Cellular integrity is critically dependent on maintaining protein homeostasis. Proteotoxic stresses challenging this balance elicit regulated protective responses essential for organismal fitness. Approximately one-third of synthesized proteins are secreted from cells and undergo biogenesis in the endoplasmic reticulum (ER). Upon import into the ER, these proteins are engaged by chaperone systems to facilitate either folding or triage and removal through pathways of ER quality control (1). The activity of ER quality control is precisely regulated in response to protein folding stress through the unfolded protein response (UPR) (2). The UPR relies on three transmembrane signaling proteins, IRE1, PERK, and ATF6, each of which senses protein misfolding in the ER through a luminal domain and transmits to the cytoplasm and nucleus in order to induce the expression of distinct subsets of ER chaperones and other factors that ameliorate ER stress (3). In PNAS, Belyy et al. (4) employ advanced quantitative imaging strategies to reveal the intricacies of the regulation of IRE1 during ER stress in the cellular context.
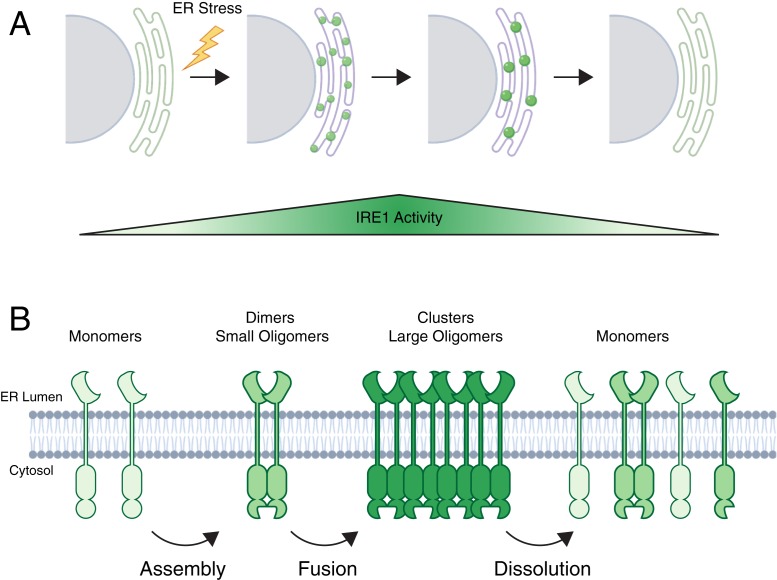
The most conserved of the three UPR sensors is IRE1, which is present in organisms from yeast to mammals. IRE1 is a transmembrane protein composed of three distinct domains, a sensing domain in the ER lumen, connected through a transmembrane segment to a cytosolic regulatory kinase domain, and finally a C-terminal RNase domain. Upon ER stress, IRE1 is activated to ultimately result in the noncanonical splicing of the mRNA encoding the transcription factor XBP1. After translation, the resulting XBP1-spliced transcription factor drives the expression of ER chaperones and lipid biosynthesis genes as a part of transcriptional program to resolve protein misfolding in the ER (2). In higher eukaryotes, IRE1 also down-regulates translation of ER-targeted proteins through direct cleavage of ER-associated mRNAs in a process known as RIDD (5). Evidence from yeast and mammalian systems suggests two mechanisms through which IRE1 senses increased levels of protein misfolding in the ER. The luminal domain includes a segment that has been shown to bind directly to the ER Hsp70 chaperone BiP, facilitated by its cochaperone ERdj4 (6). The IRE1 luminal domain can also bind directly to misfolded proteins in the ER through an MHC-like peptide binding cleft (7). Misfolding sensed through these mechanisms leads to IRE1 activation, which occurs through dimerization at interfaces in both its ER luminal and cytosolic domains that have been observed in crystal structures (8, 9). Beyond this, early microscopy studies in yeast and mammalian cells with fluorescently tagged IRE1 demonstrated the formation of large foci during ER stress, suggesting the formation of higher-order oligomers of IRE1 (Fig. 1A) (10, 11). While the formation of these IRE1 clusters is well documented, their nature has remained ambiguous since they are not amenable to purification for biochemistry or structural studies. What is the architecture of IRE1 clusters? Are they static or mobile? How are they assembled and cleared?
Fig. 1.
(A) In response to acute ER stress, IRE1 rapidly forms many small oligomers (small green orbs) throughout the ER membrane. These oligomers fuse and assemble into a few large clusters (large green orbs) during the first hours of ER stress. Upon sustained ER stress, IRE1 clusters are eventually resolved by dissolution back into the free IRE1 pool in the ER membrane. Cluster assembly closely correlates with the induction of IRE1 activities such as XBP1 splicing while dissolution occurs parallel to the shutoff of the IRE1 arm of the UPR. (B) Depiction of the molecular events occurring during IRE1 cluster formation. First, IRE1 is activated by assembly into dimers and small oligomers. Then these oligomers fuse to form large clusters that promote IRE1 activity. Finally, clusters are resolved by dissolution of IRE1 back into free monomers.
Belyy et al. explore the dynamics of IRE1 oligomers in their cellular context. To this end, they developed an IRE1α-mNeonGreen reporter leveraging the extreme brightness and improved photostability of this next-generation florescent protein. High-resolution confocal microscopy revealed that IRE1 clusters are asymmetric, prompting an investigation at superresolution using structured illumination microscopy (SIM). SIM imaging of cells stressed with tunicamycin, an inhibitor of N-linked glycosylation in the ER, revealed that IRE1 clusters have complex and intricate architectures including structures forming loops and coils that localize to branching points in the ER network. Interestingly, colocalization with the general ER membrane marker Sec61β was incomplete, suggesting that IRE1 clusters may exclude other ER components and create an IRE1-rich microdomain. This could be accomplished through local perturbation of the ER lipid membrane, for instance by compression of the lipid bilayer in the ER that has been attributed to the IRE1 oligomer (12). These observations suggest the intriguing possibility that IRE1 creates localized platforms that facilitate its function in sensing ER stress and inducing the UPR.
Next, Belyy et al. investigated the dynamics of IRE1 clusters across the timeline of acute ER stress. To this end, they developed a clonal U2OS cell line expressing inducible tagged IRE1 and leveraged automated imaging and analysis pipelines to move well beyond prior microscopy studies of IRE1 clustering in scope and depth. Live imaging of single-cell trajectories of IRE1 clustering revealed significant heterogeneity in response to stress, despite analyzing a clonal cell population. Notably, nearly one-half of cells never formed detectable IRE1 clusters. Inherent variability in stress responses could reflect differences in cellular states, such as cell cycle progression or microenvironment, dictating how cells experience stress; alternatively, they could reflect stochastic variability in stress response transcription factor activity (13). The observed latent diversity in how cells respond to stress suggests the intriguing possibility that the UPR is not a monolithic response. The extent and consequences of such diversity in cellular response to ER stress will be of great interest to further explore.
Following only those cells that formed IRE1 clusters, Belyy et al. investigated the dynamics and life cycle of these structures upon prolonged ER stress. This analysis confirms prior observations that many small IRE1 clusters form initially, and then coalesce into a few large clusters per cell, which ultimately dissipate during sustained ER stress (Fig. 1B). The apparent fusion events occurring between IRE1 clusters is reminiscent of the behavior of assemblies undergoing liquid–liquid phase separation (14). In recent years, numerous protein assemblies have been shown to form condensates and coalesce through demixing by liquid–liquid phase separation to regulate a wide variety of activities such as splicing and signal transduction (15). Common features of phase-separated structures are their dynamic exchange of monomers with bulk solution and their fluid droplet-like fusion behaviors. To reveal the nature of IRE1 cluster dynamics, the authors performed fluorescence recovery after photobleaching experiments, which surprisingly demonstrated that, despite their fusion behavior, IRE1 clusters are not phase-separated droplets since most IRE1 monomers in clusters do not readily exchange with the free pool of IRE1 in the ER. In fact, only a small percentage of IRE1 in clusters is available for exchange, suggesting that perhaps IRE1 forms a lattice-like structure where exchange may only happen at the boundaries. This is reminiscent of the lattices formed by other signaling proteins such as the bacterial chemotaxis system where stable assembly is thought to promote receptor cooperativity and enhance system sensitivity (16). Such structural cooperativity could contribute to sustaining the splicing activity of IRE1 during ER stress or perhaps alter RIDD substrate selection.
An interesting feature of IRE1 clusters revealed by the automated imaging approach of Belyy et al. is their extensive motility. Analysis of
In PNAS, Belyy et al. employ advanced quantitative imaging strategies to reveal the intricacies of the regulation of IRE1 during ER stress in the cellular context.
the trajectories of thousands of IRE1 clusters suggests their motion is largely diffusive rather than the result of active transport along the cytoskeleton. Perhaps most surprising is the observation that IRE1 cluster diffusion is largely independent of the size of clusters, suggesting their motion is constrained by other cellular features.
One possibility is that IRE1 favors particular subdomains of the ER. This is supported by previous observations that IRE1 accumulates at mitochondria–ER contact sites, a domain of the ER referred to as mitochondrial-associated membranes (MAMs) (17, 18). Enrichment of IRE1 clusters to MAMs could facilitate signaling from mitochondrial sources for integration during the ER stress response in order to coordinate adaptation across organelles. Furthermore, IRE1 has also been shown to scaffold filamin A and impact cytoskeleton dynamics through which clustering of IRE1 into discrete ER domains could impact the fate of migrating cells (19). It will be interesting to explore the interactions of clustered IRE1 with other cellular structures through microscopy or proximity labeling approaches and further investigate how IRE1 clusters may influence organelle cross talk during ER stress.
Sustained ER stress eventually leads to a shift in the UPR from a prosurvival to procell death state (20). This shift is paralleled by a decline in IRE1 activation and XBP1 splicing that correlates with a decrease in clustered IRE1. This down-regulation of IRE1 clusters could either be accomplished through selective targeting by degradation pathways or dispersal of the clusters and return of IRE1 to the free monomer pool in ER. In order to definitively test the mechanism of IRE1 cluster resolution, the authors employed an optical pulse-chase approach using the photoconvertible florescent protein mEos4b fused to IRE1. These photoconversion experiments further confirmed that IRE1 clusters are not liquid-like condensates, since their largely immobile cores remain unmixed upon fusion. Imaging cluster dissolution upon continued stress indicated that IRE1 clusters neither break up into smaller clusters nor are they degraded. Instead, IRE1 molecules melt back into the ER network in a sharp transition indicative of an external regulatory signal (Fig. 1B). The dissolution cue remains to be determined, but likely candidates are PERK-regulated dephosphorylation or the increase in free levels of chaperone ERdj4 on the luminal face of the ER, which returns IRE1 to an inactive state (6, 21). IRE1 cluster dissolution would terminate the transcriptional and likely also RIDD activity of activated IRE1, leaving the cell to return to homeostasis or, in the case of continues stress, to shift the response to additional proapoptotic branches of the UPR.
Therapeutic targeting of IRE1 is gaining great interest to treat a wide range of diseases, from cancers such as multiple myeloma to metabolic disorders such as diabetes (22). However, as of yet, small-molecule inhibitors or activators of IRE1 have not successfully transitioned to the clinic. The revealing characterization of IRE1’s life cycle during ER stress presented in Belyy et al. demonstrates the intricate complexity and dynamics regulating IRE1 activity. Combined with the tools the authors have developed, this study provides a springboard to realize a more thorough understanding of the function and regulation of IRE1, which could aid in developing future therapeutic strategies targeting IRE1 to ameliorate a variety of diseases.
Acknowledgments
We were supported by grants from the NIH (AG056290 and GM056433) and the CHDI Foundation (to J.F.). T.K.R. was supported by a NIH National Research Service Award (GM120947).
Footnotes
The authors declare no competing interest.
See companion article on page 1533 in issue 3 of volume 117.
References
- 1.Braakman I., Bulleid N. J., Protein folding and modification in the mammalian endoplasmic reticulum. Annu. Rev. Biochem. 80, 71–99 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Walter P., Ron D., The unfolded protein response: From stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Korennykh A., Walter P., Structural basis of the unfolded protein response. Annu. Rev. Cell Dev. Biol. 28, 251–277 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Belyy V., Tran N.-H., Walter P., Quantitative microscopy reveals dynamics and fate of clustered IRE1α. Proc. Natl. Acad. Sci. U.S.A. 117, 1533–1542 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maurel M., Chevet E., Tavernier J., Gerlo S., Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 39, 245–254 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Amin-Wetzel N., et al. , A J-protein co-chaperone recruits BiP to monomerize IRE1 and repress the unfolded protein response. Cell 171, 1625–1637.e13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karagöz G. E., et al. , An unfolded protein-induced conformational switch activates mammalian IRE1. eLife 6, e30700 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korennykh A. V., et al. , The unfolded protein response signals through high-order assembly of Ire1. Nature 457, 687–693 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou J., et al. , The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc. Natl. Acad. Sci. U.S.A. 103, 14343–14348 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimata Y., et al. , Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins. J. Cell Biol. 179, 75–86 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H., Korennykh A. V., Behrman S. L., Walter P., Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc. Natl. Acad. Sci. U.S.A. 107, 16113–16118 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halbleib K., et al. , Activation of the unfolded protein response by lipid bilayer stress. Mol. Cell 67, 673–684.e8 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Popovic D., Koch B., Kueblbeck M., Ellenberg J., Pelkmans L., Multivariate control of transcript to protein variability in single mammalian cells. Cell Syst. 7, 398–411.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Ricci D., et al. , Clustering of IRE1α depends on sensing ER stress but not on its RNase activity. FASEB J. 33, 9811–9827 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberti S., Phase separation in biology. Curr. Biol. 27, R1097–R1102 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Erbse A. H., Falke J. J., The core signaling proteins of bacterial chemotaxis assemble to form an ultrastable complex. Biochemistry 48, 6975–6987 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carreras-Sureda A., et al. , Non-canonical function of IRE1α determines mitochondria-associated endoplasmic reticulum composition to control calcium transfer and bioenergetics. Nat. Cell Biol. 21, 755–767 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori T., Hayashi T., Hayashi E., Su T. P., Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS One 8, e76941 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urra H., et al. , IRE1α governs cytoskeleton remodelling and cell migration through a direct interaction with filamin A. Nat. Cell Biol. 20, 942–953 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Jäger R., Bertrand M. J., Gorman A. M., Vandenabeele P., Samali A., The unfolded protein response at the crossroads of cellular life and death during endoplasmic reticulum stress. Biol. Cell 104, 259–270 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Chang T. K., et al. , Coordination between two branches of the unfolded protein response determines apoptotic cell fate. Mol. Cell 71, 629–636.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Hetz C., Chevet E., Harding H. P., Targeting the unfolded protein response in disease. Nat. Rev. Drug Discov. 12, 703–719 (2013). [DOI] [PubMed] [Google Scholar]