Abstract
OBJECTIVE
Assess the efficacy of inControl AP, a mobile closed-loop control (CLC) system.
RESEARCH DESIGN AND METHODS
This protocol, NCT02985866, is a 3-month parallel-group, multicenter, randomized unblinded trial designed to compare mobile CLC with sensor-augmented pump (SAP) therapy. Eligibility criteria were type 1 diabetes for at least 1 year, use of insulin pumps for at least 6 months, age ≥14 years, and baseline HbA1c <10.5% (91 mmol/mol). The study was designed to assess two coprimary outcomes: superiority of CLC over SAP in continuous glucose monitor (CGM)–measured time below 3.9 mmol/L and noninferiority in CGM-measured time above 10 mmol/L.
RESULTS
Between November 2017 and May 2018, 127 participants were randomly assigned 1:1 to CLC (n = 65) versus SAP (n = 62); 125 participants completed the study. CGM time below 3.9 mmol/L was 5.0% at baseline and 2.4% during follow-up in the CLC group vs. 4.7% and 4.0%, respectively, in the SAP group (mean difference −1.7% [95% CI −2.4, −1.0]; P < 0.0001 for superiority). CGM time above 10 mmol/L was 40% at baseline and 34% during follow-up in the CLC group vs. 43% and 39%, respectively, in the SAP group (mean difference −3.0% [95% CI −6.1, 0.1]; P < 0.0001 for noninferiority). One severe hypoglycemic event occurred in the CLC group, which was unrelated to the study device.
CONCLUSIONS
In meeting its coprimary end points, superiority of CLC over SAP in CGM-measured time below 3.9 mmol/L and noninferiority in CGM-measured time above 10 mmol/L, the study has demonstrated that mobile CLC is feasible and could offer certain usability advantages over embedded systems, provided the connectivity between system components is stable.
Introduction
People with type 1 diabetes face a life-long optimization problem: limiting their exposure to hyperglycemia while simultaneously avoiding hypoglycemia (1). Classic studies have shown that many complications from diabetes are predicted by average glycemia, typically assessed by hemoglobin A1c (HbA1c), and can be reduced with intensive insulin therapy (2,3); however, the risk for hypoglycemia remains the primary barrier to optimal glycemic control (1). At present, closed-loop control (CLC), known as the artificial pancreas, offers the best solution to this optimization problem: day-and-night real-time fine-tuning of insulin delivery by an automated system.
In the past few years, the volume of CLC clinical trials increased dramatically. In 2018, the National Library of Medicine included 132 publications in the CLC field, and in the first 6 weeks of 2019 alone, 25 new articles were published. Research results are being translated into clinical practice: following a pivotal trial and regulatory approval in 2017, the Medtronic MiniMed 670G is now commercially available and used clinically (4). This is a hybrid CLC system, which automatically modulates the insulin pump basal rate to mitigate both hypo- and hyperglycemia but does not administer automated insulin boluses (e.g., corrections after a meal). Another system, the Tandem t:slim X2 with Control-IQ technology, which has completed its pivotal trial (ClinicalTrials.gov identifier NCT03563313, recently published in the New England Journal of Medicine) (5), uses a Dexcom G6 sensor that does not require fingersticks for calibration and a control algorithm that modulates basal rate, administers automated insulin corrections, and has a dedicated safety module safeguarding against hypoglycemia (5).
Both the MiniMed 670G and the Control-IQ closed-loop systems feature CLC algorithms that are embedded in the insulin pump, which is the traditional approach to closing the loop. An alternative solution could be provided by mobile CLC systems using consumer electronics (e.g., a smartphone) to run the control algorithm and transmit data to the Cloud. Mobile CLC could offer advantages: 1) smartphones are widely available and wirelessly connectable to various devices and networks—no current insulin pump offers similar capabilities; 2) the life cycle of a smartphone is months, as opposed to years for insulin pumps, and thus smartphones allow easier updates of the device form factor; and 3) psychologists share that patients, particularly youth, may be reluctant to use their insulin pump in public, but no one is reluctant to use a phone, and that may be a key to CLC adoption. The first experimental mobile CLC platform, the Diabetes Assistant (DiAs) developed at the University of Virginia (6), was pilot tested in 2012 (7). Subsequently, DiAs was used in a number of studies by >300 participants at 10 clinical centers in the U.S. and Europe (8,9), including long-term trials at home (10) and the first “stress test” of a CLC system—the Artificial Pancreas Ski Trial, where children with type 1 diabetes used DiAs during 5-day winter sport camps in Virginia, Colorado, and California (9). The next step in the use of off-the-shelf mobile technology for closed-loop insulin delivery was done by the iAPS smartphone app developed at Harvard University, which runs on an unlocked phone, communicates with Dexcom G5 and G6 continuous glucose monitors (CGMs) and Tandem Diabetes Care and Insulet insulin pumps, and uses a zone model predictive control algorithm (11). Two recent studies using different algorithms implemented in Android smartphones reported mobile CLC use of 12 weeks by a significant number of participants (86 [12] and 68 [13], respectively). It is therefore likely that embedded and mobile CLC systems could coexist in the future.
Consequently, the objective of this multicenter, randomized, parallel design study was to compare at-home day-and-night CLC using inControl AP, a direct descendant of DiAs and a prototype of Control-IQ using the same control algorithm (5,14), with sensor-augmented pump (SAP) therapy over 13 weeks. Because hypoglycemia has been repeatedly recognized as the major barrier to optimized treatment of diabetes, reducing the frequency of hypoglycemia was set as the primary objective of the study. Specifically, the study hypothesis was that CLC would be superior to SAP in terms of reduction of hypoglycemia assessed by CGM time below 3.9 mmol/L and noninferior to SAP in terms of hyperglycemia assessed by CGM time above 10 mmol/L.
Research Design and Methods
Study Design
This parallel-group, multicenter, randomized unblinded clinical trial was conducted at seven university diabetes centers in the U.S. The protocol and Health Insurance Portability and Accountability Act–compliant informed consent forms were approved by a central institutional review board. Study participants aged ≥18 years and parents or guardians of participants ≤17 years signed informed consent; written assent was obtained from minors. Ethics approval was provided by a central institutional review board in Tampa, Florida. An investigational device exemption was obtained from the U.S. Food and Drug Administration. Safety aspects were overseen by an independent data safety monitoring board appointed by the National Institutes of Health (NIH). The study was registered with ClinicalTrials.gov (NCT02985866).
Participants
Major inclusion criteria were type 1 diabetes treated with insulin for at least 1 year, continuous subcutaneous insulin infusion therapy for at least 6 months, age ≥14 years, no use of glucose-lowering agents other than insulin in the 3 months before enrollment, and HbA1c <10.5% (91 mmol/mol). A study goal was to have at least 50 participants with HbA1c <7.5% (58 mmol/mol) and at least 50 participants with HbA1c ≥7.5% (58 mmol/mol). A complete list of all inclusion and exclusion criteria is provided in Supplementary Table 1.
Randomization
The trial consisted of a run-in phase to collect baseline CGM data and to train participants on the use of study devices followed by randomization to either CLC or SAP for 13 weeks. Randomization was performed on the study website using a computer-generated sequence with a permuted block design, stratified by site to the CLC or SAP group in a 1:1 ratio.
Procedures
Study Technology
The CLC group was provided with a system that included an Accu-Chek Spirit Combo insulin pump (Roche Diabetes Care, Indianapolis, IN), a Dexcom G4 or G5 study CGM system (Dexcom, San Diego, CA), and the inControl AP software developed by TypeZero Technologies, running on a Google Nexus 5X smartphone and using Bluetooth and Bluetooth Low Energy to communicate with the study CGM and insulin pump, respectively (Supplementary Fig. 1). The SAP group participants were provided with the study CGM and used their personal continuous subcutaneous insulin infusion. The CGM required calibration using a fingerstick blood glucose (BG) measurement every 12 h. Thus, participants in both groups were also provided with a CONTOUR NEXT ONE Blood Glucose Monitoring System (Ascensia Diabetes Care, Basel, Switzerland) meter and test strips and with Precision Xtra (Abbott Diabetes Care, Alameda, CA) ketone meter and strips. The inControl AP application was based on a modular control algorithm originally developed at the University of Virginia, which automates basal insulin rate delivery and delivers insulin correction boluses to bring the user into a predefined target range. In addition, the algorithm uses a dedicated safety module to minimize the risk for hypoglycemia (14) and adjusts insulin delivery every 5 min. Overnight, inControl AP takes advantage of the relatively steady resting state of the participants to intensify basal rate modulation with the goal of achieving near-normoglycemia by narrowing its target range to ∼6.1–6.7 mmol/L, or 110–120 mg/dL, by the morning. As noted above, this algorithm is essentially the same as the algorithms used by DiAs (6) and Control-IQ (5); thus, further description of the control strategy can be found in previous publications. A network service communicated with inControl Cloud, the Cloud component of the system, which allowed data from any device to be monitored remotely in real time; however, for this study, the system was not configured to send automated notifications to study personnel regarding potential glycemic risks or system malfunctions. A user profile was entered into inControl to initialize the system, including date of birth, sex, height, weight, total daily insulin, 2-h insulin limit, low alert threshold, and high alert threshold. The participant’s parameters for basal rate(s), correction factor(s), and carbohydrate ratio(s) were also entered. The start and end times of the participant’s usual sleep period were used to set the sleep mode timing. Information about the specific phone, pump, and CGM was also included in the user profile. Participants initiated a prandial bolus or manual correction using the meal screen and entering information for BG value and carbohydrate content of the meal. The bolus calculator gave consideration to the current insulin on board and recommended a bolus amount that could be accepted, changed, or used as an extended bolus.
Run-in Phase
Participants who used a personal Dexcom G4 or G5 CGM prestudy for at least 21 of the prior 28 days proceeded directly to randomization. For these participants, the data downloaded from the personal CGM provided the 2-week baseline data. All others wore a blinded study CGM for 2 weeks to collect baseline data, followed by 2 weeks of training using an unblinded study CGM. Successful completion of the run-in was followed by randomization.
Randomized Trial
After randomization, all participants completed an initial 2-week training period with a call at 1 week, followed by clinic visits at 2, 5, 9, and 13 weeks and phone contact at 3 weeks. inControl AP training for participants randomly assigned to the CLC group could extend to a second clinic visit during the 1st week of the training period. When this occurred, the participants used the study pump and CGM in open loop before completing training and starting to use the inControl system. The study insulin pump, CGM, BG meter, and ketone meter data were downloaded at each visit. HbA1c was measured locally at screening, randomization, and the 13-week visit and also at a central laboratory at randomization and 13 weeks. Age-appropriate background questionnaires were administered to participants and guardians of participants aged ≤17 years at randomization. The occurrence of adverse events was solicited throughout the study. Reportable adverse events included serious adverse events, adverse events occurring in association with a study device or procedure, severe hypoglycemia (defined as hypoglycemic events that require the assistance of another person because of altered consciousness), and diabetic ketoacidosis (as defined by the Diabetes Control and Complications Trial [2]).
In January 2018, use of closed-loop mode by the CLC group was temporarily suspended as a precaution (no serious adverse events occurred) after a problem was discovered with the transfer of CGM data. In certain instances, the controller calculated insulin needs on the basis of noncurrent CGM values, leading to erroneous under- or overdelivery of insulin. Participants continued to use the system in open-loop mode until the problem was resolved. This issue affected 30 participants in the CLC group for up to 2 weeks. The analyses included all the CGM data recorded during this period, even though closed-loop mode was not in use.
Outcomes
Two coprimary end points were the proportion of time spent with glucose below 3.9 mmol/L (70 mg/dL) (superiority) and time spent with glucose above 10 mmol/L (180 mg/dL) (noninferiority; limit = 5% difference) on the basis of CGM glucose levels, excluding the first 2 weeks postrandomization (i.e., calculated using weeks 3–13 following randomization). Participants with any amount of follow-up CGM data were included in CGM tabulations and analyses. Secondary end points included HbA1c; insulin requirements; body weight at 13 weeks; CGM-measured time below 3 and 3.3 mmol/L (54 and 60 mg/dL); low BG index (LBGI) (15); discrete events below 3.9 mmol/L (70 mg/dL), defined as ≥15 consecutive min with a sensor glucose value in that range; time above 13.9 and 16.7 mmol/L (250 and 300 mg/dL); high BG index (15); time within 3.9–7.8 mmol/L (70–140 mg/dL); mean CGM glucose; and coefficient of variation. Selected CGM-measured end points were analyzed separately during self-reported awake and sleep periods. Safety outcomes included the frequency of severe hypoglycemic and hyperglycemic events and the nature and severity of other adverse events.
Statistical Analysis
Sample Size
The total sample size was computed to be 110 participants, assuming 1) 1:1 randomization; 2) 90% power, with adjustment to account for two coprimary analyses; 3) a 50% relative reduction in time spent below 3.9 mmol/L (absolute reduction of 2.0%, assuming baseline of 4.0%) with an effective SD of 2.8% and two-sided type I error of 5%; and 4) a noninferiority limit of 5% for the treatment group comparison of time spent above 10 mmol/L and assuming there is a true difference of 2.5% favoring the CLC group, with an effective SD of 11% and a one-sided type I error of 2.5%. The sample size was increased to 126 to account for dropouts.
Analytical Methods
Statistical analyses were performed on an intention-to-treat basis, and all participants with any amount of postrandomization data were included in all primary and secondary analyses. Means with SDs and medians with interquartile ranges (IQRs) are reported for primary and secondary end points with skewed distributions. The treatment groups are compared using linear models while adjusting for age, corresponding baseline value, previous CGM use, and a random center effect. Missing baseline data were handled by direct likelihood method (16). Model residuals were confirmed to be approximately normally distributed. To address the issue of multiple comparisons with two primary outcomes, the intervention was considered effective only if both were statistically significant at the 5% level. All secondary analyses were corrected for multiple comparisons using the false discovery rate at a 10% level for statistical significance. All secondary P values reported are two-sided. Statistical analyses were carried out using SAS 9.4 software.
Results
Between November 2017 and May 2018, 132 participants were screened and 127 entered the randomized trial: 65 participants were randomly assigned to the CLC group and 62 to the SAP group. The trial was completed by 125 (98.4%) participants; 1 participant in each group was dropped before the 3-month outcome and did not have any CGM data 3–13 weeks postrandomization to be included in analyses (Supplementary Fig. 2).
Mean ± SD age was 32 ± 15 years, median (IQR) diabetes duration was 18 (10, 27) years, and mean HbA1c was 7.4 ± 0.9% (57 ± 9.8 mmol/mol). The treatment groups appeared balanced on baseline characteristics (Table 1).
Table 1.
Characteristics of the study participants at enrolment or randomization
| CLC group (n = 65) | SAP group (n = 62) | |
|---|---|---|
| Age at randomization (years) | ||
| <21 | 18 (28) | 17 (27) |
| 21 to <35 | 19 (29) | 21 (34) |
| 35 to <65 | 25 (38) | 21 (34) |
| ≥65 | 3 (5) | 3 (5) |
| Mean ± SD | 33 ± 16 | 32 ± 14 |
| Range | 14–70 | 14–75 |
| Diabetes duration at randomization (years) | ||
| 1 to <5 | 10 (15) | 3 (5) |
| 5 to <10 | 10 (15) | 5 (8) |
| 10 to <20 | 13 (20) | 29 (47) |
| 20 to <30 | 18 (28) | 13 (21) |
| 30 to <40 | 6 (9) | 9 (15) |
| ≥40 | 8 (12) | 3 (5) |
| Median (IQR) | 19 (7, 27) | 16 (11, 27) |
| Range | 2–52 | 2–47 |
| Prior CGM use | ||
| Never | 1 (2) | 4 (6) |
| In past, but not current | 16 (25) | 14 (23) |
| Current | 48 (74) | 44 (71) |
| BMI at enrollment (kg/m2) | ||
| <18 | 2 (3) | 0 |
| 18 to <25 | 22 (34) | 29 (47) |
| 25 to <30 | 23 (35) | 22 (35) |
| ≥30 | 18 (28) | 11 (18) |
| Median (IQR) | 27 (24, 31) | 25 (23, 29) |
| Range | 17–54 | 19–41 |
| Female sex | 32 (49) | 28 (45) |
| Race | ||
| White | 55 (85) | 55 (89) |
| Black/African American | 1 (2) | 1 (2) |
| Asian | 3 (5) | 2 (3) |
| More than one race | 3 (5) | 4 (6) |
| Unknown/not reported | 3 (5) | 0 |
| Hispanic or Latino ethnicity | ||
| Yes | 6 (9) | 3 (5) |
| No | 59 (91) | 59 (95) |
| HbA1c at randomization: laboratory | ||
| <7.0% (53 mmol/mol) | 22 (34) | 21 (34) |
| 7.0% to <7.5% (53 to <58 mmol/mol) | 11 (17) | 12 (19) |
| 7.5% to <8.0% (58 to <64 mmol/mol) | 13 (20) | 15 (24) |
| 8.0% to ≤10.5% (64 to ≤91 mmol/mol) | 19 (29) | 14 (23) |
| Mean ± SD | ||
| % | 7.4 ± 0.9 | 7.4 ± 0.8 |
| mmol/mol | 57 ± 9.8 | 57 ± 8.7 |
| Range | ||
| % | 5.5–9.5 | 5.9–9.4 |
| mmol/mol | 37–80 | 41–79 |
| C-peptide at randomization (nmol/L): laboratory | ||
| <0.003 | 48 (74) | 45 (73) |
| 0.003 to <0.1 | 9 (14) | 13 (21) |
| 0.1 to <0.5 | 6 (9) | 4 (6) |
| ≥0.5 | 2 (3) | 0 |
| Maximum | 0.647 | 0.188 |
| Diabetic ketoacidosis events in past 12 months | ||
| 0 | 64 (98) | 61 (98) |
| 1 | 1 (2) | 0 |
| 2 | 0 | 1 (2) |
| Severe hypoglycemic events in past 12 months | ||
| 0 | 63 (97) | 58 (94) |
| 1 | 2 (3) | 4 (6) |
Data are n (%) unless otherwise indicated.
In the CLC group, the median (IQR) percentage of time the system was in active closed-loop mode was 69% (50%, 80%). The median (IQR) proportion of CGM use during the study was 91% (83%, 95%) in the CLC group and 94% (81%, 96%) in the SAP group. Study participants performed a median of three BG measurements per day in each treatment group. There were 31 unscheduled visits in the CLC group and 4 in the SAP group, with the CLC group visits principally related to device issues, device training, and study supplies. Among 19 CLC participants with at least one unscheduled visit, there was 1 participant with four visits, 3 participants with three visits, 3 participants with two visits, and 12 participants with one visit. There were 107 unscheduled phone call, text, or email contacts in the CLC group and 35 in the SAP group, with the CLC group contacts principally related to device issues, device training, and diabetes management.
Primary End Points
Mean ± SD percent time below 3.9 mmol/L (70 mg/dL) was 5.0 ± 4.2% at baseline and 2.4 ± 1.7% during follow-up in the CLC group vs. 4.7 ± 4.9% and 4.0 ± 3.4%, respectively, in the SAP group (risk-adjusted mean difference −1.7% [95% CI −2.4, −1.0]; P < 0.0001 for superiority). Mean percent above 10 mmol/L (180 mg/dL) was 40 ± 17% at baseline and 34 ± 11% during follow-up in the CLC group vs. 43 ± 18% and 39 ± 15%, respectively, in the SAP group (risk-adjusted mean difference −3.0% [−6.1, 0.1]; P < 0.0001 for the noninferiority limit of 5%) (Table 2 and Supplementary Fig. 3).
Table 2.
Primary end points
| Baseline (2 weeks) | Postrandomization (final 11 weeks) | |||||
|---|---|---|---|---|---|---|
| CLC (n = 63)† | SAP (n = 61)† | CLC (n = 64)† | SAP (n = 61)† | Difference (95% CI)‡ | P value‡ | |
| Hours of sensor data, median (IQR) | 304 (267, 323) | 311 (294, 322) | 1,656 (1,464, 1,781) | 1,683 (1,565, 1,780) | NA | NA |
| CGM-measured % below 70 mg/dL (3.9 mmol/L),¥ median (IQR) | 4.3 (1.6, 7.4) | 3.6 (1.7, 5.9) | 2.1 (1.2, 3.5) | 3.0 (1.7, 5.7) | ||
| Mean ± SD | 5.0 ± 4.2 | 4.7 ± 4.9 | 2.4 ± 1.7 | 4.0 ± 3.4 | −1.7 (−2.4, −1.0) | <0.0001 |
| CGM-measured % above 180 mg/dL (10 mmol/L),¥ mean ± SD | 40 ± 17 | 43 ± 18 | 34 ± 11 | 39 ± 15 | −3.0 (−6.1, 0.1) | <0.0001 NI* |
NA, not applicable; NI, noninferiority.
Excludes one participant from the CLC group and one participant from the SAP group who dropped out postrandomization and did not have any data 3–13 weeks postrandomization to be included in analyses. The baseline CGM data for another CLC participant were unavailable, so their follow-up CGM data were included in the regression models using the direct likelihood method (16).
Adjusted for baseline values, age, prior CGM use, and clinical center (random effects).
Small imbalances by chance were observed between the two treatment groups at randomization for BMI and type 1 diabetes duration (see Table 1). Results for both primary outcomes were similar after additionally adjusting for baseline BMI and duration (data not shown).
P value for NI (prespecified NI limit = 5%).
Secondary End Points
Secondary hypoglycemic end points were lower in the CLC group compared with the SAP group. The mean proportion of time when sensor glucose was in the target range of 3.9–10 mmol/L (70–180 mg/dL) was 64 ± 11% in the CLC group vs. 57 ± 14% in the SAP group (P = 0.0074). Treatment group differences in mean glucose, time in range of 3.9–7.8 mmol/L (70–140 mg/dL), and glucose coefficient of variation were small and not statistically significant (Table 3 and Supplementary Fig. 3).
Table 3.
Secondary CGM-measured glucose end points
| Baseline (2 weeks) | Postrandomization (final 11 weeks) | |||||
|---|---|---|---|---|---|---|
| CLC (n = 63)† | SAP (n = 61)† | CLC (n = 64)† | SAP (n = 61)† | Risk-adjusted difference (90% CI)‡ | P value‡ | |
| Hours of sensor data, median (IQR) | 304 (267, 323) | 311 (294, 322) | 1,656 (1,464, 1,781) | 1,683 (1,565, 1,780) | NA | NA |
| Hypoglycemia | ||||||
| CGM-measured % below 54 mg/dL (3.0 mmol/L), median (IQR) | 1.1 (0.3, 2.1) | 0.9 (0.2, 1.9) | 0.5 (0.2, 0.8) | 0.6 (0.2, 1.4) | ||
| Mean ± SD | 1.7 ± 2.0 | 1.6 ± 2.4 | 0.6 ± 0.6 | 1.1 ± 1.2 | −0.5 (−0.8, −0.2) | 0.0009 |
| CGM-measured % below 60 mg/dL (3.3 mmol/L), median (IQR) | 2.0 (0.6, 3.8) | 1.7 (0.6, 3.1) | 0.9 (0.4, 1.4) | 1.2 (0.5, 2.4) | ||
| Mean ± SD | 2.8 ± 2.8 | 2.6 ± 3.3 | 1.1 ± 1.0 | 1.9 ± 1.9 | −0.8 (−1.3, −0.4) | 0.0003 |
| CGM-measured LBGI, median (IQR) | 1.0 (0.6, 1.7) | 0.9 (0.4, 1.5) | 0.6 (0.4, 0.9) | 0.8 (0.5, 1.4) | ||
| Mean ± SD | 1.3 ± 1.0 | 1.2 ± 1.2 | 0.7 ± 0.4 | 1.0 ± 0.8 | −0.4 (−0.6, −0.2) | <0.0001 |
| CGM-measured hypoglycemic events,a median (IQR) | 5.9 (3.5, 9.2) | 5.5 (2.6, 8.3) | 4.2 (2.5, 6.3) | 4.8 (3.1, 8.2) | ||
| Mean ± SD | 6.5 ± 4.3 | 5.7 ± 4.0 | 4.5 ± 2.7 | 5.9 ± 4.0 | −1.9 (−2.9, −1.0) | <0.0001 |
| Overall control | ||||||
| Glucose (mg/dL), mean ± SD | 172 ± 30 | 177 ± 33 | 166 ± 19 | 170 ± 28 | −1.3 (−7.8, 5.2) | 0.82 |
| CGM-measured % in range 70–180 mg/dL (3.9–10 mmol/L), mean ± SD | 55 ± 16 | 53 ± 16 | 64 ± 11 | 57 ± 14 | 4.8 (1.4, 8.3) | 0.0074 |
| CGM-measured % in range 3.9–7.8 mmol/L (70–140 mg/dL), mean ± SD | 34 ± 14 | 32 ± 14 | 39 ± 10 | 35 ± 12 | 3.0 (0, 6.0) | 0.1047 |
| CGM-measured CV (%), median (IQR) | 39 (34, 44) | 39 (35, 43) | 37 (34, 41) | 38 (35, 42) | ||
| Mean ± SD | 39 ± 7 | 39 ± 7 | 37 ± 5 | 38 ± 5 | −1.0 (−2.5, 0.5) | 0.67 |
| Hyperglycemia | ||||||
| CGM-measured % above 250 mg/dL (13.9 mmol/L), median (IQR) | 13.5 (5.0, 23.7) | 13.9 (6.8, 26.6) | 10.9 (5.3, 15.7) | 11.3 (5.0, 17.3) | ||
| Mean ± SD | 15.4 ± 11.8 | 17.5 ± 12.8 | 11.2 ± 7.4 | 13.7 ± 11.4 | −1.2 (−4.0, 1.6) | 0.82 |
| CGM-measured % above 300 mg/dL (16.7 mmol/L), median (IQR) | 4.4 (1.3, 12.3) | 5.7 (1.5, 12.0) | 3.4 (1.5, 6.1) | 3.2 (1.1, 6.9) | ||
| Mean ± SD | 6.8 ± 6.6 | 8.0 ± 8.2 | 4.4 ± 4.2 | 5.7 ± 7.1 | −0.4 (−2.1, 1.3) | 0.82 |
| CGM-measured HBGI, median (IQR) | 9.1 (5.6, 13.5) | 9.6 (6.6, 14.0) | 8.2 (5.9, 9.9) | 8.6 (6.1, 10.6) | ||
| Mean ± SD | 9.9 ± 5.0 | 10.8 ± 5.6 | 8.2 ± 3.3 | 9.3 ± 4.9 | −0.6 (−1.7, 0.6) | 0.82 |
CV, coefficient of variation; HBGI, high BG index; NA, not applicable.
Excludes one participant from the CLC group and one participant from the SAP group who dropped out postrandomization and did not have any data 3–13 weeks postrandomization to be included in analyses. The baseline CGM data for another CLC participant were unavailable, so their follow-up CGM data were included in both models using the direct likelihood method.
Adjusted for baseline values, age, prior CGM use, and clinical center (random effects). P values and CIs adjusted for multiplicity using the false discovery rate, with 0.10 as the threshold for statistical significance.
At least 15 consecutive min <70 mg/dL.
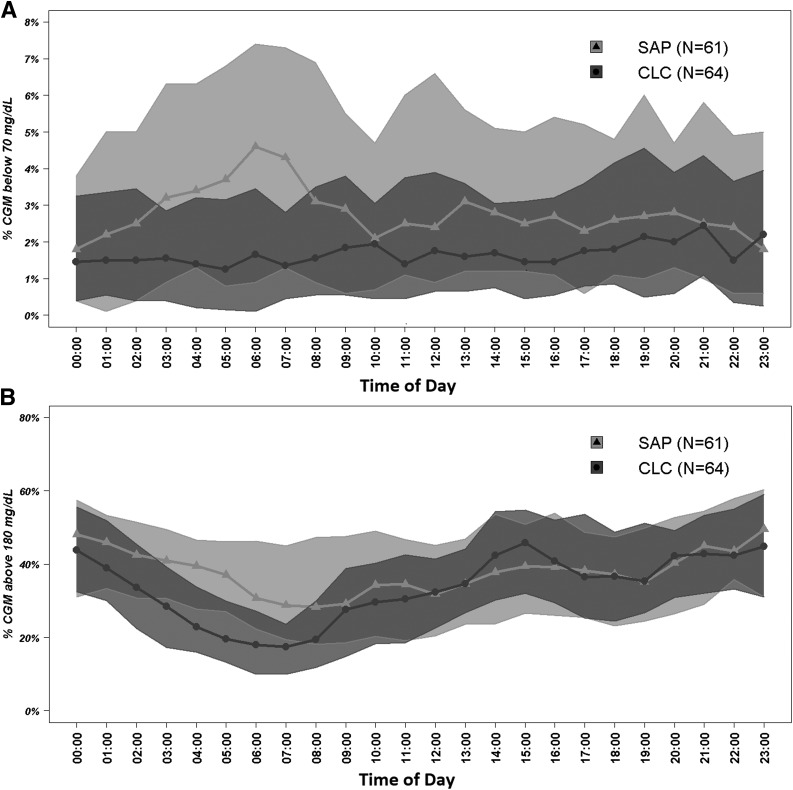
Figure 1 presents daily profiles of CGM-based times in range, which show that the benefits of CLC were particularly evident during nighttime. During self-reported sleep periods, outcomes improved for the CLC group compared with the SAP group for time below 3.9 mmol/L (70 mg/dL) (mean difference −2.3%; P < 0.0001), time in range 3.9–10 mmol/L (70–180 mg/dL) (+9.2%; P < 0.0001), time in range 3.9–7.8 mmol/L (70–140 mg/dL) (+6.6%; P = 0.0002), and time above 10 mmol/L (180 mg/dL) (−6.9%; P = 0.0015 superiority) (Supplementary Table 2). Corresponding improvements were less pronounced or not present during self-reported awake periods for time below 3.9 mmol/L (70 mg/dL) (−1.4%; P = 0.0003), time in range 3.9–10 mmol/L (70–180 mg/dL) (+2.5%; P = 0.58), time above 10 mmol/L (180 mg/dL) (−1.0%; P = 0.82), and other glucose metrics (Supplementary Table 3).
Figure 1.
Postrandomization hourly proportion of time when sensor glucose was below 70 mg/dL (A) and above 180 mg/dL (B).
Mean ± SD HbA1c was similar in both treatment groups at 13 weeks: 7.2 ± 0.7% (55 ± 7.7 mmol/mol) in the CLC group vs. 7.2 ± 0.9% (55 ± 9.8 mmol/mol) in the SAP group (P = 0.86) (Supplementary Table 4). No significant differences were detected for insulin requirements or body weight at 13 weeks (Supplementary Table 5). Prespecified subgroup analyses (Supplementary Table 6) suggested a greater reduction in CLC for time below 3.9 mmol/L (70 mg/dL) among participants with low baseline C-peptide (P value for interaction = 0.0601) and high baseline time below 3.9 mmol/L (70 mg/dL) (P = 0.0406). For time above 10 mmol/L (180 mg/dL), the benefit from CLC appeared to be limited to females (34% vs. 45%), with no observed benefit for males (34% vs. 34%; P value for interaction between treatment and sex = 0.0181). The latter is consistent with the higher compliance observed in females than males (74% vs. 64% median system use).
Adverse Events
During the randomized trial, one severe hypoglycemia event occurred in the CLC group (unrelated to the study device) and none in the SAP group. Three other transient adverse events unrelated to the study device occurred in the CLC group (skin surgery, gastrointestinal disorder, and dehydration during an ultramarathon). There were no episodes of diabetic ketoacidosis; however, there were 14 days in the CLC group and 4 days in the SAP group on which participants observed ketone readings >1.0 mmol/L.
Conclusions
Mobile closed-loop control can be an appealing alternative to artificial pancreas systems with control algorithms embedded in the insulin pump, offering certain potential benefits such as more elaborate user interface, portability across devices, and improved user experience. However, one outcome of this study suggests that reaching such a conclusion would require further improvement in between-device connectivity: in the experimental group, the CLC system was active only 69% of the time, and the disruptions were primarily due to lost wireless signal. Nevertheless, with an intention-to-treat analysis, this study achieved its predefined primary outcomes, showing the superiority of a mobile closed-loop system over SAP therapy in terms of reduced risk for hypoglycemia, accompanied by noninferiority in terms of exposure to hyperglycemia. Thus, mobile CLC is a feasible and promising approach, especially with a more refined system that would ensure better device connectivity.
Systematic reviews of artificial pancreas field research place this study in context (17) and provide meta-analyses of the results from CLC trials to date, which confirm that the findings of this study are consistent with the expectations for a closed-loop system: improved time in a target range and reduced risk/exposure to hypo- and hyperglycemia (18,19). Other trials comparing mobile CLC to SAP and using different algorithms included a 12-week trial reporting 10.8% improvement in time within 3.9–10.0 mmol/L and 0.8% improvement in time below 3.9 mmol/L (12) and a 12-week trial reporting 9.2% improvement in time within 3.9–10.0 mmol/L but with five severe hypoglycemic episodes occurring in the closed-loop treatment group (13). In this context, we refer to our report of a 6-month multicenter randomized trial, which enrolled 168 participants to test Control-IQ, a system using the same inControl algorithm presented here, embedded in the insulin pump. This trial resulted in 11% improvement in time within 3.9–10.0 mmol/L and 0.9% improvement in time below 3.9 mmol/L, without any severe hypoglycemic episodes (5), a result that underscores the importance of the system form factor and connectivity for the quality of glycemic outcomes.
The use of smartphones took CLC to various outpatient environments (7–10,20,21), but the prevailing opinion in the CLC field is that the real clinical application of CLC would only be possible if the control algorithm were embedded in the insulin pump—the path taken by contemporary commercial systems. Our thesis is that various algorithmic approaches and system configurations can coexist, and it is therefore imperative to test a variety of options offering different functionalities. The study reported here was designed to test a mobile CLC system, inControl AP, in the largest multicenter randomized clinical trial to date. The primary objective was to compare the glycemic optimization achieved by mobile CLC to the control achieved by SAP therapy. Treatment optimization was understood in its classical sense: reduction of the risk for hypoglycemia without increasing the exposure to hyperglycemia (1). Consistent with this notion, the study achieved its predefined primary outcomes, affecting favorably both the CGM-measured time spent below 3.9 mmol/L (70 mg/dL) and the CGM-measured time above 10 mmol/L (180 mg/dL) (Table 2). Several CGM-based secondary outcomes, primarily those related to hypoglycemia, improved as well (e.g., times below 3 mmol/L [54 mg/dL]) and so did the overall risk for hypoglycemia as reflected by the LBGI (Table 3). The latter is consistent with the engineering design of this particular control algorithm (i.e., with the action of its safety module dedicated to prevention of hypoglycemia [14]). The time in range, traditionally assessed by percent CGM readings within 3.9–10 mmol/L (70–180 mg/dL), improved significantly as well, which could be of clinical importance in the near future when metrics such as time in range become benchmarks for guiding clinical practice. The changes in metrics of average glycemia, however, did not differ significantly between the CLC and SAP groups; in both groups, HbA1c went slightly down, as did average CGM glucose (Table 3 and Supplementary Table 4). This effect was consistent with the study design and objectives: reduction of hypoglycemia without increase in hyperglycemia.
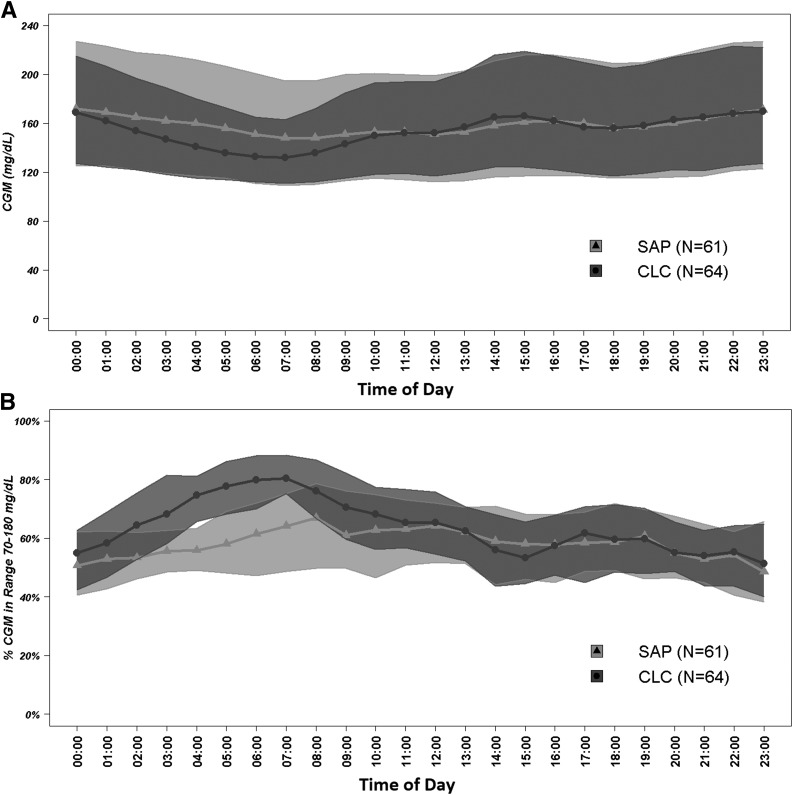
As evident from Fig. 2 and confirmed by comparing Supplementary Tables 2 and 3, the difference between CLC and SAP therapies was most prominent overnight, which is also consistent with the engineering design of the control algorithm: a gradual intensification of basal rate modulation overnight with the goal of achieving near-normoglycemia every morning. This effect is particularly well depicted by Fig. 2B. Finally, during the randomized trial, there were more days with high ketones (14 vs. 4) in the treatment group than in the control group. Because the overall rate of hyperglycemia was lower on closed-loop treatment, we speculate that this may reflect differential event reporting between the groups.
Figure 2.
Postrandomization hourly mean sensor glucose (A) and proportion of time when sensor glucose was between 70 and 180 mg/dL (B).
In meeting its coprimary end points, the study has demonstrated that automated insulin delivery by a closed-loop system can be a feasible treatment for type 1 diabetes, superior to contemporary sensor and pump therapy, with glycemic control benefits that are particularly evident overnight. However, the form factor of the closed-loop system, mobile or embedded, may influence system reliability and usability. Our results suggest that mobile closed-loop systems are likely to be viable options in the future, provided that system connectivity can be reliably achieved.
Supplementary Material
Article Information
Funding and Duality of Interest. Funding was provided by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant UC4-DK-108483. Material support was provided by TypeZero Technologies (CLC and Cloud monitoring systems), Dexcom (CGM sensors), Roche Diabetes Care (insulin pumps), and Ascensia Diabetes Care (blood glucose meters and test strips). B.K. reports patents and patent applications related to diabetes technology managed by the University of Virginia Licensing and Ventures group; research support managed by the University of Virginia from Dexcom, Roche Diagnostics, Sanofi, and Tandem Diabetes Care; and speaking engagement, advisory panel, and consultant fees from Dexcom, Sanofi, and Tandem Diabetes Care. During this study, B.K. was a shareholder in TypeZero Technologies; this relationship expired in August 2018. S.M.A. reports research support from Medtronic. Y.C.K. reports grants from NIDDK during the conduct of the study and nonfinancial support from Dexcom, Tandem Diabetes Care, and Roche Diabetes Care and other support from Medtronic Diabetes outside the submitted work. In addition, Y.C.K. has a patent issued on “Estimation of insulin sensitivity from CGM and subcutaneous insulin delivery in type 1 diabetes.” L.M.L. reports grants from NIH, NIDDK, during the conduct of the study and personal fees from Eli Lilly, Novo Nordisk, Sanofi, Merck, AstraZeneca, Boehringer Ingelheim, Roche, Insulet, Dexcom, Convatec, and Insulinogic outside the submitted work. C.L. reports grants from Abbott Diabetes, grants from Senseonics paid to her institution, nonfinancial support from Dexcom, service as a consultant for Sanofi, and other support from Novo Nordisk outside the submitted work. J.E.P. reports grants and personal fees from Tandem Diabetes Care, grants from Medtronic and Eli Lilly, grants and nonfinancial support from Insulet, and nonfinancial support from Dexcom outside the submitted work. R.P.W. reports grants and personal fees from Dexcom and grants from Tandem Diabetes Care outside the submitted work. B.B. reports personal fees and other support from Tandem Diabetes Care during the conduct of the study; grants, personal fees, and nonfinancial support from Medtronic Diabetes; grants and personal fees from Convatec and Insulet; and nonfinancial support from Dexcom. In addition, B.B. has a patent pending on a hypoglycemia prediction algorithm. F.J.D. reports grants from NIH; personal fees, nonfinancial support, and other support from Roche; and nonfinancial and other support from Dexcom during the conduct of the study. He also reports grants from NIH, JDRF, and the Helmsley Charitable Trust; nonfinancial and other support from Tandem Diabetes Care, Insulet, LifeScan, Dexcom, and Xeris; and personal fees, nonfinancial support, and other support from Roche outside the submitted work. In addition, F.J.D. has U.S. Patents 9,984,773, 9,907,515, 9,700,708, and 8,762,070 with royalties paid; U.S. Provisional Patent Application 14/734,994 pending; and U.S. Provisional Patent Applications 61/903,965, 61/751,942, and 61/751,941 with royalties paid. S.A.B. reports grants and nonfinancial support from Tandem Diabetes Care and nonfinancial support from Roche Diagnostics and Dexcom outside the submitted work. E.D. reports grants from NIH; personal fees, nonfinancial support, and other support from Roche; and nonfinancial and other support from Dexcom during the conduct of the study. He also reports grants from NIH, JDRF, and the Helmsley Charitable Trust; nonfinancial and other support from Tandem Diabetes Care, Lifescan, Dexcom, and Xeris; personal fees, nonfinancial support, and other support from Insulet and Roche; and personal fees from Eli Lily outside the submitted work. In addition, E.D. has U.S. Patents 9,984,773, 9,907,515, 9,700,708, and 8,762,070 with royalties paid; U.S. Provisional Patent Application 14/734,994 pending; and U.S. Provisional Patent Applications 61/903,965, 61/751,942, and 61/751,941 with royalties paid. G.P.F. reports grants and personal fees from Medtronic, Dexcom, Abbott, Tandem Diabetes Care, and Insulet outside the submitted work. D.W.L. reports grants from Senseonics Eversense outside the submitted work. J.L. reports grants from NIDDK, JDRF, and the Helmsley Charitable Trust during the conduct of the study and other support from Animas Corporation, Bigfoot Biomedical, Tandem Diabetes Care, and Eli Lilly outside the submitted work. R.W.B. reports grants from NIH and nonfinancial support from Dexcom, Roche, and Ascensia during the conduct of the study and other support from Insulet, Bigfoot Biomedical, and Eli Lilly and grants and nonfinancial support from Tandem Diabetes Care and Dexcom outside the submitted work. No other potential conflicts of interest relevant to this article were reported.
Funding and support sources did not have any involvement in the study.
Author Contributions. B.K. was involved in the study design, sponsored the U.S. Food and Drug Administration investigational device exemption application, and wrote the first draft of the manuscript. S.M.A. was the protocol chair and University of Virginia site principal investigator, contributed to the study design, answered queries from the data safety and monitoring board and Food and Drug Administration, and was involved in writing and editing the manuscript. D.R. performed statistical analyses and wrote and edited the manuscript. Y.C.K., L.M.L., C.L., J.E.P., R.P.W., B.B., F.J.D., S.A.B., M.M.C., V.D., E.D., L.E., G.P.F., E.I., D.W.L., J.L., and R.W.B. researched data, contributed to the discussion, and reviewed and edited the manuscript. J.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT02985866, clinicaltrials.gov.
This article contains Supplementary Data online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-1310/-/DC1.
A complete list of the iDCL Study Group can be found online in the Supplementary Data.
B.K. and S.M.A. share equal authorship.
Contributor Information
Collaborators: iDCL Study Group, Boris Kovatchev, Stacey M. Anderson, Sue A. Brown, Emma Emory, Mary Voelmle, Katie Conshafter, Kim Morris, Mary Oliveri, Harry Mitchell, Kayla Calvo, Christian Wakeman, Marc Breton, Lori M. Laffel, Elvira Isganaitis, Louise Ambler-Osborn, Emily Flint, Alan Schultz, Kenny Kim, Jordan E. Pinsker, Mei Mei Church, Camille Andre, Carol Levy, David W. Lam, Grenye O’Malley, Camilla Levister, Selassie Ogyaadu, Yogish C. Kudva, Vikash Dadlani, Vinaya Simha, Shelly McCrady-Spitzer, Corey Reid, R. Paul Wadwa, Gregory P. Forlenza, Emily Jost, Laurel Messer, Cari Berget, Lindsey Towers, Bruce Buckingham, Laya Ekhlaspour, Liana Hsu, Sarah Loebner, Francis J. Doyle, III, Eyal Dassau, John Lum, Roy W. Beck, Tiffany Campos, Samantha Passman, Carlos Murphy, Nandan Patibandla, Dan Raghinaru, and Craig Kollman
References
- 1.Cryer PE. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes 2014;63:2188–2195 [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 3.Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN; DCCT/EDIC Research Group . Effect of glycemic exposure on the risk of microvascular complications in the Diabetes Control and Complications Trial--revisited. Diabetes 2008;57:995–1001 [DOI] [PubMed] [Google Scholar]
- 4.Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 [DOI] [PubMed] [Google Scholar]
- 5.Brown SA, Kovatchev BP, Raghinaru D, et al.; iDCL Trial Research Group . Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keith-Hynes P, Guerlain S, Mize B, et al. DiAs user interface: a patient-centric interface for mobile artificial pancreas systems. J Diabetes Sci Technol 2013;7:1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cobelli C, Renard E, Kovatchev BP, et al. Pilot studies of wearable outpatient artificial pancreas in type 1 diabetes. Diabetes Care 2012;35:e65–e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson SM, Raghinaru D, Pinsker JE, et al.; Control to Range Study Group . Multinational home use of closed-loop control is safe and effective. Diabetes Care 2016;39:1143–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breton MD, Cherñavvsky DR, Forlenza GP, et al. Closed-loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artificial pancreas ski study. Diabetes Care 2017;40:1644–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovatchev B, Cheng P, Anderson SM, et al. Feasibility of long-term closed-loop control: a multicenter 6-month trial of 24/7 automated insulin delivery. Diabetes Technol Ther 2017;19:18–24 [DOI] [PubMed] [Google Scholar]
- 11.Deshpande S, Pinsker JE, Zavitsanou S, et al. Design and clinical evaluation of the Interoperable Artificial Pancreas System (iAPS) smartphone app: interoperable components with modular design for progressive artificial pancreas research and development. Diabetes Technol Ther 2019;21:35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tauschmann M, Thabit H, Bally L, et al.; APCam11 Consortium . Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 2018;392:1321–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benhamou PY, Franc S, Reznik Y, et al. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digital Health 2019;1:e17–e25 [DOI] [PubMed] [Google Scholar]
- 14.Patek SD, Magni L, Dassau E, et al.; International Artificial Pancreas (iAP) Study Group . Modular closed-loop control of diabetes. IEEE Trans Biomed Eng 2012;59:2986–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovatchev BP. Metrics for glycaemic control - from HbA1c to continuous glucose monitoring. Nat Rev Endocrinol 2017;13:425–436 [DOI] [PubMed] [Google Scholar]
- 16.Beunckens C, Molenberghs G, Kenward MG. Direct likelihood analysis versus simple forms of imputation for missing data in randomized clinical trials. Clin Trials 2005;2:379–386 [DOI] [PubMed] [Google Scholar]
- 17.Kovatchev B. Automated closed-loop control of diabetes: the artificial pancreas. Bioelectron Med 2018;4:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ 2018;361:k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:501–512 [DOI] [PubMed] [Google Scholar]
- 20.Del Favero S, Boscari F, Messori M, et al. Randomized summer camp crossover trial in 5- to 9-year-old children: outpatient wearable artificial pancreas is feasible and safe. Diabetes Care 2016;39:1180–1185 [DOI] [PubMed] [Google Scholar]
- 21.Renard E, Farret A, Kropff J, et al.; AP@home Consortium . Day-and-night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: results of a single-arm 1-month experience compared with a previously reported feasibility study of evening and night at home. Diabetes Care 2016;39:1151–1160 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.