAMP method
Since its introduction, the LAMP technique has been applied for detecting infections in which the amplification of the pathogen genome is required. The LAMP method has rarely been used for genotyping and detecting point mutations. LAMP is a sensitive technique that can quickly amplify the target DNA in an isothermal condition. Thus, designing new LAMP methods that could be used for genotyping is very important because the introduced methods could be a suitable alternative to other genotyping techniques. Moreover, the LAMP technique is a very suitable, easy to use, and cost-effective approach for POCT or on-site testing.
The current article was aimed to study the existing evidence for genotype detection using the LAMP technique as an isothermal approach. In addition, the advantages and properties of the LAMP technique were also discussed.
AS-LAMP
In the Allele-Specific (AS)–LAMP method, two sets of LAMP primers are provided for distinguishing between two diverse nucleotides in the sequence corresponding to a target gene. Specific primers, two primers of BIP or FIP, are planned with the mutation point at the 3′ end corresponding to the B2 primer (5′ end corresponding to the BIP primer) and an extra mismatched nucleotide (lower case letter) to enhance the specificity to every target nucleotide site. In the other two primer sets, the primers of F3, B3, and FIP or BIP, are the same. For each sample, two LAMP reactions are applied with each set of primers.
Various studies used the AS-LAMP method for genotyping. For example, the method was used for the identification of the West African kdr (kdr-w; L1014F) 27 and G119S ace-1R mutations 28 in field-collected Anopheles gambiae.
Yongkiettrakul et al also used an SNP-LAMP assay for detecting the N51I mutation on the dhfr gene, which is related to pyrimethamine resistance in Plasmodium falciparum 29. The SNP-LAMP method show-ed 100% specificity 29.
Ikeda et al designed an Amplification-Refractory Mutation System (ARMS)-LAMP approach to detect an L858R mutation of the Epidermal Growth Factor Receptor (EGFR) 30. They used an in situ LAMP reaction on paraffin-embedded tissues. The FIP and BIP primers were labeled with Fluorescein Isothiocyanate (FITC). The comparison between outcomes of the in situ LAMP reaction with the results of PCR-Restriction Fragment Length Polymorphism (RFLP) showed that while the PCR-RFLP method could detect the mutation in 12 of 26 patients, the in situ LAMP technique detected the mutation in 15 patients 30.
Nakamura et al used the LAMP method combined with an electrochemical DNA chip to simultaneously identify the six polymorphisms related with Rheumatoid Arthritis (RA), including N-acetyltransferase2 (N-AT2) gene polymorphisms T341C, G590A, and G857-A, Methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms C677T and A1298C, and Serum Amyloid A1 (SAA1) gene promoter polymorphism −13 C>T 31. In the study, 31 samples were genotyped using the combined LAMP technique and the outcomes were found to be in a good agreement with the results from the PCR-RFLP 31.
Tamura et al developed a LAMP technique for detecting the N526K ftsI mutation corresponding to β-Lactamase-Negative Ampicillin-Resistant (BLNAR) Haemophilus influenzae (H. influenzae) 32. They combined the LAMP method with the ARMS approach to exactly identify a diverse single nucleotide in the objective sequence. The method could perform a species–specific identification of a nucleotide (1578T) in the ftsI gene of H. influenzae with no amplification of the point mutations (T1578G/A) in the BLNAR strains. The limit of detection was obtained (10.0 pg of genomic DNA per reaction) 32.
Duan et al developed a fast and effective approach with great specificity on the basis of LAMP for detecting the point mutation at codon 200 (TTC&x2192;TAC, F200Y) of the β2-tubulin gene, which shows resistance to benzimidazole fungicide in Fusarium asiaticum (F. asiaticum) 33. A comparison was performed between the outcomes of LAMP test and the outcomes of PCR test. The researchers found that the LAMP test could effectively identify the F200Y mutant genotype in carbendazim-resistant separates of F. asiaticum in agricultural crops 33. Lin et al used the AS-LAMP in order to distinguish between wild-type and vaccine strains corresponding to Mink enteritis virus 34.
Kwong et al applied SNP genotyping-LAMP method for identification of CYP2C19*2 and CYP2C19*3 SNPs on CYP2C19 gene on 100 samples and the results were compared with the results of real-time PCR melting curve analysis. SYBR Safe DNA Gel Stain (a DNA intercalating dye) was added directly to the reaction tube for the visualizing of LAMP products. This dye would turn green under ambient light if the targeted LAMP products were amplified. It would remain orange if the products were not amplified. The real-time PCR melting curve analysis and SNP genotyping-LAMP assay had concordant results and both methods could successfully detect CYP2C19*2 and CYPC19*3 SNPs. Besides, they were able to differentiate heterozygous CYP2C19*2 variants from homozygous ones 35.
LAMP-RFLP
A common method for genotyping in molecular biology involves the digestion of specific sequences using restriction enzymes. RFLP on the PCR product is one of the most common methods used for detecting SNPs in a genome 36–38. LAMP products can also be digested with specific restriction enzymes in order to identify the SNPs.
Shao et al developed a multiplex LAMP-RFLP (mLAMP-RFLP) for detecting Salmonella strains and Shigella strains in milk simultaneously 39. They used two sets of LAMP primers to definitely aim ipaH of Shigella spp and invA of Salmonella spp.
Yoshida et al developed a Reverse Transcription (RT)-LAMP technique mixed with RFLP to distinguish the Hoshino vaccine strain of the mumps virus from circulating wild strains 40. First, the Hemagglutinin Neuraminidase (HN) area corresponding to the virus was amplified using AMV reverse transcriptase and Bst DNA polymerase. In the HN area, the strain of vaccine has a particular restriction enzyme site of ScaI. The sensitive and differential method proved to be effective for the laboratory surveillance corresponding to vaccine-adverse events 40.
LAMP method combined with an electrochemical oligonucleotide chip
In order to determine the copies of CYP2D6 gene correctly, Nakamura et al planned a primer set that co-amplified the CYP2D6 gene, but not the CYP2D6*36 and the CYP2D8P gene 41. The copies of CYP2D6 gene were determined through comparison of the quantity of the amplified products of the CYP2D6 gene with the CYP2D8P gene. For confirmation of the amplification product specificity, the digestion of products was performed with NcoI and Hpy99 restriction enzymes. The amplified products were hybridized on a chip containing probes that were complementary to the CYP2D6 and CYP2D8P genes. The copies of CYP2D6 gene were in agreement with earlier tests. The introduced method lasted just for 1.5 hr 41.
Exo-proofreading LAMP
Kuzuhara et al developed a SNP detecting version of LAMP combined with the 3′–5′ exonuclease proofreading activity corresponding to DNA polymerase called PF LAMP 42. A primer was designed so that its 3′ terminus was aligned at the polymorphic base of the DNA target and labeled with a detectable fluorescence tag on the 3′ nucleotide base. When the 3′ end corresponding to the primer was complementary to the objective, the labeled nucleotide remained and was combined with the extension product. When the primer did not match with the template, the proofreading activity of the polymerase removed the labeled nucleotide and no tag was combined with the extension product. The detection of the genotype was accomplished using fluorescence polarization with no extra cleanup. Using this method, the researchers could successfully detect the G1951A SNP in the human Aldehyde Dehydrogenase 2 (ALDH2) gene 42.
PNA-LNA mediated LAMP
Itogana et al designed a Peptide Nucleic Acid–Locked Nucleic Acid (PNA-LNA) mediated LAMP method for detecting a KRAS mutation 43. In the PNA-LNA mediated LAMP method, a specific clamping PNA probe for the wild type nucleotide and extra LNA primers corresponding to the mutant type nucleotide were planned for the looped area corresponding to the main products of LAMP. The LAMP reactions were carried out under isothermal conditions at temperature of 65°C using a strand-displacement DNA polymerase. In the method, the FIP and B3 primers anneal and extend on the target DNA and the freshly prepared DNA chains are moved through extending F3 and B3, respectively. In the wild type samples, the PNA probe generates moved products with stem loop structures that prevent the annealing and extending the LNA primer. In the case of mutation of the objective gene, the PNA cannot form a clamp with the displaced DNA because of a single-base mismatch; as a result, the LNA primer attaches and anneals to the objective area and subsequently continues extending (Figure 2). The amplified product real-time PCR equipment was detected by agarose gel electrophoresis with the naked eye. A direct sequencing test and PNA-clamping PCR were compared with the PNA-LNA mediated LAMP technique in terms of the Limit of Detection (LOD) of the KRAS point mutation. Detection of mutant alleles could be reproducibly performed with a mutant-to-wild type ratio of 30% with direct sequencing and a ratio of 1% with PNA-clamping PCR, while the detection of mutant alleles could be performed over 50 min in specimens diluted to a mutant-to-wild type ratio of 0.1% using the LAMP method. Cao et al developed PNA-LNA clamping LAMP for the quick identification of CALR type 1 (CALR-1) and type 2 (CALR-2) mutations in Philadelphia chromosome-negative MPN subjects 44.
Figure 2.
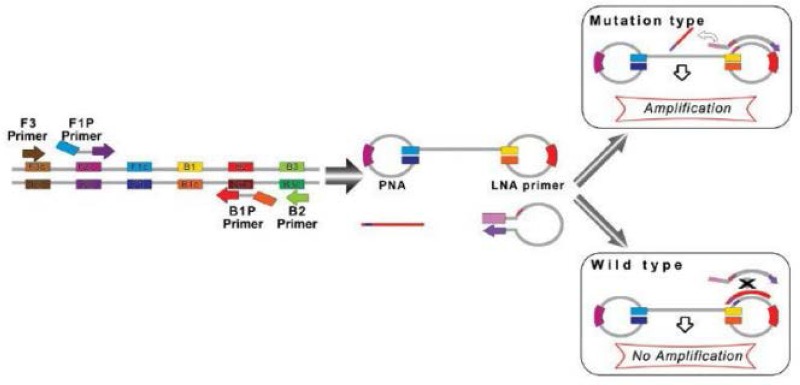
A schematic picture of PNA-LNA mediated LAMP. When the target is wild-type sequence, the 450 clamping PNA probe forms a stable duplex with the dumbbell structure, and interferes with the 451 annealing and elongation of the LNA primer. When the target DNA has mutated sequence, the 452 clamping PNA probe does not anneal with the DNA because of the single-base mismatch, and the 453 elongation reaction proceeds.
Invasive reaction combined with oligonucleotide probe-modified gold nanoparticles
Lu et al introduced a new technique derived from the LAMP method for SNP detection 45. The introduced method had a complicated structure. First, multiple fragments comprising the relevant SNPs were amplified using a set of three pairs of specific primers. Then the targeted base in the amplicon was recognized using two allele specific probes including an Upstream Probe (UP) and a Downstream Probe (DP). It should be mentioned that there were two designs of DP probes, one for a normal allele and the other for a mutated allele. Next, a subsequent invasive reaction using Afu Flap Endonuclease (FEN) was performed. In the presence of the target allele, the DP allele forms an overlapping structure that is identified and cleaved by FEN; the FEN cleaves the 5′ end of the DP probe. A hairpin should also be designed to capture the cleaved DP probes; the hairpin itself is cleaved by FEN in the presence of the cleaved DP probes. A pair of ends of the hairpin probe is designed for capturing gold nanoparticles (AuNPs) coated probes. Because of different sequences of these two ends, two types of AuNPs coated in two different probes should be applied. If the hairpin probe is not cleaved, the AuNPs on the probes captured by the hairpin probes cause the aggregation of AuNPs, which forms a precipitate in the test tubes. When the hairpin is cleaved because of the presence of the target sequence, the free AuNPs cause the solution to turn red (Figure 3). The researchers used naked-eye detection to identify the SNPs in the isothermal condition. Due to the sensitivity of the allele-specific probe to detect one base difference, they argued that the mLAMP method can be used to produce amplicon mixes for several SNP detections.
Figure 3.
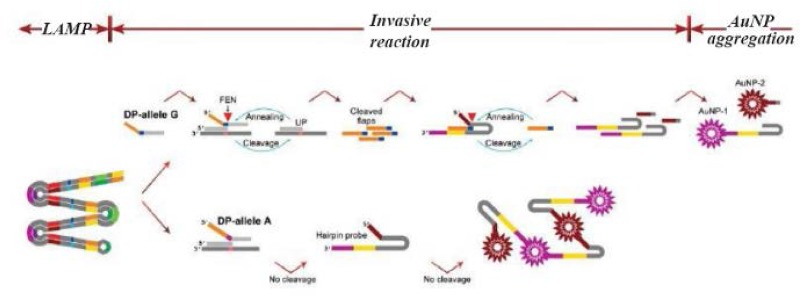
A schematic image of AS-LAMP coupled with invasive reaction and hybridization-induced AuNP 467 aggregation. The designed method contains three steps: LAMP, invasive reaction and AuNP aggregation. 468 LAMP amplifies the targeted sequence at first. Afu flap endonuclease specifically differentiate the invasive 469 structure formed by an upstream probe (UP), a downstream probe (DP), and a targeted sequence in the 470 amplicon, yielding the flaps to initiate the cleavage of hairpin probes. If the targeted allele is present, hairpin 471 probes is cleaved into two fragments, giving red color of the tube. While, if the targeted allele is absent, 472 cleavage of the hairpin probes does not occur, leading to AuNPs aggregation-induced precipitation.
Lu et al then designed a mLAMP protocol for genotyping three diverse SNPs (CYP2C19*2, CYP2C19*3 and MDR1-C3435T), which are important in guiding the dose of clopidogrel 45. In fact, they used the 3-plex LAMP for objective amplification and the subsequent individual invader reaction for discrimination of every allele in the objective area. They indicated that 100 copies of genomic DNA are sufficient for giving a correct typing outcome for all three SNPs. Comparing the outcomes of the protocol with the outcomes of the pyrosequencing method on clinical samples showed identical typing results.
Conclusion
In this review, different studies that used the LAMP method for genotyping and SNPs detection were analyzed. These studies combined the LAMP method with various technologies in order to produce a technique that was suitable for genotyping. Although the LAMP technique has mainly been applied to detect the genomes of infectious agents without the determination of the SNPs, the reviewed studies showed that the LAMP method could also be a good approach for the genotyping of different SNPs. Unlike the conventional LAMP method which is widely used for pathogen detection, the AS-LAMP is a newly introduced method and further studies are required to make it popular for researchers.
Because the LAMP method is a sharp, fast, and robust technique, it is also suitable for use in POC situations. Thus, the LAMP method combined with other approaches for genotyping is highly recommended for POC testing.
Acknowledgement
We would like to thank staffs of the Thalassemia Research Center for support of this study.
References
- 1.Moore P. PCR: replicating success. Nature 2005;435 (7039):235–238. [DOI] [PubMed] [Google Scholar]
- 2.Aliyu SH, Marriot RK, Curran MD, Parmar S, Bentley N, Brown NM, et al. Real-time PCR investigation into the importance of Fusobacterium necrophorum as a cause of acute pharyngitis in general practice. J Med Microbiol 2004;53(Pt 10):1029–1035. [DOI] [PubMed] [Google Scholar]
- 3.Ashimoto A, Chen C, Bakker I, Slots J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol 1996;11 (4):266–273. [DOI] [PubMed] [Google Scholar]
- 4.Atkins SD, Clark IM. Fungal molecular diagnostics: a mini review. J Appl Genet 2004;45(1):3–15. [PubMed] [Google Scholar]
- 5.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal PCR in medical diagnostic fields association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000;92(9):709–720. [DOI] [PubMed] [Google Scholar]
- 6.Levin RE. The application of real-time PCR to food and agricultural systems. a review. Food Biotechnol 2007;18 (1):97–133. [Google Scholar]
- 7.Tsai SM, Chana KW, Hsu WL, Chang TJ, Wong ML, Wang CY. Development of a loop-mediated isothermal amplification for rapid detection of orf virus. J Virol Methods 2009;157(2):200–204. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Song D, He W, Bao Y, Lu R, Su G, et al. Rapid detection of orf virus by loop-mediated isothermal amplification based on the DNA polymerase gene. Arch Virol 2013;158(4):793–798. [DOI] [PubMed] [Google Scholar]
- 9.Gill P, Ghaemi A. Nucleic acid isothermal amplification technologies: a review. Nucleosides Nucleotides Nucleic Acids 2008;27(3):224–243. [DOI] [PubMed] [Google Scholar]
- 10.Karami A, Gill P, Motamedi MH, Saghafinia M. A review of the current isothermal amplification techniques: applications, advantages and disadvantages. J Global Infect Dis 2011(3):293–302. [Google Scholar]
- 11.Guichón A, Chiparelli H, Martínez A, Rodríguez C, Tre-nto A, Russi JC, et al. Evaluation of a new NASBA assay for the qualitative detection of hepatitis C virus based on the NucliSense® Basic Kit reagents. J Clin Virol 2004; 29(2):84–91. [DOI] [PubMed] [Google Scholar]
- 12.Bremer J, Nowicki M, Beckner S, Brambilla D, Cronin M, Herman S, et al. Comparison of two amplification technologies for detection and quantitation of human immunodeficiency virus type 1 RNA in the female genital tract. J Clin Microbiol 2000;38(7):2665–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent M, Xu Y, Kong H. Helicase-dependent isothermal DNA amplification. EMBO Rep 2004;5(8):795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fire A, Xu SQ. Rolling replication of short DNA circles. Proc Natl Acad Sci USA 1995;92(10):4641–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lizardi PM, Huang X, Zhu Z, Bray-Ward P, Thomas DC, Ward DC. Mutation detection and single-molecule counting using isothermal rolling circle amplification. Nat Genet 1998;19(3):225–232. [DOI] [PubMed] [Google Scholar]
- 16.Dean FB, Hosono S, Fang L, Wu X, Faruqi AF, Bray-Ward P, et al. Comprehensive human genome amplification using multiple displacement amplification. Proc Natl Acad Sci USA 2002;99(8):5261–5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 2000;28(12): E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rafati A, Gill P. Microfluidic method for rapid turbidimetric detection of the DNA of mycobacterium tuberculosis using loop-mediated isothermal amplification in capillary tubes. Microchim Acta 2015;182(3–4):523–530. [Google Scholar]
- 19.Li Y, Fan P, Zhou S, Zhang L. Loop-mediated isothermal amplification (LAMP): a novel rapid detection platform for pathogens. Microb Pathog 2017;107:54–61. [DOI] [PubMed] [Google Scholar]
- 20.Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP): recent progress in research and development. J Infect Chemother 2013;19(3):404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori Y, Notomi T. Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J Infect Chemother 2009;15(2):62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gill P, Ranjbar B, Saber R, Khajeh K, Mohammadian M. Biomolecular and structural analyses of cauliflower-like DNAs by ultraviolet, circular dichroism, and fluorescence spectroscopies in comparison with natural DNA. J Biomol Tech 2011;22(2):60–66. [PMC free article] [PubMed] [Google Scholar]
- 23.Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loopmediated isothermal amplification by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun 2001;289:150–154. [DOI] [PubMed] [Google Scholar]
- 24.Mori Y, Kitao M, Tomita N, Notomi T. Real-time turbidimetry of LAMP reaction for quantifying template DNA. J Biochem Biophys Methods 2004;59(2):145–157. [DOI] [PubMed] [Google Scholar]
- 25.Zhao X, Lin CW, Wang J, Oh DH. Advances in rapid detection methods for foodborne pathogens. J Microbiol Biotechnol 2014;24(3):297–312. [DOI] [PubMed] [Google Scholar]
- 26.Law JW, Ab Mutalib NS, Chan KG, Lee LH. Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol 2015;5:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Namountougou M, Diabate A, Etang J, Bass C, Sawado-go SP, Gnankinie O, et al. First report of the L1014S kdr mutation in wild populations of Anopheles gambiae M and S molecular forms in Burkina Faso (West Africa). Acta Trop 2013;125(2):123–127. [DOI] [PubMed] [Google Scholar]
- 28.Badolo A, Bando H, Traoré A, Ko-Ketsu M, Guelbeogo WM, Kanuka H, et al. Detection of G119S ace-1 (R) mutation in field-collected Anopheles gambiae mosquitoes using allele-specific loop-mediated isothermal amplification (AS-LAMP) method. Malar J 2015;14:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yongkiettrakul S, Kampeera J, Chareanchim W, Rattanajak R, Pornthanakasem W, Kiatpathomchai W, et al. Simple detection of single nucleotide polymorphism in Plasmodium falciparum by SNP-LAMP assay combined with lateral flow dipstick. Parasitol Int 2017;66(1):964–971. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda S, Takabe K, Inagaki M, Funakoshi N, Suzuki K. Detection of gene point mutation in paraffin sections using in situ loop-mediated isothermal amplification. Pathol Int 2007;57(9):594–599. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura N, Ito K, Takahashi M, Hashimoto K, Kawamoto M, Yamanaka M, et al. Detection of six single-nucleotide polymorphisms associated with rheumatoid arthritis by a loop-mediated isothermal amplification method and an electrochemical DNA chip. Anal Chem 2007;79(24):9484–9493. [DOI] [PubMed] [Google Scholar]
- 32.Tamura S, Maeda T, Misawa K, Osa M, Hamamoto T, Yuki A, et al. Development of a highly resolved loop mediated isothermal amplification method to detect the N526K ftsI mutation of β-lactamase-negative ampicillin-resistant Haemophilus influenzae. J Microbiol Methods 2017;141:108–114. [DOI] [PubMed] [Google Scholar]
- 33.Duan Y, Yang Y, Li T, Zhao D, Cao J, Shi Y, et al. Development of a rapid and high-throughput molecular method for detecting the F200Y mutant genotype in benzimidazole-resistant isolates of Fusarium asiaticum. Pest Manag Sci 2016;72(11):2128–2135. [DOI] [PubMed] [Google Scholar]
- 34.Lin P, Wang H, Cheng Y, Song S, Sun Y, Zhang M, et al. Loop-mediated isothermal amplification-single nucleotide polymorphism analysis for detection and differentiation of wild-type and vaccine strains of mink enteritis virus. Sci Rep 2018;8(1):8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwong KM, Tam CC, Chan R, Lee SWL, Ip P, Kwok J. Comparison of single nucleotide polymorphism genotyping of CYP2C19 by loop-mediated isothermal amplification and real-time PCR melting curve analysis. Clin Chim Acta 2018;478:45–50. [DOI] [PubMed] [Google Scholar]
- 36.Chuang LY, Yang CH, Tsui KH, Cheng YH, Chang PL, Wen CH, et al. Restriction enzyme mining for SNPs in genomes. Anticancer Res 2008;28(4A):2001–2007. [PubMed] [Google Scholar]
- 37.Ota M, Asamura H, Oki T, Sada M. Restriction enzyme analysis of PCR products. Methods Mol Biol 2009;578: 405–414. [DOI] [PubMed] [Google Scholar]
- 38.Chang HW, Yang CH, Chang PL, Cheng YH, Chuang LY. SNP-RFLPing: restriction enzyme mining for SNPs in genomes. BMC Genomics 2006;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shao Y, Zhu S, Jin C, Chen F. Development of multiplex loop-mediated isothermal amplification-RFLP (mLAMP-RFLP) to detect Salmonella spp. and Shigella spp. in milk. Int J Food Microbiol 2011;148(2):75–79. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida N, Fujino M, Ota Y, Notomi T, Nakayama T. Simple differentiation method of mumps Hoshino vaccine strain from wild strains by reverse transcription loop-mediated isothermal amplification (RT-LAMP). Vaccine 2007;25(7):1281–1286. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura N, Fukuda T, Nonen S, Hashimoto K, Azuma J, Gemma N. Simple and accurate determination of CYP2D6 gene copy number by a loop-mediated isothermalamplification method and an electrochemical DNA chip. Clin Chim Acta 2010;411(7–8):568–573. [DOI] [PubMed] [Google Scholar]
- 42.Kuzuhara Y, Yonekawa T, Iwasaki M, Kadota T, Kanda H, Horigome T, et al. Homogeneous assays for single-nucleotide polymorphism genotyping: Exo-proofreading assay based on loop-mediated isothermal amplification. Yokohama Med J 2005;56(1):9–16. [Google Scholar]
- 43.Itonaga M, Matsuzaki I, Warigaya K, Tamura T, Shimizu Y, Fujimoto M, et al. Novel methodology for rapid detection of KRAS mutation using PNA-LNA mediated loop-mediated isothermal amplification. PLoS One 2016; 11(3):e0151654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao G, Kong J, Xing Z, Tang Y, Zhang X, Xu X, et al. Rapid detection of CALR type 1 and type 2 mutations using PNA-LNA clamping loop-mediated isothermal amplification on a CD-like microfluidic chip. Anal Chim Acta 2018;1024:123–135. [DOI] [PubMed] [Google Scholar]
- 45.Lu Y, Ma X, Wang J, Sheng N, Dong T, Song Q, et al. Visualized detection of single base difference in multiplexed loop mediated isothermal amplification amplicons by invasive reaction coupled with oligonucleotide probe-modified gold nanoparticles. Biosens Bioelectron 2017; 90:388–393. [DOI] [PubMed] [Google Scholar]


